Surface Alloying and Improved Property of Nb on TC4 Induced by High Current Pulsed Electron Beam
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
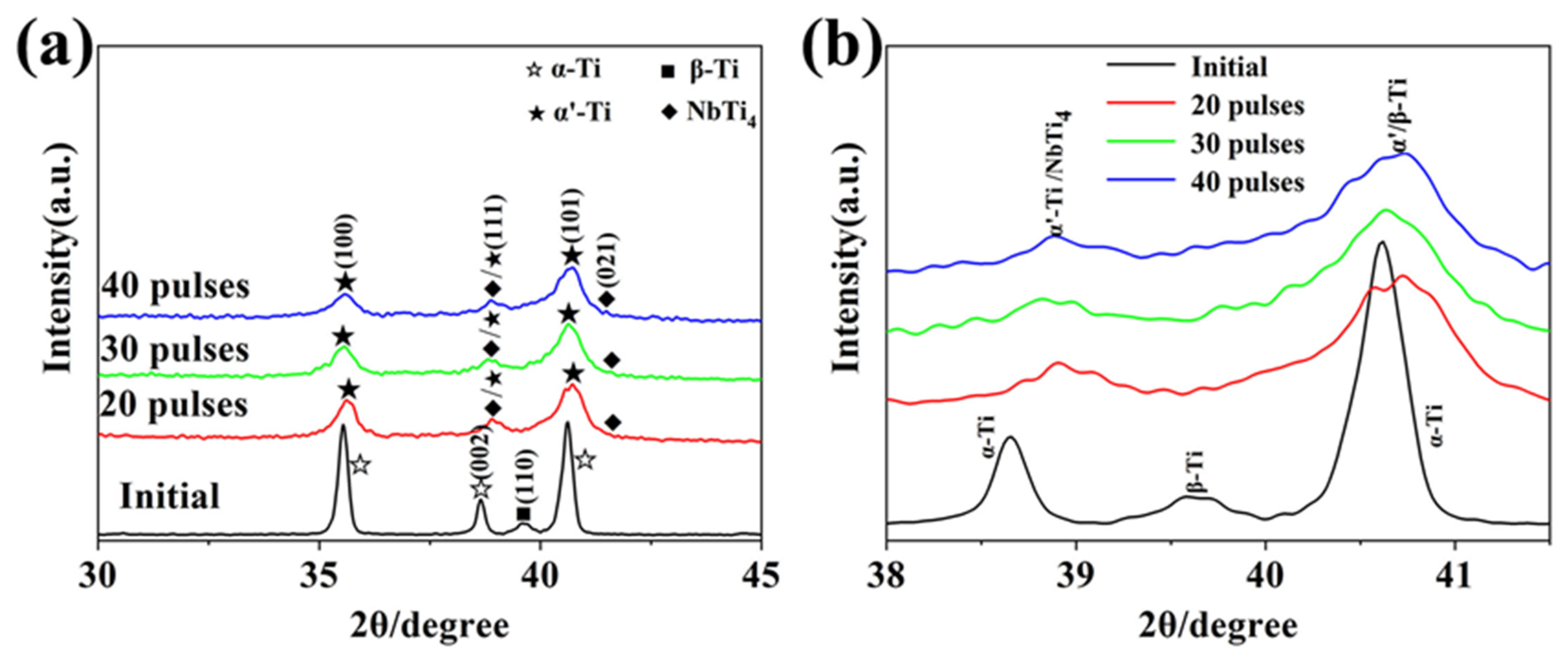
3.1. Phase Identification
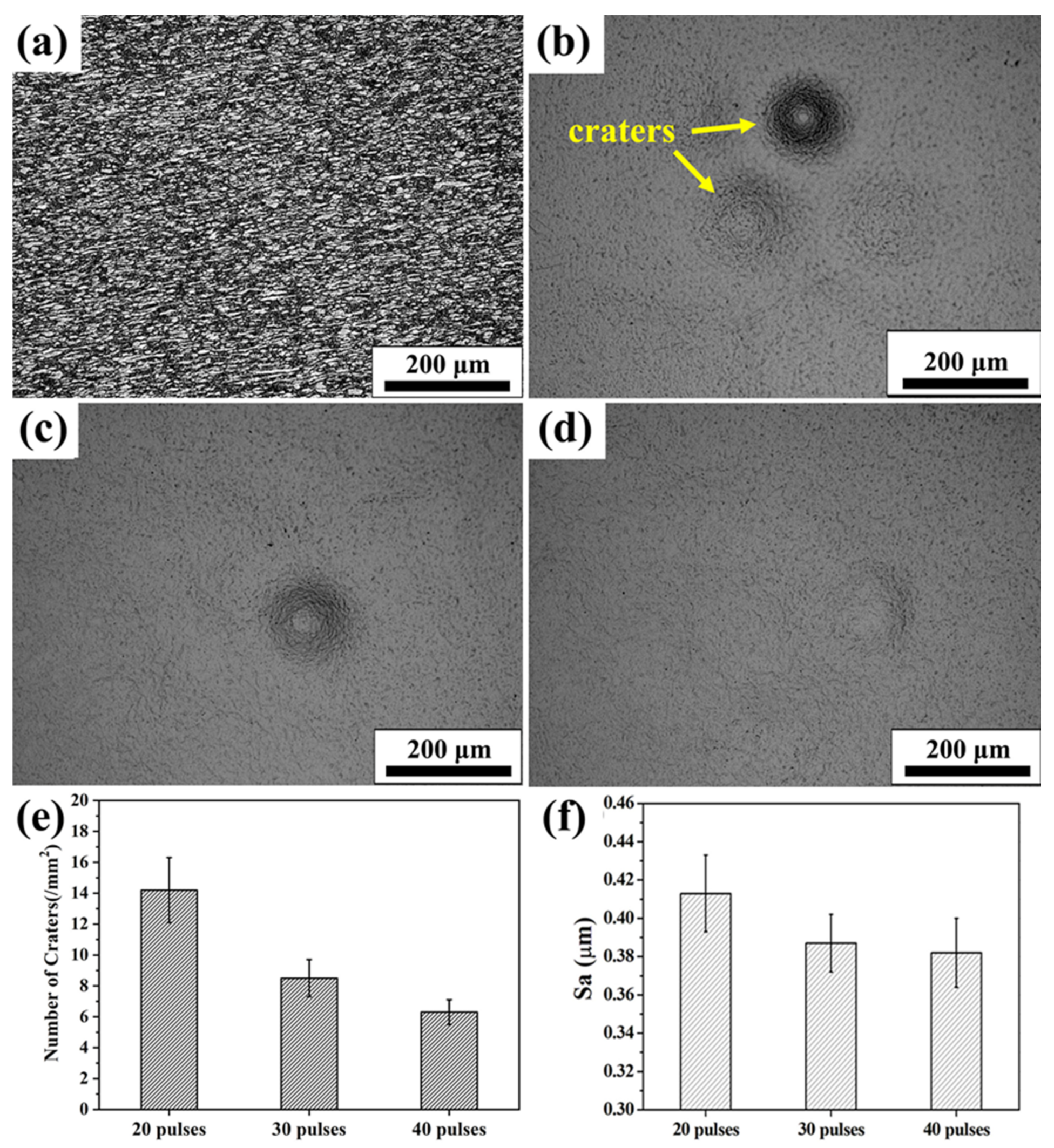
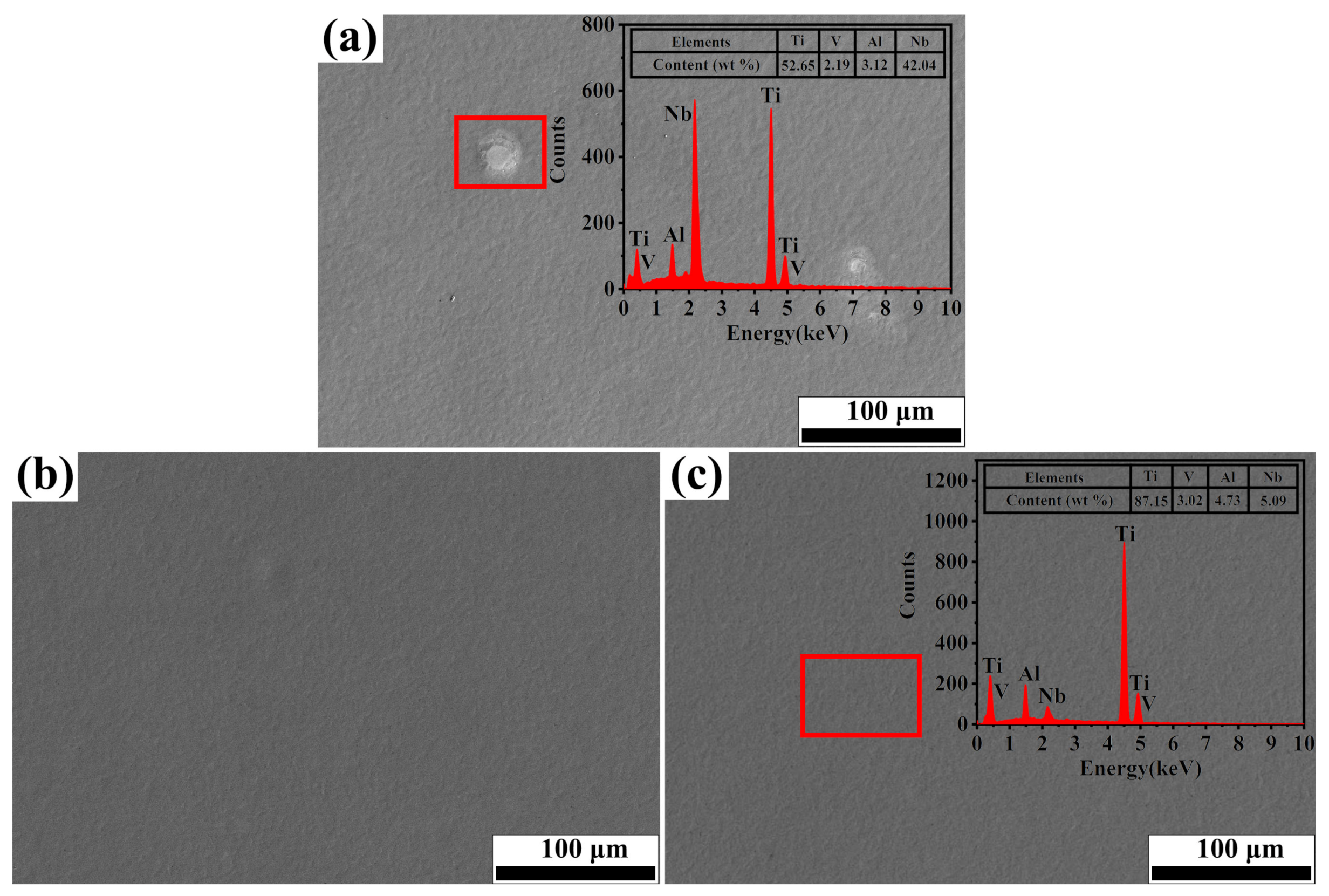
3.2. Surface Topography
3.3. Microhardness
- (1).
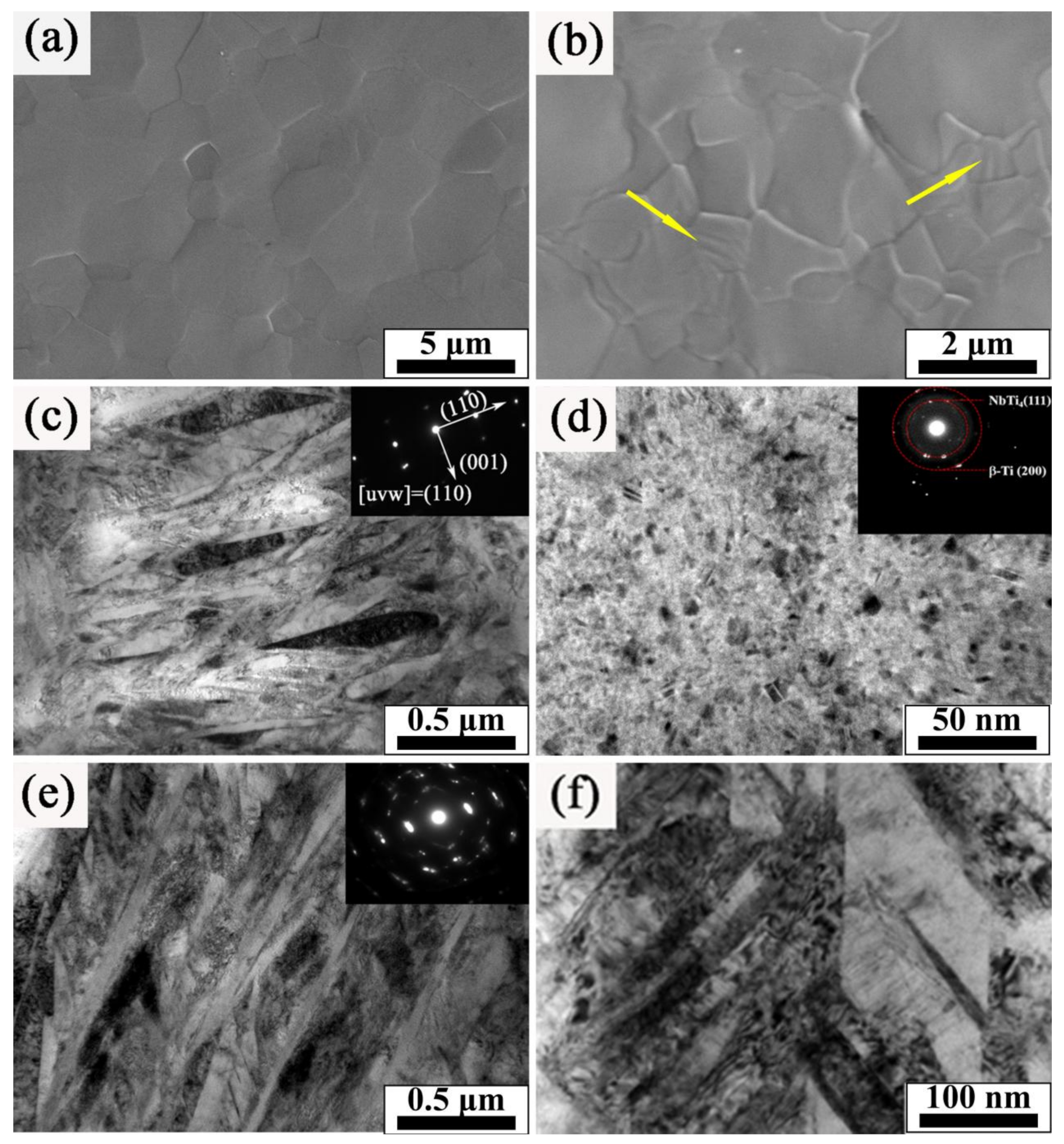
- The temperature gradient of the cooling process was high after HCPEB irradiation, causing the rapid solidification of surface and thereby forming a fine uniform structure. Although the hardness of α′ generated by HCPEB irradiation is lower than that of α martensite, the size of α′ laths was found to be very tiny and accompanied by high density dislocation and twins.
- (2).
- According to Guo’s work [37], the main factor for increasing hardness should be the Nb solid solution strengthening. In this study, the Nb and matrix element diffused each other due to the deposition of the surface energy during irradiation, and it is suggested that the Nb atoms can act as obstacles for the dislocation motion. To illustrate, once the stress required to activate the dislocation increases, the yield increases, leading to a further increase in microhardness, known as solid solution strengthening.
- (3).
- The enhancement of the β phase and the presence of hardening NbTi4 phase played a significant role in dispersion strengthening, which is the key to improving the surface hardness of TC4-Nb alloyed samples. In addition, the HCPEB irradiated alloy layer formed an abundant deformation structure (twin crystals and dislocation), which could play the role of splitting martensite, further refining grain, strengthening dislocation and promoting the improvement of surface hardness [5,38].

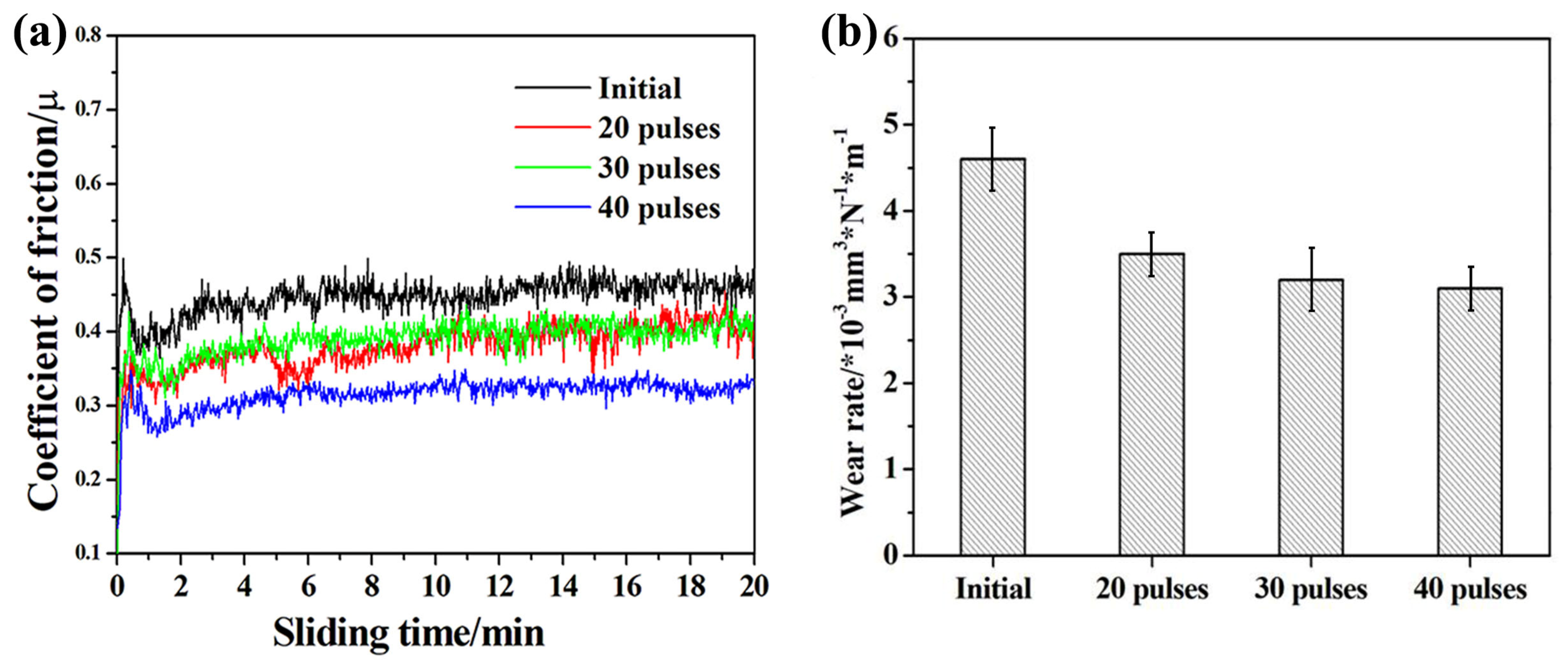
3.4. Wear Resistance
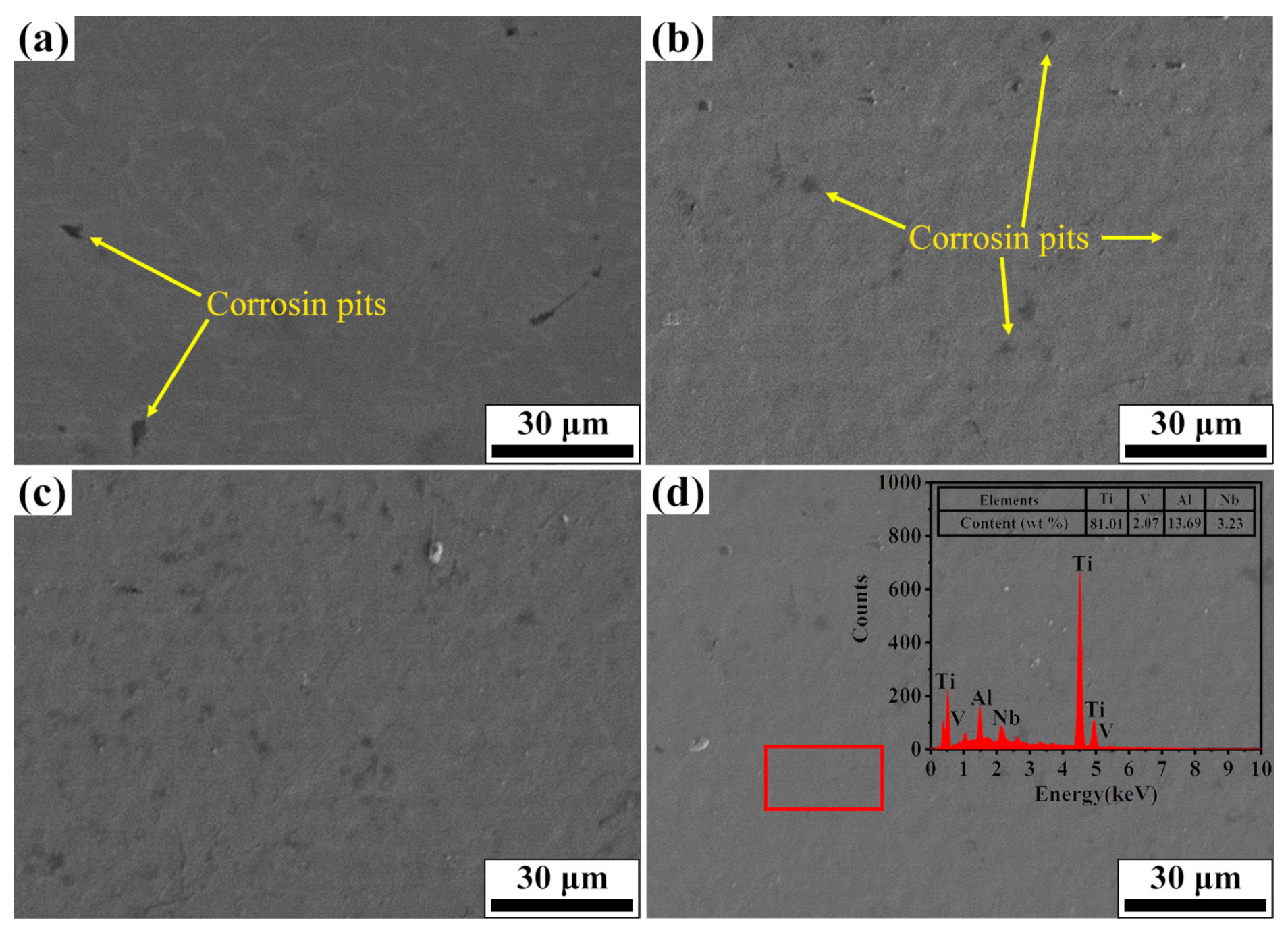
3.5. Corrosion Resistance
- (1).
- The addition of Nb promotes the nucleation of Nb2O5 on the surface of the alloy. According to the literature [46,47], when the oxides are formed in the corrosive medium, the oxides are embedded in the matrix of the alloy as independent clusters. When alloying elements are uniformly distributed in each component phase of the alloy, these oxide clusters can be uniformly distributed in the matrix of the alloy, thus forming a stable passivation film on the surface of the alloy, so that the alloy shows good corrosion resistance.
- (2).
- The grains within the Nb-alloyed layer were refined during the irradiation process. The high increase in the number of grain boundaries provided more channels for the rapid diffusion of atoms, which results in the formation of denser oxide film on the top layer of the sample. Moreover, the melting pit and the composition uniformity on the surface have important effects on the compactness of the oxide film [48].
- (3).
- Phase transition of the irradiated sample surface will also affect the corrosion resistance. Previous research showed that the formation of martensite is helpful to improve the corrosion resistance of the samples [44,49]. To sum up, the improvement of surface corrosion performance of Nb-alloyed samples is the combined effect of the above factors.
4. Conclusions
- (1).
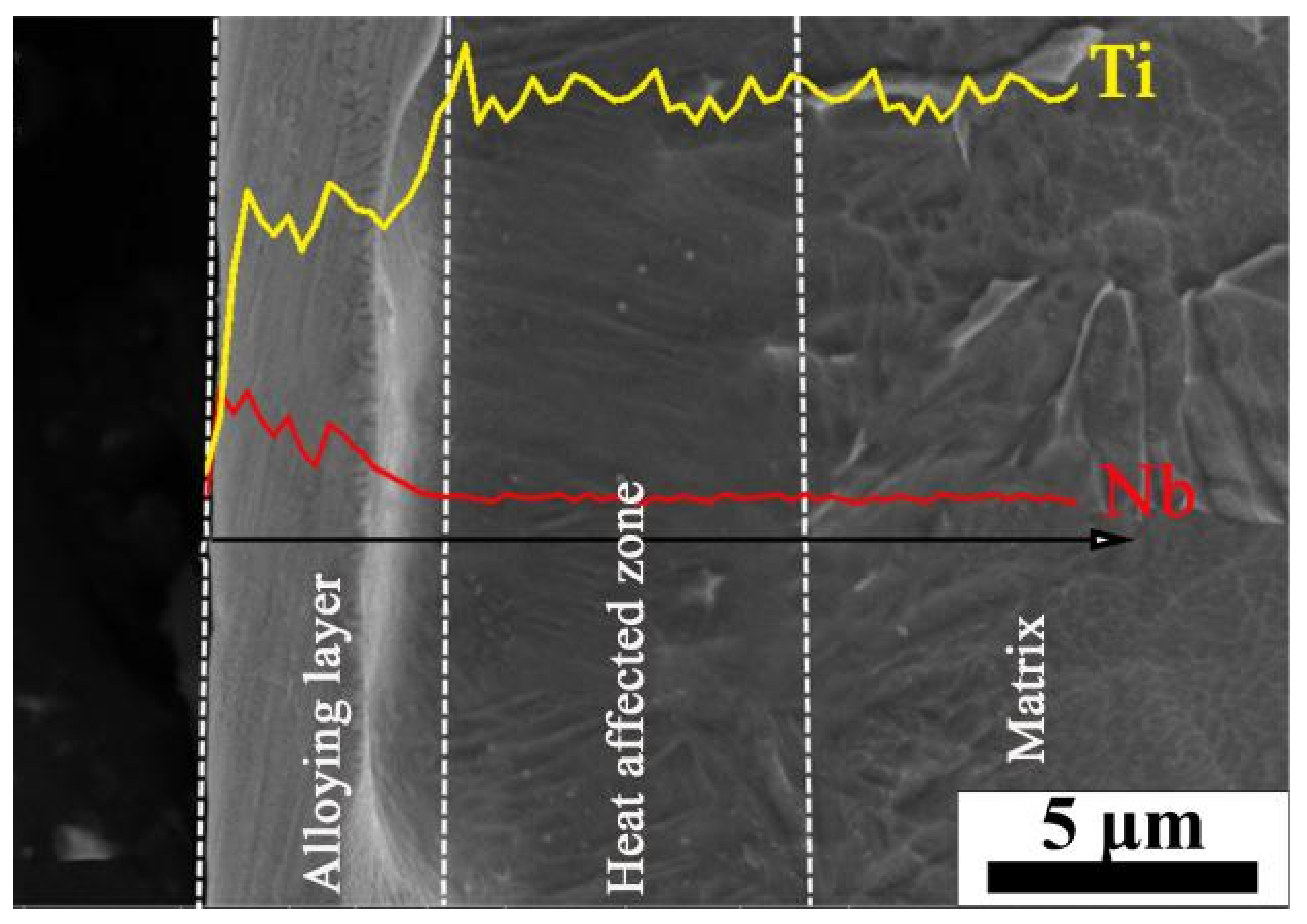
- Extremely rapid heating, cooling and solidifying during HCPEB irradiation make Nb powder to dissolve into TC4 matrix and form an alloying layer on the surface of the TC4 alloy. A typical pit structure appeared on the surface layer, and the density and roughness of the pit decreased with the increase of irradiation pulses.
- (2).
- Martensitic transformation occurred on the surface of TC4 alloy during HCPEB irradiation, forming a fine strip α′ martensite phase in some β phase grains. Many NbTi4 particles with about 10 nm in size were formed in the β phase.
- (3).
- The microhardness and wear resistance of the sample surface were improved, which was mainly due to the joint action of several strengthening mechanisms such as solid solution strengthening, dispersion strengthening and fine crystal strengthening.
- (4).
- The corrosion resistance of TC4-Nb alloys was significantly improved after irradiation, which is the result of Nb alloying, grain refining and surface homogenization.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| wear rate of material | |
| hardness | |
| fraction of oxygen in oxide | |
| density of oxide | |
| sliding velocity | |
| oxidation rate constant | |
| ζc | critical thickness of oxide layer |
References
- Yan, J.; Zhang, Z.; Xu, J.; Wu, Y.; Zhao, X.; Xue, Y.; Liu, H. Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation. High Temp. Mater. Process. 2020, 39, 457–465. [Google Scholar] [CrossRef]
- Gurrappa, I. Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater. Charact. 2003, 51, 131–139. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, C.T.; Yao, X.; Guan, Q.; Lu, R. Surface alloying of Cr on Ti6Al4V alloy induced by high-current pulse electron beam. Surf. Coat. Technol. 2019, 370, 288–297. [Google Scholar] [CrossRef]
- Yonekura, D.; Fujita, J.; Miki, K. Fatigue and wear properties of Ti-6Al-4V alloy with Cr/CrN multilayer coating. Surf. Coat. Technol. 2015, 275, 232–238. [Google Scholar] [CrossRef]
- Yu, S.; Liu, D.; Cui, T.; Zhang, X. Formation process and cross-sectional hardness of a Cr-alloyed layer on Ti6Al4V alloy. Mater. Lett. 2015, 161, 724–726. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Lin, N.; Yao, X.; He, Z.; Liu, X. High-temperature tribological behaviors of TiNi/Ti2Ni alloyed layer on surface of Ti6Al4V alloy. Surf. Rev. Lett. 2017, 24, 1–7. [Google Scholar] [CrossRef]
- Tang, C.; Liu, D.; Li, F. Plasma niobium alloying on Ti6Al4V alloy surface and its wear resistance. Heat Treat. Met. 2010, 35, 1–6. [Google Scholar]
- Qin, L.; Yang, K.; Liu, C.; Tang, B. Enhanced plasma boriding with molybdenum using double glow plasma surface alloying technique. Mater. Lett. 2012, 82, 127–129. [Google Scholar] [CrossRef]
- Lyu, P.; Chen, Y.; Liu, Z.; Cai, J.; Zhang, C.; Jin, Y.; Guan, Q.; Zhao, N. Surface modification of CrFeCoNiMo high entropy alloy induced by high-current pulsed electron beam. Appl. Surf. Sci. 2020, 504, 144453. [Google Scholar] [CrossRef]
- Ozur, G.E.; Proskurovsky, D.I.; Rotshtein, V.P. A mechanism of microcrater formation in metallic material irradiated by a low-energy high-current electron beam. Tech. Phys. Lett. 2016, 42, 328–331. [Google Scholar] [CrossRef]
- Rotshtein, V.P.; Proskurovsky, D.I.; Ozur, G.E.; Ivanov, Y.F.; Markov, A.B. Surface modification and alloying of metallic materials with low-energy high-current electron beams. Surf. Coat. Technol. 2004, 180, 377–381. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, P.; Xia, H.; Yang, Z.; Konovalov, S.; Chen, X.; Guan, Q. The microstructure and properties of nanostructured Cr-Al alloying layer fabricated by high-current pulsed electron beam. Vacuum 2019, 167, 263–270. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, L.; Wang, X. Studies on surface topography and mechanical properties of TiN coating irradiated by high current pulsed electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2018, 436, 63–67. [Google Scholar] [CrossRef]
- Rotshtein, V.P.; Ivanov, Y.F.; Markov, A.B.; Proskurovsky, D.I.; Karlik, K.V.; Oskomov, K.V.; Uglov, B.V.; Kuleshov, A.K.; Novitskaya, M.V.; Dub, S.N.; et al. Surface alloying of stainless steel 316 with copper using pulsed electron-beam melting of film-substrate system. Surf. Coat. Technol. 2006, 200, 6378–6383. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, C.T.; Guan, J.; Lv, P.; Guan, Q.; Lu, R. Nanocrystalline Cr-Ni alloying layer induced by high-current pulsed electron beam. Nanomaterials 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Guo, Z.; Zhao, L.; Guan, Q.; Zhao, L.; Liu, Y. Microstructures and corrosion resistance of Zircaloy-4 after surface alloying with copper by high-current pulsed electron beam. Appl. Surf. Sci. 2020, 501, 144222. [Google Scholar] [CrossRef]
- Proskurovsky, D.I.; Rotshtein, V.P.; Ozur, G.E.; Markov, A.B.; Nazarov, D.S.; Shulov, V.A.; Ivanov, Y.F.; Buchheit, R.G. Pulsed electron-beam technology for surface modification of metallic materials. J. Vac. Sci. Technol. A Vacuum Surf. Films 1998, 16, 2480–2488. [Google Scholar] [CrossRef]
- Liang, W.p.; Miao, Q.; Ben, N.j.; Ren, B.l. Tribological behaviors of Ti-6Al-4V alloy with surface plasma molybdenized layer. Surf. Coat. Technol. 2013, 228, S249–S253. [Google Scholar] [CrossRef]
- Zou, J.X.; Zhang, K.M.; Hao, S.Z.; Dong, C.; Grosdidier, T. Mechanisms of hardening, wear and corrosion improvement of 316 L stainless steel by low energy high current pulsed electron beam surface treatment. Thin Solid Films 2010, 519, 1404–1415. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, J.; Lv, P.; Zhang, Y.; Xia, H.; Guan, Q. Surface microstructure and properties of Cu-C powder metallurgical alloy induced by high-current pulsed electron beam. J. Alloy. Compd. 2017, 697, 96–103. [Google Scholar] [CrossRef]
- Qin, Y.; Dong, C.; Wang, X.; Hao, S.; Wu, A.; Zou, J.; Liu, Y. Temperature profile and crater formation induced in high-current pulsed electron beam processing. J. Vac. Sci. Technol. A Vacuum Surf. Films 2003, 21, 1934–1938. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Q.; Lv, P.; Cai, J.; Peng, C.T.; Jin, Y.; Guan, Q. Surface modification of Cu-W powder metallurgical alloy induced by high-current pulsed electron beam. Powder Technol. 2018, 325, 340–346. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.W. Influence of a large pulsed electron beam (LPEB) on the corrosion resistance of Ti-6Al-7Nb alloys. Corros. Sci. 2015, 90, 153–160. [Google Scholar] [CrossRef]
- Meisner, L.L.; Semin, V.O.; Mironov, Y.P.; Meisner, S.N.; D′yachenko, F.A. Cross-sectional analysis of the graded microstructure and residual stress distribution in a TiNi alloy treated with low energy high-current pulsed electron beam. Mater. Today Commun. 2018, 17, 169–179. [Google Scholar] [CrossRef]
- Grosdidier, T.; Philippe, M.J. Deformation induced martensite and superelasticity in a β-metastable titanium alloy. Mater. Sci. Eng. A 2000, 291, 218–223. [Google Scholar] [CrossRef]
- Ghosh, C.; Basu, J.; Ramachandran, D.; Mohandas, E. Phase separation and ω transformation in binary V-Ti and ternary V-Ti-Cr alloys. Acta Mater. 2016, 121, 310–324. [Google Scholar] [CrossRef]
- Cai, J.; Wan, M.; Zou, Y.; Lv, P.; Han, Z.; Wang, Z.; Guan, Q. Surface modification of pure titanium by high current pulsed electron beam. Adv. Mater. Res. 2012, 560, 994–999. [Google Scholar] [CrossRef]
- Zhang, X.D.; Hao, S.Z.; Li, X.N.; Dong, C.; Grosdidier, T. Surface modification of pure titanium by pulsed electron beam. Appl. Surf. Sci. 2011, 257, 5899–5902. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Wang, X.; Cai, J.; Guan, Q. Surface alloys of 0.45 C carbon steel produced by high current pulsed electron beam. High Temp. Mater. Process. 2019, 38, 444–451. [Google Scholar] [CrossRef]
- Salvo, C.; Aguilar, C.; Cardoso-Gil, R.; Medina, A.; Bejar, L.; Mangalaraja, R.V. Study on the microstructural evolution of Ti-Nb based alloy obtained by high-energy ball milling. J. Alloy. Compd. 2017, 720, 254–263. [Google Scholar] [CrossRef]
- Banerjee, R.; Collins, P.C.; Fraser, H.L. Phase evolution in laser-deposited titanium-chromium alloys. Metal. Mater. Trans. A 2002, 33, 2129–2138. [Google Scholar] [CrossRef]
- Xian, W.H.; Li, D.G.; Chen, D.R. Investigation on ultrasonic cavitation erosion of TiMo and TiNb alloys in sulfuric acid solution. Ultrason. Sonochem. 2020, 62, 104877. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.H.; Lee, J.K. Deformation twinning in h.c.p. metals and alloys. Philos. Mag. A Phys. Condens. Matter Struct. Defects Mech. Prop. 1991, 63, 987–1000. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y. Twins formation and their role in nanostructuring of zirconium. Mater. Sci. Eng. A 2009, 523, 130–133. [Google Scholar] [CrossRef]
- Neiman, A.A.; Semin, V.O.; Meisner, L.L.; Ostapenko, M.G. Structural decomposition and phase changes in TiNi surface layer modified by low-energy high-current pulsed electron beam. J. Alloy. Compd. 2019, 803, 721–729. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, C.; Zhang, L.; Cai, J.; Lv, P.; Jin, Y.; Guan, Q. Microstructure and properties of Cu-Cr powder metallurgical alloy induced by high-current pulsed electron beam. J. Alloy. Compd. 2018, 755, 251–256. [Google Scholar] [CrossRef]
- Guo, G.; Tang, G.; Ma, X.; Sun, M.; Ozur, G.E. Effect of high current pulsed electron beam irradiation on wear and corrosion resistance of Ti6Al4V. Surf. Coat. Technol. 2013, 229, 140–145. [Google Scholar] [CrossRef]
- Cai, J.; Lv, P.; Zhang, C.L.; Wu, J.; Li, C.; Guan, Q.F. Microstructure and properties of low carbon steel after surface alloying induced by high current pulsed electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 410, 47–52. [Google Scholar] [CrossRef]
- Straffelini, G.; Molinari, A. Dry sliding wear of Ti-6Al-4V alloy as influenced by the counterface and sliding conditions. Wear 1999, 236, 328–338. [Google Scholar] [CrossRef]
- Molinari, A.; Straffelini, G.; Tesi, B.; Bacci, T. Dry sliding wear mechanisms of the Ti6A14V alloy. Wear 1997, 208, 105–112. [Google Scholar] [CrossRef]
- Krupp, U.; Christ, H.J. Internal nitridation of nickel-base alloys. Part, I. Behavior of binary and ternary alloys of the Ni-Cr-Al-Ti system. Oxid. Met. 1999, 52, 277–298. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, W.; Chu, K.; Zhao, C. Tribological and corrosion behaviors of bulk Cu-W nanocomposites fabricated by mechanical alloying and warm pressing. J. Alloys Compd. 2016, 676, 164–172. [Google Scholar] [CrossRef]
- Wu, P.P.; Deng, K.K.; Nie, K.B.; Zhang, Z.Z. Corrosion resistance of AZ91 Mg alloy modified by high-current pulsed electron beam. Acta Metall. Sin. Eng. Lett. 2019, 32, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.; Wang, H.; Zhao, L. Surface modification of 40CrNiMo7 steel with high current pulsed electron beam treatment. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 368, 81–85. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, J.; Grosdidier, T.; Dong, C.; Yang, D. Improved pitting corrosion resistance of AISI 316L stainless steel treated by high current pulsed electron beam. Surf. Coat. Technol. 2006, 201, 1393–1400. [Google Scholar] [CrossRef]
- Morant, C.; López, M.F.; Gutiérrez, A.; Jiménez, J.A. AFM and SEM characterization of non-toxic vanadium-free Ti alloys used as biomaterials. Appl. Surf. Sci. 2003, 220, 79–87. [Google Scholar] [CrossRef]
- Lv, P.; Xu, J.; Yang, R.; Zhang, C.; Zhang, F.; Dong, S.; Guan, Q. Effect of thermocycling on the microstructure of Ti-6Al-4V alloy in simulated low Earth orbit space environment. Sci. China Mater. 2016, 59, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Gao, C.; Lv, P.; Zhang, C.; Guan, Q.; Lu, J.; Xu, X. Hot corrosion behaviour of thermally sprayed CoCrAlY coating irradiated by high-current pulsed electron beam. J. Alloy. Compd. 2019, 784, 1221–1233. [Google Scholar] [CrossRef]
- Yan, P.; Zou, J.; Zhang, C.; Grosdidier, T. Surface modifications of a cold rolled 2024 Al alloy by high current pulsed electron beams. Appl. Surf. Sci. 2020, 504, 144382. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Tian, N.; Zhang, C.; Lyu, P.; Cai, J.; Guan, Q. Surface Alloying and Improved Property of Nb on TC4 Induced by High Current Pulsed Electron Beam. Nanomaterials 2021, 11, 2906. https://doi.org/10.3390/nano11112906
Du X, Tian N, Zhang C, Lyu P, Cai J, Guan Q. Surface Alloying and Improved Property of Nb on TC4 Induced by High Current Pulsed Electron Beam. Nanomaterials. 2021; 11(11):2906. https://doi.org/10.3390/nano11112906
Chicago/Turabian StyleDu, Xueze, Nana Tian, Conglin Zhang, Peng Lyu, Jie Cai, and Qingfeng Guan. 2021. "Surface Alloying and Improved Property of Nb on TC4 Induced by High Current Pulsed Electron Beam" Nanomaterials 11, no. 11: 2906. https://doi.org/10.3390/nano11112906
APA StyleDu, X., Tian, N., Zhang, C., Lyu, P., Cai, J., & Guan, Q. (2021). Surface Alloying and Improved Property of Nb on TC4 Induced by High Current Pulsed Electron Beam. Nanomaterials, 11(11), 2906. https://doi.org/10.3390/nano11112906





