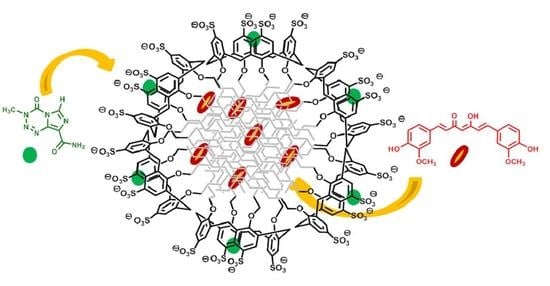
Co-Loading of Temozolomide and Curcumin into a Calix[4]arene-Based Nanocontainer for Potential Combined Chemotherapy: Binding Features, Enhanced Drug Solubility and Stability in Aqueous Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
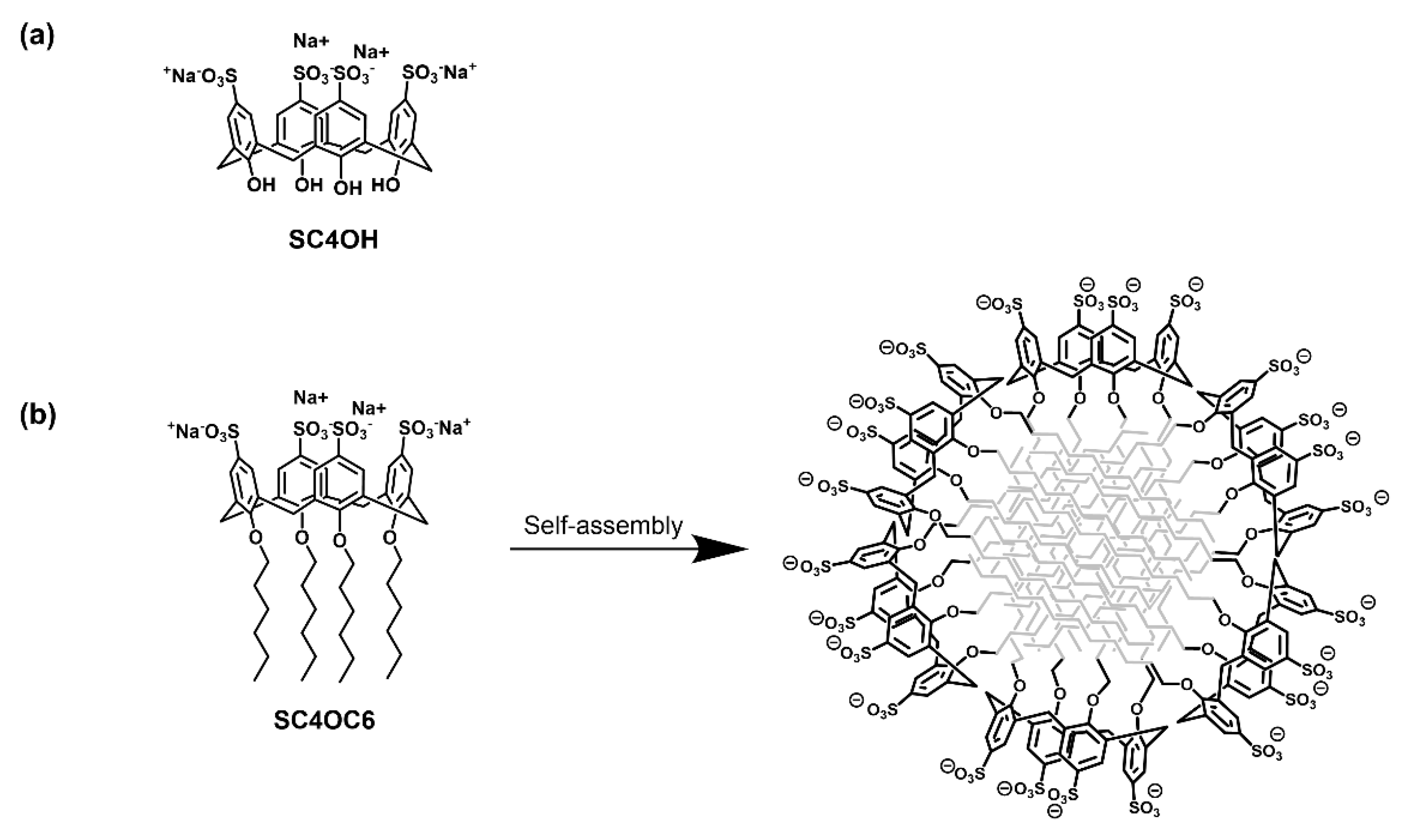
2.3. Synthesis and Characterization of SC4OC6
2.4. Preparation and Characterization of the Micellar SC4OC6 Nanoparticles
2.5. ITC Titrations
2.6. Loading of TMZ in Micellar SC4OC6 Nanoparticles
2.7. Loading of CUR in Micellar SC4OC6 Nanoparticles
2.8. Loading of TMZ in SC4OC6 /CUR Nanoassembly
2.9. Determination of Loaded TMZ and CUR
2.10. CUR Loading Capacity% (LC%)
2.11. TMZ and CUR Stability
3. Results and Discussion
3.1. Preparation and Characterization of the Micellar SC4OC6 Nanoassembly
3.2. Preparation and Characterization of the SC4OC6/CUR Nanoassembly
3.2.1. Solubility of CUR in the Presence of Micellar SC4OC6 Nanoparticle
3.2.2. Stability of CUR in the SC4OC6 Nanoassembly
3.2.3. Dimensional Analysis and Zeta Potential of the SC4OC6/CUR Nanoassembly
3.3. Preparation and Characterization of the SC4OC6/TMZ Nanoassembly
3.3.1. Solubility of TMZ in the Presence of the Micellar SC4OC6 Nanoparticle
3.3.2. Characterization of SC4OC6/TMZ Inclusion Complex
3.3.3. Dimensional Analysis and Zeta Potential of the SC4OC6/TMZ Complex
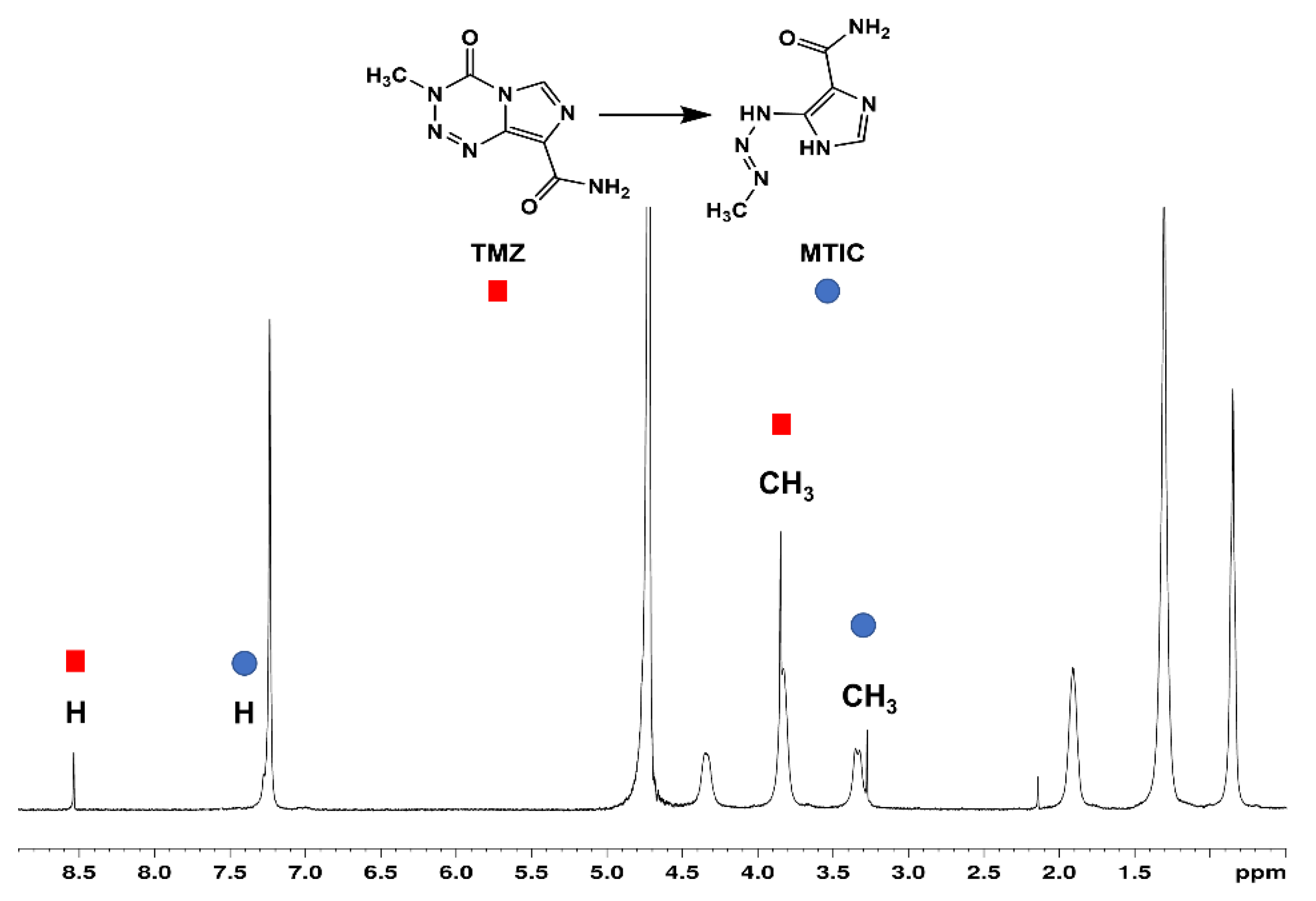
3.3.4. Stability of TMZ when Complexed with SC4OC6
3.4. Co-Entrapment of TMZ and CUR in the SC4OC6 Nanocontainer
Dimensional and Zeta Potential Analysis of SC4OC6/CUR/TMZ
3.5. Solution Thermodynamics of the SC4OC6/TMZ and SC4OC6/CUR/TMZ Complexes Formation
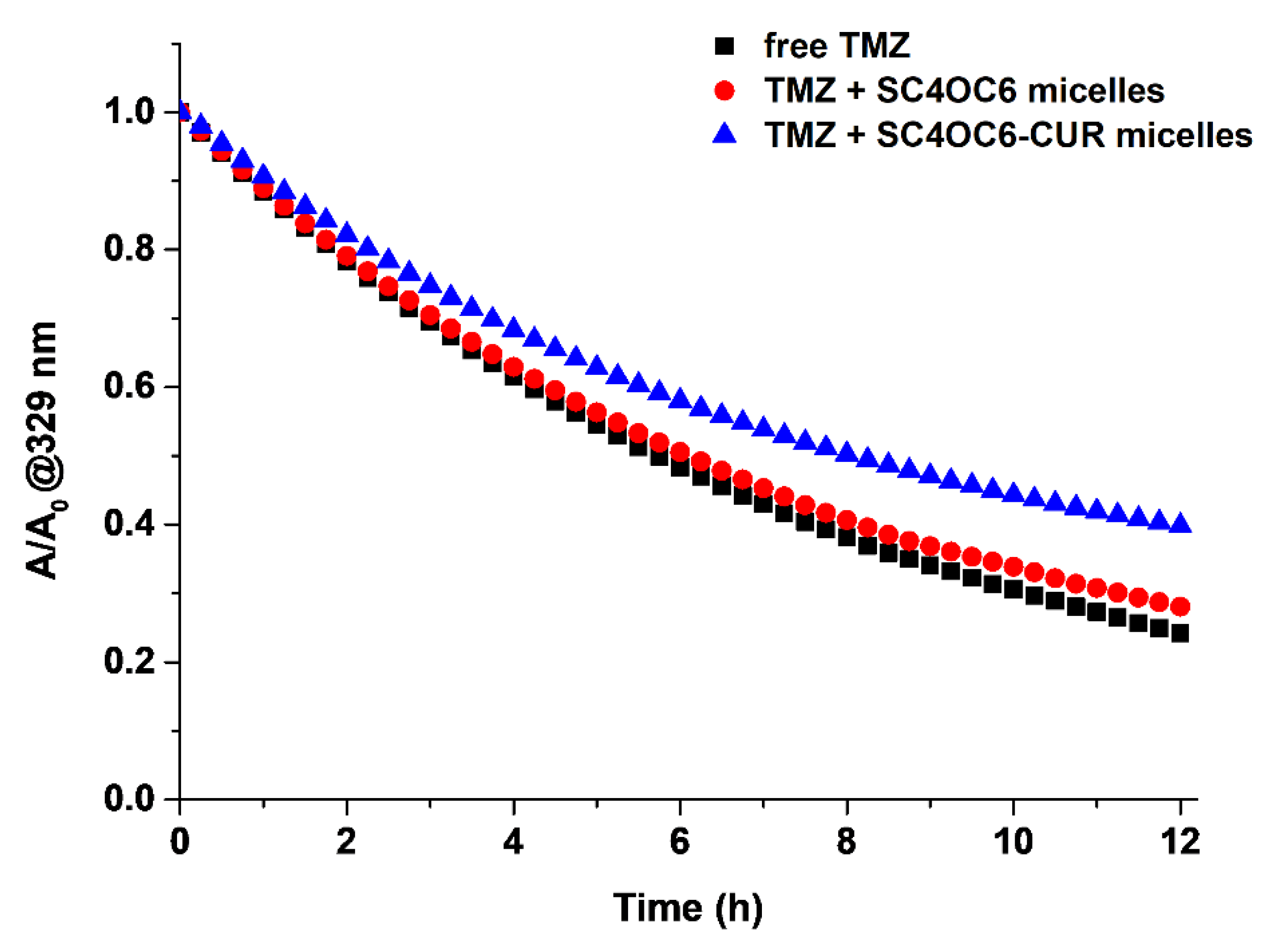
3.6. Stability of TMZ in the Ternary SC4OC6/CUR/TMZ Assembly
3.7. Potential of SC4OC6/CUR/TMZ in Nanocarrier-Based Combined Chemotherapy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug Combination in Cancer Treatment—From Cocktails to Conjugate Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mat. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent Advances of Cocktail Chemotherapy by Combination Drug Delivery Systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Zhou, Y.; Wen, C.; Zhou, C.; Zhang, W.; Hu, X.; Wang, L.; You, C.; Shao, J. Curcumin sensitizes glioblastoma to temozolomide by simultaneously generating ROS and disrupting AKT/mTOR signaling. Oncol. Rep. 2014, 32, 1610–1616. [Google Scholar] [CrossRef]
- Huang, B.-R.; Tsai, C.-H.; Chen, C.-C.; Way, T.-D.; Kao, J.-Y.; Liu, Y.S.; Lin, H.-Y.; Lai, S.-W.; Lu, D.-Y. Curcumin Promotes Connexin 43 Degradation and Temozolomide-Induced Apoptosis in Glioblastoma Cells. Am. J. Chin. Med. 2019, 47, 657–674. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Figueiró, K.F.; Terra, S.R.; Paludo, F.J.; Morrone, M.; Bristot, I.J.; Battastini, A.M.; Forcelini, C.M.; Bishop, A.J.R.; et al. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015, 358, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.; Rohani, S.; Gillies, E.R. Curcumin, a promising anti-cancer therapeutic: A review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Adv. 2014, 4, 10815–10829. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Moody, C.L.; Wheelhouse, R.T. The Medicinal Chemistry of Imidazotetrazine Prodrugs. Pharmaceuticals 2014, 7, 797–838. [Google Scholar] [CrossRef] [Green Version]
- Newlands, E.S.; Stevens, M.F.G.; Wedge, S.R.; Wheelhouse, R.T.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef]
- Lopes, I.C.; de Oliveira, S.C.B.; Oliveira-Brett, A.M. Temozolomide chemical degradation to 5-aminoimidazole-4-carboxamide–electrochemical study. J. Electroanal. Chem. 2013, 704, 183–189. [Google Scholar] [CrossRef]
- Newlands, E.S.; Blackledge, G.R.P.; Slack, J.A.; Rustin, G.J.; Smith, D.B.; Stuart, N.S.; Quarterman, C.P.; Hoffman, R.; Stevens, M.F.G.; Brampton, M.H.; et al. Phase i trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br. J. Cancer 1992, 65, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Gürten, B.; Yenigül, E.; Sezer, A.D.; Malta, S. Complexation and enhancement of temozolomide solubility with cyclodextrins. Braz. J. Pharm. Sci. 2018, 54, e17513. [Google Scholar] [CrossRef]
- Krzak, A.; Bilewicz, R. Voltammetric/UV–Vis study of temozolomide inclusion complexes with cyclodextrin derivative. Bioelectrochemistry 2020, 136, 107587. [Google Scholar] [CrossRef]
- Appel, E.A.; Rowland, M.J.; Loh, X.J.; Heywood, R.M.; Watts, C.; Scherman, O.A. Enhanced stability and activity of temozolomide in primary glioblastoma multiforme cells with cucurbit[n]uril. Chem. Commun. 2012, 48, 9843–9845. [Google Scholar] [CrossRef]
- Renziehausen, A.; Tsiailanis, A.D.; Perryman, R.; Stylos, E.K.; Chatzigiannis, C.; O’Neill, K.; Crook, T.; Tzakos, A.G.; Syed, N. Encapsulation of Temozolomide in a Calixarene Nanocapsule Improves Its Stability and Enhances Its Therapeutic Efficacy against Glioblastoma. Mol. Cancer Ther. 2019, 18, 1497–1505. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Li, R.-J.; Huang, C.-Y.; Wei, K.-C.; Chen, P.-Y. Controlled release of liposome-encapsulated temozolomide for brain tumour treatment by convection-enhanced delivery. J. Drug Target. 2018, 36, 325–332. [Google Scholar] [CrossRef]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Li, G.; Zhang, H.; Chen, X.; Li, Y.; Yao, Q.; Xie, M. Sequential delivery of dual drugs with nanostructured lipid carriers for improving synergistic tumor treatment effect. Drug Deliv. 2020, 27, 983–995. [Google Scholar] [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Purcar, V. Curcumin: Modern Applications for a Versatile Additive. Coatings 2021, 11, 519. [Google Scholar] [CrossRef]
- Shabaninejad, Z.; Pourhanifeh, M.H.; Movahedpour, A.; Mottaghi, R.; Nickdasti, A.; Mortezapour, E.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; Sadeghian, M.; et al. Therapeutic potentials of curcumin in the treatment of glioblastoma. Eur. J. Med. Chem. 2020, 188, 112040. [Google Scholar] [CrossRef]
- Batra, H.; Pawar, S.; Bahld, D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharmacol. Res. 2019, 139, 91–105. [Google Scholar] [CrossRef]
- Nasery, M.M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Guo, X. Development of calixarene-based drug nanocarriers. J. Mol. Liq. 2021, 325, 115246. [Google Scholar] [CrossRef]
- Hoskins, C.; Curtis, A.D.M. Simple Calix[n]arenes and Calix[4]resorcinarenes as Drug Solubilizing Agents. J. Nanomed. Res. 2015, 2, 00028. [Google Scholar] [CrossRef]
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.M.L. Potential Eye Drop based on a Calix[4]arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability and Anti-Inflammatory Effect. Mol. Pharm. 2017, 14, 1610–1622. [Google Scholar] [CrossRef]
- Noruzi, E.B.; Molaparast, M.; Zarei, M.; Shaabani, B.; Kariminezhad, Z.; Ebadi, B.; Shafiei-Irannejad, V.; Rahimi, M.; Pietrasik, J. Para-sulfonatocalix[n]arene-based biomaterials: Recent progress in pharmaceutical and biological applications. Eur. J. Med. Chem. 2020, 190, 112121. [Google Scholar] [CrossRef]
- Coleman, A.W.; Jebors, S.; Cecillon, S.; Perret, P.; Garin, D.; Marti-Battle, D.; Moulin, M. Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J. Chem. 2008, 32, 780–782. [Google Scholar] [CrossRef]
- Fraix, A.; Afonso, D.; Consoli, G.M.L.; Sortino, S. A calix[4]arene-based ternary supramolecular nanoassembly with improved fluoroquinolone photostability and enhanced NO photorelease. Photochem. Photobiol. Sci. 2019, 18, 2216–2224. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Guo, D.-S.; Duan, Y.-C.; Wang, Y.-J.; Liu, Y. Amphiphilic p-Sulfonatocalix[4]arene as ‘‘Drug Chaperone’’ for Escorting Anticancer Drugs. Sci. Rep. 2015, 5, 9019. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Fujii, F.; Sakata, H.; Tamura, M.; Kinjo, M. Amphiphilic p-sulfonatocalix[4]arene-coated CdSe/ZnS quantum dots for the optical detection of the neurotransmitter acetylcholine. Chem. Commun. 2005, 34, 4300–4302. [Google Scholar] [CrossRef]
- Hansen, L.D.; Fellingham, G.W.; Russell, D.J. Simultaneous determination of equilibrium constants and enthalpy changes by titration calorimetry: Methods, instruments, and uncertainties. Anal. Biochem. 2011, 409, 220–229. [Google Scholar] [CrossRef]
- Sgarlata, C.; Zito, V.; Arena, G. Conditions for calibration of an isothermal titration calorimeter using chemical reactions. Anal. Bioanal. Chem. 2013, 405, 1085–1094. [Google Scholar] [CrossRef]
- Loh, W.; Brinatti, C.; Tam, K.C. Use of isothermal titration calorimetry to study surfactant aggregation in colloidal systems. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 999–1016. [Google Scholar] [CrossRef]
- Arena, G.; Gans, P.; Sgarlata, C. HypCal, a general-purpose computer program for the determination of standard reaction enthalpy and binding constant values by means of calorimetry. Anal. Bioanal. Chem. 2016, 408, 6413–6422. [Google Scholar] [CrossRef]
- Ukhatskaya, E.V.; Kurkov, S.V.; Matthews, S.E.; El Fagui, A.; Amiel, C.; Dalmas, F.; Loftsson, T. Evaluation of a cationic calix[4]arene: Solubilization and self-aggregation ability. Int. J. Pharm. 2010, 402, 10–19. [Google Scholar] [CrossRef]
- Savva, M. Pharmaceutical Calculations; Springer International Publishing: Cham, Switzerland, 2019; pp. 275–284. [Google Scholar] [CrossRef]
- Kellermann, M.; Bauer, W.; Hirsch, A.; Schade, B.; Ludwig, K.; Böttcher, C. The First Account of a Structurally Persistent Micelle. Angew. Chem. Int. Ed. 2004, 43, 2959–2962. [Google Scholar] [CrossRef]
- Basilio, N.; García-Río, L.; Martín-Pastor, M. NMR Evidence of Slow Monomer—Micelle Exchange in a Calixarene-Based Surfactant. J. Phys. Chem. B 2010, 114, 4816–4820. [Google Scholar] [CrossRef]
- Basílio, N.; Garcia-Rio, L. Calixarene-Based Surfactants: Conformational-Dependent Solvation Shells for the Alkyl Chains. ChemPhysChem 2012, 13, 2368–2376. [Google Scholar] [CrossRef]
- Basilio, N.; García-Río, L.; Martin-Pastor, M. Calixarene-based surfactants: Evidence of structural reorganization upon micellization. Langmuir 2012, 28, 2404–2414. [Google Scholar] [CrossRef]
- Bouchemal, K.; Agnely, F.; Koffi, A.; Ponchel, G. A concise analysis of the effect of temperature and propanediol-1, 2 on Pluronic F127 micellization using isothermal titration microcalorimetry. J. Colloid Interface Sci. 2009, 338, 169–176. [Google Scholar] [CrossRef]
- Kamboj, R.; Bharmoria, P.; Chauhan, V.; Singh, S.; Kumar, A.; Mithu, V.S.; Kang, T.S. Micellization behavior of morpholinium-based amide-functionalized ionic liquids in aqueous media. Langmuir 2014, 30, 9920–9930. [Google Scholar] [CrossRef]
- Amado, E.; Augsten, C.; Mäder, K.; Blume, A.; Kressler, J. Amphiphilic water soluble triblock copolymers based on poly (2, 3-dihydroxypropyl methacrylate) and poly (propylene oxide): Synthesis by atom transfer radical polymerization and micellization in aqueous solutions. Macromolecules 2006, 39, 9486–9496. [Google Scholar] [CrossRef]
- Archer, W.R.; Schulz, M.D. Isothermal titration calorimetry: Practical approaches and current applications in soft matter. Soft Matter 2020, 16, 8760–8774. [Google Scholar] [CrossRef]
- Thongngam, M.; McClements, D.J. Influence of pH, ionic strength, and temperature on self-association and interactions of sodium dodecyl sulfate in the absence and presence of chitosan. Langmuir 2005, 21, 79–86. [Google Scholar] [CrossRef]
- Šarac, B.; Bešter-Rogač, M. Temperature and salt-induced micellization of dodecyltrimethylammonium chloride in aqueous solution: A thermodynamic study. J. Colloid Interface Sci. 2009, 338, 216–221. [Google Scholar] [CrossRef]
- Garidel, P.; Hildebrand, A. Thermodynamic properties of association colloids. J. Therm. Anal. Calorim. 2005, 82, 483–489. [Google Scholar] [CrossRef]
- Garidel, P.; Hildebrand, A.; Neubert, R.; Blume, A. Thermodynamic characterization of bile salt aggregation as a function of temperature and ionic strength using isothermal titration calorimetry. Langmuir 2000, 16, 5267–5275. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, R.; Medoš, Z.; Tošner, Z.; Zhigunov, A.; Uchman, M.; Hervø-Hansen, S.; Lund, M.; Bešter-Rogač, M.; Matĕjíček, P. Total Description of Intrinsic Amphiphile Aggregation: Calorimetry Study and Molecular Probing. Langmuir 2018, 34, 14448–14457. [Google Scholar] [CrossRef]
- De Neve, L.; York, M.; Dickens, J.; Leys, J.; Meurs, G.; Sinnaeve, D.; Van der Meeren, P. Molecular structure and ionic strength both affect the micellization and solubilization behavior of PEO-PPO-PEO surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 172–179. [Google Scholar] [CrossRef]
- He, Y.; Shang, Y.; Shao, S.; Liu, H.; Hu, Y. Micellization of cationic gemini surfactant and its interaction with DNA in dilute brine. J. Colloid Interface Sci. 2011, 358, 513–520. [Google Scholar] [CrossRef]
- Raju, B.B.; Winnik, F.M.; Morishima, Y. A look at the thermodynamics of the association of amphiphilic polyelectrolytes in aqueous solutions: Strengths and limitations of isothermal titration calorimetry. Langmuir 2001, 17, 4416–4421. [Google Scholar] [CrossRef]
- Makowska, J.; Wyrzykowski, D.; Pilarski, B.; Chmurzyński, L. Thermodynamics of sodium dodecyl sulphate (SDS) micellization in the presence of some biologically relevant pH buffers. J. Therm. Anal. Calorim. 2015, 121, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.X.; Tan, R.C.; Li, Y.L.; Yang, Y.Q.; Yu, L.; He, Q.C. Effect of salts on the formation of C8-lecithin micelles in aqueous solution. J. Colloid Interface Sci. 2001, 236, 28–34. [Google Scholar] [CrossRef]
- Mareeswaran, P.M.; Babu, E.; Sathish, V.; Kim, B.; Woo, S.I.; Rajagopal, S. p-Sulfonatocalix[4]arene as a carrier for curcumin. New J. Chem. 2014, 38, 1336–1345. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: UV–visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef]
- Migliore, R.; Granata, G.; Rivoli, A.; Consoli, G.M.L.; Sgarlata, C. Binding affinity and driving forces for the interaction of calixarene-based micellar aggregates with model antibiotics in neutral aqueous solution. Front. Chem. 2020, 8, 1227. [Google Scholar] [CrossRef]
- Kaur, R.; Rani, A.; Banipal, P.K.; Banipal, T.S. Study on interactions of vitamin B1 with sodium dodecyl sulfate for potential food applications: Conductometric, volumetric, calorimetric and spectroscopic approach. J. Mol. Liq. 2019, 285, 616–625. [Google Scholar] [CrossRef]
- Banipal, T.S.; Kaur, H.; Banipal, P.K. Studies on the binding ability of diclofenac sodium to cationic surfactants micelles in aqueous ethanol solutions. J. Therm. Anal. Calorim. 2017, 128, 501–511. [Google Scholar] [CrossRef]
- Kaur, R.; Banipal, P.K.; Banipal, T.S. Binding ability of sodium valproate with cationic surfactants and effect on micellization: Calorimetric, surface tension, light scattering and spectroscopic approach. J. Therm. Anal. Calorim. 2020, 140, 2833–2847. [Google Scholar] [CrossRef]
- Dasgupta, M.; Judy, E.; Kishore, N. Partitioning of anticancer drug 5-fluorouracil in micellar media explored by physicochemical properties and energetics of interactions: Quantitative insights for implications in drug delivery. Colloids Surf. B 2020, 187, 110730. [Google Scholar] [CrossRef] [PubMed]
- Maity, B.; Chatterjee, A.; Ahmed, S.A.; Seth, D. Interaction of the nonsteroidal anti-inflammatory drug indomethacin with micelles and its release. J. Phys. Chem. B 2015, 119, 3776–3785. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Brancatelli, G.; Forte, G.; Arena, G.; Geremia, S.; Sciotto, D.; Sgarlata, C. Factors driving the self-assembly of water-soluble calix[4]arene and gemini guests: A combined solution, computational and solid-state study. RSC Adv. 2014, 4, 53575–53587. [Google Scholar] [CrossRef]
- Giglio, V.; Sgarlata, C.; Vecchio, G. Novel amino-cyclodextrin crosslinked oligomer as efficient carrier for anionic drugs: A spectroscopic and nanocalorimetric investigation. RSC Adv. 2015, 5, 16664–16671. [Google Scholar] [CrossRef]
- Sgarlata, C.; Bonaccorso, C.; Gulino, F.G.; Zito, V.; Arena, G.; Sciotto, D. Stereochemistry and thermodynamics of the inclusion of aliphatic and aromatic anionic guests in a tetracationic calix[4]arene in acidic and neutral aqueous solutions. New J. Chem. 2009, 33, 991–997. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Ciadamidaro, A.; Zito, V.; Sgarlata, C.; Sciotto, D.; Arena, G. Molecular recognition of organic anions by a water-soluble calix[4]arene: Evidence for enthalpy–entropy compensation. Thermochim. Acta 2012, 530, 107–115. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Migliore, R.; Volkova, M.A.; Arena, G.; Sgarlata, C. Self-assembling of supramolecular adducts by sulfonato-calix[4]arene and pyridinium gemini guests in neutral aqueous solution. Thermochim. Acta 2017, 656, 47–52. [Google Scholar] [CrossRef]
- Mukhija, A.; Kishore, N. Partitioning of drugs in micelles and effect on micellization: Physicochemical insights with tryptophan and diclofenac sodium. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 204–214. [Google Scholar] [CrossRef]
- Choudhary, S.; Talele, P.; Kishore, N. Thermodynamic insights into drug–surfactant interactions: Study of the interactions of naproxen, diclofenac sodium, neomycin, and lincomycin with hexadecytrimethylammonium bromide by using isothermal titration calorimetry. Colloids Surf. B 2015, 132, 313–321. [Google Scholar] [CrossRef]
- Maeda, H.; Matsumura, Y. EPR effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 129–130. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Jin, Q.; Deng, Y.; Chen, X.; Ji, J. Rational Design of Cancer Nanomedicine for Simultaneous Stealth Surface and Enhanced Cellular Uptake. ACS Nano 2019, 13, 954–977. [Google Scholar] [CrossRef]
- Chen, M.X.; Li, T.; Peng, S.; Tao, D. Supramolecular nanocapsules from the self-assembly of amphiphilic calixarene as a carrier for paclitaxel. New J. Chem. 2016, 40, 9923–9929. [Google Scholar] [CrossRef]
- Jin, T.; Tsuboi, S.; Komatsuzaki, A.; Imamura, Y.; Muranaka, Y.; Sakata, T.; Yasuda, H. Enhancement of aqueous stability and fluorescence brightness of indocyanine green using small calix[4]arene micelles for near-infrared fluorescence imaging. Med. Chem. Commun. 2016, 7, 623–631. [Google Scholar] [CrossRef]





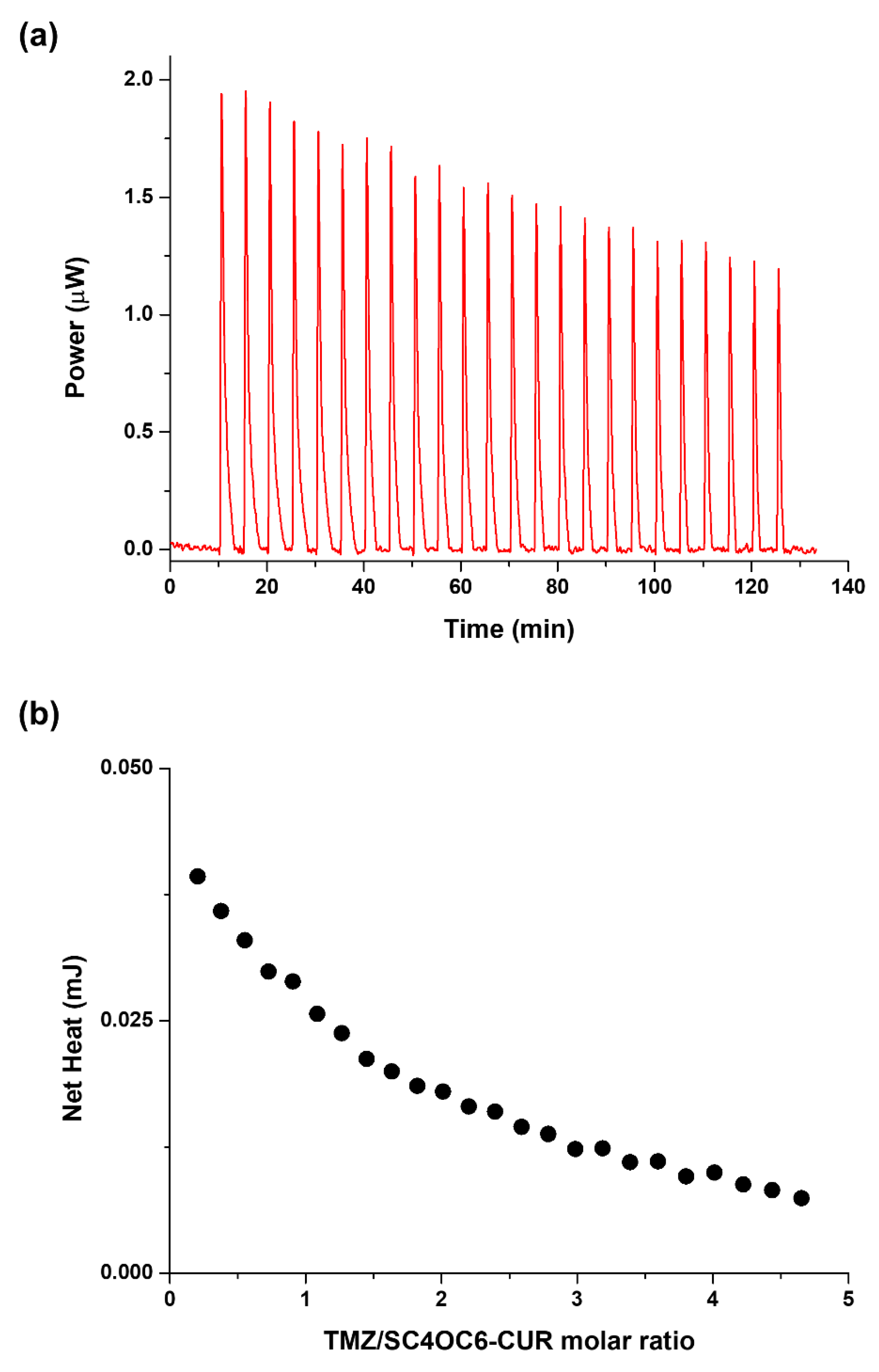
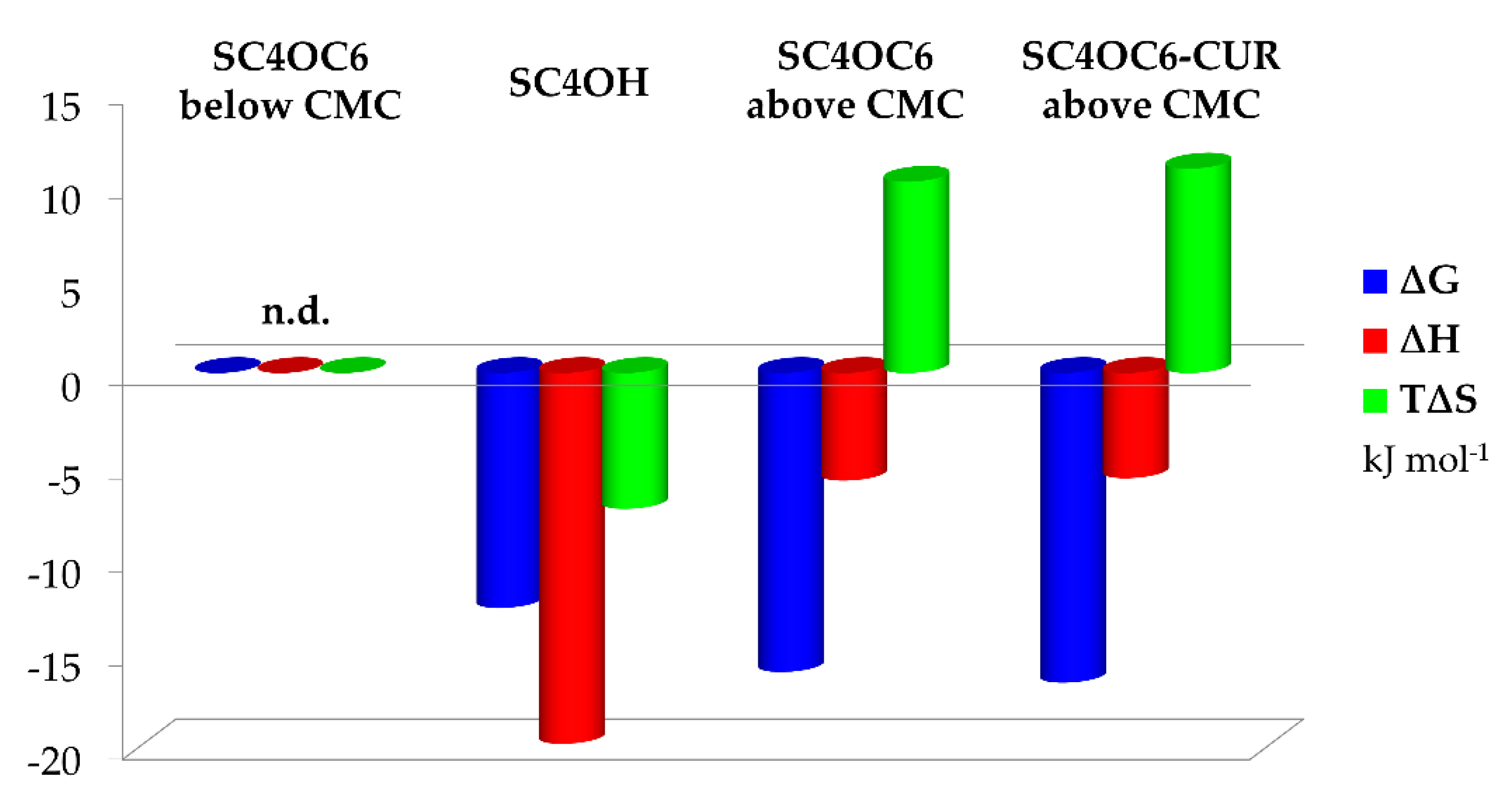
| log K | ΔH (kJ mol−1) | ΔS (J K−1 mol−1) | |
|---|---|---|---|
| (TMZ)(SC4OC6)below CMC | n.d. | n.d. | n.d. |
| (TMZ)(SC4OC6)above CMC | 2.8 (1) | −5.75 (6) | 34 (2) |
| (TMZ)(SC4OC6-CUR)above CMC | 2.9 (1) | −5.62 (4) | 36 (2) |
| (TMZ)(SC4OH) | 2.2 (1) | −15.82 (7) | −25 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliore, R.; D’Antona, N.; Sgarlata, C.; Consoli, G.M.L. Co-Loading of Temozolomide and Curcumin into a Calix[4]arene-Based Nanocontainer for Potential Combined Chemotherapy: Binding Features, Enhanced Drug Solubility and Stability in Aqueous Medium. Nanomaterials 2021, 11, 2930. https://doi.org/10.3390/nano11112930
Migliore R, D’Antona N, Sgarlata C, Consoli GML. Co-Loading of Temozolomide and Curcumin into a Calix[4]arene-Based Nanocontainer for Potential Combined Chemotherapy: Binding Features, Enhanced Drug Solubility and Stability in Aqueous Medium. Nanomaterials. 2021; 11(11):2930. https://doi.org/10.3390/nano11112930
Chicago/Turabian StyleMigliore, Rossella, Nicola D’Antona, Carmelo Sgarlata, and Grazia M. L. Consoli. 2021. "Co-Loading of Temozolomide and Curcumin into a Calix[4]arene-Based Nanocontainer for Potential Combined Chemotherapy: Binding Features, Enhanced Drug Solubility and Stability in Aqueous Medium" Nanomaterials 11, no. 11: 2930. https://doi.org/10.3390/nano11112930
APA StyleMigliore, R., D’Antona, N., Sgarlata, C., & Consoli, G. M. L. (2021). Co-Loading of Temozolomide and Curcumin into a Calix[4]arene-Based Nanocontainer for Potential Combined Chemotherapy: Binding Features, Enhanced Drug Solubility and Stability in Aqueous Medium. Nanomaterials, 11(11), 2930. https://doi.org/10.3390/nano11112930