Structure–Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanomaterials and Physicochemical Characterization
2.2. Toxicity Assessment
2.2.1. Cell Culture
2.2.2. Cell Exposure to Nanomaterials
- Vortex TopMix FB15024 Fisher Scientific (Hampton, VA, USA)—Mode: continuous—Frequency: 40 Hz—Room temperature—Time: 60 s.
- Ultrasonicate Bath Fisher Scientific Bioblock (Hampton, VA, USA)—Temperature: 20 °C—Frequency: 130 Hz—Power: 100%—Time: 15 min.
- Branson S-450 Sonicator, without probe (Emerson, Saint Louis, MO, USA)—Program: 2 s pulse + 2 s inter—70% for 10 min then 85% for 5 min.
2.2.3. Cytotoxicity
2.2.4. Pro-Inflammatory Response
2.2.5. ROS Production
2.2.6. FRAS Measurement
2.2.7. Toxicity Classification
- No toxicity: no significant response compared to negative control even at the highest dose (120 µg/mL).
- High LOAEL (Lowest Observed Adverse Effect Level): significant response compared to negative control when the exposure dose is high: 120 µg/mL or 60 µg/mL. Please note that for simplification purposes, this class is referred to as “moderate toxicity”.
- Low LOAEL: significant response compared to negative control when the exposure dose is low: 15 µg/mL or 30 µg/mL. Please note that for simplification purposes, this class is referred to as “high toxicity”.
- Low FRAS effect was declared when the response at the lowest exposure dose (5 g/L) did not exceed 30 mMTEU.
- Moderate FRAS effect was declared when the response at the lowest exposure dose (5 g/L) was between 30 and 60 mMTEU.
- High FRAS effect was declared when the response at the lower exposure (5 g/L) exceeded 60 mMTEU.
3. Results
3.1. Physicochemical Characterization of the Nanomaterials
3.2. Toxicity Assessment
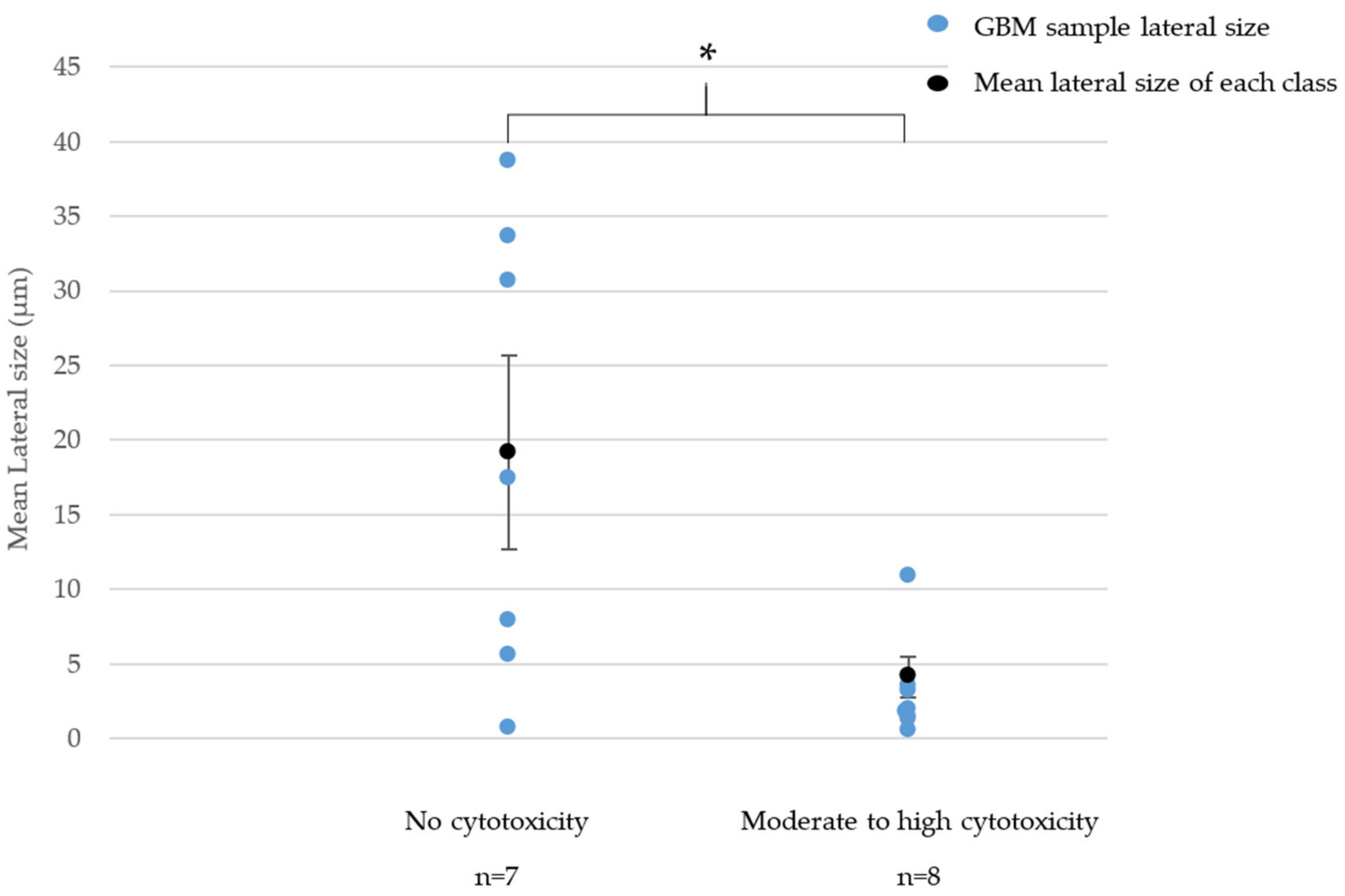
3.2.1. Cytotoxicity (LDH Release Assay)
3.2.2. Pro-Inflammatory Response (TNF-α ELISA Assay)
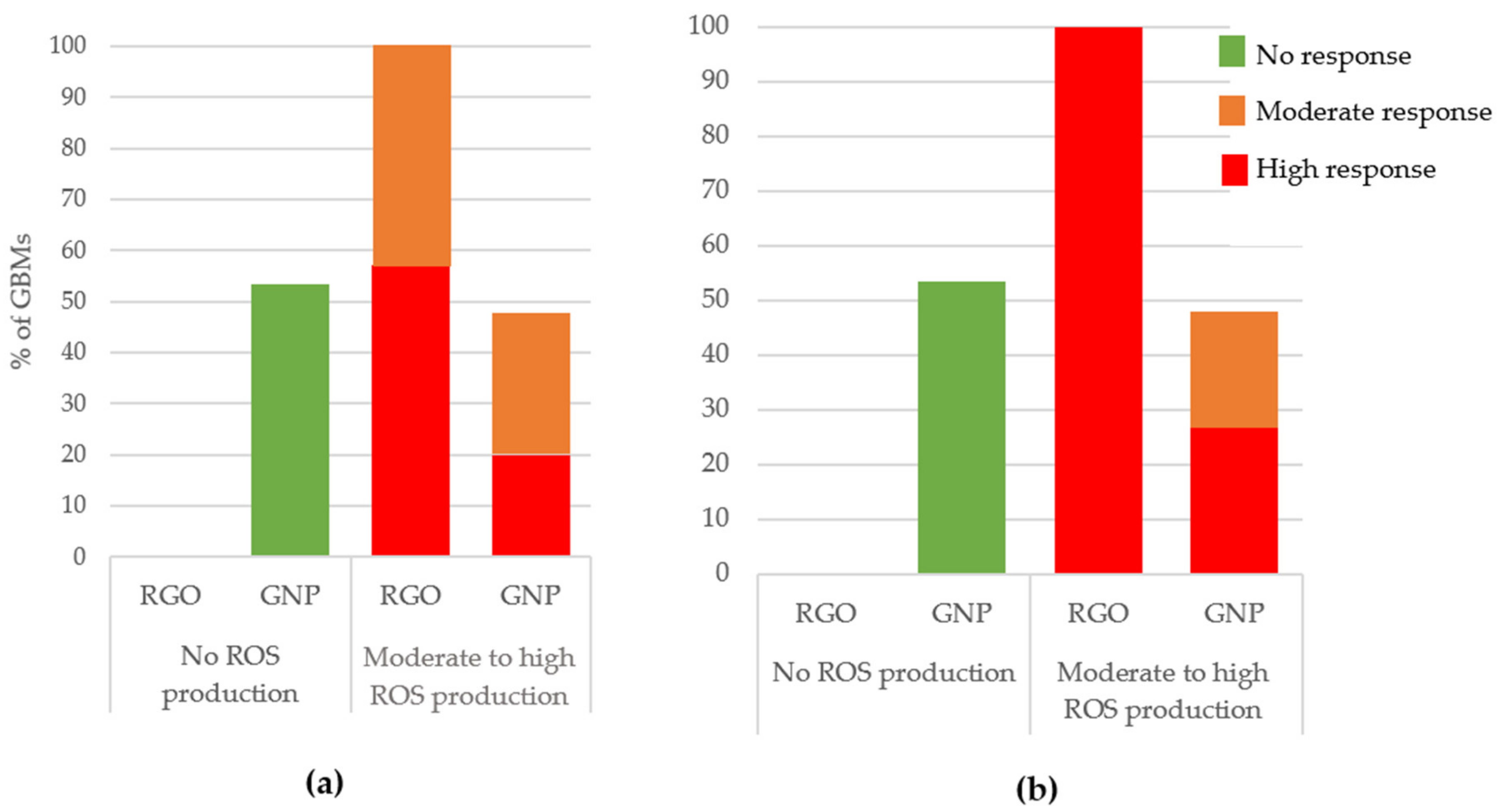
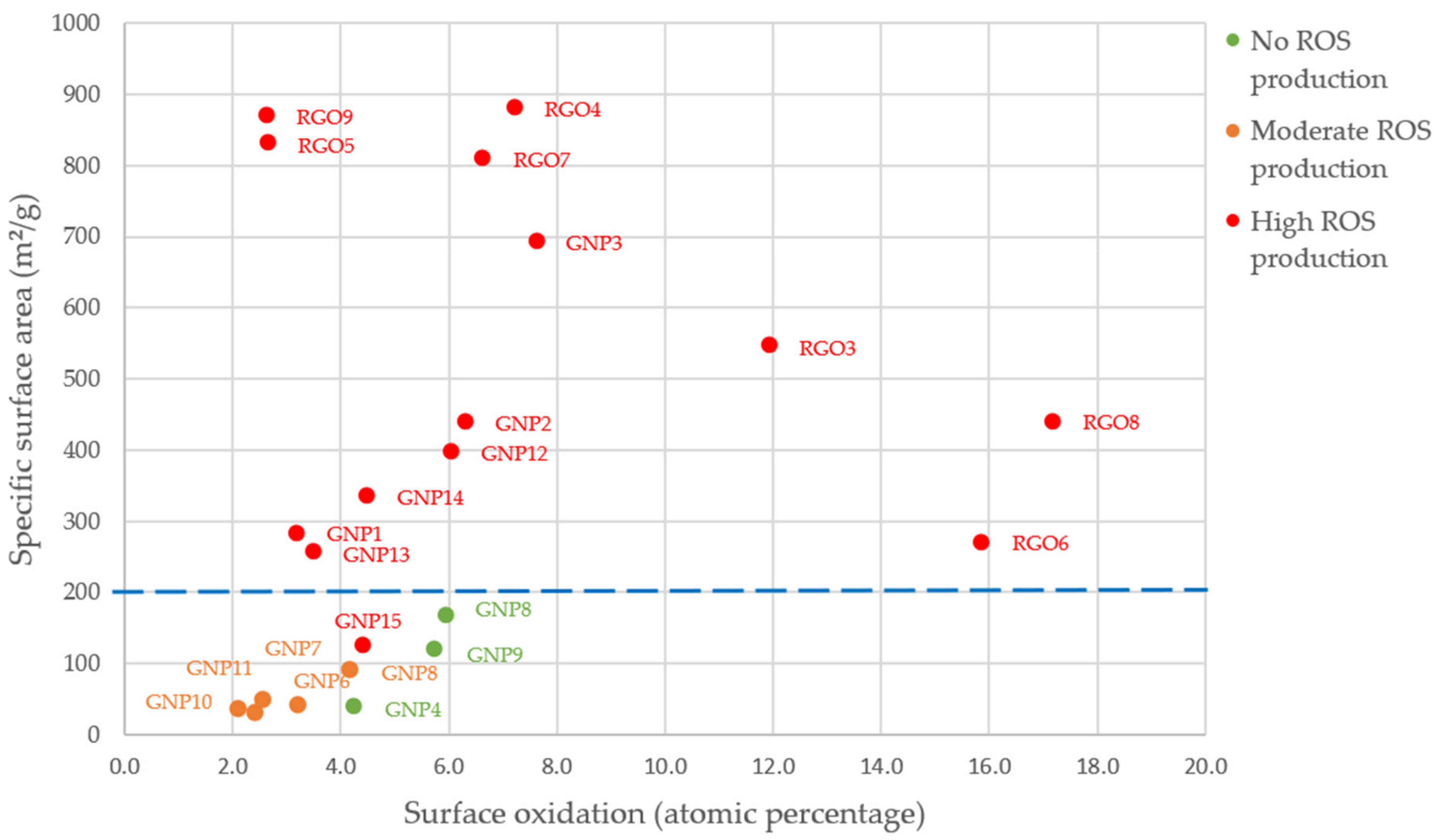
3.2.3. Oxidative Stress
ROS Production (DCFH-DA Assay)
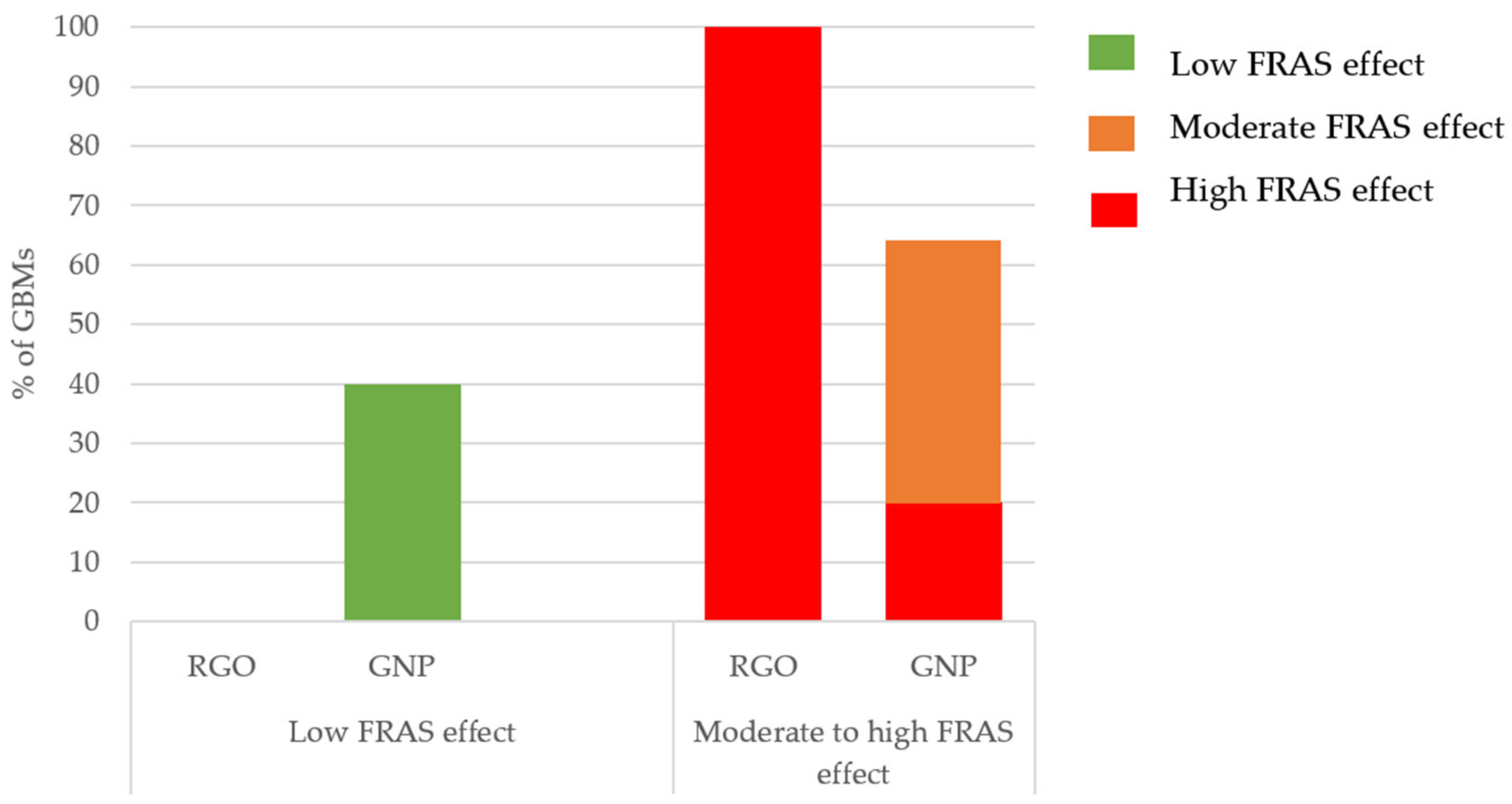
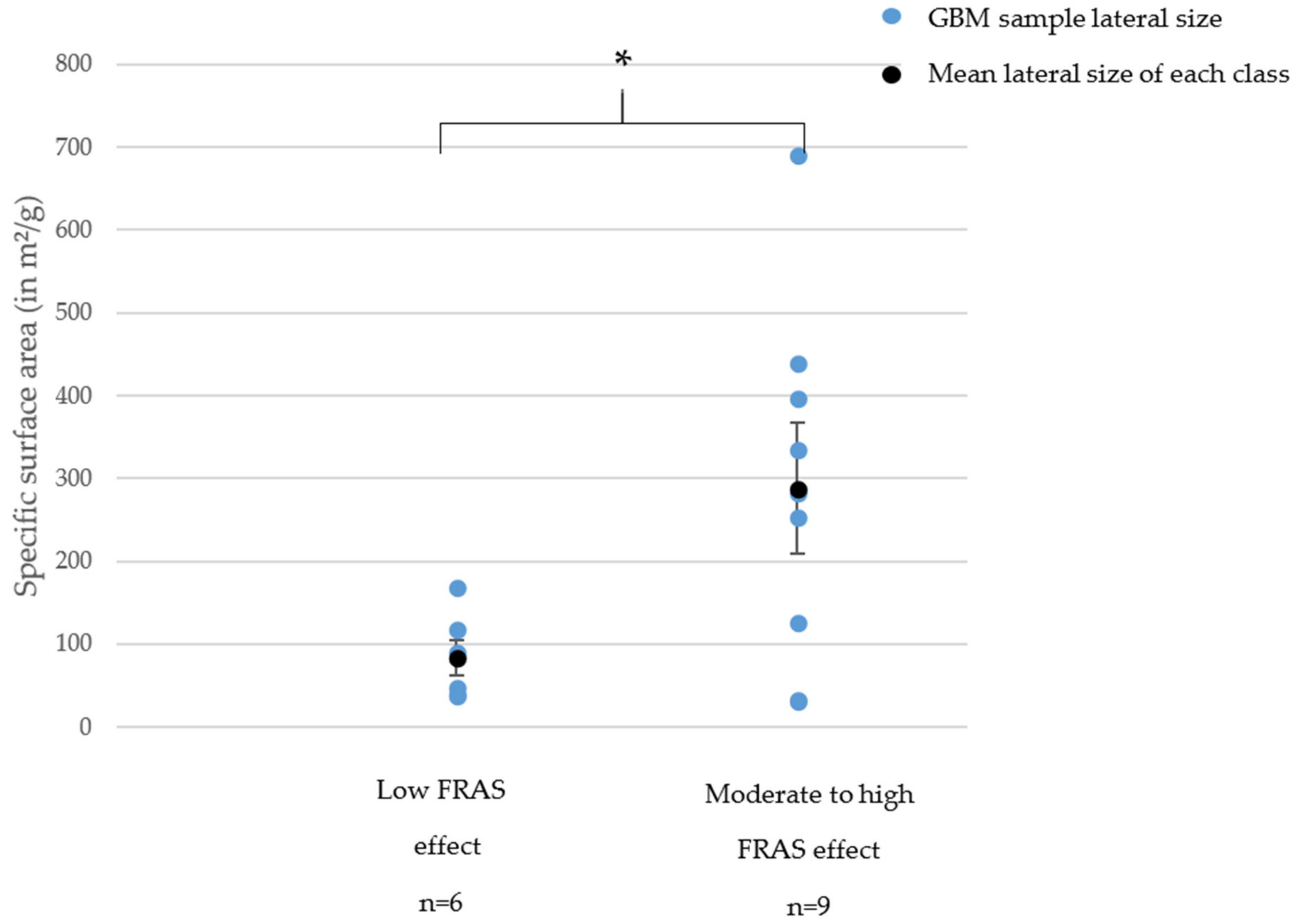
Acellular Biological Oxidative Damage (FRAS Assay)
4. Discussion
- RGOs and GNPs did not show the same toxicity: RGOs generally appeared to have higher toxicity impacts.
- For GNPs, the cytotoxicity significantly increased when the lateral size decreased.
- For GNPs, the oxidative stress (cellular or acellular) significantly increased when the specific surface area increased, we could note a threshold of 200 m²/g. Below this limit, the samples mostly showed no ROS production and only a low FRAS effect.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J.A. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Kleinstreuer, N.C.; Tetko, I.V.; Tong, W. Introduction to Special Issue: Computational Toxicology. Chem. Res. Toxicol. 2021, 34, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Inshakova, E.; Inshakov, O. World Market for Nanomaterials: Structure and Trends. In MATEC Web of Conferences, Proceedings of the International Conference on Modern Trends in Manufacturing Technologies and Equipment (ICMTMTE 2017), Sevastopol, Russia, 11–15 September 2017; EDP Sciences: Les Ulis, France, 2017; Volume 129, p. 02013. [Google Scholar] [CrossRef]
- Li, T.; Galli, G. Electronic properties of MoS 2 nanoparticles. J. Phys. Chem. C 2007, 111, 16192–16196. [Google Scholar] [CrossRef]
- Rechberger, W.; Hohenau, A.; Leitner, A.; Krenn, J.R.; Lamprecht, B.; Aussenegg, F.R. Optical properties of two interacting gold nanoparticles. Opt. Commun. 2003, 220, 137–141. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Huxford, R.C.; Lin, W. Hybrid nanomaterials for biomedical applications. Chem. Commun. 2010, 46, 5832–5849. [Google Scholar] [CrossRef]
- Bergamaschi, E. Occupational exposure to nanomaterials: Present knowledge and future development. Nanotoxicology 2009, 3, 194–201. [Google Scholar] [CrossRef]
- Schulte, P.; Geraci, C.; Zumwalde, R.; Hoover, M.; Kuempe, E. Occupational risk management of engineered nanoparticles. J. Occup. Environ. Hyg. 2008, 5, 239–249. [Google Scholar] [CrossRef]
- Oomen, A.G.; Steinhäuser, K.G.; Bleeker, E.A.J.; Van Broekhuizen, F.; Sips, A.; Dekkers, S.; Wijnhoven, S.W.P.; Sayre, P.G. Risk assessment frameworks for nanomaterials: Scope, link to regulations, applicability, and outline for future directions in view of needed increase in efficiency. NanoImpact 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Sewell, F.; Edwards, J.; Prior, H.; Robinson, S. Opportunities to apply the 3Rs in safety assessment programs. ILAR J. 2016, 57, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Wohlleben, W.; Hellack, B.; Nickel, C.; Herrchen, M.; Hund-Rinke, K.; Kettler, K.; Riebeling, C.; Haase, A.; Funk, B.; Kühnel, D.; et al. The nanoGRAVUR framework to group (nano)materials for their occupational, consumer, environmental risks based on a harmonized set of material properties, applied to 34 case studies. Nanoscale 2019, 11, 17637–17654. [Google Scholar] [CrossRef]
- Dekkers, S.; Oomen, A.G.; Bleeker, E.A.J.; Vandebriel, R.J.; Micheletti, C.; Cabellos, J.; Janer, G.; Fuentes, N.; Vázquez-Campos, S.; Borges, T.; et al. Towards a nanospecific approach for risk assessment. Regul. Toxicol. Pharmacol. 2016, 80, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Organisation for Economic Co-operation and Development. OECD Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)Sar] Models. Transport 2007, 2, 1–154. [Google Scholar]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. Biomed. Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 1–35. [Google Scholar] [CrossRef]
- Kraegeloh, A.; Suarez-Merino, B.; Sluijters, T.; Micheletti, C. Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach. Nanomaterials 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Schwarz-Plaschg, C.; Kallhoff, A.; Eisenberger, I. Making Nanomaterials Safer by Design? Nanoethics 2017, 11, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Lai, L.; Shen, Z.; Lin, J. Graphene and graphene-based composites as Li-ion battery electrode materials and their application in full cells. J. Mater. Chem. A 2017, 5, 15423–15446. [Google Scholar] [CrossRef]
- Li, G.; Huang, B.; Pan, Z.; Su, X.; Shao, Z.; An, L. Advances in three-dimensional graphene-based materials: Configurations, preparation and application in secondary metal (Li, Na, K, Mg, Al)-ion batteries. Energy Environ. Sci. 2019, 12, 2030–2053. [Google Scholar] [CrossRef]
- Naghibi, S.; Kargar, F.; Wright, D.; Huang, C.Y.T.; Mohammadzadeh, A.; Barani, Z.; Salgado, R.; Balandin, A.A. Noncuring Graphene Thermal Interface Materials for Advanced Electronics. Adv. Electron. Mater. 2020, 6, 1901303. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Y.; Tian, H.; Li, M.; Jian, J.; Wei, Y.; Tian, Y.; Wang, D.-Y.; Pang, Y.; Geng, X.; et al. Multilayer Graphene Epidermal Electronic Skin. ACS Nano 2018, 12, 8839–8846. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials Open Archive TOULOUSE Archive Ouverte (OATAO) Classification Framework for Graphene-Based Materials classification graphene nanotechnology structure-activity relationship toxicology. Angew. Chemie Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Take, S.; Tani, R.; Maru, J.; Obara, S.; Endoh, S. Assessment of cytotoxicity and mutagenicity of exfoliated graphene. Toxicol. Vitr. 2018, 52, 195–202. [Google Scholar] [CrossRef]
- De Marzi, L.; Ottaviano, L.; Perrozzi, F.; Nardone, M.; Santucci, S.; De Lapuente, J.; Borras, M.; Treossi, E.; Palermo, V.; Poma, A. Flake size-dependent cyto and genotoxic evaluation of graphene oxide on in vitro A549, CaCo2 and vero cell lines. J. Biol. Regul. Homeost. Agents 2014, 28, 281–289. [Google Scholar]
- Yan, J.; Chen, L.; Huang, C.C.; Lung, S.C.C.; Yang, L.; Wang, W.C.; Lin, P.H.; Suo, G.; Lin, C.H. Consecutive evaluation of graphene oxide and reduced graphene oxide nanoplatelets immunotoxicity on monocytes. Colloids Surf. B Biointerfaces 2017, 153, 300–309. [Google Scholar] [CrossRef]
- Tabish, T.A.; Narayan, R.J. Crossing the blood–brain barrier with graphene nanostructures. Mater. Today 2021, in press. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.J.; Gao, X.; Nie, G. Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef]
- Achawi, S.; Pourchez, J.; Feneon, B.; Forest, V. Graphene-Based Materials In vitro Toxicity and Their Structure–Activity Relationships: A Systematic Literature Review. Chem. Res. Toxicol. 2021, 34, 2003–2018. [Google Scholar] [CrossRef]
- Palomäki, J.; Sund, J.; Vippola, M.; Kinaret, P.; Greco, D.; Savolainen, K.; Puustinen, A.; Alenius, H. A secretomics analysis reveals major differences in the macrophage responses towards different types of carbon nanotubes. Nanotoxicology 2015, 9, 719–728. [Google Scholar] [CrossRef]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In Vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Piątek-Guziewicz, A.; Zagrodzki, P.; Paśko, P.; Krośniak, M.; Mach, T.; Zwolińska-Wcisło, M. Ferric reducing ability of plasma and assessment of selected plasma antioxidants in adults with celiac disease. Folia Med. Cracov. 2017, 4, 13–26. [Google Scholar]
- Gandon, A.; Werle, K.; Neubauer, N.; Wohlleben, W. Surface reactivity measurements as required for grouping and read-across: An advanced FRAS protocol. J. Phys. Conf. Ser. Inst. Phys. Publ. 2017, 838, 12033. [Google Scholar] [CrossRef] [Green Version]
- Achawi, S.; Feneon, B.; Pourchez, J.; Forest, V. Assessing biological oxidative damage induced by graphene-based materials: An asset for grouping approaches using the FRAS assay. Regul. Toxicol. Pharmacol. 2021, 127, 105067. [Google Scholar] [CrossRef] [PubMed]
- Fusco, L.; Garrido, M.; Martín, C.; Sosa, S.; Ponti, C.; Centeno, A.; Alonso, B.; Zurutuza, A.; Vázquez, E.; Tubaro, A.; et al. Skin irritation potential of graphene-based materials using a non-animal test. Nanoscale 2020, 12, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Pelin, M.; Sosa, S.; Prato, M.; Tubaro, A. Occupational exposure to graphene based nanomaterials: Risk assessment. Nanoscale 2018, 10, 15894–15903. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.; Kumar, V.; Dhiman, N.; Chauhan, L.K.S.; Pasricha, R.; Pandey, A.K. Physico-chemical properties based differential toxicity of graphene oxide/reduced graphene oxide in human lung cells mediated through oxidative stress. Sci. Rep. 2016, 6, 39548. [Google Scholar] [CrossRef]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.P.; Jiang, W.; Sun, B.; Hersam, M.C.; et al. Surface Oxidation of Graphene Oxide Determines Membrane Damage, Lipid Peroxidation, and Cytotoxicity in Macrophages in a Pulmonary Toxicity Model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.-P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.-B.; Cui, Z.-S.; Wei, D.-P.; Shi, H.-F.; Pei, D.-S. Nanotoxicity of different sizes of graphene (G) and graphene oxide (GO) in vitro and in vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef]
- Russier, J.; Treossi, E.; Scarsi, A.; Perrozzi, F.; Dumortier, H.; Ottaviano, L.; Meneghetti, M.; Palermo, V.; Bianco, A. Evidencing the mask effect of graphene oxide: A comparative study on primary human and murine phagocytic cells. Nanoscale 2013, 5, 11234–11247. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-inflammatory Responses in Cells and Animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef]
- Vranic, S.; Rodrigues, A.F.; Buggio, M.; Newman, L.; White, M.R.H.; Spiller, D.G.; Bussy, C.; Kostarelos, K. Live Imaging of Label-Free Graphene Oxide Reveals Critical Factors Causing Oxidative-Stress-Mediated Cellular Responses. ACS Nano 2018, 12, 1373–1389. [Google Scholar] [CrossRef] [Green Version]
- Dziewięcka, M.; Pawlyta, M.; Majchrzycki, Ł.; Balin, K.; Barteczko, S.; Czerkawska, M.; Augustyniak, M. The Structure-Properties-Cytotoxicity Interplay: A Crucial Pathway to Determining Graphene Oxide Biocompatibility. Int J. Mol. Sci. 2021, 22, 5401. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; Von Dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, J.; Wu, J.; Tong, C.; Zhang, T. Meta-analysis of cellular toxicity for graphene via data-mining the literature and machine learning. Sci. Total Environ. 2021, 793, 148532. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, G.H.; Han, B.S.; Lee, B.S.; Lee, S.; Cho, M.H.; Kim, J.H.; Kim, D.W. Toxic response of graphene nanoplatelets in vivo and in vitro. Arch. Toxicol. 2015, 89, 1557–1568. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87. [Google Scholar] [CrossRef]
- Tabish, T.A.; Pranjol, M.Z.I.; Jabeen, F.; Abdullah, T.; Latif, A.; Khalid, A.; Ali, M.; Hayat, H.; Winyard, P.G.; Whatmore, J.L.; et al. Investigation into the toxic effects of graphene nanopores on lung cancer cells and biological tissues. Appl. Mater. Today 2018, 12, 389–401. [Google Scholar] [CrossRef]
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 2016, 99, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Jo, M.S.; Kim, J.K.; Shin, J.H.; Baek, J.E.; Park, H.S.; An, H.J.; Lee, J.S.; Kim, B.W.; Kim, H.P.; et al. Short-term inhalation study of graphene oxide nanoplates. Nanotoxicology 2018, 12, 224–238. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Strauss, V.; Treumann, S.; Küttler, K.; Wohlleben, W.; Hofmann, T.; Gröters, S.; Wiench, K.; van Ravenzwaay, B.; Landsiedel, R. Comparative inhalation toxicity of multi-wall carbon nanotubes, graphene, graphite nanoplatelets and low surface carbon black. Part. Fibre Toxicol. 2013, 10, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.R.; Mercer, R.R.; Stefaniak, A.B.; Seehra, M.S.; Geddam, U.K.; Chaudhuri, I.S.; Kyrlidis, A.; Kodali, V.K.; Sager, T.; Kenyon, A.; et al. Evaluation of pulmonary and systemic toxicity following lung exposure to graphite nanoplates: A member of the graphene-based nanomaterial family. Part. Fibre Toxicol. 2016, 13, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef]
- Jarosz, A.; Skoda, M.; Dudek, I.; Szukiewicz, D. Oxidative Stress and Mitochondrial Activation as the Main Mechanisms Underlying Graphene Toxicity against Human Cancer Cells. Oxid. Med. Cell. Longev. 2016, 2016, 5851035. [Google Scholar] [CrossRef] [Green Version]
- Jaworski, S.; Strojny, B.; Sawosz, E.; Wierzbicki, M.; Grodzik, M.; Kutwin, M.; Daniluk, K.; Chwalibog, A. Degradation of mitochondria and oxidative stress as the main mechanism of toxicity of pristine graphene on U87 glioblastoma cells and tumors and HS-5 cells. Int. J. Mol. Sci. 2019, 20, 650. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, L.; Shi, X.; Wang, X.; Yang, Y.; Yang, K.; Liu, T.; Yang, G.; Liu, Z. Surface coating-dependent cytotoxicity and degradation of graphene derivatives: Towards the design of non-toxic, degradable nano-graphene. Small 2014, 10, 1544–1554. [Google Scholar] [CrossRef] [PubMed]



| Surface Oxidation (% O) | Mean Lateral Size (µm) | Surface Defects (ID/IG) | Specific Surface Area (m²/g) | |
|---|---|---|---|---|
| GNP1 | 3.2 | 1.25 | 0.369 | 283 |
| GNP2 | 6.3 | 0.66 | 0.470 | 439 |
| GNP3 | 7.6 | 0.53 | 0.724 | 692 |
| GNP4 | 4.3 | 3.56 | 0.340 | 38 |
| GNP5 | 3.3 | 5.58 | 0.062 | 41 |
| GNP6 | 2.6 | 7.91 | 0.146 | 48 |
| GNP7 | 4.2 | 10.86 | 0.101 | 89 |
| GNP8 | 5.9 | 17.34 | 0.634 | 168 |
| GNP9 | 5.7 | 38.57 | 0.068 | 119 |
| GNP10 | 2.1 | 33.54 | 0.132 | 34 |
| GNP11 | 2.5 | 30.70 | 0.225 | 31 |
| GNP12 | 6.1 | 1.63 | 0.348 | 396 |
| GNP13 | 4.4 | 3.16 | 0.645 | 125 |
| GNP14 | 4.5 | 1.51 | 0.346 | 335 |
| GNP15 | 3.5 | 2.02 | 0.321 | 255 |
| rGO3 | 11.9 | 8.26 | 1.038 | 545 |
| rGO4 | 7.2 | 31.56 | 0.937 | 880 |
| rGO5 | 2.7 | 6.99 | 1.066 | 830 |
| rGO6 | 15.9 | 32.01 | 0.905 | 270 |
| rGO7 | 6.7 | 15.1 | 0.957 | 810 |
| rGO8 | 17.2 | 1.04 | 0.908 | 440 |
| rGO9 | 2.6 | 1.11 | 1.066 | 870 |
| CB1 | 2.6 | 0.36 | NA | 112 |
| CB2 | 2.3 | 0.9 | NA | 85 |
| Amorphous Silica | 70.0 | 0.09 | NA | 160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achawi, S.; Feneon, B.; Pourchez, J.; Forest, V. Structure–Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity. Nanomaterials 2021, 11, 2963. https://doi.org/10.3390/nano11112963
Achawi S, Feneon B, Pourchez J, Forest V. Structure–Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity. Nanomaterials. 2021; 11(11):2963. https://doi.org/10.3390/nano11112963
Chicago/Turabian StyleAchawi, Salma, Bruno Feneon, Jérémie Pourchez, and Valérie Forest. 2021. "Structure–Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity" Nanomaterials 11, no. 11: 2963. https://doi.org/10.3390/nano11112963
APA StyleAchawi, S., Feneon, B., Pourchez, J., & Forest, V. (2021). Structure–Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity. Nanomaterials, 11(11), 2963. https://doi.org/10.3390/nano11112963






