Carbon-Based Nanomaterials Increase Reactivity of Primary Monocytes towards Various Bacteria and Modulate Their Differentiation into Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanomaterials
Biological Contamination
2.2. Monocyte Isolation and Culture
2.3. Monocyte Exposition to C-BNM
2.3.1. Oxidative Stress
2.3.2. Intracellular Localization of C-BNM
2.4. Monocyte Exposition to Bacteria
2.5. Cytokine Secretion
2.5.1. IL-6 and IL-10 Production
2.5.2. TNF-α Production
2.6. Evaluation of Phagocytosis
2.7. Monocyte Differentiation to Macrophages
2.8. Statistical Analysis
3. Results
3.1. Nanomaterials
3.2. Monocyte Exposition to C-BNM
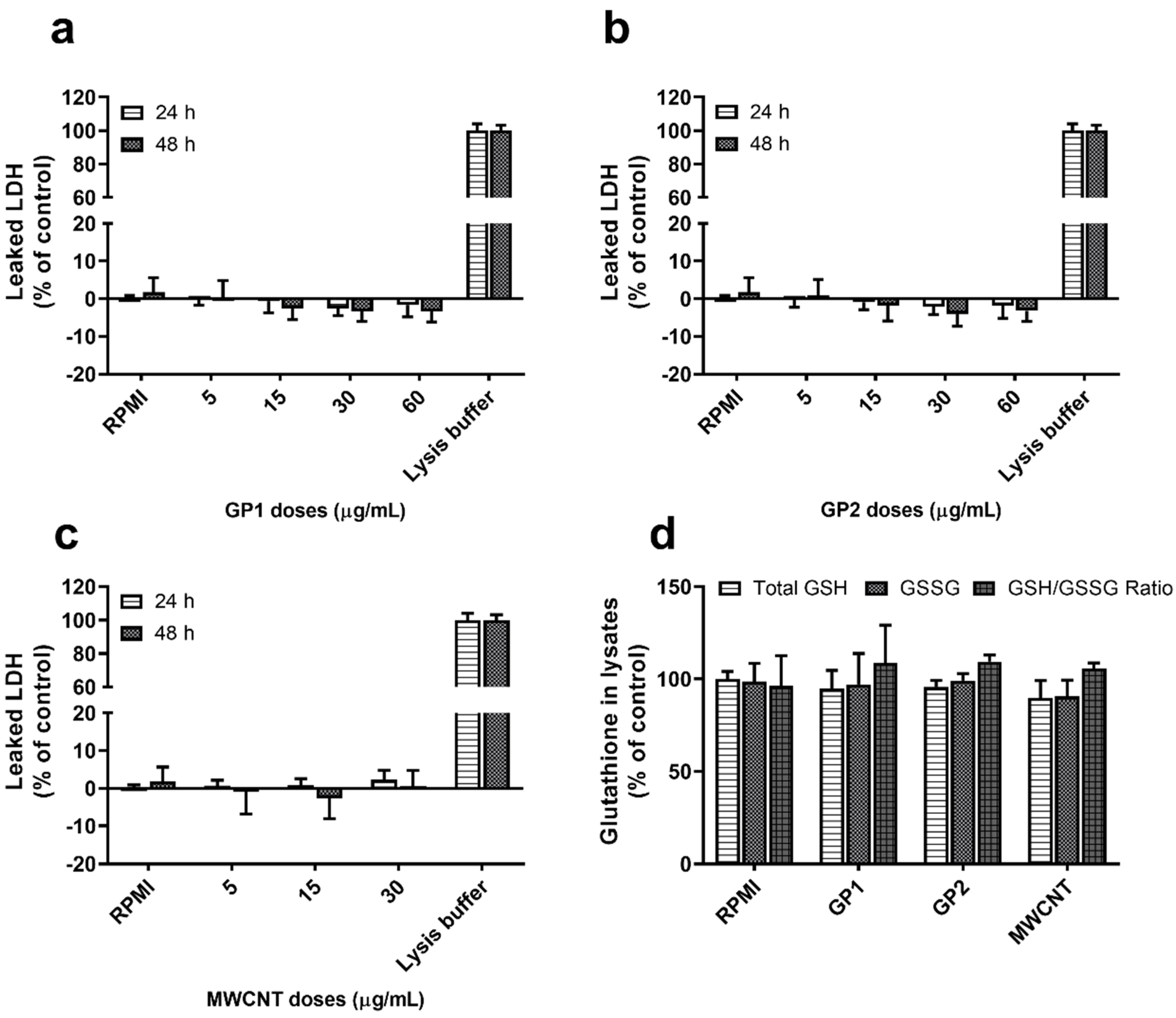
3.2.1. Viability
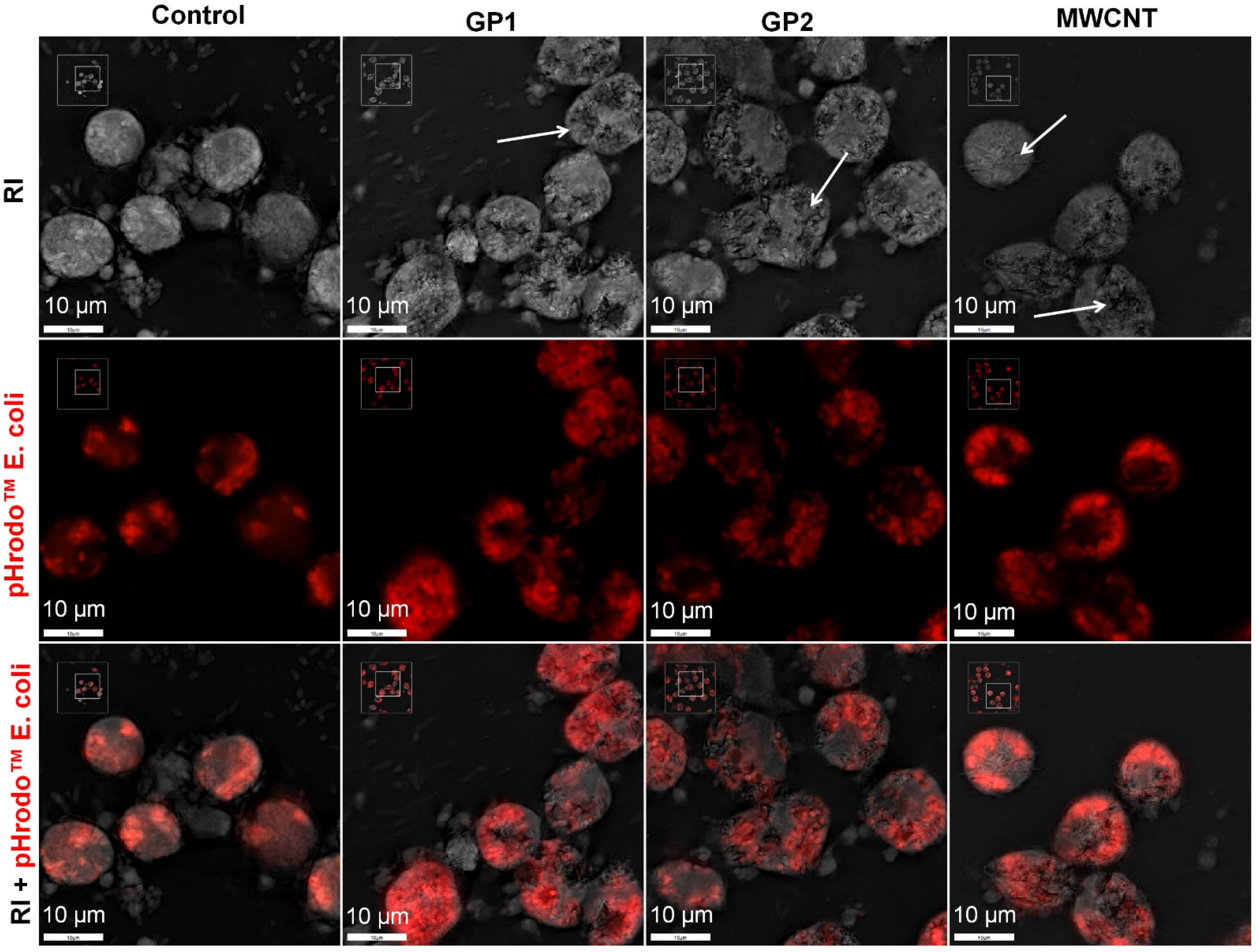
3.2.2. Intracellular Localization of C-BNM
3.3. Monocyte Exposition to Bacteria
3.3.1. Cytokine Production
3.3.2. Viability
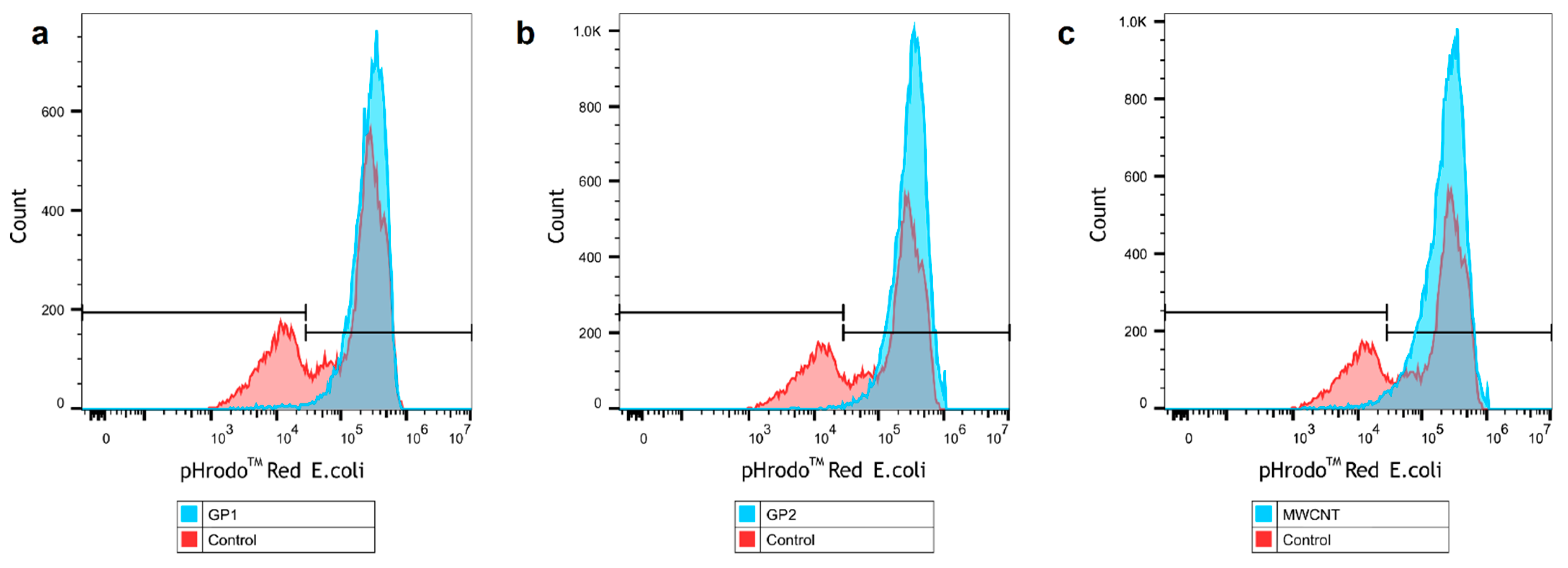
3.4. Evaluation of Phagocytosis
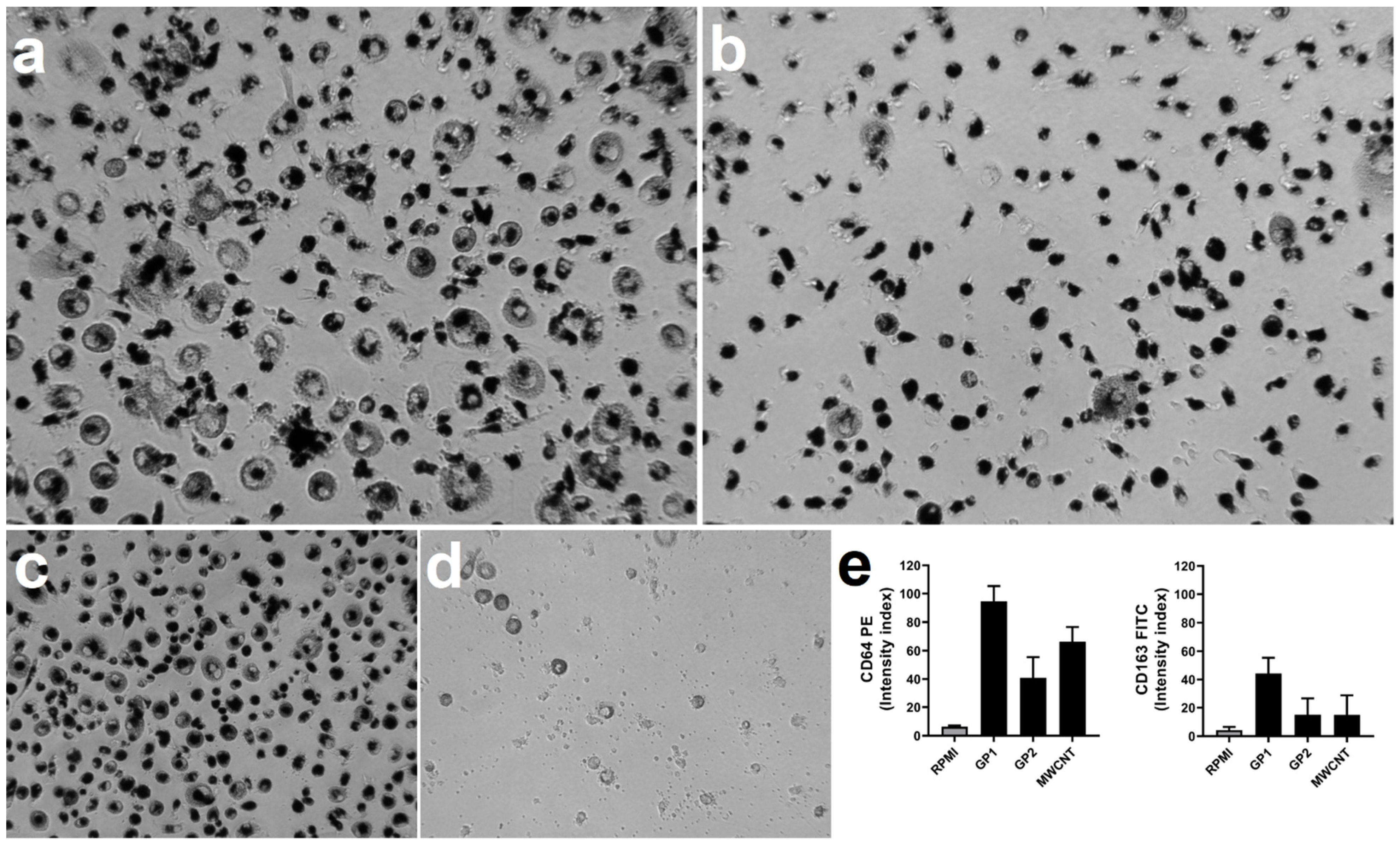
3.5. Monocyte Differentiation to Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-based nanocomposites for neural tissue engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-nanoparticle-based self-healing hydrogel in preventing postoperative recurrence of breast cancer. ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carter, D.; Goode, A.E.; Kiryushko, D.; Masuda, S.; Hu, S.; Lopes-Rodrigues, R.; Dexter, D.T.; Shaffer, M.S.P.; Porter, A.E. Quantification of blood–brain barrier transport and neuronal toxicity of unlabelled multiwalled carbon nanotubes as a function of surface charge. Nanoscale 2019, 11, 22054–22069. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Jiang, J.; Wang, Y.; Jiang, H.; Zhang, J.; Nie, X.; Liu, B. Systematic assessment of the toxicity and potential mechanism of graphene derivatives in vitro and in vivo. Toxicol. Sci. 2019, 167, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Barosova, H.; Karakocak, B.B.; Septiadi, D.; Petri-Fink, A.; Stone, V.; Rothen-Rutishauser, B. An in vitro lung system to assess the proinflammatory hazard of carbon nanotube aerosols. Int. J. Mol. Sci. 2020, 21, 5335. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Ghanbari, F.; Nasarzadeh, P.; Seydi, E.; Ghasemi, A.; Taghi Joghataei, M.; Ashtari, K.; Akbari, M. Mitochondrial oxidative stress and dysfunction induced by single- and multiwall carbon nanotubes: A comparative study. J. Biomed. Mater. Res. Part A 2017, 105, 2047–2055. [Google Scholar] [CrossRef]
- Wan, B.; Wang, Z.X.; Lv, Q.Y.; Dong, P.X.; Zhao, L.X.; Yang, Y.; Guo, L.H. Single-walled carbon nanotubes and graphene oxides induce autophagosome accumulation and lysosome impairment in primarily cultured murine peritoneal macrophages. Toxicol. Lett. 2013, 221, 118–127. [Google Scholar] [CrossRef]
- Svadlakova, T.; Hubatka, F.; Turanek Knotigova, P.; Kulich, P.; Masek, J.; Kotoucek, J.; Macak, J.; Motola, M.; Kalbac, M.; Kolackova, M.; et al. Proinflammatory effect of carbon-based nanomaterials: In vitro study on stimulation of inflammasome NLRP3 via destabilisation of lysosomes. Nanomaterials 2020, 10, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drasler, B.; Kucki, M.; Delhaes, F.; Buerki-Thurnherr, T.; Vanhecke, D.; Korejwo, D.; Chortarea, S.; Barosova, H.; Hirsch, C.; Petri-Fink, A.; et al. Single exposure to aerosolized graphene oxide and graphene nanoplatelets did not initiate an acute biological response in a 3D human lung model. Carbon 2018, 137, 125–135. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, S.J.; Lee, K.; Choi, Y.C.; Lee, B.S.; Lee, G.H.; Kim, D.W. Pulmonary persistence of graphene nanoplatelets may disturb physiological and immunological homeostasis. J. Appl. Toxicol. 2017, 37, 296–309. [Google Scholar] [CrossRef]
- Jaworski, S.; Sawosz, E.; Grodzik, M.; Winnicka, A.; Prasek, M.; Wierzbicki, M.; Chwalibog, A. In vitro evaluation of the effects of graphene platelets on glioblastoma multiforme cells. Int. J. Nanomed. 2013, 8, 413–420. [Google Scholar]
- Malanagahalli, S.; Murera, D.; Martín, C.; Lin, H.; Wadier, N.; Dumortier, H.; Vázquez, E.; Bianco, A. Few layer graphene does not affect cellular homeostasis of mouse macrophages. Nanomaterials 2020, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2016, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, A.; Swaroop, S.; Koduri, C.K.; Girish, C.M.; Chandran, P.; Panchakarla, L.S.; Somasundaram, V.H.; Gowd, G.S.; Nair, S.; Koyakutty, M. Comparative in vivo toxicity, organ biodistribution and immune response of pristine, carboxylated and PEGylated few-layer graphene sheets in Swiss albino mice: A three month study. Carbon 2015, 95, 511–524. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; Møller, P.; Clausen, P.A.; Saber, A.T.; Micheletti, C.; Jensen, K.A.; Wallin, H.; Vogel, U. Biodistribution of carbon nanotubes in animal models. Basic Clin. Pharmacol. Toxicol. 2017, 121, 30–43. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Luo, Y. Pharmacological and toxicological aspects of carbon nanotubes (CNTs) to vascular system: A review. Toxicol. Appl. Pharmacol. 2019, 385, 114801. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical applications of graphene-based structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [Green Version]
- Dale, D.C.; Boxer, L.; Liles, W.C. The phagocytes: Neutrophils and monocytes. Blood 2008, 112, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Meunier, E.; Coste, A.; Olagnier, D.; Authier, H.; Lefevre, L.; Dardenne, C.; Bernad, J.; Beraud, M.; Flahaut, E.; Pipy, B. Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine 2012, 8, 987–995. [Google Scholar] [CrossRef] [Green Version]
- Delogu, L.G.; Venturelli, E.; Manetti, R.; Pinna, G.A.; Carru, C.; Madeddu, R.; Murgia, L.; Sgarrella, F.; Dumortier, H.; Bianco, A. Ex vivo impact of functionalized carbon nanotubes on human immune cells. Nanomedicine 2012, 7, 231–243. [Google Scholar] [CrossRef] [Green Version]
- David, C.A.W.; Barrow, M.; Murray, P.; Rosseinsky, M.J.; Owen, A.; Liptrott, N.J. In vitro determination of the immunogenic impact of nanomaterials on primary peripheral blood mononuclear cells. Int. J. Mol. Sci. 2020, 21, 5610. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.; Stenvik, J.; Nilsen, A.M. Iron oxide nanoparticles modulate lipopolysaccharide-induced inflammatory responses in primary human monocytes. Int. J. Nanomed. 2016, 11, 4625–4642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverny, G.; Casset, A.; Purohit, A.; Schaeffer, E.; Spiegelhalter, C.; de Blay, F.; Pons, F. Immunomodulatory properties of multi-walled carbon nanotubes in peripheral blood mononuclear cells from healthy subjects and allergic patients. Toxicol. Lett. 2013, 217, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Lebre, F.; Boland, J.B.; Gouveia, P.; Gorman, A.L.; Lundahl, M.L.E.; Lynch, R.I.; O’Brien, F.J.; Coleman, J.; Lavelle, E.C. Pristine graphene induces innate immune training. Nanoscale 2020, 12, 11192–11200. [Google Scholar] [CrossRef] [PubMed]
- Kinaret, P.A.S.; Scala, G.; Federico, A.; Sund, J.; Greco, D. Carbon nanomaterials promote M1/M2 macrophage activation. Small 2020, 16, 1907609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öner, D.; Moisse, M.; Ghosh, M.; Duca, R.C.; Poels, K.; Luyts, K.; Putzeys, E.; Cokic, S.M.; Van Landuyt, K.; Vanoirbeek, J.; et al. Epigenetic effects of carbon nanotubes in human monocytic cells. Mutagenesis 2017, 32, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, L.; Huang, C.-C.; Lung, S.-C.C.; Yang, L.; Wang, W.-C.; Lin, P.-H.; Suo, G.; Lin, C.-H. Consecutive evaluation of graphene oxide and reduced graphene oxide nanoplatelets immunotoxicity on monocytes. Colloids Surf. B Biointerfaces 2017, 153, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Fadeel, B. It takes two to tango: Understanding the interactions between engineered nanomaterials and the immune system. Eur. J. Pharm. Biopharm. 2015, 95, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Knötigová, P.T.; Mašek, J.; Hubatka, F.; Kotouček, J.; Kulich, P.; Šimečková, P.; Bartheldyová, E.; Machala, M.; Švadláková, T.; Krejsek, J.; et al. Application of advanced microscopic methods to study the interaction of carboxylated fluorescent nanodiamonds with membrane structures in THP-1 cells: Activation of inflammasome NLRP3 as the result of lysosome destabilization. Mol. Pharm. 2019, 16, 3441–3451. [Google Scholar] [CrossRef]
- Brown, D.M.; Kinloch, I.A.; Bangert, U.; Windle, A.H.; Walter, D.M.; Walker, G.S.; Scotchford, C.A.; Donaldson, K.; Stone, V. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosis. Carbon 2007, 45, 1743–1756. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Kostarelos, K.; Fadeel, B. Cytokine profiling of primary human macrophages exposed to endotoxin-free graphene oxide: Size-independent NLRP3 inflammasome activation. Adv. Healthc. Mater. 2018, 7, 1700815. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Ji, Z.; Wang, M.; Liao, Y.-P.; Chang, C.H.; Li, R.; Zhang, H.; Nel, A.E.; Xia, T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small 2015, 11, 2087–2097. [Google Scholar] [CrossRef] [Green Version]
- Katsumiti, A.; Tomovska, R.; Cajaraville, M.P. Intracellular localization and toxicity of graphene oxide and reduced graphene oxide nanoplatelets to mussel hemocytes in vitro. Aquat. Toxicol. 2017, 188, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shin, J.H.; Lee, J.S.; Hwang, J.H.; Lee, J.H.; Baek, J.E.; Kim, T.G.; Kim, B.W.; Kim, J.S.; Lee, G.H.; et al. 28-Day inhalation toxicity of graphene nanoplatelets in Sprague-Dawley rats. Nanotoxicology 2016, 10, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Schinwald, A.; Murphy, F.; Askounis, A.; Koutsos, V.; Sefiane, K.; Donaldson, K.; Campbell, C.J. Minimal oxidation and inflammogenicity of pristine graphene with residence in the lung. Nanotoxicology 2014, 8, 824–832. [Google Scholar] [CrossRef]
- Schinwald, A.; Murphy, F.A.; Jones, A.; MacNee, W.; Donaldson, K. Graphene-based nanoplatelets: A new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS Nano 2012, 6, 736–746. [Google Scholar] [CrossRef]
- Vallhov, H.; Qin, J.; Johansson, S.M.; Ahlborg, N.; Muhammed, M.A.; Scheynius, A.; Gabrielsson, S. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 2006, 6, 1682–1686. [Google Scholar] [CrossRef]
- Oostingh, G.J.; Casals, E.; Italiani, P.; Colognato, R.; Stritzinger, R.; Ponti, J.; Pfaller, T.; Kohl, Y.; Ooms, D.; Favilli, F.; et al. Problems and challenges in the development and validation of human cell-based assays to determine nanoparticle-induced immunomodulatory effects. Part. Fibre Toxicol. 2011, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smulders, S.; Kaiser, J.P.; Zuin, S.; Van Landuyt, K.L.; Golanski, L.; Vanoirbeek, J.; Wick, P.; Hoet, P.H. Contamination of nanoparticles by endotoxin: Evaluation of different test methods. Part. Fibre Toxicol. 2012, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and non-canonical activation of NLRP3 inflammasome at the crossroad between immune tolerance and intestinal inflammation. Front. Immunol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Wang, X.; Ji, Z.; Li, R.; Xia, T. NLRP3 inflammasome activation induced by engineered nanomaterials. Small 2013, 9, 1595–1607. [Google Scholar] [CrossRef]
- Yimin; Kohanawa, M. A regulatory effect of the balance between TNF-α and IL-6 in the granulomatous and inflammatory response to Rhodococcus aurantiacus infection in mice. J. Immunol. 2006, 177, 642–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.P.; Bottini, M.; Fadeel, B. Graphene and the Immune System: A romance of many dimensions. Front. Immunol. 2017, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, H.; von dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef] [Green Version]
- Park, E.-J.; Lee, G.-H.; Han, B.S.; Lee, B.-S.; Lee, S.; Cho, M.-H.; Kim, J.-H.; Kim, D.-W. Toxic response of graphene nanoplatelets in vivo and in vitro. Arch. Toxicol. 2015, 89, 1557–1568. [Google Scholar] [CrossRef]
- Di Cristo, L.; Mc Carthy, S.; Paton, K.; Movia, D.; Prina-Mello, A. Interplay between oxidative stress and endoplasmic reticulum stress mediated- autophagy in unfunctionalised few-layer graphene-exposed macrophages. 2D Mater. 2018, 5, 045033. [Google Scholar] [CrossRef]
- Boyles, M.S.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.; Proudfoot, L.; Windle, A.H.; et al. Multi-walled carbon nanotube induced frustrated phagocytosis, cytotoxicity and pro-inflammatory conditions in macrophages are length dependent and greater than that of asbestos. Toxicol. Vitr. 2015, 29, 1513–1528. [Google Scholar] [CrossRef]
- Moujaber, O.; Stochaj, U. The cytoskeleton as regulator of cell signaling pathways. Trends Biochem. Sci. 2020, 45, 96–107. [Google Scholar] [CrossRef]
- Hohmann, T.; Dehghani, F. The cytoskeleton-a complex interacting meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In vitro experimental model of trained innate immunity in human primary monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Morgan, M.J.; Chen, K.; Choksi, S.; Liu, Z.G. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 2012, 119, 2895–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, C.; Cattaneo, M.G.; Molteni, R.; De Palma, C. Autophagy in the regulation of tissue differentiation and homeostasis. Front. Cell Dev. Biol. 2020, 8, 1563. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.J.; Simon, A.K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 2019, 19, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Yang, X.; Feng, Y.; Yu, J. Trained immunity: An underlying driver of inflammatory atherosclerosis. Front. Immunol. 2020, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Usui, F.; Karasawa, T.; Kawashima, A.; Kimura, H.; Mizushina, Y.; Shirasuna, K.; Mizukami, H.; Kasahara, T.; Hasebe, N.; et al. NLRP3 deficiency reduces macrophage interleukin-10 production and enhances the susceptibility to doxorubicin-induced cardiotoxicity. Sci. Rep. 2016, 6, 26489. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J. Neuroinflamm. 2019, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.K.; O’Carroll, C.E.; Wells, C.A.; Carmody, R.J. Toll-like receptors drive specific patterns of tolerance and training on restimulation of macrophages. Front. Immunol. 2018, 9, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiecień, I.; Polubiec-Kownacka, M.; Dziedzic, D.; Wołosz, D.; Rzepecki, P.; Domagała-Kulawik, J. CD163 and CCR7 as markers for macrophage polarization in lung cancer microenvironment. Cent.-Eur. J. Immunol. 2019, 44, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, Z.; Duan, G.; Wu, A.; Gu, Z.; Zhang, L.; Chen, C.; Chai, Z.; Ge, C.; Zhou, R. Graphene oxide nanosheets retard cellular migration via disruption of actin cytoskeleton. Small 2017, 13, 1602133. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; He, Y.; Liu, X.; Wang, S.; Ma, C.; Tian, X.; Wang, J.; Wu, X. Graphene oxide inhibits cell migration and invasion by destroying actin cytoskeleton in cervical cancer cells. Aging 2020, 12, 17625–17633. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.; Svadlakova, T.; Singh, A.; Kolackova, M.; Vankova, R.; Borsky, P.; Holmannova, D.; Karas, A.; Borska, L.; Fiala, Z. In vitro assessment of the genotoxic potential of pristine graphene platelets. Nanomaterials 2021, 11, 2210. [Google Scholar] [CrossRef]



| Nanomaterial | Particle Size (nm) (Z-Average) | PdI | Particle Size (nm) (Diameter) | Particle Size (nm) (Length) | Average ζ-Potential (mV) (in full RPMI) |
|---|---|---|---|---|---|
| GP1 | 178.5 ± 103 | 0.188 | −8.52 | ||
| GP2 | 332 ± 85 | 0.293 | −10.8 | ||
| MWCNT | 110~200 | ≤10,000 | −13.1 |
| Pre-Treatment | Monocytes with EC (%) | Monocytes without EC (%) | Viability (%) |
|---|---|---|---|
| Control | 62–72 | 27–38 | 96–97 |
| GP1 | 85–99 | 2–18 | 98–99 |
| GP2 | 82–98 | 1–15 | 98–99 |
| MWCNT | 82–98 | 3–18 | 95–98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svadlakova, T.; Kolackova, M.; Vankova, R.; Karakale, R.; Malkova, A.; Kulich, P.; Hubatka, F.; Turanek-Knotigova, P.; Kratochvilova, I.; Raska, M.; et al. Carbon-Based Nanomaterials Increase Reactivity of Primary Monocytes towards Various Bacteria and Modulate Their Differentiation into Macrophages. Nanomaterials 2021, 11, 2510. https://doi.org/10.3390/nano11102510
Svadlakova T, Kolackova M, Vankova R, Karakale R, Malkova A, Kulich P, Hubatka F, Turanek-Knotigova P, Kratochvilova I, Raska M, et al. Carbon-Based Nanomaterials Increase Reactivity of Primary Monocytes towards Various Bacteria and Modulate Their Differentiation into Macrophages. Nanomaterials. 2021; 11(10):2510. https://doi.org/10.3390/nano11102510
Chicago/Turabian StyleSvadlakova, Tereza, Martina Kolackova, Radka Vankova, Rumeysa Karakale, Andrea Malkova, Pavel Kulich, Frantisek Hubatka, Pavlina Turanek-Knotigova, Irena Kratochvilova, Milan Raska, and et al. 2021. "Carbon-Based Nanomaterials Increase Reactivity of Primary Monocytes towards Various Bacteria and Modulate Their Differentiation into Macrophages" Nanomaterials 11, no. 10: 2510. https://doi.org/10.3390/nano11102510
APA StyleSvadlakova, T., Kolackova, M., Vankova, R., Karakale, R., Malkova, A., Kulich, P., Hubatka, F., Turanek-Knotigova, P., Kratochvilova, I., Raska, M., Krejsek, J., & Turanek, J. (2021). Carbon-Based Nanomaterials Increase Reactivity of Primary Monocytes towards Various Bacteria and Modulate Their Differentiation into Macrophages. Nanomaterials, 11(10), 2510. https://doi.org/10.3390/nano11102510







