Electromagnetic Interference Shielding of 2D Transition Metal Carbide (MXene)/Metal Ion Composites
Abstract
:1. Introduction
2. Methods
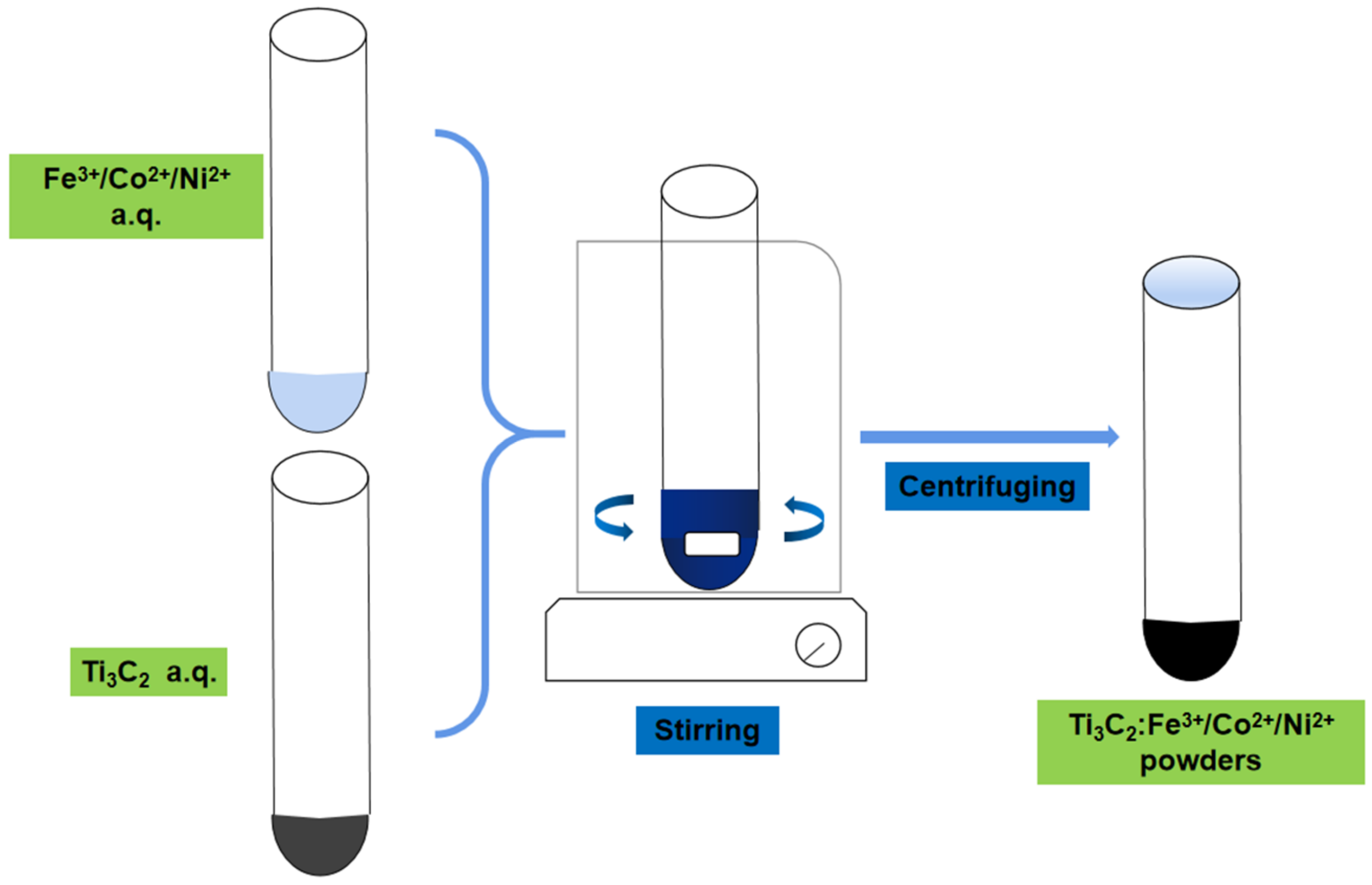
2.1. Material Synthesis
2.1.1. Preparation of Ti3C2 Powder
2.1.2. Incorporation of Metal Ions
2.1.3. Preparation of Ti3C2 and Ti3C2:Fe3+/Co2+/Ni2+ Films
2.2. Characterization Methods
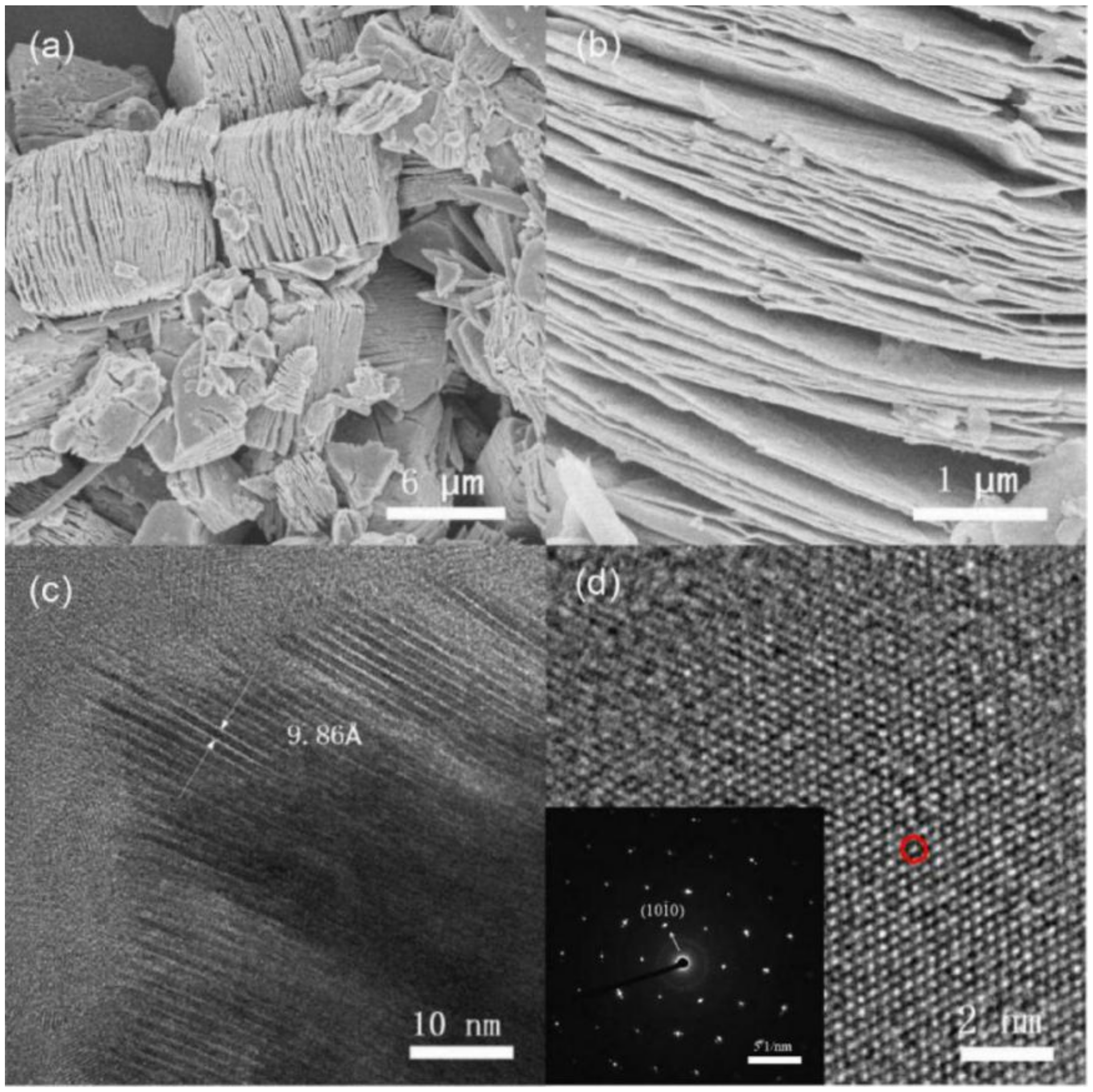
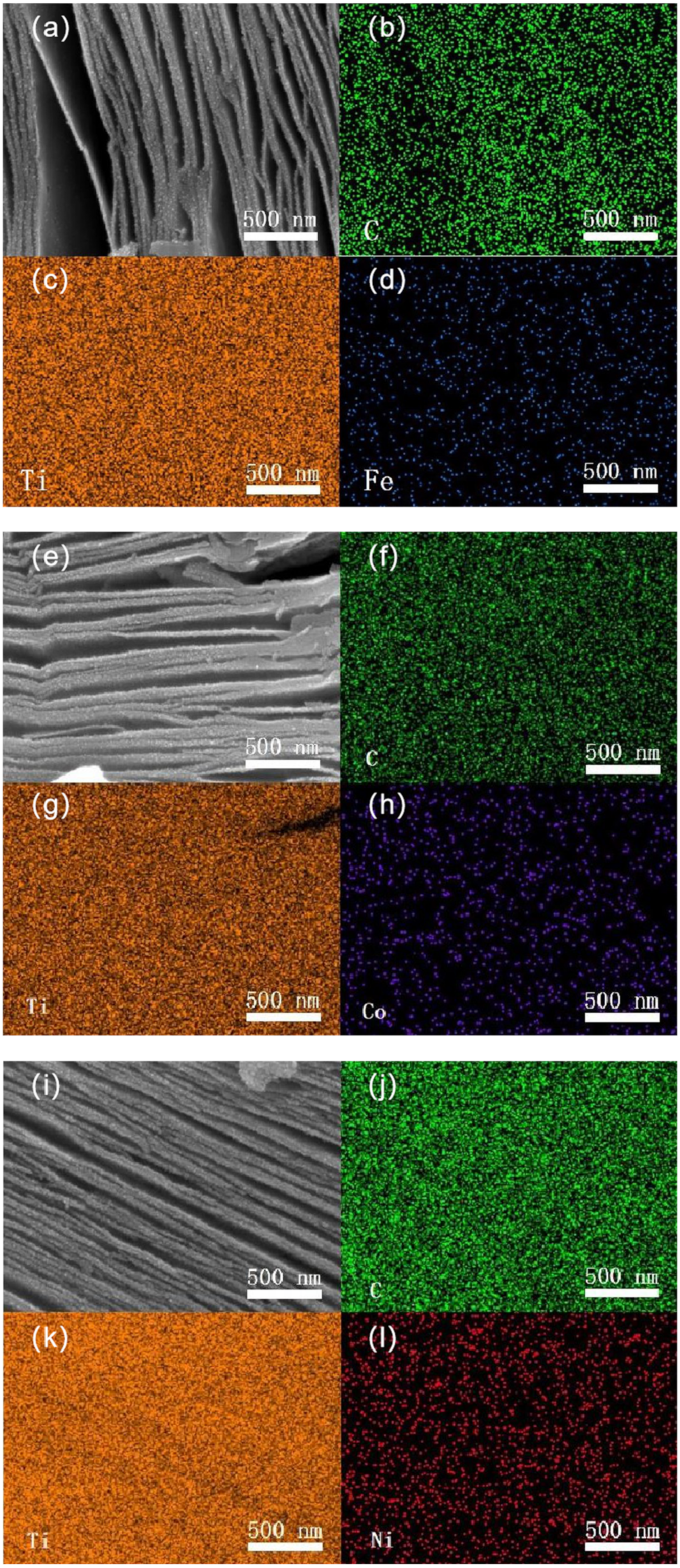
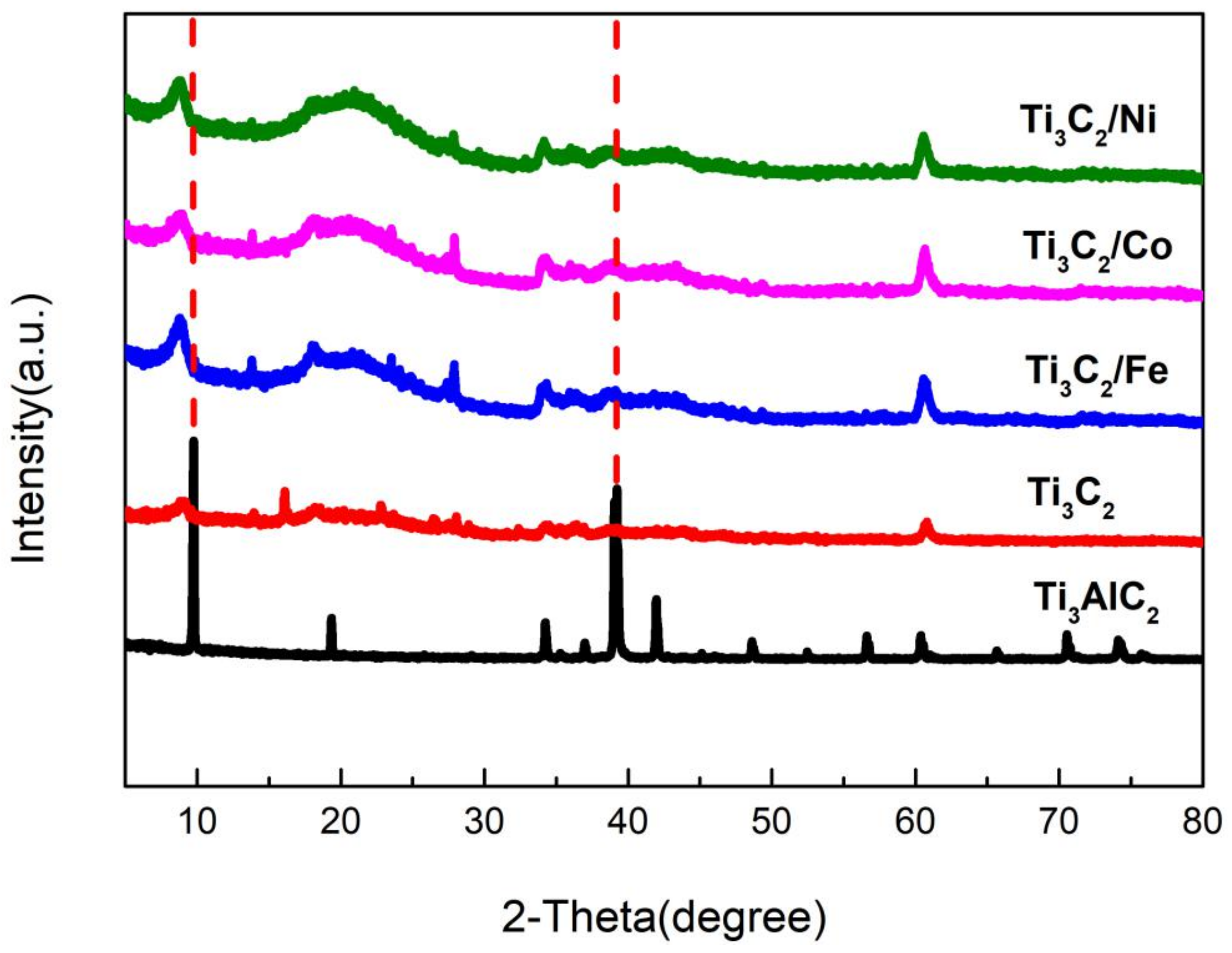
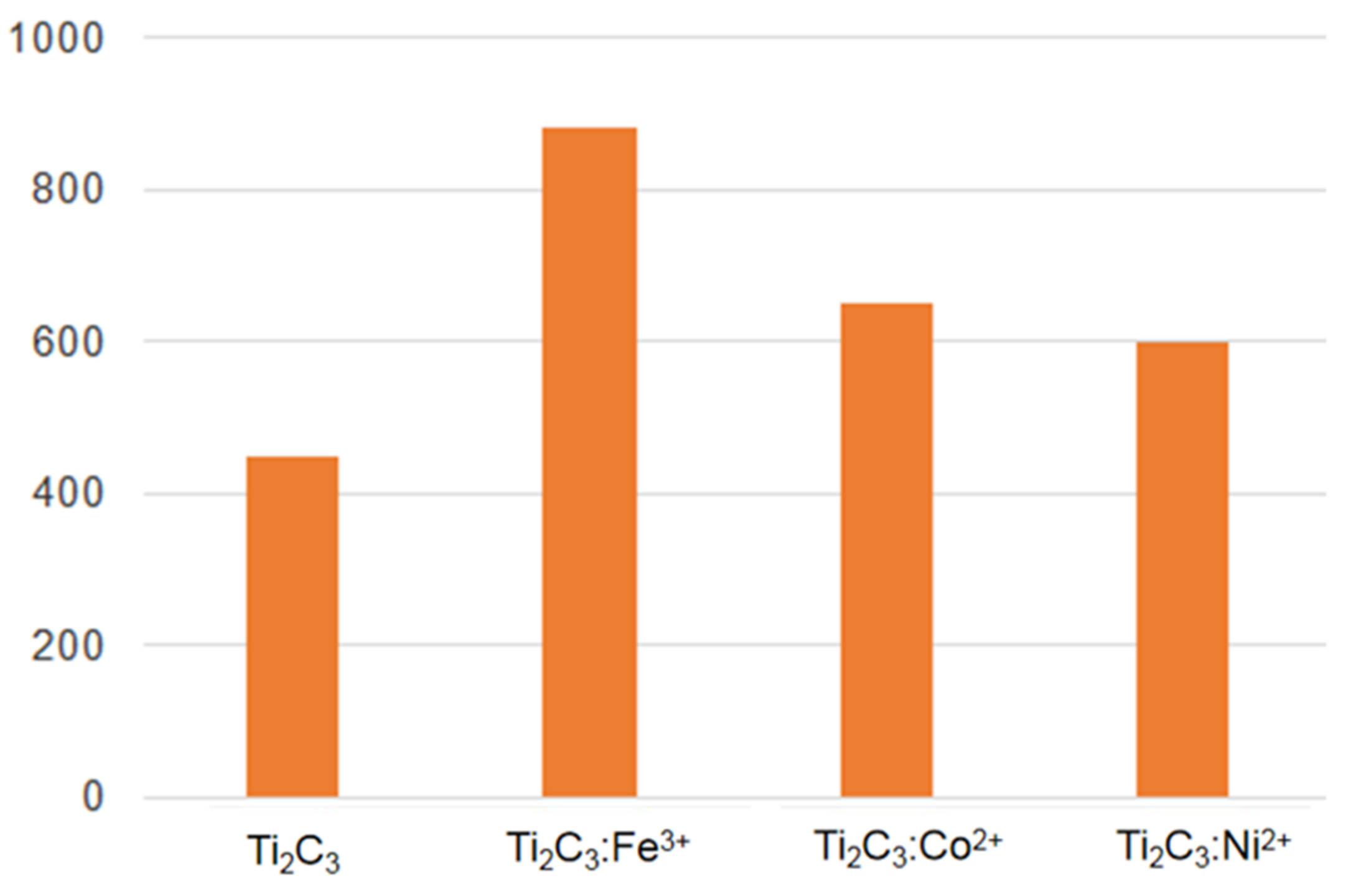
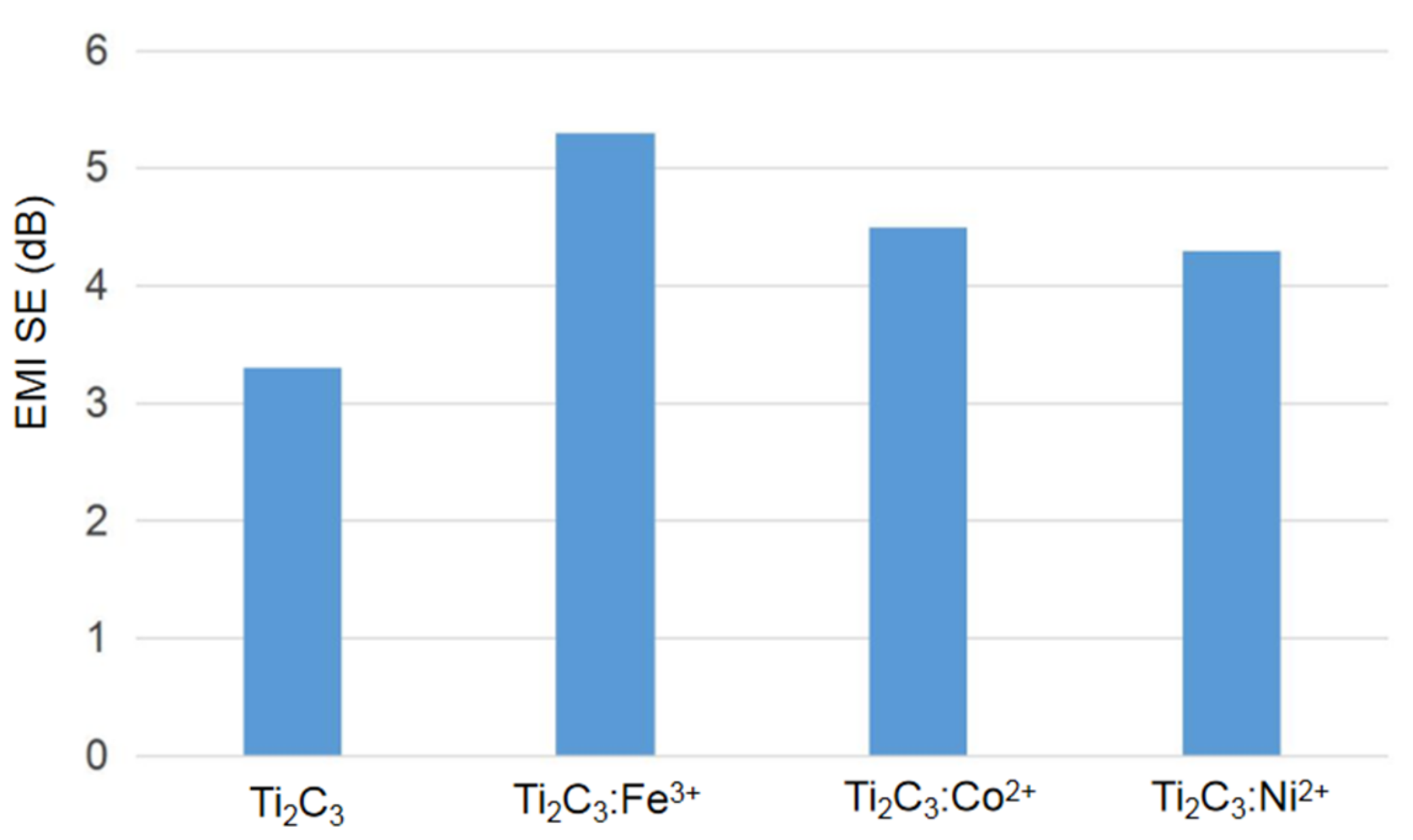
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jalali, M.; Dauterstedt, S.; Michaud, A.; Wuthrich, R. Electromagnetic shielding of polymer-matrix composites with metallic nanoparticles. Compos. Part B Eng. 2011, 42, 1420–1426. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.C.; Zhu, J.; Wang, M.K.; Hu, L.P.; Tang, Y.F.; Shu, Y.Q.; Xie, Z.J.; Zhang, H. Emerging mono-elemental bismuth nanostructures: Controlled synthesis and their versatile applications. Adv. Funct. Mater. 2021, 31, 2007584. [Google Scholar] [CrossRef]
- Song, Q.; Ye, F.; Yin, X.W.; Li, W.; Li, H.J.; Liu, Y.S.; Xie, K.Y.; Li, X.H.; Fu, Q.G.; Cheng, L.F.; et al. Carbon nanotube-multilayered graphene edge plane core-shell hybrid foams for ultrahigh-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1701583. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wang, M.K.; Hu, L.P.; Wang, C.; Xie, Z.J.; Zhang, H. Recent advances in semiconducting monoelemental selenium nanostructures for device applications. Adv. Funct. Mater. 2020, 30, 2003301. [Google Scholar] [CrossRef]
- Xiong, D.B.; Li, X.F.; Bai, Z.M.; Lu, S.G. Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, H.B.; Sun, R.H.; Liu, Y.F.; Liu, Z.S.; Zhou, A.G.; Yu, Z.Z. Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Liu, J.Z.; Zhang, X.X.; Tian, D.; Zhan, Z.Y.; Lu, C.H. Ultrathin MXene/calcium alginate aerogel film for high-performance electromagnetic interference shielding. Adv. Mater. Interfaces 2019, 6, 1802040. [Google Scholar] [CrossRef]
- Wang, M.K.; Zhu, J.; Zi, Y.; Wu, Z.G.; Hu, H.G.; Xie, Z.J.; Zhang, Y.; Hu, L.P.; Huang, W.C. Functional two-dimensional black phosphorus nanostructures towards next-generation devices. J. Mater. Chem. A 2021, 9, 12433–12473. [Google Scholar] [CrossRef]
- Das, T.K.; Ghosh, P.; Das, N.C. Preparation, development, outcomes, and application versatility of carbon fiber-based polymer composites: A review. Adv. Compos. Hybrid Mater. 2019, 1, 1–20. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Das, T.K.; Mondal, S.; Nayak, L.; Das, N.C. A comparative study of physico-mechanical and electrical properties of polymer-carbon nanofiber in wet and melt mixing methods. Mater. Sci. Eng. B 2019, 245, 95–106. [Google Scholar] [CrossRef]
- Mondal, S.; Revindren, R.; Shin, B.; Kim, S.; Lee, H.; Ganguly, S.; Das, N.C.; Nah, C. Electrical conductivity and electromagnetic interference shielding effectiveness of nano-structured carbon assisted poly(methyl methacrylate) nanocomposites. Polym. Eng. Sci. 2020, 60, 2414–2427. [Google Scholar] [CrossRef]
- Ling, J.Q.; Zhai, W.T.; Feng, W.W.; Shen, B.; Zhang, J.F.; Zheng, W.G. Facile preparation of lightweight microcellular polyetherimide/graphene composite foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef]
- Ganguly, S.; Ghosh, S.; Das, P.; Das, T.K.; Ghosh, S.K.; Das, N.C. Poly(N-vinylpyrrolidone)-stabilized colloidal graphene-reinforced poly(ethylene-co-methyl acrylate) to mitigate electromagnetic radiation pollution. Polym. Bull. 2020, 77, 2923–2943. [Google Scholar] [CrossRef]
- Li, N.; Huang, Y.; Du, F.; He, H.B.; Lin, X.; Gao, H.J.; Ma, Y.F.; Li, F.F.; Chen, Y.S.; Eklund, P.C. Electromagnetic interference (EMI) shielding of single-walled carbon nanotube epoxy composites. Nano Lett. 2006, 6, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Ravindren, R.; Mondal, S.; Bhawal, P.; Ali, S.M.N.; Das, N.C. Superior electromagnetic interference shielding effectiveness and low percolation threshold through the preferential distribution of carbon black in the highly flexible polymer blend composites. Polym. Compos. 2019, 40, 1404–1418. [Google Scholar] [CrossRef]
- Zou, L.H.; Zhang, S.L.; Li, X.P.; Lan, C.T.; Qiu, Y.P.; Ma, Y. Step-by-step strategy for constructing multilayer structured coatings toward high-efficiency electromagnetic interference shielding. Adv. Mater. Interfaces 2016, 3, 1500476. [Google Scholar] [CrossRef]
- Lan, C.T.; Guo, M.; Li, C.L.; Qiu, Y.P.; Ma, Y.; Sun, J.Q. Axial alignment of carbon nanotubes on fibers to enable highly conductive fabrics for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2020, 12, 7477–7485. [Google Scholar] [CrossRef]
- Shin, B.; Mondal, S.; Lee, M.; Kim, S.; Huh, Y.; Nah, C. Flexible thermoplastic polyurethane-carbon nanotube composites for electromagnetic interference shielding and thermal management. Chem. Eng. J. 2021, 418, 129282. [Google Scholar] [CrossRef]
- Kong, F.Y.; He, X.D.; Liu, Q.Q.; Qi, X.X.; Zhang, Y.T.; Wang, R.G.; Bai, Y.L. Improving the electrochemical properties of MXene Ti3C2, multilayer for Li-ion batteries by vacuum calcination. Electrochim. Acta 2018, 265, 140–150. [Google Scholar] [CrossRef]
- Huang, W.C.; Hu, L.P.; Tang, Y.F.; Xie, Z.J.; Zhang, H. Recent advances in functional 2D MXene-based nanostructures for next-generation devices. Adv. Funct. Mater. 2020, 30, 2005223. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Zhao, M.Q.; Urbankowski, P.; Halim, J.; Anasori, B.; Kota, S.; Ren, C.E.; Barsoum, M.W.; Gogotsi, Y. Fabrication of Ti3C2Tx MXene transparent thin films with tunable optoelectronic properties. Adv. Electron. Mater. 2016, 2, 1600050. [Google Scholar] [CrossRef]
- Ghidiu, M.; Kota, S.; Drozd, V.; Barsoum, M.W. Pressure-induced shear and interlayer expansion in Ti3C2 MXene in the presence of water. Sci. Adv. 2018, 4, eaao65850. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Ma, C.Y.; Li, C.; Zhang, Y.; Hu, L.P.; Chen, T.T.; Tang, Y.F.; Ju, J.F.; Zhang, H. Highly stable MXene (V2CTx)-based harmonic pulse generation. Nanophotonics 2020, 9, 2577–2585. [Google Scholar] [CrossRef]
- Peng, C.; Yang, X.F.; Li, Y.H.; Yu, H.; Wang, H.J.; Peng, F. Hybrids of two-dimensional Ti3C2 and TiO2 exposing {001} facets toward enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2016, 8, 6051–6060. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.M.; Tang, H.; Du, F.; Guo, Y.H.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl + LiF etchant: Enhanced exfoliation and delamination. J. Alloy. Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary strengthening and toughening of MXene/cellulose nanofiber composite paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Autere, A.; Jussila, H.; Dai, Y.Y.; Wang, Y.D.; Lipsanen, H.; Sun, Z.P. Nonlinear optics with 2D layered materials. Adv. Mater. 2018, 30, 1705963. [Google Scholar] [CrossRef] [Green Version]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Agnese, Y.D.; Rozier, P.; Taber, P.L. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Srimuk, P.; Halim, J.; Lee, J.; Tao, Q.Z.; Rosen, J.; Presser, V. Two-dimensional molybdenum carbide (MXene) with divacancy ordering for brackish and seawater desalination via cation and anion intercalation. ACS Sustain. Chem. Eng. 2018, 6, 3739–3747. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.F.; Anasori, B.; Ascaso, A.S.; Park, S.H.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, flexible, and conductive 2D titanium carbide (MXene) films with high volumetric capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef]
- Zhang, C.F.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.H.; Ascaso, A.S.; Shmeliov, A.; Krishnan, D.; et al. Oxidation stability of colloidal two-dimensional titanium carbides (MXenes). Chem. Mater. A Publ. Am. Chem. Soc. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.Y.; Wang, Y.J.; Chen, H.B.; Caro, J.; Wang, H.H. A two-dimensional lamellar membrane: MXene nanosheet stacks. Angew. Chem. 2017, 56, 1825–1829. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.K.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic shielding of monolayer MXene assemblies. Adv. Mater. 2020, 32, 1906769. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, A.; Sambyal, P.; Koo, C.M. 2D MXenes for electromagnetic shielding: A review. Adv. Funct. Mater. 2020, 30, 2000883. [Google Scholar] [CrossRef]
- Hu, T.; Wang, J.M.; Zhang, H.; Li, Z.J.; Hu, M.M.; Wang, X.H. Vibrational properties of Ti3C2 and Ti3C2T2 (T = O, F, OH) monosheets by first-principles calculations: A comparative study. Phys. Chem. Chem. Phys. 2015, 15, 1–7. [Google Scholar]
- Das, P.; Ganguly, S.; Saha, A.; Noked, M.; Margel, S.; Gedanken, A. Carbon-dots-initiated photopolymerization: An in situ synthetic approach for MXene/poly(norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl. Mater. Interfaces 2021, 13, 31038–31050. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.K.; Xu, W.; Pan, G.C. Ratiometric photoluminescence sensing based on Ti3C2 MXene quantum dots for the intracellular pH sensor. Nanoscale 2018, 3, 1–8. [Google Scholar]
- Xue, Y.H.; Zhang, Q.; Wang, W.J.; Cao, H.; Yang, Q.H.; Fu, L. Opening two-dimensional materials for energy conversion and storage: A concept. Adv. Energy Mater. 2017, 7, 1602684. [Google Scholar] [CrossRef]
- Yuan, H.T.; Wang, H.T.; Cui, Y. Two-dimensional layered chalcogenides: From rational synthesis to property control via orbital occupation and electron filling. Acc. Chem. Res. 2015, 48, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Yin, X.W.; Wu, H.; Hou, Z.X.; Song, C.Q.; Li, X.L.; Zhang, L.T.; Cheng, L.F. Ti3C2 MXenes with modified surface for high-performance electromagnetic absorption and shielding in the X-Band. ACS Appl. Mater. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef]
- Li, H.; Lian, P.C.; Lu, Q.J.; Chen, J.C.; Hou, R.R.; Mei, Y. Excellent air and water stability of two-dimensional black phosphorene/MXene heterostructure. Mater. Res. Express 2019, 6, 065504. [Google Scholar] [CrossRef]
- Zhang, L.X.; Su, W.T.; Huang, Y.W.; Li, H.; Fu, L.; Song, K.X.; Huang, X.W.; Yu, J.H.; Lin, C.T. In situ high-pressure X-ray diffraction and raman spectroscopy study of Ti3C2Tx MXene. Nanoscale Res. Lett. 2018, 13, 343. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Hu, M.M.; Li, Z.J.; Zhang, H.; Zhang, C.; Wang, J.M.; Wang, X.H. Covalency-dependent vibrational dynamics in two-dimensional titanium carbides. J. Phys. Chem. A 2015, 119, 12977–12984. [Google Scholar] [CrossRef]
- Jiang, X.T.; Liu, S.X.; Liang, W.Y.; Luo, S.J.; He, Z.L.; Ge, Y.Q.; Wang, H.D.; Cao, R.; Zhang, F.; Wen, Q.; et al. Broadband nonlinear photonics in few-layer MXene Ti3C2Tx (T = F, O, or OH). Laser Photonics Rev. 2018, 12, 1700229. [Google Scholar] [CrossRef]
- Shi, C.Y.; Beidaghi, M.; Naguib, M.; Mashtalir, O.; Gogotsi, Y.; Billinge, S.J.L. Structure of nanocrystalline Ti3C2 MXene using atomic pair distribution function. Phys. Rev. Lett. 2014, 112, 125501. [Google Scholar] [CrossRef] [Green Version]
- Bhawal, P.; Ganguly, S.; Das, T.K.; Mondal, S.; Das, N.C. Mechanically robust conductive carbon clusters confined ethylene methyl acrylate-based flexible composites for superior shielding effectiveness. Polym. Adv. Technol. 2018, 29, 95–110. [Google Scholar] [CrossRef]
- Mondal, S.; Ganguly, S.; Das, P.; Bhawal, P.; Das, T.K.; Nayak, L.; Khastgir, D.; Das, N.C. High-performance carbon nanofiber coated cellulose filter paper for electromagnetic interference shielding. Cellulose 2017, 24, 5117–5131. [Google Scholar] [CrossRef]
- Zou, L.H.; Lan, C.T.; Li, X.P.; Zhang, S.L. Superhydrophobization of cotton fabric with multiwalled carbon nanotubes for durable electromagnetic interference shielding. Fibers Polym. 2015, 16, 2158–2164. [Google Scholar] [CrossRef]
- Liang, L.Y.; Han, G.J.; Li, Y.; Zhao, B.; Zhou, B.; Feng, Y.Z.; Ma, J.M.; Wang, Y.M.; Zhang, R.; Liu, C.T. Promising Ti3C2Tx MXene/Ni chain hybrid with excellent electromagnetic wave absorption and shielding capacity. ACS Appl. Mater. Interfaces 2019, 11, 25399–25409. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.M.; Li, J.; Alhabeb, M.; Karpovich, C.; Wang, H.; Lipton, J.; Maleski, K.; Kong, J.; Shaulsky, E.; Elimelech, M.; et al. Layer-by-layer assembly of cross-functional semi-transparent MXene-carbon nanotubes composite films for next-generation electromagnetic interference shielding. Adv. Funct. Mater. 2018, 28, 1803360. [Google Scholar] [CrossRef]
- Wang, Q.W.; Zhang, H.B.; Liu, J.; Zhao, S.; Xie, X.; Liu, L.; Yang, R.; Koratkar, N.; Yu, Z.Z. Multifunctional and water-resistant MXene-decorated polyester textiles with outstanding electromagnetic interference shielding and joule heating performances an efficient and scalable dip-coating multifunctional textiles. Adv. Funct. Mater. 2019, 29, 1806819. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Hantanasirisakul, K.; Li, X.; Iqbal, A.; Hatter, C.B.; Anasori, B.; Koo, C.M.; Torita, T.; Soda, Y.; et al. Anisotroic MXene aerogels with a mechanically tunable ratio of electromagnetic wave reflection to absorption. Adv. Opt. Mater. 2019, 7, 1900267. [Google Scholar] [CrossRef]
- Xu, H.L.; Yin, X.W.; Li, X.L.; Li, M.H.; Liang, S.; Zhang, L.T.; Cheng, L.F. Lightweight Ti2CTx MXene/poly(vinyl alcohol) composite foams for electromagnetic wave shielding with absorption-dominated feature. ACS Appl. Mater. Interfaces 2019, 11, 10198–10207. [Google Scholar] [CrossRef]
- Luo, J.Q.; Zhao, S.; Zhang, H.B.; Deng, Z.; Li, L.; Yu, Z.Z. Flexible, stretchable and electrically conductive MXene/natural rubber nanocomposite films for efficient electromagnetic interference shielding. Compos. Sci. Technol. 2019, 182, 107754. [Google Scholar] [CrossRef]


| Structural Forms | Composition | Thickness (mm) | SE (dB) | Ref. |
|---|---|---|---|---|
| Compact & laminates structures | Ti3C2Tx | 0.045 | 92 | [36] |
| Ti3C2Tx/CNF | 0.047 | 24 | [27] | |
| Ti3C2Tx/Ni | 1.3 | 33.8 | [52] | |
| Layer-by-layer | Ti3C2Tx/CNT | 0.0002 | 2.9 | [53] |
| Ti3C2Tx/PPy | 0.45 | 42 | [54] | |
| Porous | Ti3C2Tx | 1 | 70.6 | [55] |
| Ti3C2Tx/PVA | 5 | 33 | [56] | |
| Segregated | Ti3C2Tx/NR | 0.25 | 53.6 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Xiao, Q. Electromagnetic Interference Shielding of 2D Transition Metal Carbide (MXene)/Metal Ion Composites. Nanomaterials 2021, 11, 2929. https://doi.org/10.3390/nano11112929
Xia X, Xiao Q. Electromagnetic Interference Shielding of 2D Transition Metal Carbide (MXene)/Metal Ion Composites. Nanomaterials. 2021; 11(11):2929. https://doi.org/10.3390/nano11112929
Chicago/Turabian StyleXia, Xuefeng, and Quanlan Xiao. 2021. "Electromagnetic Interference Shielding of 2D Transition Metal Carbide (MXene)/Metal Ion Composites" Nanomaterials 11, no. 11: 2929. https://doi.org/10.3390/nano11112929
APA StyleXia, X., & Xiao, Q. (2021). Electromagnetic Interference Shielding of 2D Transition Metal Carbide (MXene)/Metal Ion Composites. Nanomaterials, 11(11), 2929. https://doi.org/10.3390/nano11112929





