Lithium-Based Upconversion Nanoparticles for High Performance Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LiYF4:Yb,Er (18/2%) Nanocrystals
2.2. Preparation of Ligand-Free Ln-UCNPs for the Solar Cell Experiment
2.3. Preparation of Mesoporous Layer
2.4. Perovskite Solar Cells Devices Fabrication
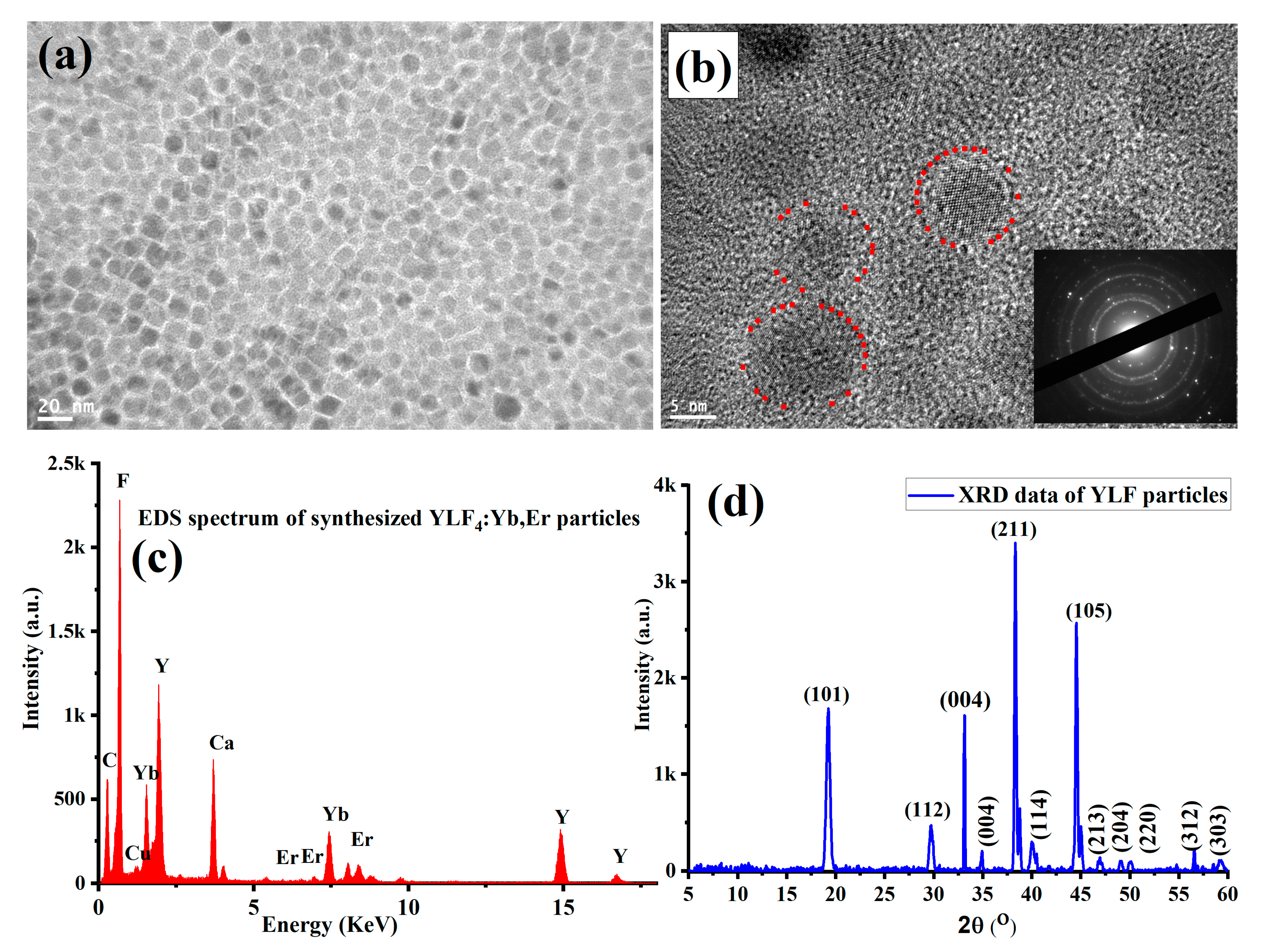
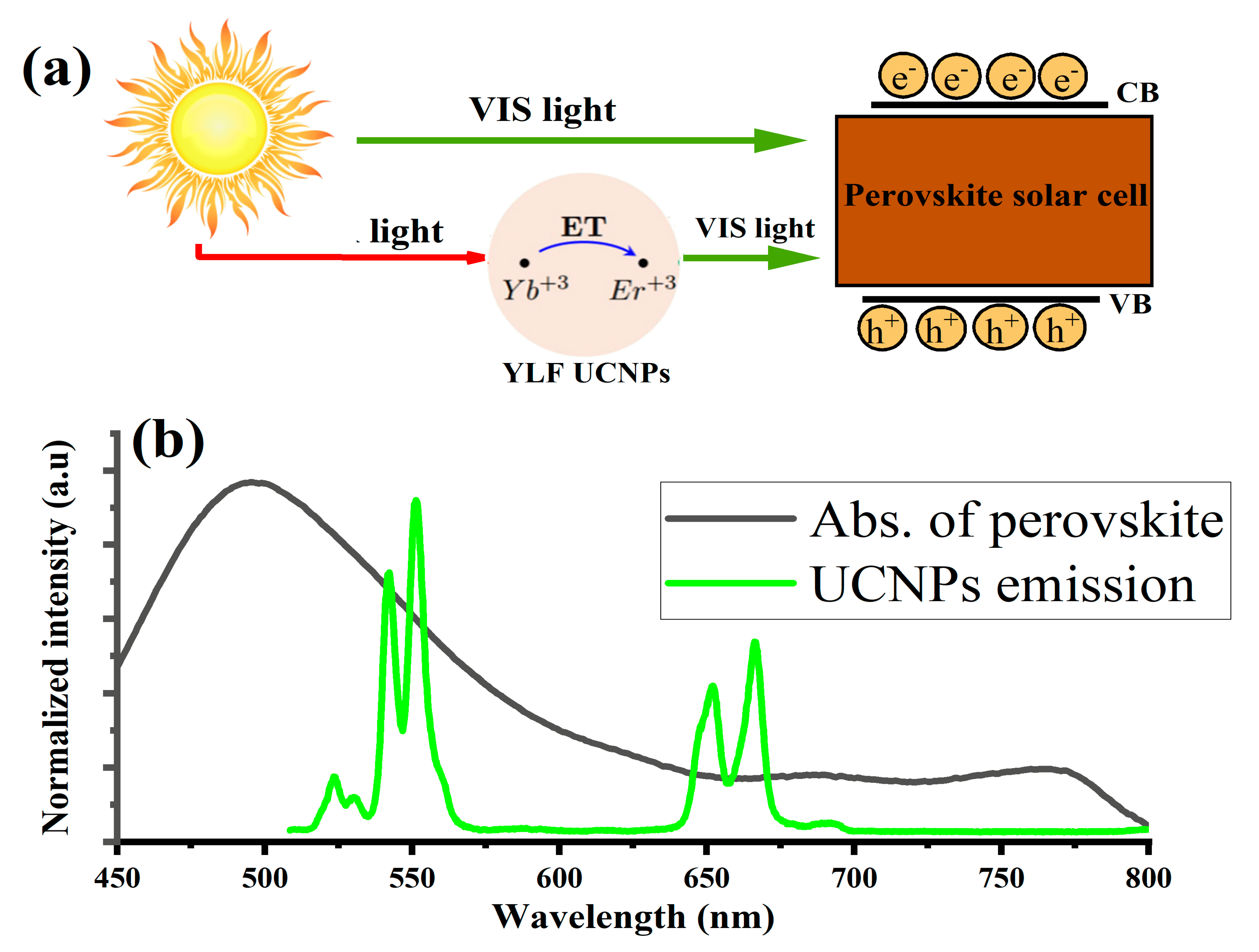
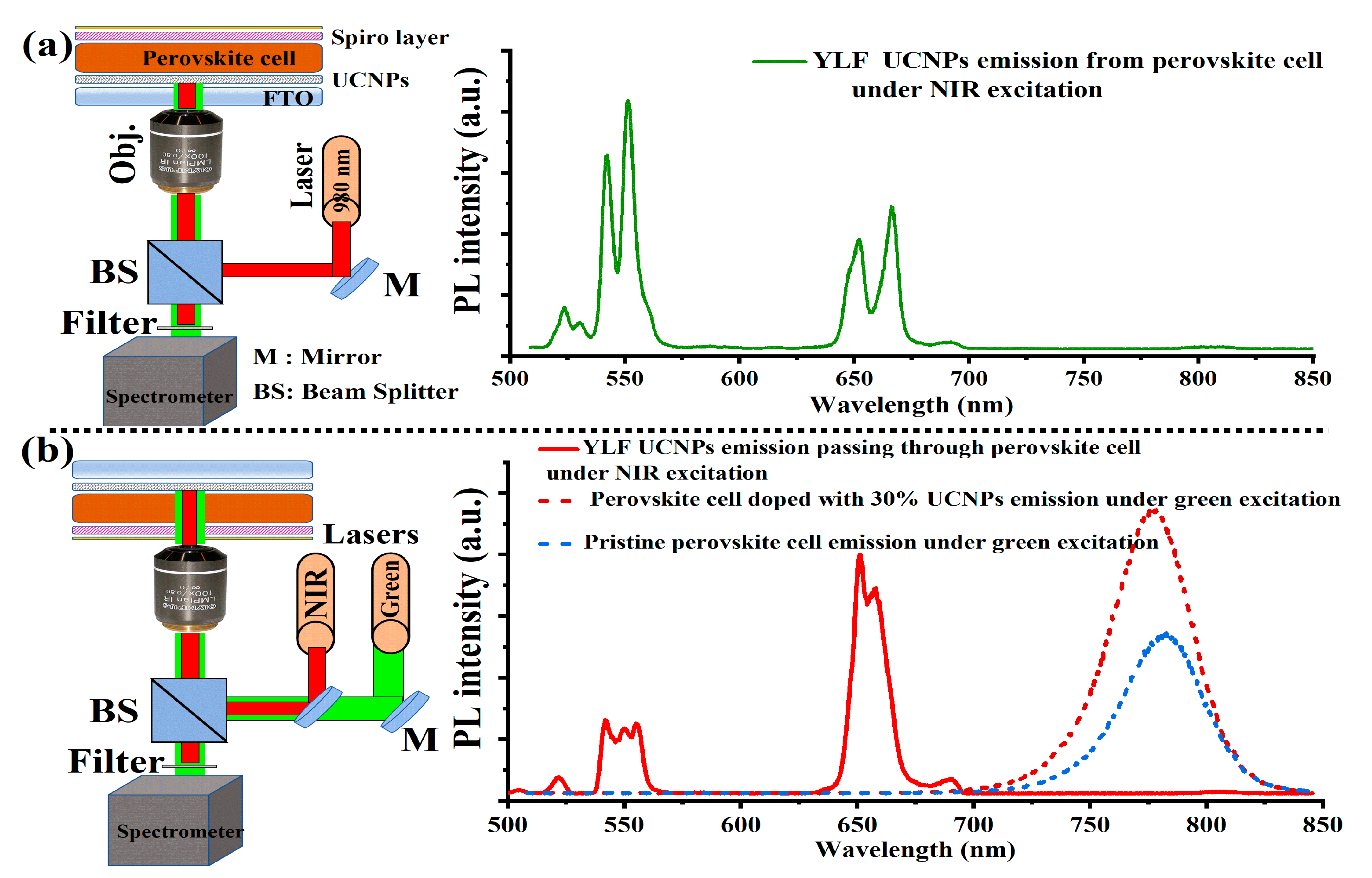
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, N.S. Toward Cost-Effective Solar Energy Use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef]
- Shang, Y.; Hao, S.; Yang, C.; Chen, G. Enhancing Solar Cell Efficiency Using Photon Upconversion Materials. Nanomaterials 2015, 5, 1782–1809. [Google Scholar] [CrossRef] [Green Version]
- Vidal, R.; Alberola-Borràs, J.-A.; Sánchez-Pantoja, N.; Mora-Seró, I. Comparison of Perovskite Solar Cells with other Photovoltaics Technologies from the Point of View of Life Cycle Assessment. Adv. Energy Sustain. Res. 2021, 2, 2000088. [Google Scholar] [CrossRef]
- Saga, T. Advances in crystalline silicon solar cell technology for industrial mass production. NPG Asia Mater. 2010, 2, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, S. The Importance of New “Sand-to-Silicon” Processes for the Rapid Future Increase of Photovoltaics. ACS Energy Lett. 2020, 5, 3628–3632. [Google Scholar] [CrossRef]
- Papež, N.; Gajdoš, A.; Dallaev, R.; Sobola, D.; Sedlák, P.; Motúz, R.; Nebojsa, A.; Grmela, L. Performance analysis of GaAs based solar cells under gamma irradiation. Appl. Surf. Sci. 2020, 510, 145329. [Google Scholar] [CrossRef]
- Kim, S.H.; Saeed, M.A.; Lee, S.Y.; Shim, J.W. Investigating the Indoor Performance of Planar Heterojunction Based Organic Photovoltaics. IEEE J. Photovolt. 2021, 11, 997–1003. [Google Scholar] [CrossRef]
- You, Y.-J.; Saeed, M.A.; Shafian, S.; Kim, J.; Hyeon Kim, S.; Kim, S.H.; Kim, K.; Shim, J.W. Energy recycling under ambient illumination for internet-of-things using metal/oxide/metal-based colorful organic photovoltaics. Nanotechnology 2021, 32, 465401. [Google Scholar] [CrossRef]
- Giordano, F.; Abate, A.; Correa Baena, J.P.; Saliba, M.; Matsui, T.; Im, S.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Hagfeldt, A.; Graetzel, M. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 10379. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, J.; Yang, Y.; Liu, X.; Jia, J.; Dong, J.; Lan, Z.; Lin, J.; Huang, M.; Wei, Y.; et al. High performance perovskite solar cells based on β-NaYF4:Yb3+/Er3+/Sc3+@NaYF4 core-shell upconversion nanoparticles. J. Power Sources 2019, 426, 178–187. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Li, X.; Ibrahim Dar, M.; Yi, C.; Luo, J.; Tschumi, M.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Han, H.; Grätzel, M. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat. Chem. 2015, 7, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Wang, M.; Zang, Z.; Tang, X.; Fang, L. Two-dimensional lead-free hybrid halide perovskite using superatom anions with tunable electronic properties. Sol. Energy Mater. Sol. Cells 2019, 191, 33–38. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, T.; Leng, C.; Zang, Z.; Wang, M.; Hu, W.; Tang, X.; Lu, S.; Fang, L.; Zhou, M. Performance improvement of perovskite solar cells by employing a CdSe quantum dot/PCBM composite as an electron transport layer. J. Mater. Chem. A 2017, 5, 17499–17505. [Google Scholar] [CrossRef]
- Wang, M.; Zang, Z.; Yang, B.; Hu, X.; Sun, K.; Sun, L. Performance improvement of perovskite solar cells through enhanced hole extraction: The role of iodide concentration gradient. Sol. Energy Mater. Sol. Cells 2018, 185, 117–123. [Google Scholar] [CrossRef]
- Meng, F.-L.; Wu, J.-J.; Zhao, E.-F.; Zheng, Y.-Z.; Huang, M.-L.; Dai, L.-M.; Tao, X.; Chen, J.-F. High-efficiency near-infrared enabled planar perovskite solar cells by embedding upconversion nanocrystals. Nanoscale 2017, 9, 18535–18545. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, K.; Jo, E.-J.; Kim, W.; Kim, H.; Lee, R.; Lee, J.Y.; Jo, J.Y.; Kim, M.-G.; Jung, G.Y. Plasmon enhanced up-conversion nanoparticles in perovskite solar cells for effective utilization of near infrared light. Nanoscale 2019, 11, 22813–22819. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, J.; Shi, W.; Liu, Y.; Zhang, Y.; Liu, Y.; Gao, H.; Mao, Y. Enhanced Power Conversion Efficiency of Perovskite Solar Cells with an Up-Conversion Material of Er(3+)-Yb(3+)-Li(+) Tri-doped TiO(2). Nanoscale Res Lett 2018, 13, 147. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Ghosh, S.; Thakur, M.K.; Zhou, J.; Ostrikov, K.; Jin, D.; Chattopadhyay, S. Up-conversion hybrid nanomaterials for light- and heat-driven applications. Prog. Mater. Sci. 2021, 121, 100838. [Google Scholar] [CrossRef]
- Que, M.; Que, W.; Yin, X.; Chen, P.; Yang, Y.; Hu, J.; Yu, B.; Du, Y. Enhanced conversion efficiency in perovskite solar cells by effectively utilizing near infrared light. Nanoscale 2016, 8, 14432–14437. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Jin, J.; Chen, X.; Xu, W.; Yin, Z.; Pan, G.; Li, D.; Song, H. Semiconductor plasmon-sensitized broadband upconversion and its enhancement effect on the power conversion efficiency of perovskite solar cells. J. Mater. Chem. A 2017, 5, 16559–16567. [Google Scholar] [CrossRef]
- Xu, F.; Sun, Y.; Gao, H.; Jin, S.; Zhang, Z.; Zhang, H.; Pan, G.; Kang, M.; Ma, X.; Mao, Y. High-Performance Perovskite Solar Cells Based on NaCsWO3@ NaYF4@NaYF4:Yb,Er Upconversion Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 2674–2684. [Google Scholar] [CrossRef]
- He, M.; Pang, X.; Liu, X.; Jiang, B.; He, Y.; Snaith, H.; Lin, Z. Monodisperse Dual-Functional Upconversion Nanoparticles Enabled Near-Infrared Organolead Halide Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 4280–4284. [Google Scholar] [CrossRef]
- Qiu, L.; Yang, Y.; Dong, G.; Xia, D.; Li, M.; Fan, X.; Fan, R. Surfaces modification of MAPbI3 films with hydrophobic β-NaYF4:Yb,Er up-conversion ultrathin layers for improving the performance of perovskite solar cells. Appl. Surf. Sci. 2018, 448, 145–153. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, C.; Zheng, J.; Zhou, X.; Yu, M.; Chen, X.; Huang, S. Highly bright Li(Gd,Y)F4:Yb,Er upconverting nanocrystals incorporated hole transport layer for efficient perovskite solar cells. Appl. Surf. Sci. 2019, 485, 332–341. [Google Scholar] [CrossRef]
- Liang, J.; Gao, H.; Yi, M.; Shi, W.; Liu, Y.; Zhang, Z.; Mao, Y. β-NaYF4:Yb3+, Tm3+@TiO2 core-shell nanoparticles incorporated into the mesoporous layer for high efficiency perovskite solar cells. Electrochim. Acta 2018, 261, 14–22. [Google Scholar] [CrossRef]
- Alkahtani, M.; Alfahd, A.; Alsofyani, N.; Almuqhim, A.A.; Qassem, H.; Alshehri, A.A.; Almughem, F.A.; Hemmer, P. Photostable and Small YVO4:Yb,Er Upconversion Nanoparticles in Water. Nanomaterials 2021, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Mialon, G.; Türkcan, S.; Dantelle, G.; Collins, D.P.; Hadjipanayi, M.; Taylor, R.A.; Gacoin, T.; Alexandrou, A.; Boilot, J.-P. High Up-Conversion Efficiency of YVO4:Yb,Er Nanoparticles in Water down to the Single-Particle Level. J. Phys. Chem. C 2010, 114, 22449–22454. [Google Scholar] [CrossRef]
- Carl, F.; Birk, L.; Grauel, B.; Pons, M.; Würth, C.; Resch-Genger, U.; Haase, M. LiYF4:Yb/LiYF4 and LiYF4:Yb,Er/LiYF4 core/shell nanocrystals with luminescence decay times similar to YLF laser crystals and the upconversion quantum yield of the Yb,Er doped nanocrystals. Nano Res. 2021, 14, 797–806. [Google Scholar] [CrossRef]
- Alkahtani, M.; Alsofyani, N.; Alfahd, A.; Almuqhim, A.A.; Almughem, F.A.; Alshehri, A.A.; Qasem, H.; Hemmer, P.R. Engineering Red-Enhanced and Biocompatible Upconversion Nanoparticles. Nanomaterials 2021, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.M.; Bikhert, Y.V.; Lisitsyna, L.A.; Dauletbekova, A.K.; Reyterov, V.M.; Karipbayev, Z.T. Cathodoluminescence and Radiation-Induced Absorption in YLiF4 Crystals in Excitation by Electron Pulse. Adv. Mater. Res. 2014, 880, 13–18. [Google Scholar] [CrossRef]
- Millers, D.; Grigorjeva, L.; Pankratov, V.; Trepakov, V.A.; Kapphan, S.E. Pulsed electron beam excited transient absorption in SrTiO3. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2002, 194, 469–473. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.Y.; Cho, S.-H.; Lee, K.; Jang, H.S. Facile synthesis of multicolor tunable ultrasmall LiYF4:Yb,Tm,Er/LiGdF4 core/shell upconversion nanophosphors with sub-10 nm size. Dye. Pigment. 2017, 139, 831–838. [Google Scholar] [CrossRef]
- Lage, M.M.; Moreira, R.L.; Matinaga, F.M.; Gesland, J.-Y. Raman and Infrared Reflectivity Determination of Phonon Modes and Crystal Structure of Czochralski-Grown NaLnF4 (Ln = La, Ce, Pr, Sm, Eu, and Gd) Single Crystals. Chem. Mater. 2005, 17, 4523–4529. [Google Scholar] [CrossRef]
- Garcia, E.G.; Ryan, R.R. Structure of the Laser Host Material LiYF4. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1993, 49, 2053–2054. [Google Scholar] [CrossRef]
- Thoma, R.E.; Weaver, C.F.; Friedman, H.A.; Insley, H.; Harris, L.A.; Yakel, H.A. Phase Equilibria in the System Lif—YF3. J. Phys. Chem. 1961, 65, 1096–1099. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.-B. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Pathak, S.; Abate, A.; Lee, M.M.; Snaith, H.J. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 2013, 4, 2885. [Google Scholar] [CrossRef] [PubMed]
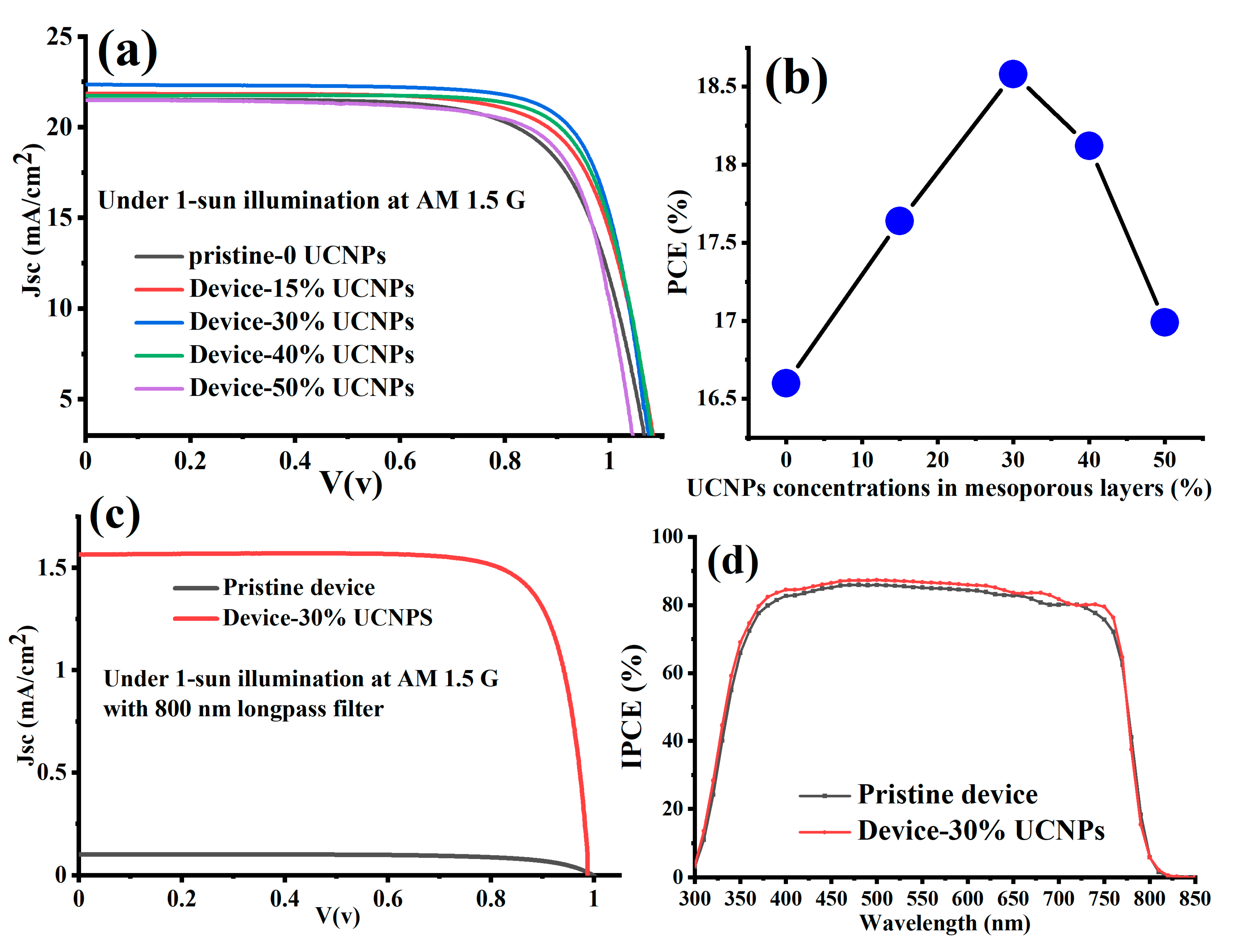
| Sample | Jsc (mA/cm2) | FF (%) | Voc (V) | PCE (%) |
|---|---|---|---|---|
| Pristine | 21.49 | 71.3 | 1.084 | 16.5 |
| Device with 15% UCNPs | 21.85 | 72.7 | 1.112 | 17.64 |
| Device with 30% UCNPs | 22.34 | 82.1 | 1.013 | 18.6 |
| Device with 40% UCNPs | 21.73 | 77.1 | 1.082 | 18.12 |
| Device with 50% UCNPs | 21.49 | 76.8 | 1.01 | 16.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkahtani, M.; Almuqhim, A.A.; Qasem, H.; Alsofyani, N.; Alfahd, A.; Alenzi, S.M.; Aljuwayr, A.; Alzahrani, Y.A.; Al-Badri, A.; Alotaibi, M.H.; et al. Lithium-Based Upconversion Nanoparticles for High Performance Perovskite Solar Cells. Nanomaterials 2021, 11, 2909. https://doi.org/10.3390/nano11112909
Alkahtani M, Almuqhim AA, Qasem H, Alsofyani N, Alfahd A, Alenzi SM, Aljuwayr A, Alzahrani YA, Al-Badri A, Alotaibi MH, et al. Lithium-Based Upconversion Nanoparticles for High Performance Perovskite Solar Cells. Nanomaterials. 2021; 11(11):2909. https://doi.org/10.3390/nano11112909
Chicago/Turabian StyleAlkahtani, Masfer, Anas Ali Almuqhim, Hussam Qasem, Najla Alsofyani, Anfal Alfahd, Sultan M. Alenzi, Abdulaziz Aljuwayr, Yahya A. Alzahrani, Abdurahman Al-Badri, Mohammad Hayal Alotaibi, and et al. 2021. "Lithium-Based Upconversion Nanoparticles for High Performance Perovskite Solar Cells" Nanomaterials 11, no. 11: 2909. https://doi.org/10.3390/nano11112909
APA StyleAlkahtani, M., Almuqhim, A. A., Qasem, H., Alsofyani, N., Alfahd, A., Alenzi, S. M., Aljuwayr, A., Alzahrani, Y. A., Al-Badri, A., Alotaibi, M. H., Bagabas, A., AlHazaa, A. N., & Hemmer, P. R. (2021). Lithium-Based Upconversion Nanoparticles for High Performance Perovskite Solar Cells. Nanomaterials, 11(11), 2909. https://doi.org/10.3390/nano11112909







