Flexible Tellurium-Based Electrode for High-Performance Lithium-Tellurium Battery
Abstract
:1. Introduction
2. Materials and Methods
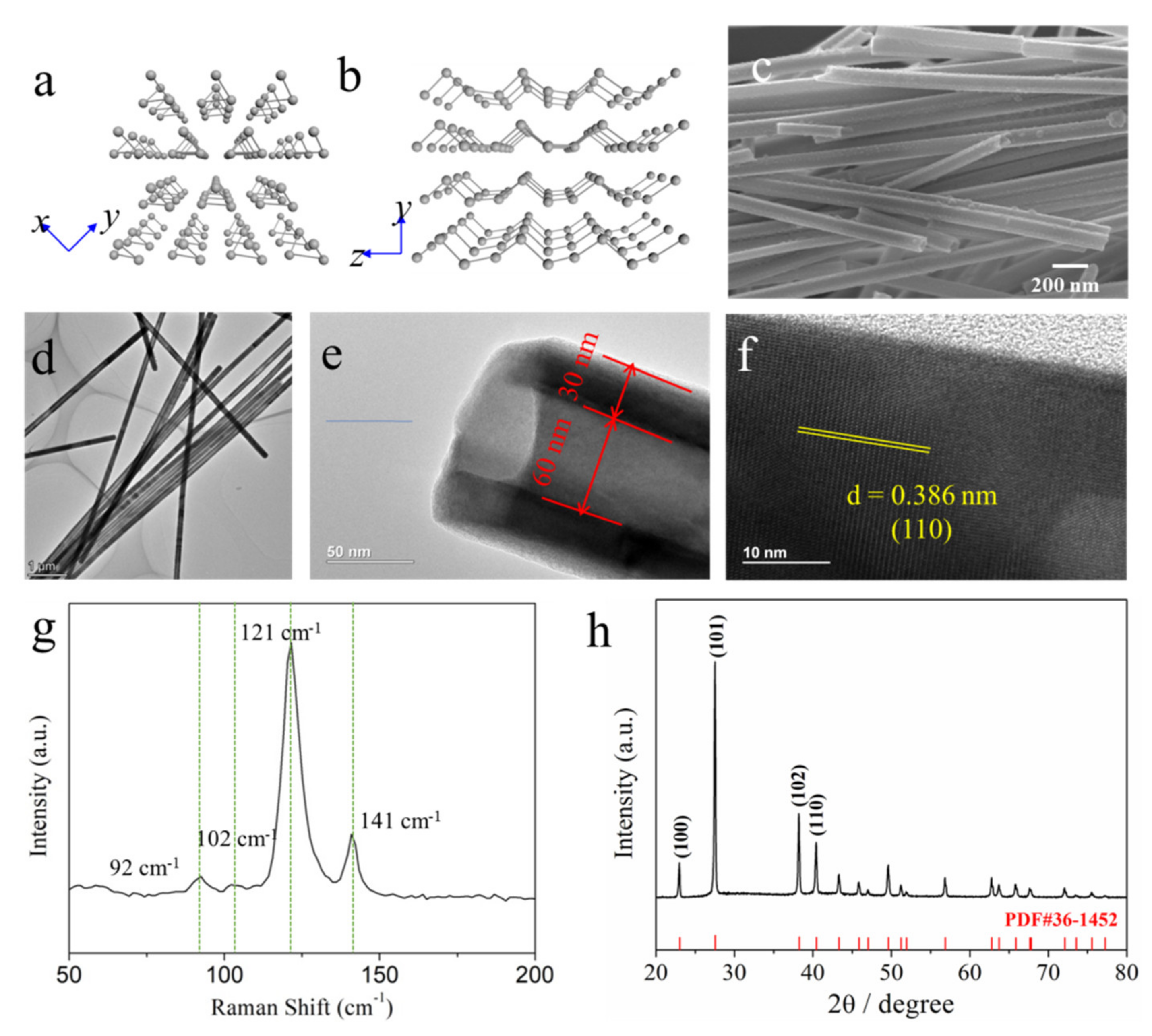
2.1. Synthesis of Te NTs
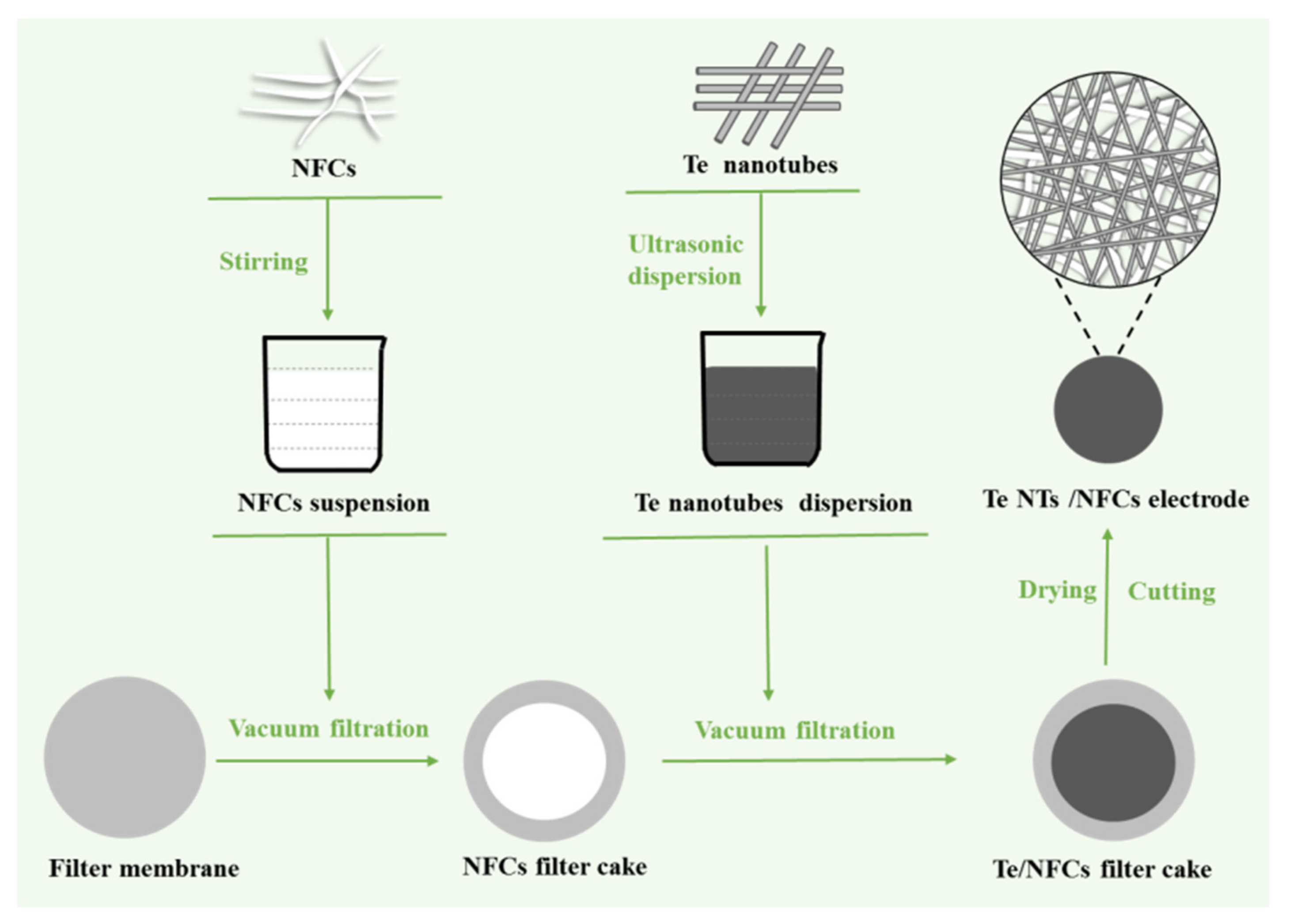
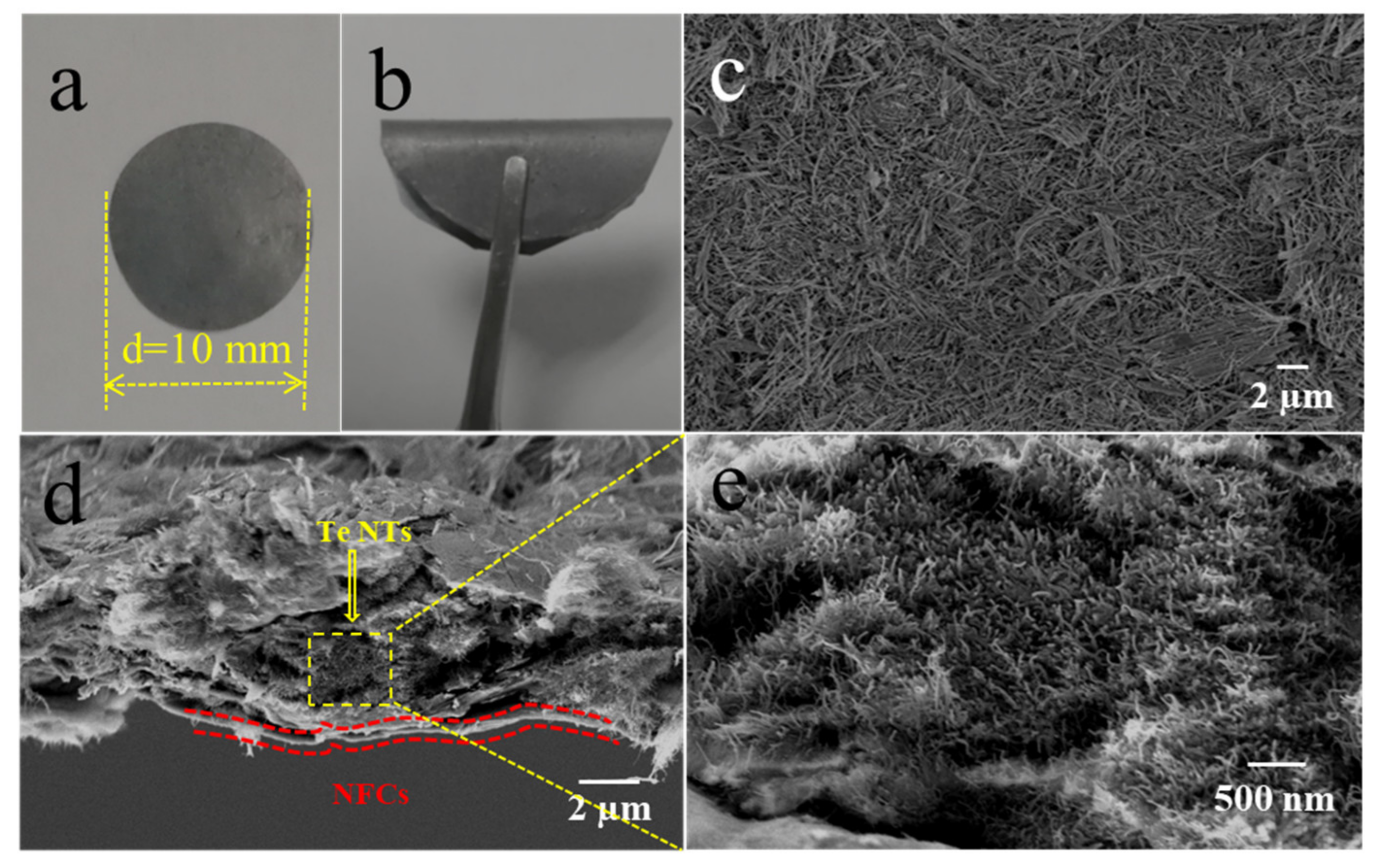
2.2. Fabrication of the Flexible and Freestanding Te-Based Electrode
2.3. Materials Characterizations
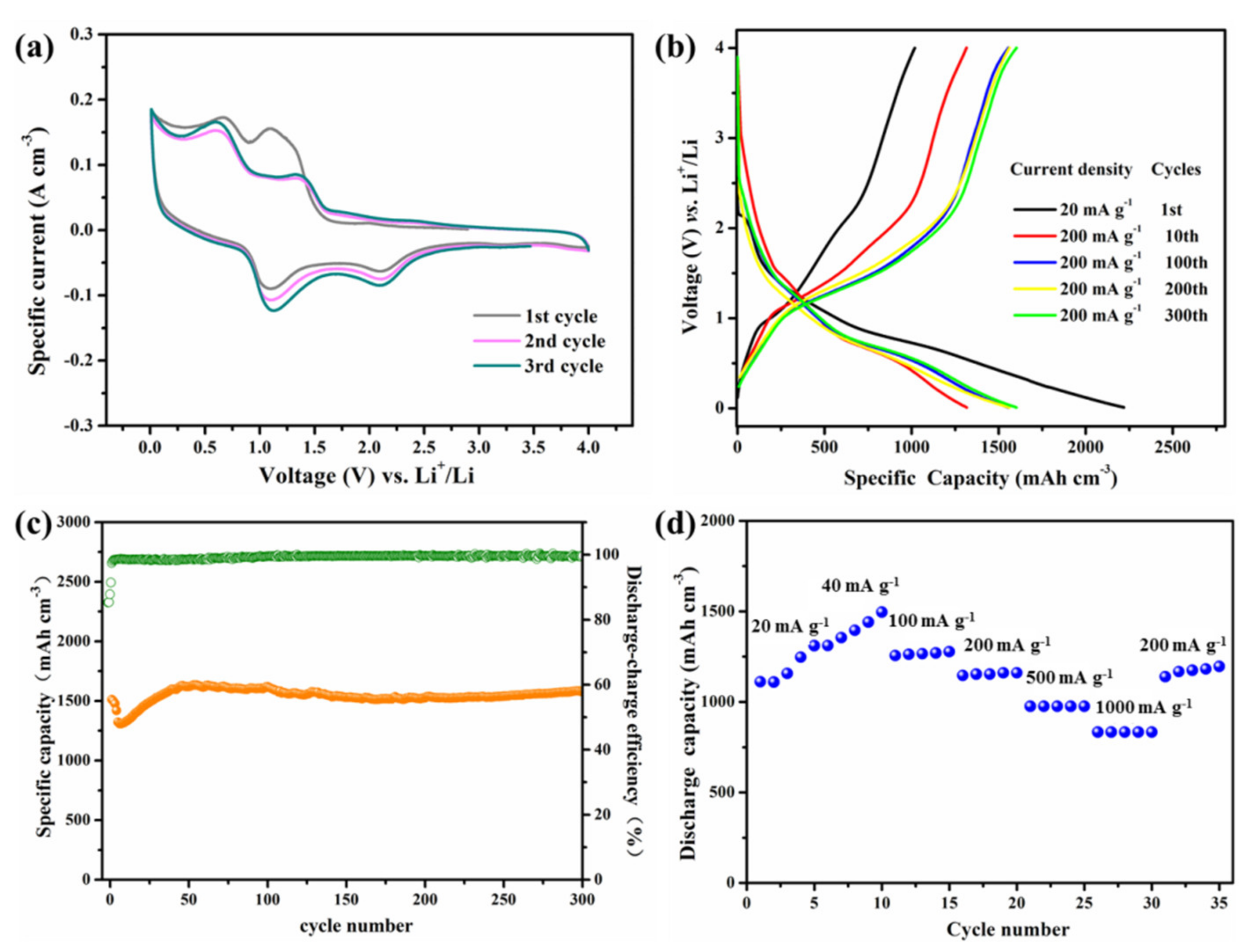
2.4. Electrochemical Measurement
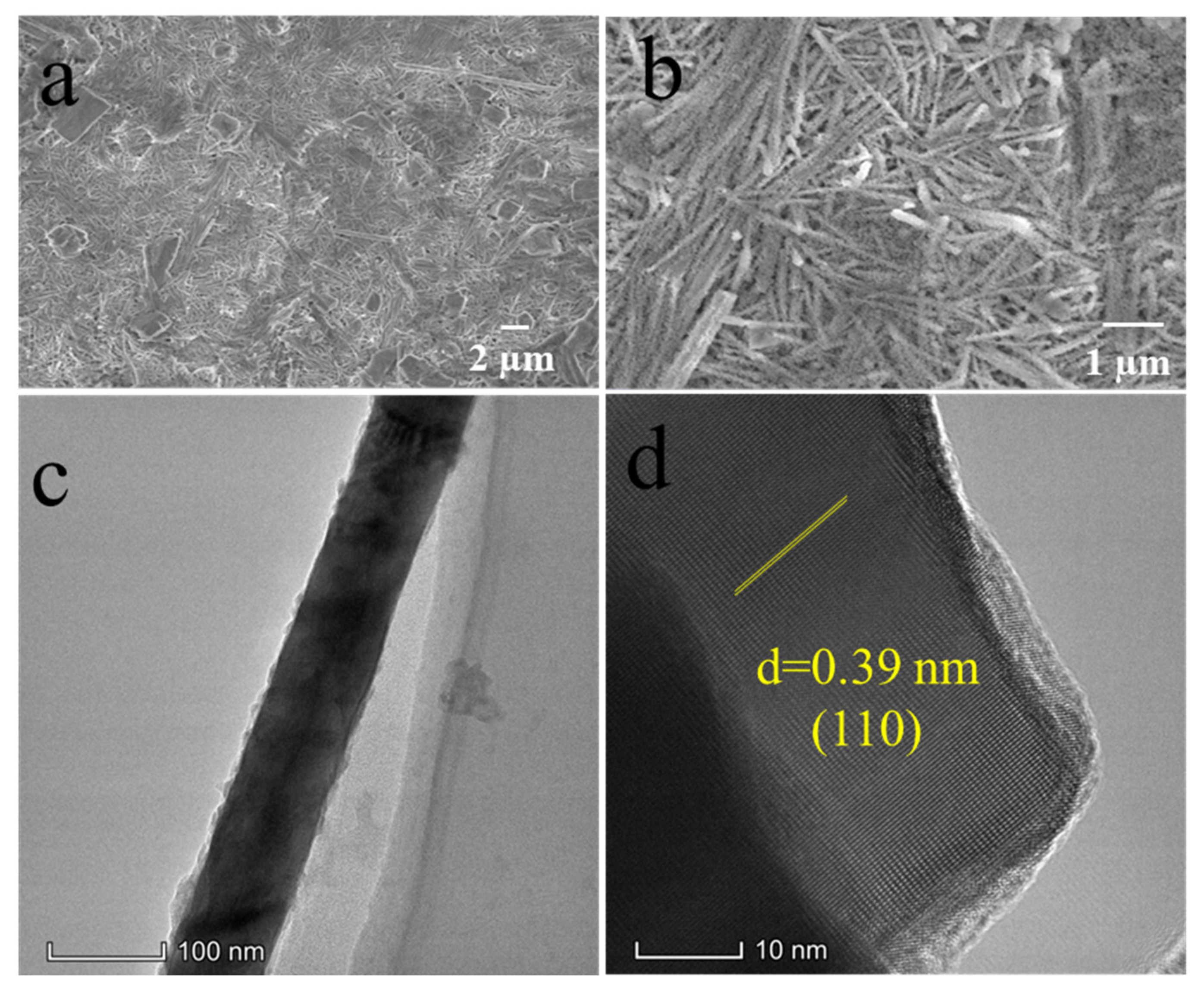
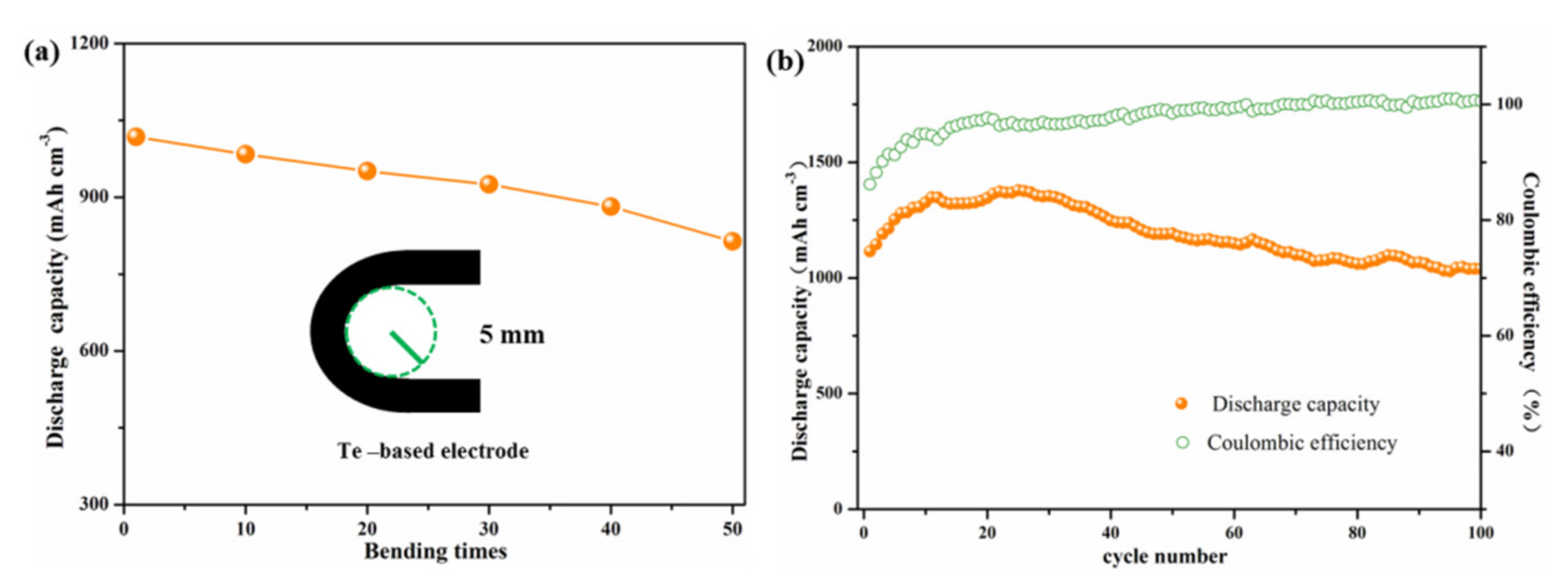
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Ren, J.; Weng, W.; Peng, H. Advances in wearable fiber-shaped lithium-ion batteries. Adv. Mater. 2016, 28, 4524–4531. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.-Q.; Peng, S.; Zhang, Q. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule 2019, 3, 2647–2661. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Dong, Y.; Tang, Y.; Wang, L.; Jia, D.; Zhao, Z.; Qiu, J. Self-assembled sulfur/reduced graphene oxide nanoribbon paper as a free-standing electrode for high performance lithium–sulfur batteries. Chem. Comm. 2016, 52, 12825–12828. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.T.; Zimmermann, T.; Hauert, R.; Caseri, W.J.C. Preparation and characterization of cationic nanofibrillated cellulose from etherification and high-shear disintegration processes. Cellulose 2011, 18, 1391–1406. [Google Scholar] [CrossRef] [Green Version]
- Aulin, C.; Salazar-Alvarez, G.; Lindström, T. High strength, flexible and transparent nanofibrillated cellulose–nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 2012, 4, 6622–6628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Z.Y.; Wang, Y.X.; Fan, C.; Liu, C.R.; Peng, Q.L.; Chen, J.J.; Feng, Z.S. Preparation and characterization of flexible lithium iron phosphate/graphene/cellulose electrode for lithium ion batteries. J. Colloid. Interface Sci. 2018, 512, 398–403. [Google Scholar] [CrossRef]
- Li, J.; Luo, H.; Zhai, B.; Lu, R.; Guo, Z.; Zhang, H.; Liu, Y. Black phosphorus: A two-dimension saturable absorption material for mid-infrared Q-switched and mode-locked fiber lasers. Sci. Rep. 2016, 6, 30361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, R.; Pal, A.; Pal, T. 2D Materials for Renewable Energy Storage Devices: Outlook and Challenges. Chem. Comm. 2016, 52, 13528–13542. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Guo, Z.; Xiao, Z.; Wang, H.; Luo, X.; Zhang, H. Emerging two-dimensional noncarbon nanomaterials for flexible lithium-ion batteries: Opportunities and challenges. J. Mater. Chem. A 2019, 7, 25227–25246. [Google Scholar] [CrossRef]
- Ning, G.; Xu, C.; Cao, Y.; Zhu, X.; Jiang, Z.; Fan, Z.; Qian, W.; Wei, F.; Gao, J. Chemical vapor deposition derived flexible graphene paper and its application as high performance anodes for lithium rechargeable batteries. J. Mater. Chem. A 2013, 1, 408–414. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, G.; Liu, Z.; Ma, X.; Chen, J.; Zhang, Z.; Ma, X.; Li, F.; Cheng, H.M.; Wencai, R. Scalable Clean Exfoliation of High-Quality Few-Layer Black Phosphorus for a Flexible Lithium Ion Battery. Adv. Mater. 2016, 28, 510–517. [Google Scholar] [CrossRef]
- Yue, Z.; Dan, M.; Freschi, D.J.; Jian, L. Materials Design and Fundamental Understanding of Tellurium-based Electrochemistry for Rechargeable Batteries. Energy Storage Mater. 2021, 40, 166–188. [Google Scholar]
- He, J.; Chen, Y.; Lv, W.; Wen, K.; Wang, Z.; Zhang, W.; Li, Y.; Qin, W.; He, W. Three-Dimensional Hierarchical Reduced Graphene Oxide/Tellurium Nanowires: A High-Performance Freestanding Cathode for Li–Te Batteries. ACS Nano 2016, 10, 8837–8842. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, H.; Eom, K.S. Enhancing the electrochemical performances of a tellurium-based cathode for a high-volumetric capacity Li battery via a high-energy ball mill with sulfur edge-functionalized carbon. J. Power Sources 2019, 430, 112–119. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, G.; Wang, R.; Huang, S.; Wang, Q.; Liu, Y.; Du, Y.; Goddard, W.A.; Kim, M.J.; Xu, X.; et al. Field-effect transistors made from solution-grown two-dimensional tellurene. Nat. Electron. 2018, 1, 228–236. [Google Scholar] [CrossRef]
- He, Z.; Yang, Y.; Liu, J.-W.; Yu, S.-H. Emerging tellurium nanostructures: Controllable synthesis and their applications. Chem. Soc. Rev. 2017, 46, 2732–2753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, F.; Xu, Y.; Huang, W.; Zhang, H. Epitaxial Growth of Topological Insulators on Semiconductors (Bi2Se3/Te@Se) toward High㏄erformance Photodetectors. Small Methods 2019, 3, 1900349. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Xu, Y.; Huang, W.; Li, C.; Gao, L.; Wu, L.; Shi, Z.; Ma, C.; Ge, Y.; et al. Synthesis and optoelectronics of mixed-dimensional Bi/Te binary heterostructures. Nanoscale Horiz. 2020, 5, 847–856. [Google Scholar] [CrossRef]
- Amani, M.; Tan, C.; Zhang, G.; Zhao, C.; Bullock, J.; Song, X.; Kim, H.; Shrestha, V.R.; Gao, Y.; Crozier, K.B.; et al. Solution synthesized high mobility tellurium nanoflakes for short-wave infrared photodetectors. ACS Nano 2018, 12, 7253–7263. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; You, Q.; Huang, P.; Wang, Y.; Huang, Z.N.; Ge, Y.; Wu, L.; Dong, Z.; Dai, X.; et al. Enhanced Photodetection Properties of Tellurium@Selenium Roll-to-Roll Nanotube Heterojunctions. Small Methods 2019, 15, 1900902. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, Y.; Wang, H.; Zeng, Y.; Zhao, J.; Zhang, L.; Li, J.; Meng, F.; Shi, Z.; Fan, D.; et al. Solar-blind deep-ultraviolet photodetectors based on solution-synthesized quasi-2D Te nanosheets. Nanophotonics 2020, 9. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Liang, H.W.; Liu, G.Q.; Feng, M.; Xu, L.; Liu, J.W.; Wang, J.L.; Yu, S.H. A new generation of alloyed/multimetal chalcogenide nanowires by chemical transformation. Sci. Adv. 2015, 1, 1500714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, F.; Wu, L.; Zhang, Y.; Huang, W.; Tang, Y.; Hu, L.; Huang, P.; Zhang, X.; Zhang, H. Van der Waals Integration of Bismuth Quantum Dots–Decorated Tellurium Nanotubes (Te@Bi) Heterojunctions and Plasma-Enhanced Optoelectronic Applications. Small 2019, 15, 1903233. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Y.; Yuan, X.; Zhang, F.; Huang, W.; Ma, D.; Zhao, J.; Wang, Y.; Ge, Y.; Huang, H.; et al. 1D@0D hybrid dimensional heterojunction-based photonics logical gate and isolator. Appl. Mater. Today 2020, 19, 100589. [Google Scholar] [CrossRef]
- Seo, J.U.; Seong, G.K.; Park, C.M. Te/C nanocomposites for Li-Te Secondary Batteries. Sci. Rep. 2015, 5, 7969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, B.; Fan, H.; Cheng, X.-B.; Yan, X.; Hong, S.; Dong, Q.; Gao, C.; Zhang, Z.; Lai, Y.; Zhang, Q. Spatially Uniform Deposition of Lithium Metal in 3D Janus Hosts. Energy Storage Mater. 2019, 16, 259–266. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.; Yoon, J.; Um, J.H.; Lee, W.; Kim, J.; Cabana, J.; Yoon, W.S. Exploring Anomalous Charge Storage in Anode Materials for Next-Generation Li Rechargeable Batteries. Chem. Rev. 2020, 120, 6934–6976. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y. Flexible Tellurium-Based Electrode for High-Performance Lithium-Tellurium Battery. Nanomaterials 2021, 11, 2903. https://doi.org/10.3390/nano11112903
Li Y, Zhang Y. Flexible Tellurium-Based Electrode for High-Performance Lithium-Tellurium Battery. Nanomaterials. 2021; 11(11):2903. https://doi.org/10.3390/nano11112903
Chicago/Turabian StyleLi, Yan, and Ye Zhang. 2021. "Flexible Tellurium-Based Electrode for High-Performance Lithium-Tellurium Battery" Nanomaterials 11, no. 11: 2903. https://doi.org/10.3390/nano11112903
APA StyleLi, Y., & Zhang, Y. (2021). Flexible Tellurium-Based Electrode for High-Performance Lithium-Tellurium Battery. Nanomaterials, 11(11), 2903. https://doi.org/10.3390/nano11112903






