Bacitracin-Ag Nanoclusters as a Novel Antibacterial Agent Combats Shigella flexneri by Disrupting Cell Membrane and Inhibiting Biofilm Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Materials
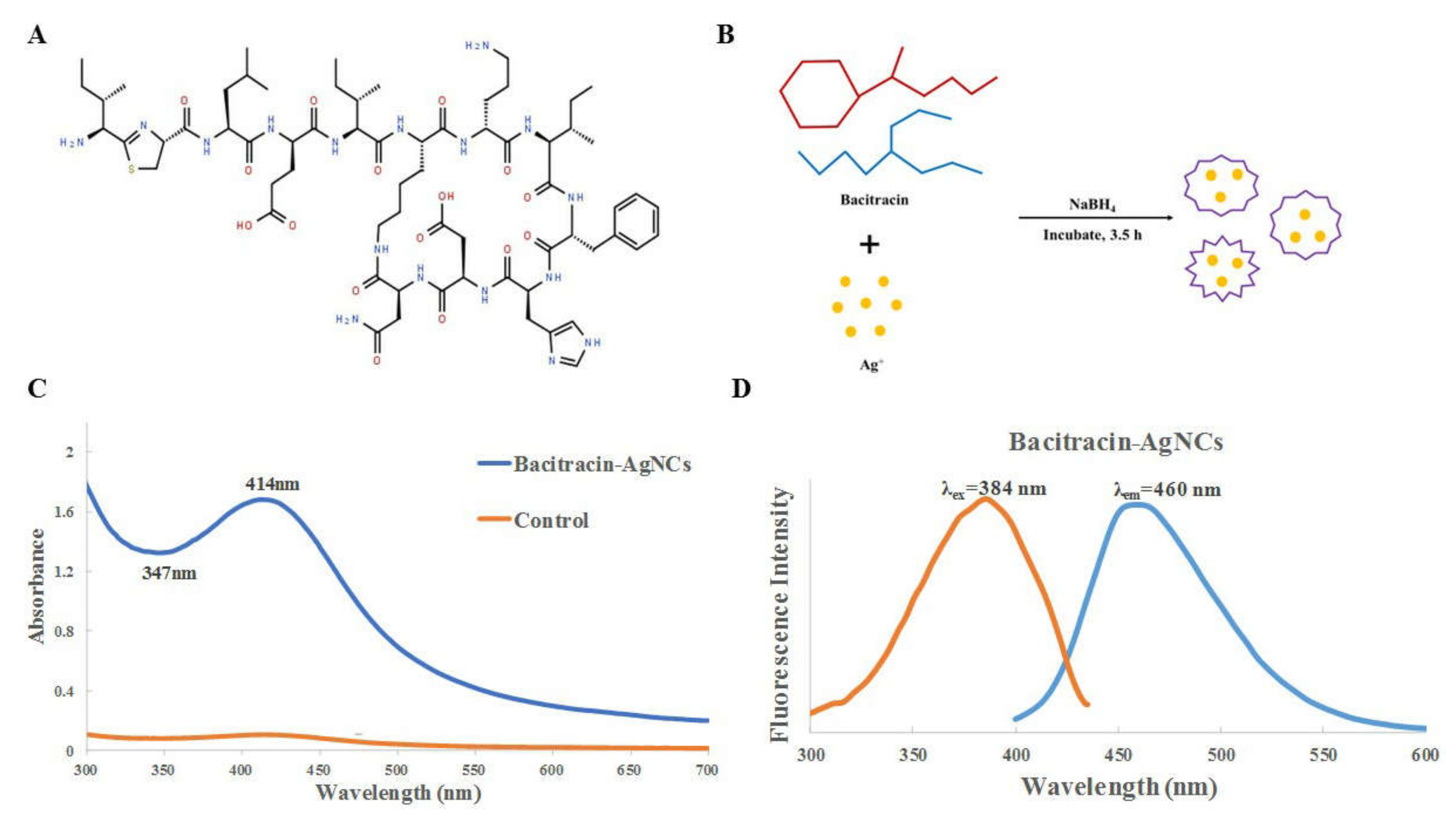
2.2. Synthesis, Purification and Characterization of Bacitracin-AgNCs
2.3. Antibacterial Activity on S. flexneri
2.3.1. LB Liquid Medium Turbidity Assay
2.3.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.3.3. Growth Curve Assay
2.4. Antibacterial Mechanism of Bacitracin-AgNCs against S. flexneri
2.4.1. Release of Cell Constituents
2.4.2. Membrane Permeability Assays
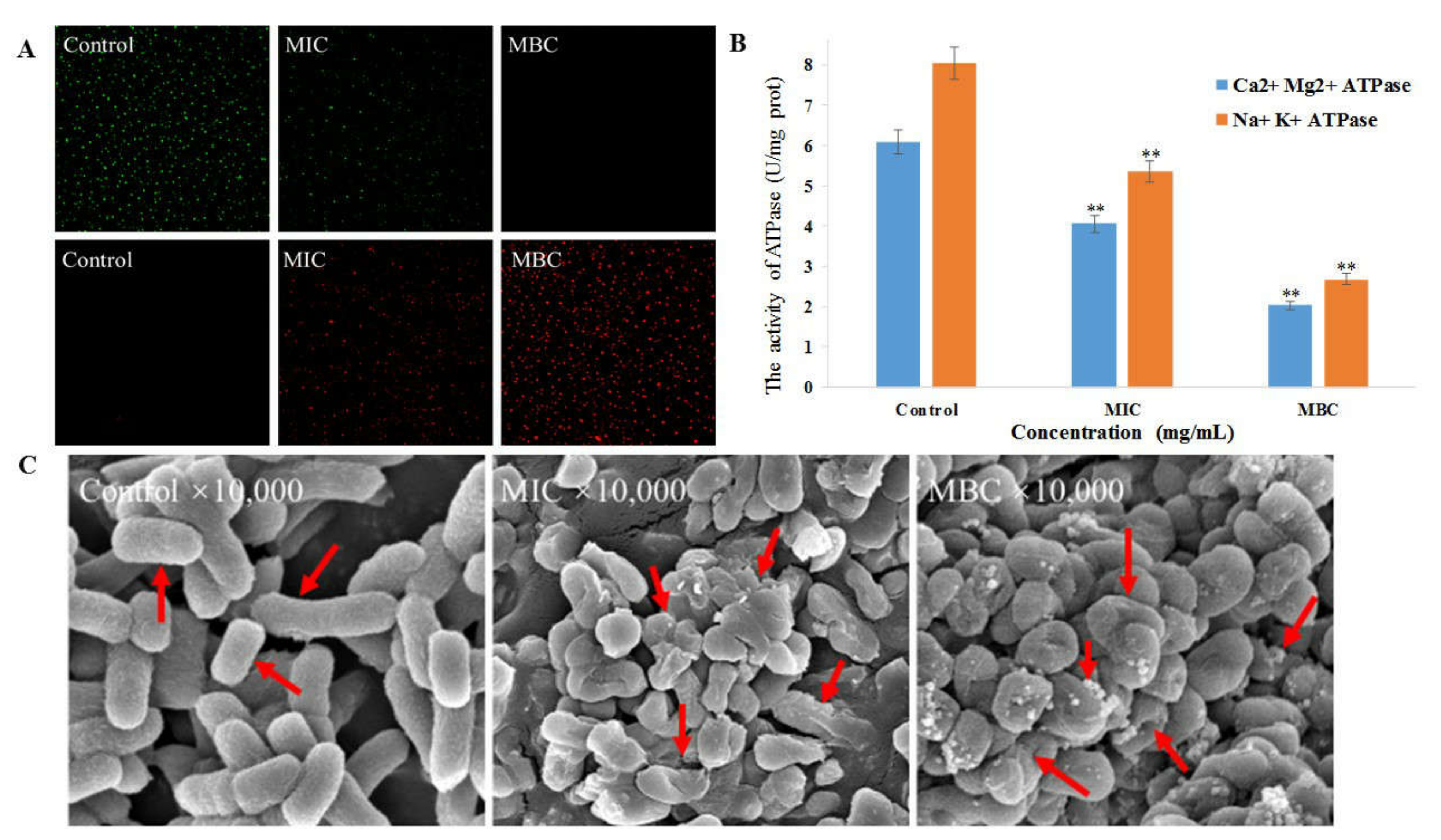
2.4.3. Confocal Laser Scanning Microscopy (CLSM) Assay
2.4.4. Determination of Cellular Na+ K+- and Ca2+ Mg2+-ATPase Activity
2.4.5. Field-Emission Scanning Electron Microscopy (FESEM) Analyses
2.5. Inhibition of Biofilm Formation by Bacitracin-AgNCs
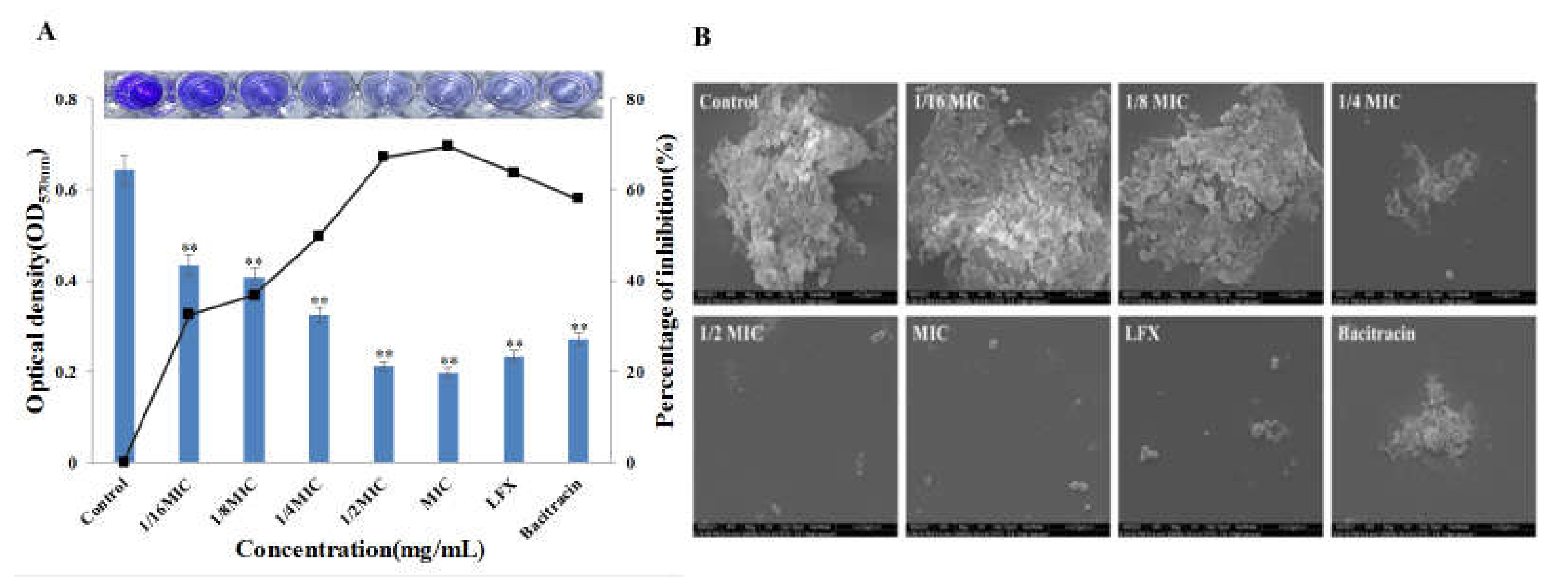
2.5.1. Biofilm Formation and Quantitative Crystal Violet Assay
2.5.2. Environment Scanning Electron Microscopy (ESEM) Analysis
2.6. Statistical Analysis
3. Results
3.1. Synthesis and Fabrication of Bacitracin-AgNCs
3.2. Characterization of Bacitracin-AgNCs
3.2.1. UV-VIS Spectra and Fluorescence Spectroscopy
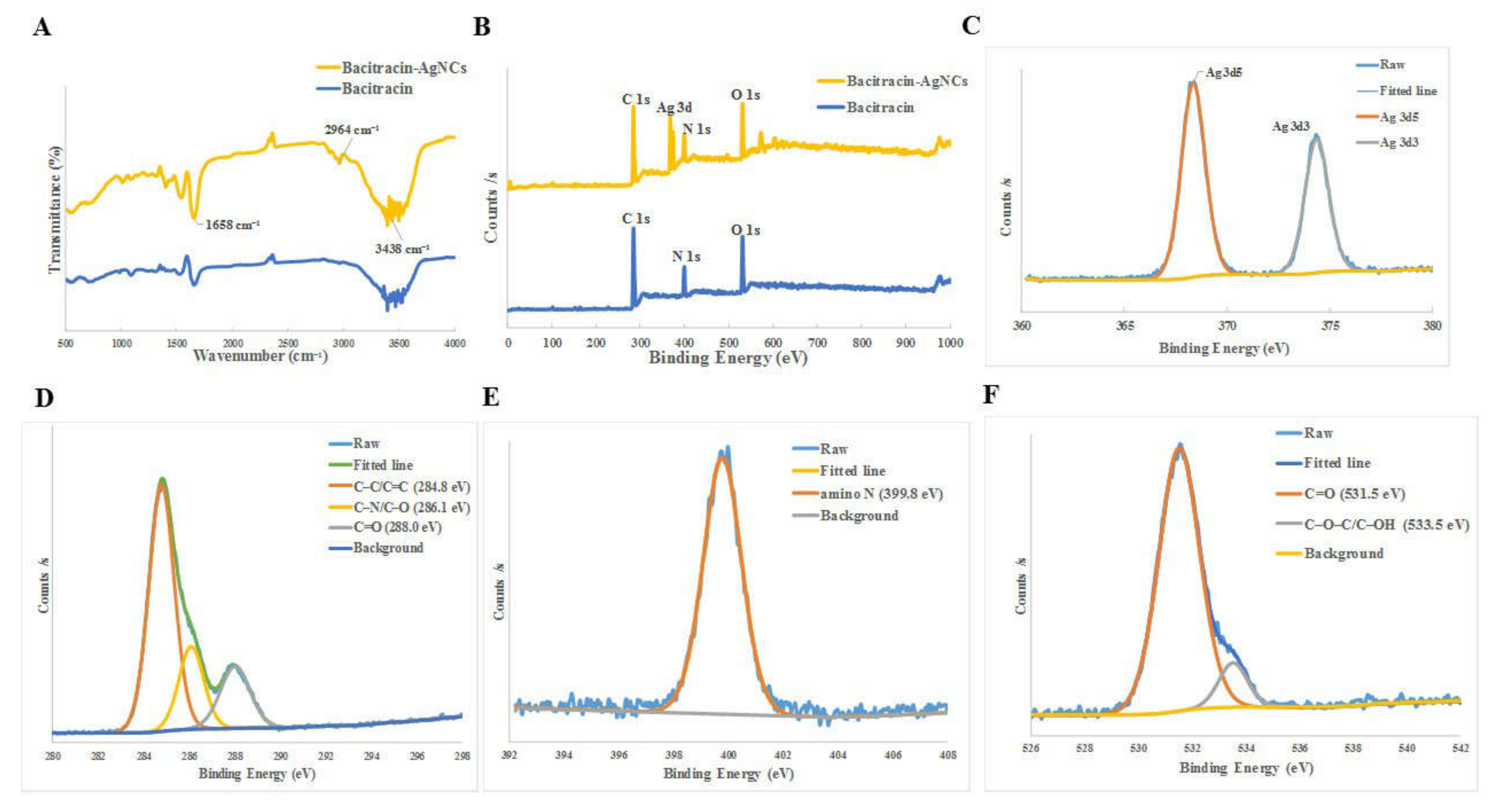
3.2.2. Fourier Transform Infrared Spectrometer (FT-IR) and X-ray Photoelectron Spectroscopy (XPS)
3.2.3. FESEM and HR-TEM of Bacitracin-AgNCs
3.2.4. Thermogravimetric Analysis (TGA)
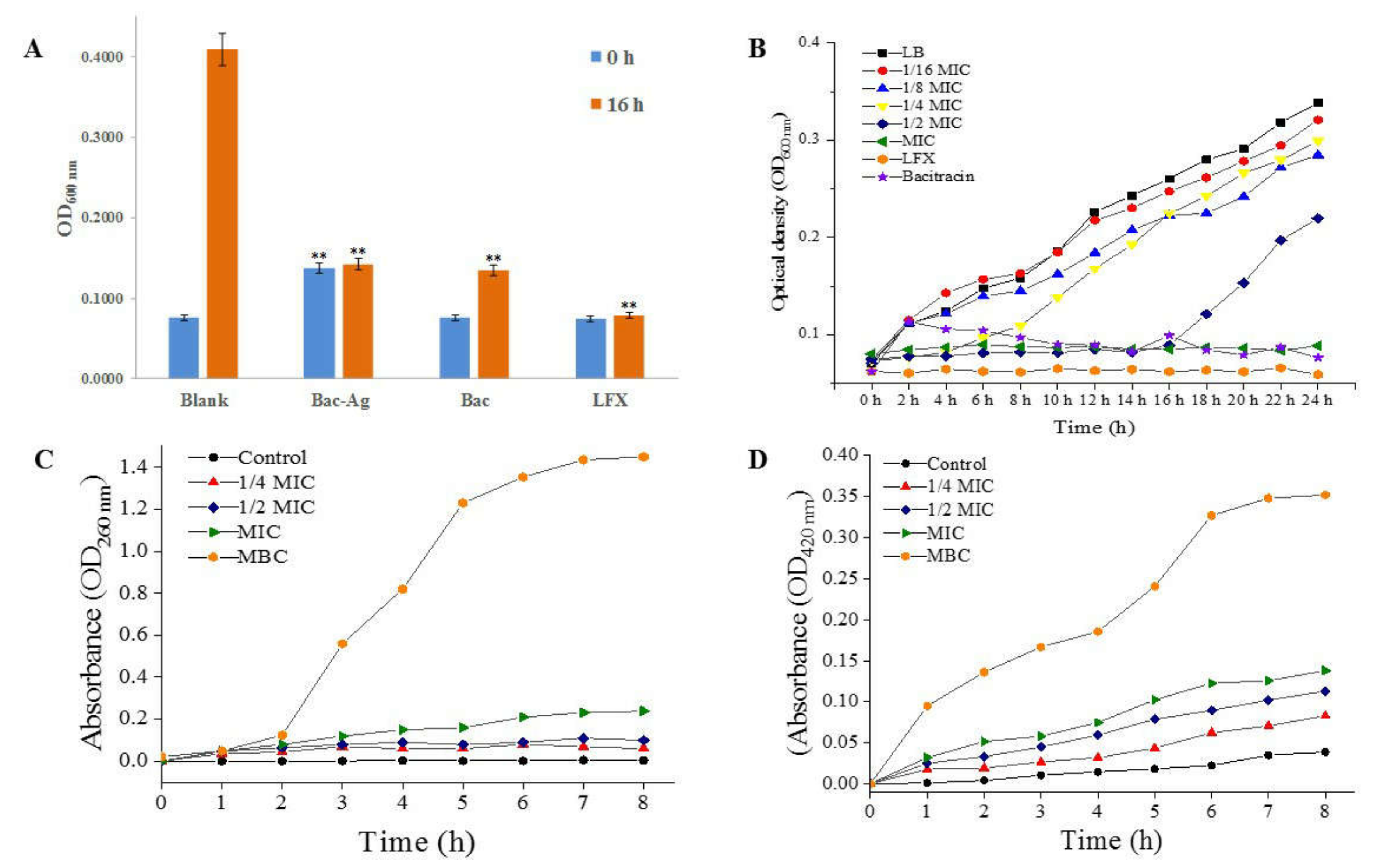
3.3. Antibacterial Activity of Bacitracin-AgNCs against S. flexneri
3.3.1. Turbidity Test Results
3.3.2. MIC, MBC of Bacitracin-AgNCs and Growth Curves
3.4. Antibacterial Mechanism
3.4.1. The Leakage of Intracellular Nucleotide
3.4.2. Effect of Bacitracin-AgNCs on S. flexneri Cell Membrane Permeability
3.4.3. CLSM Observation of Bacitracin-AgNCs on S. flexneri Cell
3.4.4. Cellular Na+ K+- and Ca2+ Mg2+-ATPase Activity
3.4.5. Field-Emission Scanning Electron Microscopy (FESEM) Analyses
3.5. Effect of Bacitracin-AgNCs on Formation of S. flexneri Biofilm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella Isolates From the Global Enteric Multicenter Study Inform Vaccine Development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef]
- Rahimi, E.; Shirazi, F.; Khamesipour, F. Isolation and Study of the Antibiotic Resistance Properties of Shigella Species in Meat and Meat Products. J. Food Process. Preserv. 2016, 41, e12947. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Molecular characterization of multidrug-resistant Shigella spp. of food origin. Int. J. Food Microbiol. 2015, 194, 108252. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Laverty, G.; Mccloskey, A.P.; Gilmore, B.F.; Jones, D.S.; Zhou, J.; Xu, B. Ultrashort cationic naphthalene-derived self-assembled peptides as antimicrobial nanomaterials. Biomacromolecules 2014, 15, 3429. [Google Scholar] [CrossRef] [Green Version]
- Nemamcha, A.; Rehspringer, J.-L.; Khatmi, D. Synthesis of palladium nanoparticles by sonochemical reduction of palladium (II) nitrate in aqueous solution. J. Phys. Chem. B 2006, 110, 383–387. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y. Synthesis of calcium phosphate microcapsules using yeast-based biotemplate. J. Mater. Chem. 2012, 22, 626–630. [Google Scholar] [CrossRef]
- Guo, C.; Irudayaraj, J. Fluorescent Ag clusters via a protein-directed approach as a Hg (II) ion sensor. Anal. Chem. 2011, 83, 2883–2889. [Google Scholar] [CrossRef]
- Wang, C.; Xing, K.; Zhang, G.; Yuan, M.; Xu, S.; Liu, D.; Chen, W.; Peng, J.; Hu, S.; Lai, W.-H. Novel ELISA based on fluorescent quenching of DNA-stabilized silver nanoclusters for detecting E. coli O157: H7. Food Chem. 2019, 281, 91–96. [Google Scholar] [CrossRef]
- Xue, W.; Li, Y.; Zhou, J.; Wang, Z.; Liu, Y.; Zhang, X.; Liu, Z.; Gao, F.; Gao, D. Self-assembly of adenovirus-templated platinum nanoshells and evaluation of their biocompatibilities. Rsc Adv. 2015, 5, 86381–86386. [Google Scholar] [CrossRef]
- Mandal, S.M.; Roy, A.; Mahata, D.; Migliolo, L.; Nolasco, D.O.; Franco, O.L. Functional and structural insights on self-assembled nanofiber-based novel antibacterial ointment from antimicrobial peptides, bacitracin and gramicidin S. J. Antibiot. 2014, 67, 771–775. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Puca, V.; Traini, T.; Guarnieri, S.; Carradori, S.; Sisto, F.; Macchione, N.; Muraro, R.; Mincione, G.; Grande, R. The antibiofilm effect of a medical device containing TIAB on microorganisms associated with surgical site infection. Molecules 2019, 24, 2280. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Y.; Peng, Y.; Yang, X. Exploring the antibacteria performance of multicolor Ag, Au, and Cu nanoclusters. ACS Appl. Mater. Interfaces 2019, 11, 8461–8469. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Jiang, X. Aminosaccharide–gold nanoparticle assemblies as narrow-spectrum antibiotics against methicillin-resistant Staphylococcus aureus. Nano Res. 2018, 11, 6237–6243. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Kai, Z.; Huang, Y.; Hong, G. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Hao, T.; Li, S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Sugimoto, Y.; Aoki, T. Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim. Biophys. Acta Gen. Subj. 2000, 1523, 196–205. [Google Scholar] [CrossRef]
- Otto, C.C.; Cunningham, T.M.; Hansen, M.R.; Haydel, S.E. Effects of antibacterial mineral leachates on the cellular ultrastructure, morphology, and membrane integrity of Escherichia coli and methicillin-resistant Staphylococcus aureus. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Kulshrestha, S.; Khan, S.; Hasan, S.; Khan, M.E.; Misba, L.; Khan, A.U. Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: An in vitro and in vivo approach. Appl. Microbiol. Biotechnol. 2015, 100, 1901–1914. [Google Scholar] [CrossRef]
- Sivaranjani, M.; Prakash, M.; Gowrishankar, S.; Rathna, J.; Pandian, S.K.; Ravi, A.V. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl. Microbiol. Biotechnol. 2017, 101, 3349. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Jin, W.; Chen, Q.; Cai, Y.; Zhu, Q.; Zhang, W. Antibacterial activity of silver nanoparticles with different morphologies as well as their possible antibacterial mechanism. Appl. Phys. A 2016, 122, 874. [Google Scholar] [CrossRef]
- Yang, K.; Liu, M.; Wang, Y.; Wang, S.; Miao, H.; Yang, L.; Yang, X. Carbon dots derived from fungus for sensing hyaluronic acid and hyaluronidase. Sens. Actuators B Chem. 2017, 251, 503–508. [Google Scholar] [CrossRef]
- Ni, X.; Wang, J.; Yue, Y.; Cheng, W.; Wang, D.; Han, G. Enhanced Antibacterial Performance and Cytocompatibility of Silver Nanoparticles Stabilized by Cellulose Nanocrystal Grafted with Chito-Oligosaccharides. Materials 2018, 11, 1339. [Google Scholar] [CrossRef] [Green Version]
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Jing, X. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.-S.; Xiong, H.-M. Nitrogen and sulfur co-doped carbon dots with strong blue luminescence. Nanoscale 2014, 6, 13817–13823. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. Acs Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Basiruddin, S.; Chakraborty, A. One step synthesis of maltose functionalized red fluorescent Ag cluster for specific glycoprotein detection and cellular imaging probe. RSC Adv. 2014, 4, 43098–43104. [Google Scholar] [CrossRef]
- Lin, S.; Chen, L.; Huang, L.; Cao, S.; Luo, X.; Liu, K. Novel antimicrobial chitosan-cellulose composite films bioconjugated with silver nanoparticles. Ind. Crop. Prod. 2015, 70, 395–403. [Google Scholar] [CrossRef]
- Rao, K.; Reddy, P.R.; Lee, Y.I.; Kim, C. Synthesis and characterization of chitosan–PEG–Ag nanocomposites for antimicrobial application. Carbohydr. Polym. 2012, 87, 920–925. [Google Scholar]
- Hu, S.; Trinchi, A.; Atkin, P.; Cole, I. Tunable Photoluminescence Across the Entire Visible Spectrum from Carbon Dots Excited by White Light. Angew. Chem. Int. Ed. 2014, 54, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.J.; Epperson, J.D. Metal binding and structure-activity relationship of the metalloantibiotic peptide bacitracin. J. Inorg. Biochem. 2002, 91, 46–58. [Google Scholar] [CrossRef]
- Qi, Z.D.; Lin, Y.; Zhou, B.; Ren, X.D.; Pang, D.W.; Liu, Y. Characterization of the Mechanism of the Staphylococcus aureus Cell Envelope by Bacitracin and Bacitracin-Metal Ions. J. Membr. Biol. 2008, 225, 27. [Google Scholar] [CrossRef]
- Du, J.; Hu, Z.; Yu, Z.; Li, H.; Pan, J.; Zhao, D.; Bai, Y. Antibacterial activity of a novel Forsythia suspensa fruit mediated green silver nanoparticles against food-borne pathogens and mechanisms investigation. Mater. Sci. Eng. C 2019, 102, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; García, S.; Heredia, N. Extracts of Edible and Medicinal Plants Damage Membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Li, J.; Yang, L.; Fang, S.; Ming, Y. Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control 2016, 73, 1445–1451. [Google Scholar] [CrossRef]
- Rurián-Henares, J.; Morales, F.J. Antimicrobial activity of melanoidins against Escherichia coli is mediated by a membrane-damage mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Wang, H.; Song, Z.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Lee, D.-S.; Je, J.-Y. Gallic Acid-Grafted-Chitosan Inhibits Foodborne Pathogens by a Membrane Damage Mechanism. J. Agric. Food Chem. 2013, 61, 6574–6579. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Vadivel, V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 8279–8297. [Google Scholar] [CrossRef]
- Zawadzka, K.; Kisielewska, A.; Piwoński, I.; Kądzioła, K.; Lisowska, K. Mechanisms of antibacterial activity and stability of silver nanoparticles grown on magnetron sputtered TiO2 coatings. Bull. Mater. Sci. 2016, 39, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Pei, H.; Han, Z.; Feng, G.; Li, D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of ε-Polylysine and nisin against Bacillus subtilis. Food Control 2015, 47, 444–450. [Google Scholar] [CrossRef]
- Oh, I.; Yang, W.Y.; Park, J.; Lee, S.; Mar, W.; Oh, K.B.; Shin, J. In vitro Na+/K+-ATPase inhibitory activity and antimicrobial activity of sesquiterpenes isolated from Thujopsis dolabrata. Arch. Pharmacal Res. 2011, 34, 2141. [Google Scholar] [CrossRef]
- Serra, D.O.; Hengge, R. Bacterial Multicellularity: The Biology of Escherichia coli Building Large-Scale Biofilm Communities. Annu. Rev. Microbiol. 2021, 75, 269–290. [Google Scholar] [CrossRef]
- Mishra, S.K.; Raveendran, S.; Ferreira, J.; Kannan, S. In Situ Impregnation of Silver Nanoclusters in Microporous Chitosan-PEG Membranes as an Antibacterial and Drug Delivery Percutaneous Device. Langmuir ACS J. Surf. Colloids 2016, 32, 10305–10316. [Google Scholar] [CrossRef]
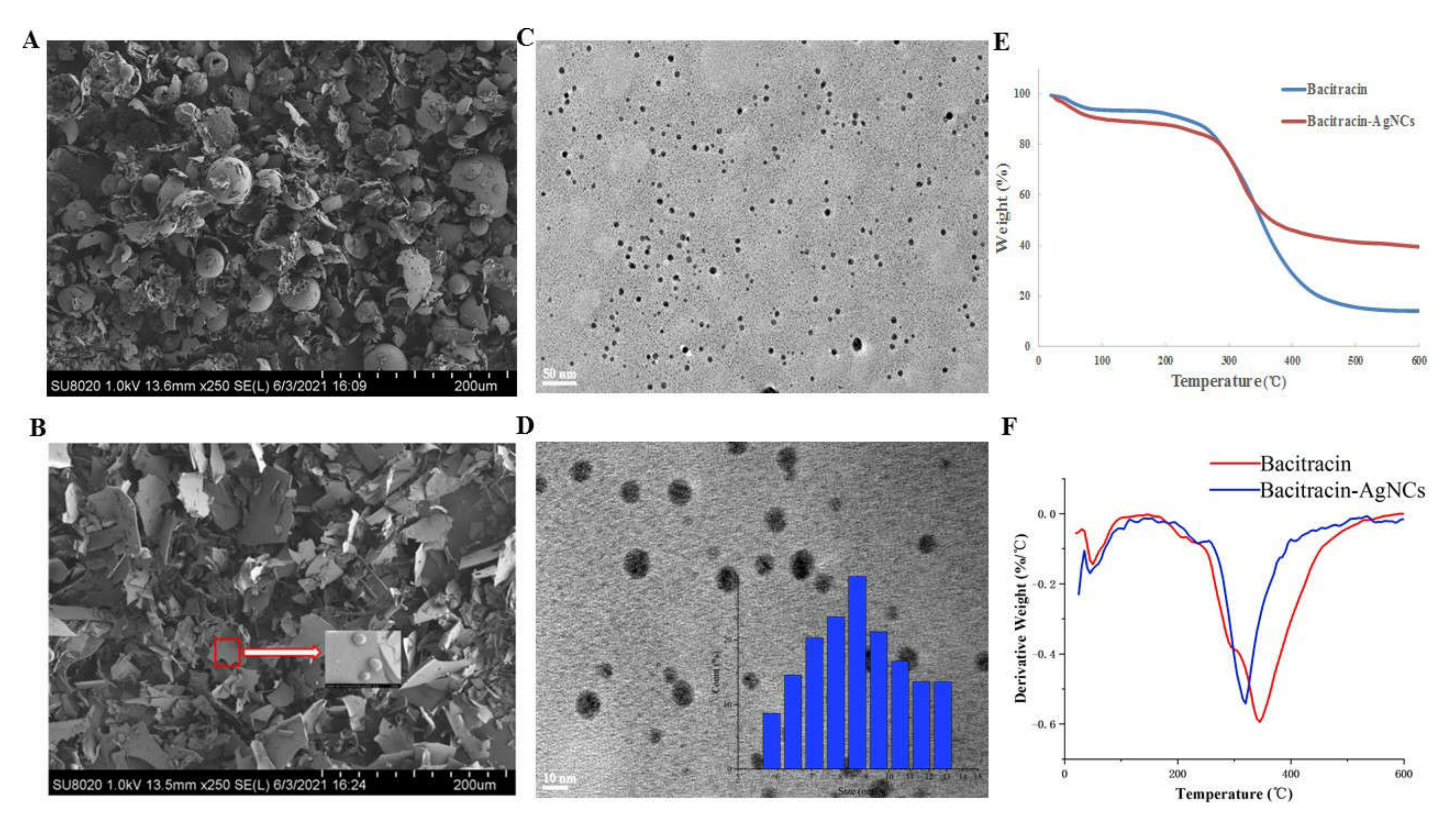
| Code | Tonset (°C) | Tmax (°C) |
|---|---|---|
| Bacitracin | 168.61 | 345.16 |
| Bacitracin-AgNCs | 155.89 | 319.71 |
| Bacteria | Concentrations of Bacitracin-AgNCs (mg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.015625 | 0.03125 | 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| Shigella flexneri | ++ | ++ | + | + | + | + | + | + | + | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, L.; Zhou, X. Bacitracin-Ag Nanoclusters as a Novel Antibacterial Agent Combats Shigella flexneri by Disrupting Cell Membrane and Inhibiting Biofilm Formation. Nanomaterials 2021, 11, 2928. https://doi.org/10.3390/nano11112928
Wang L, Liu L, Zhou X. Bacitracin-Ag Nanoclusters as a Novel Antibacterial Agent Combats Shigella flexneri by Disrupting Cell Membrane and Inhibiting Biofilm Formation. Nanomaterials. 2021; 11(11):2928. https://doi.org/10.3390/nano11112928
Chicago/Turabian StyleWang, Lin, Liu Liu, and Xiaotong Zhou. 2021. "Bacitracin-Ag Nanoclusters as a Novel Antibacterial Agent Combats Shigella flexneri by Disrupting Cell Membrane and Inhibiting Biofilm Formation" Nanomaterials 11, no. 11: 2928. https://doi.org/10.3390/nano11112928
APA StyleWang, L., Liu, L., & Zhou, X. (2021). Bacitracin-Ag Nanoclusters as a Novel Antibacterial Agent Combats Shigella flexneri by Disrupting Cell Membrane and Inhibiting Biofilm Formation. Nanomaterials, 11(11), 2928. https://doi.org/10.3390/nano11112928






