Photosensitizers Mediated Photodynamic Inactivation against Fungi
Abstract
:1. Introduction
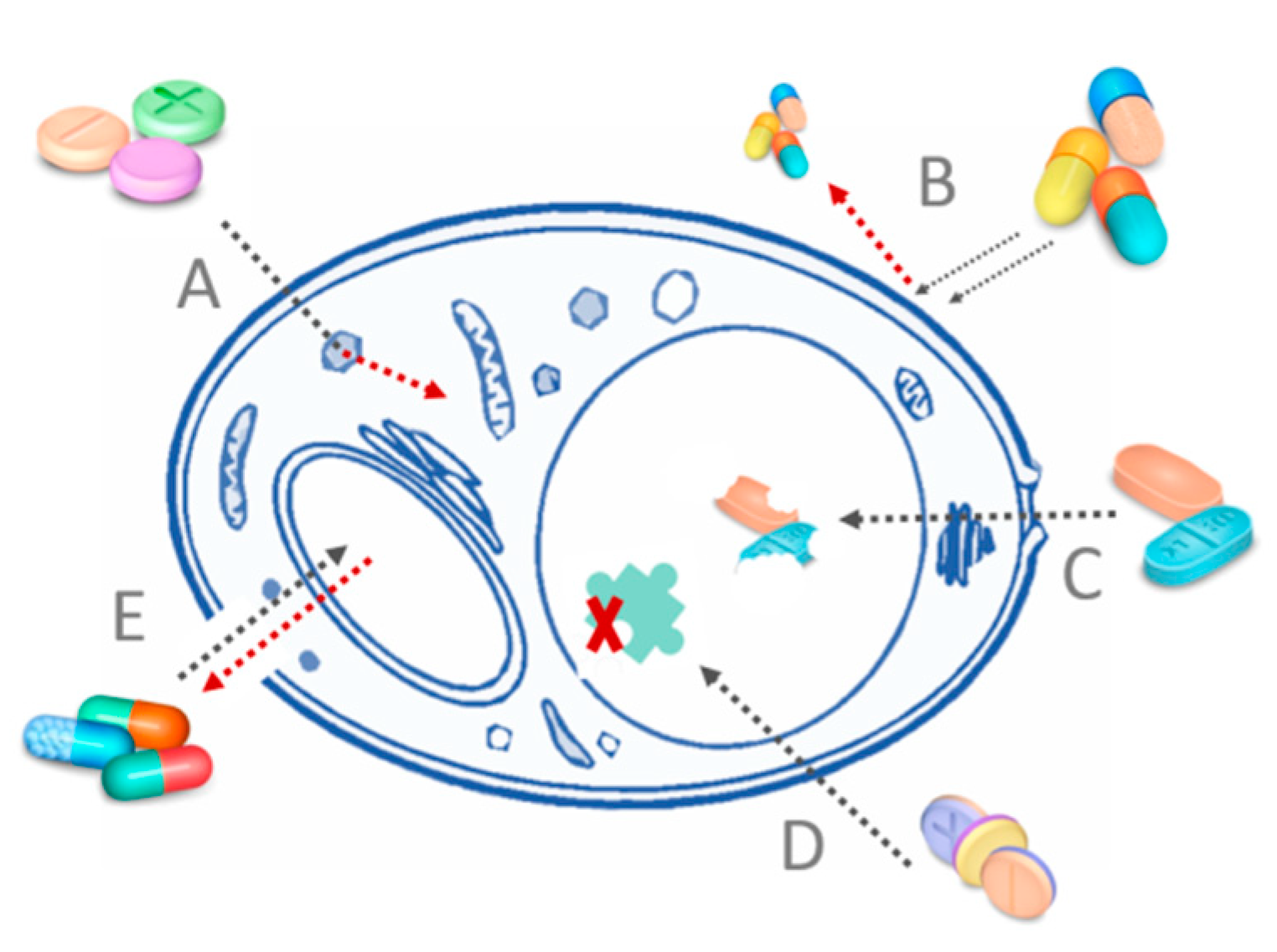
2. Mechanism of Photosensitizer Action against Fungi
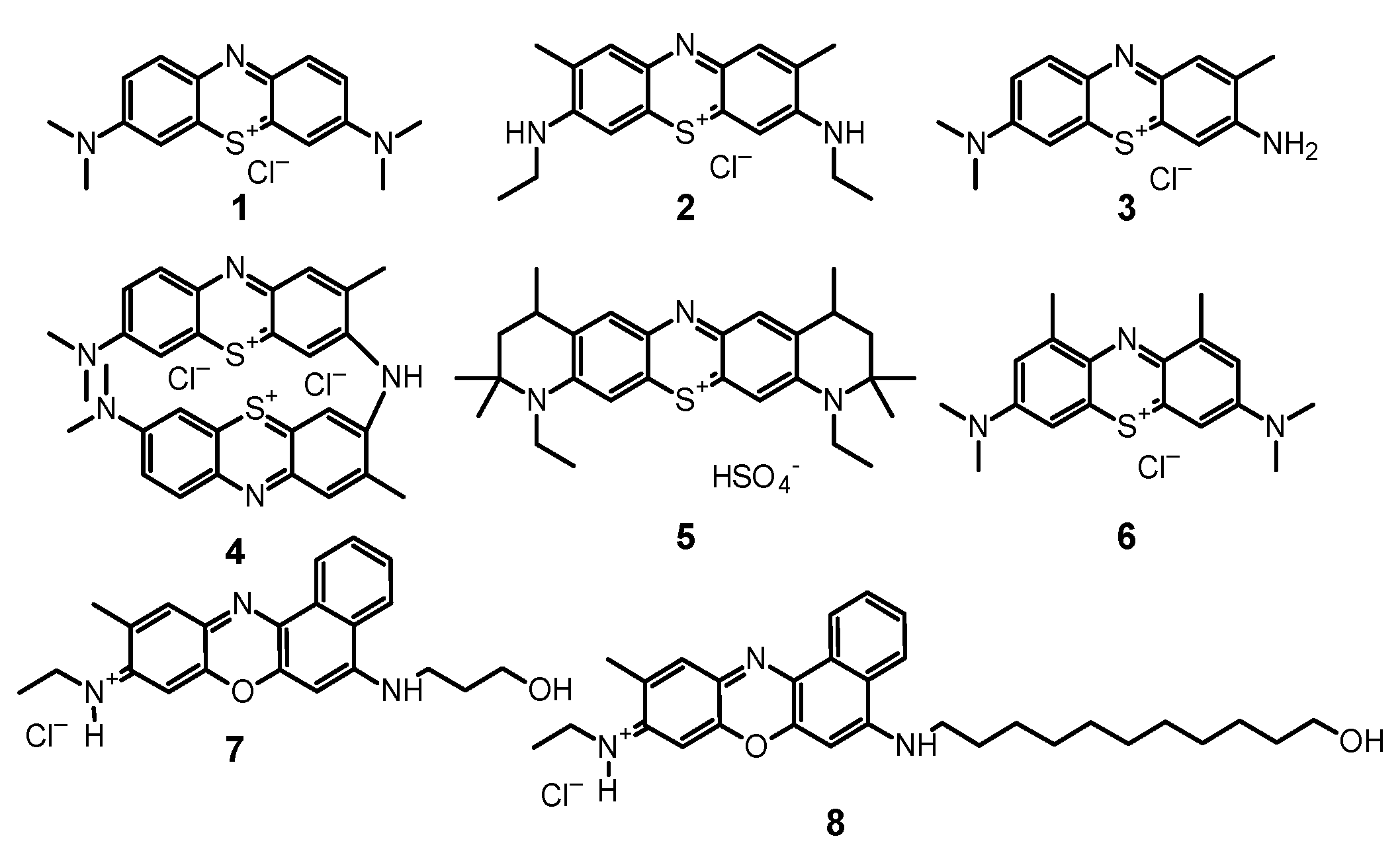
3. Phenothiazine Photosensitizers
3.1. Methylene Blue
3.2. Toluidine Blue O and New Toluidine Blue O
3.3. Combination of Phenothiazines with Antifungals
3.4. Combinations of Phenothiazines with Nanoparticles
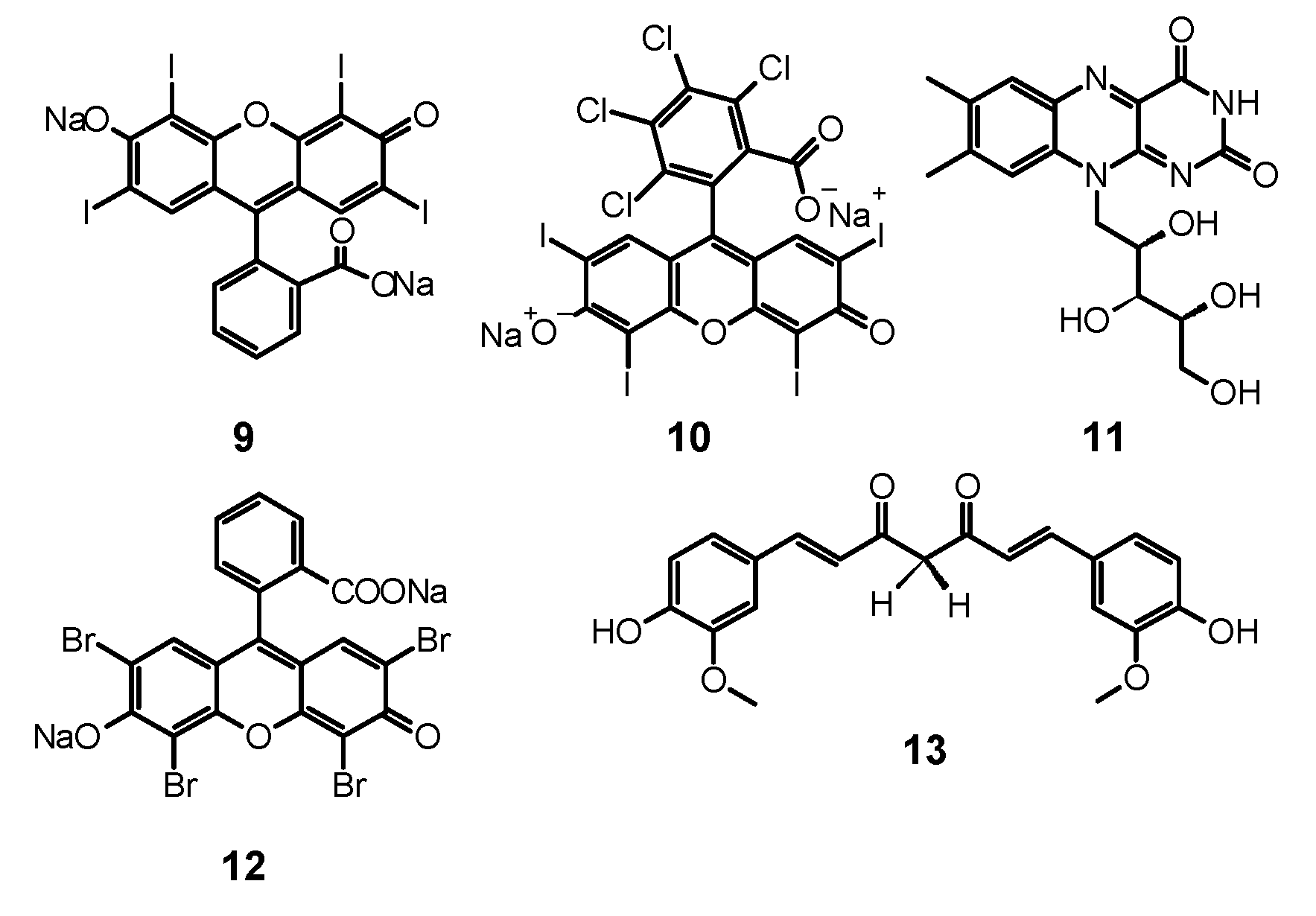
4. Xanthenes
4.1. Erythrosine
4.2. Rose Bengal
5. Curcumin
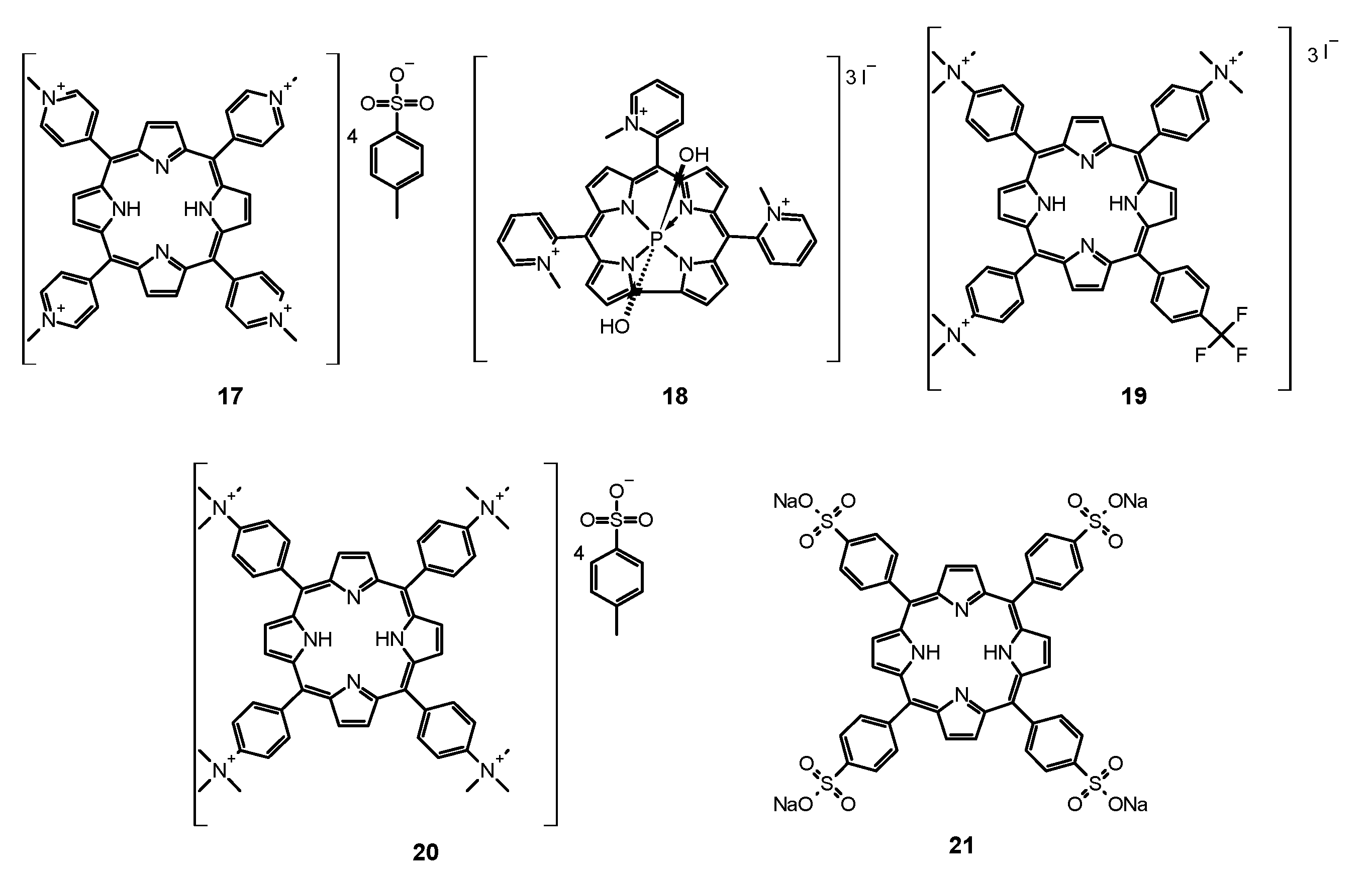
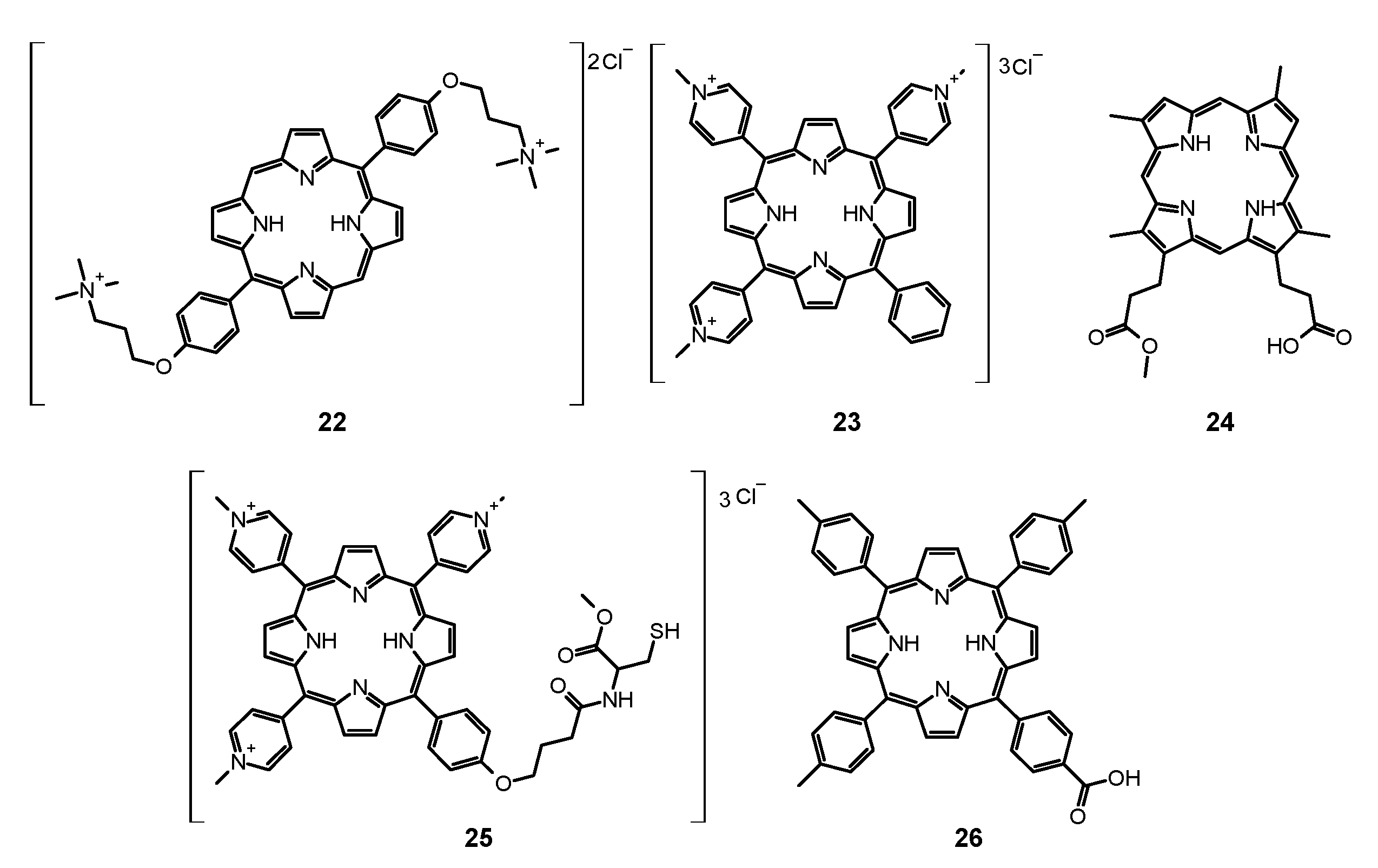
6. Porphyrins
6.1. First Generation Photosensitizers
6.1.1. Porfimer Sodium
6.1.2. Photogem®
6.1.3. Hematoporphyrin Monomethyl Ether
6.2. Second Generation Photosensitizers
6.2.1. 5-Aminolevulinic Acid and Its Derivatives
6.2.2. Others
6.3. Porphyrin Containing Materials
7. Chlorins
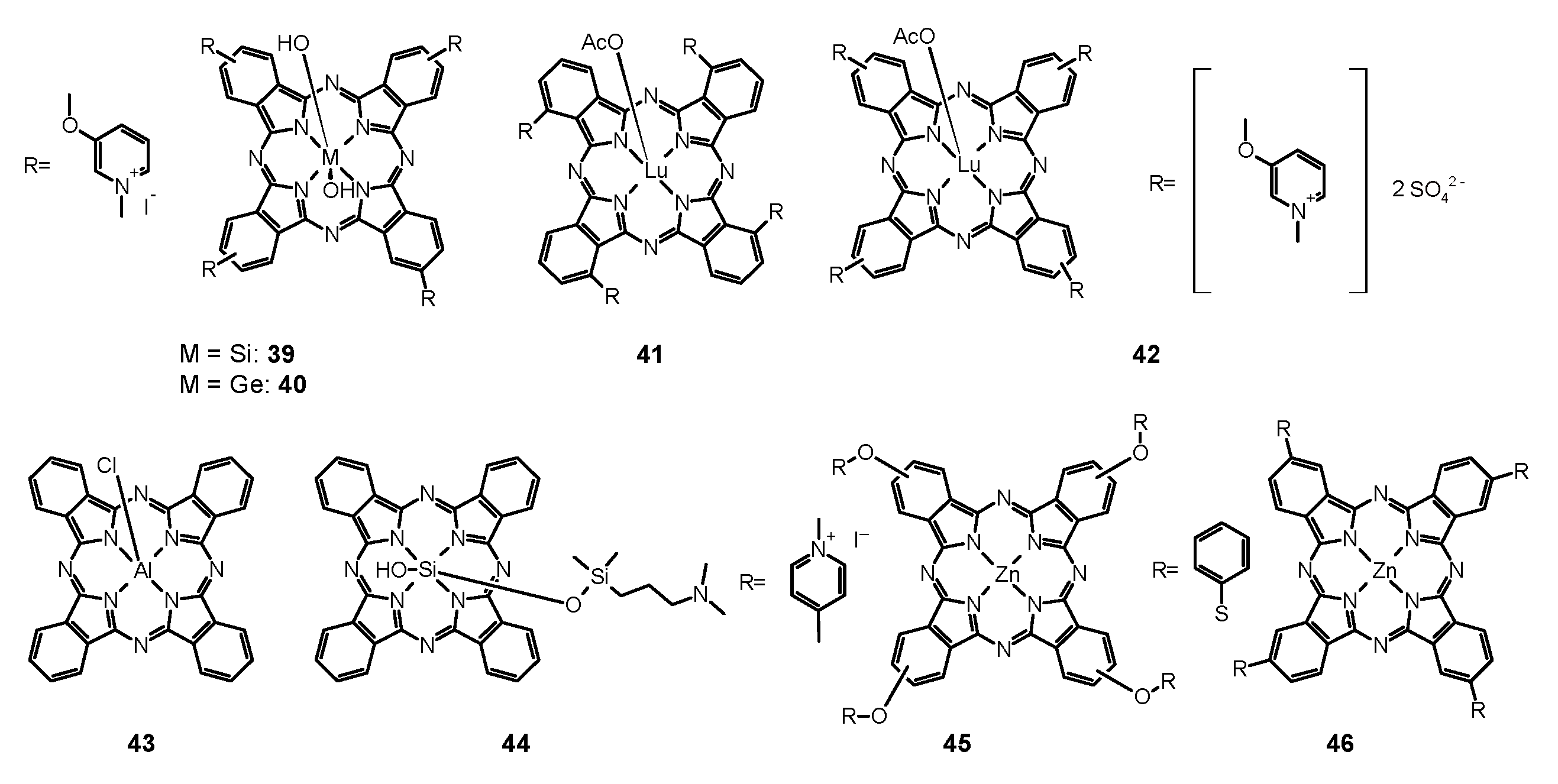
8. Porphyrazines and Phthalocyanines
9. Other Photosensitizers
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geddes-McAlister, J.; Shapiro, R.S. New Pathogens, New Tricks: Emerging, Drug-Resistant Fungal Pathogens and Future Prospects for Antifungal Therapeutics: Drug-Resistant Fungal Pathogens. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal Drugs: New Insights in Research & Development. Pharmacol. Ther. 2019, 195, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Pereira Gonzales, F.; Maisch, T. Photodynamic Inactivation for Controlling Candida Albicans Infections. Fungal Biol. 2012, 116, 1–10. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal Photodynamic Therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Fungal Infections. Front. Microbiol. 2015, 6, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmello, J.C.; Alves, F.; Basso, F.G.; de Souza Costa, C.A.; Tedesco, A.C.; Lucas Primo, F.; de Oliveira Mima, E.G.; Pavarina, A.C. Antimicrobial Photodynamic Therapy Reduces Adhesion Capacity and Biofilm Formation of Candida Albicans from Induced Oral Candidiasis in Mice. Photodiagn. Photodyn. Ther. 2019, 27, 402–407. [Google Scholar] [CrossRef]
- Fumes, A.C.; da Silva Telles, P.D.; Corona, S.A.M.; Borsatto, M.C. Effect of APDT on Streptococcus Mutans and Candida Albicans Present in the Dental Biofilm: Systematic Review. Photodiagn. Photodyn. Ther. 2018, 21, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.M.; Pavel, S. The Susceptibility of Dermatophytes to Photodynamic Treatment with Special Focus on Trichophyton Rubrum. Photochem. Photobiol. 2011, 87, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. A Comprehensive Overview of Photodynamic Therapy in the Treatment of Superficial Fungal Infections of the Skin. J. Photochem. Photobiol. B Biol. 2005, 78, 1–6. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.; Sibata, C.H. Photosensitizers in Clinical PDT. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Lyon, J.P.; Moreira, L.M.; de Moraes, P.C.G.; dos Santos, F.V.; de Resende, M.A. Photodynamic Therapy for Pathogenic Fungi: Antifungal PDT. Mycoses 2011, 54, e265–e271. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K. Functional Singlet Oxygen Generators Based on Phthalocyanines. Coord. Chem. Rev. 2012, 256, 1556–1568. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 70, pp. 343–394. ISBN 978-0-12-812834-3. [Google Scholar]
- Baier, J.; Maier, M.; Engl, R.; Landthaler, M.; Bäumler, W. Time-Resolved Investigations of Singlet Oxygen Luminescence in Water, in Phosphatidylcholine, and in Aqueous Suspensions of Phosphatidylcholine or HT29 Cells. J. Phys. Chem. B 2005, 109, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Skupin-Mrugalska, P.; Piskorz, J.; Goslinski, T.; Mielcarek, J.; Konopka, K.; Düzgüneş, N. Current Status of Liposomal Porphyrinoid Photosensitizers. Drug Discov. Today 2013, 18, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-Mrugalska, P.; Mielcarek, J.; Goslinski, T.; Balzarini, J. Photosensitizers Mediated Photodynamic Inactivation Against Virus Particles. Mini-Rev. Med. Chem. 2015, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Non-Porphyrinoid Photosensitizers Mediated Photodynamic Inactivation against Bacteria. Dye. Pigment. 2019, 163, 337–355. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid Photosensitizers Mediated Photodynamic Inactivation against Bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Liang, Y.; Lu, L.-M.; Chen, Y.; Lin, Y.-K. Photodynamic Therapy as an Antifungal Treatment. Exp. Ther. Med. 2016, 12, 23–27. [Google Scholar] [CrossRef]
- Bornstein, E.; Hermans, W.; Gridley, S.; Manni, J. Near-Infrared Photoinactivation of Bacteria and Fungi at Physiologic Temperatures. Photochem. Photobiol. 2009, 85, 1364–1374. [Google Scholar] [CrossRef]
- Biel, M.A. Photodynamic Therapy of Bacterial and Fungal Biofilm Infections. In Photodynamic Therapy; Gomer, C.J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 635, pp. 175–194. ISBN 978-1-60761-696-2. [Google Scholar]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, M. Photodynamic Antifungal Chemotherapy†. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Paardekooper, M.; De Bruijne, A.W.; Van Gompel, A.E.; Verhage, R.A.; Averbeck, D.; Dubbelman, T.M.A.R.; Van den Broek, P.J.A. Single Strand Breaks and Mutagenesis in Yeast Induced by Photodynamic Treatment with Chloroaluminum Phthalocyanine. J. Photochem. Photobiol. B Biol. 1997, 40, 132–140. [Google Scholar] [CrossRef]
- Song, L.; Zhang, F.; Yu, J.; Wei, C.; Han, Q.; Meng, X. Antifungal Effect and Possible Mechanism of Curcumin Mediated Photodynamic Technology against Penicillium Expansum. Postharvest Biol. Technol. 2020, 167, 111234. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Sobotta, L.; Kucinska, M.; Murias, M.; Mielcarek, J.; Duzgunes, N. Cellular Changes, Molecular Pathways and the Immune System Following Photodynamic Treatment. Curr. Med. Chem. 2014, 21, 4059–4073. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [Green Version]
- da Silva Martins, J.; Junqueira, J.C.; Faria, R.L.; Santiago, N.F.; Rossoni, R.D.; Colombo, C.E.D.; Jorge, A.O.C. Antimicrobial Photodynamic Therapy in Rat Experimental Candidiasis: Evaluation of Pathogenicity Factors of Candida Albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 71–77. [Google Scholar] [CrossRef]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic Enzymes as Virulence Factors of Candida Albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef]
- Paardekooper, M.; Bruune, A.W.D.; Steveninck, J.V.; Broek, P.J.V.D. Intracellular damage in yeast cells caused by photodynamic treatment with toluidine blue. Photochem. Photobiol. 1995, 61, 84–89. [Google Scholar] [CrossRef]
- Reginato, E. Immune Response after Photodynamic Therapy Increases Anti-Cancer and Anti-Bacterial Effects. WJI 2014, 4, 1. [Google Scholar] [CrossRef]
- Tanaka, M.; Mroz, P.; Dai, T.; Huang, L.; Morimoto, Y.; Kinoshita, M.; Yoshihara, Y.; Nemoto, K.; Shinomiya, N.; Seki, S.; et al. Photodynamic Therapy Can Induce a Protective Innate Immune Response against Murine Bacterial Arthritis via Neutrophil Accumulation. PLoS ONE 2012, 7, e39823. [Google Scholar] [CrossRef] [Green Version]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Sahu, K.; Sharma, M.; Kumar Gupta, P. Modulation of Inflammatory Response of Wounds by Antimicrobial Photodynamic Therapy. Laser Ther. 2015, 24, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo-Godoi, L.M.A.; Menezes, R.T.; Carvalho, J.S.; Garcia, M.T.; Segundo, A.G.; Jorge, A.O.C.; Junqueira, J.C. Exploring the Galleria Mellonella Model to Study Antifungal Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2019, 27, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Paziani, M.H.; Tonani, L.; de Menezes, H.D.; Bachmann, L.; Wainwright, M.; Braga, G.Ú.L.; von Zeska Kress, M.R. Antimicrobial Photodynamic Therapy with Phenothiazinium Photosensitizers in Non-Vertebrate Model Galleria Mellonella Infected with Fusarium Keratoplasticum and Fusarium Moniliforme. Photodiagn. Photodyn. Ther. 2019, 25, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue—Mediated Antimicrobial Photodynamic Therapy on Candida Spp. A Systematic Review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Hansberg, W.; Navarro, R. Fungal Responses to Reactive Oxygen Species. Med. Mycol. 2006, 44, S101–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briones-Martin-Del-Campo, M.; Orta-Zavalza, E.; Juarez-Cepeda, J.; Gutierrez-Escobedo, G.; Cañas-Villamar, I.; Castaño, I.; De Las Peñas, A. The oxidative stress response of the opportunistic fungal pathogen Candida glabrata. Rev. Iberoam. Micol. 2014, 31, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gessler, N.N.; Aver’yanov, A.A.; Belozerskaya, T.A. Reactive Oxygen Species in Regulation of Fungal Development. Biochem. Mosc. 2007, 72, 1091–1109. [Google Scholar] [CrossRef]
- Almeida, L.M.; Zanoelo, F.F.; Castro, K.P.; Borissevitch, I.E.; Soares, C.M.A.; Gonçalves, P.J. Cell Survival and Altered Gene Expression Following Photodynamic Inactivation of Paracoccidioides Brasiliensis: Photochemistry and Photobiology. Photochem. Photobiol. 2012, 88, 992–1000. [Google Scholar] [CrossRef]
- Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; St. Denis, T.G.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R.; Tegos, G.P. Concepts and Principles of Photodynamic Therapy as an Alternative Antifungal Discovery Platform. Front. Microbiol. 2012, 3, 120. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M.; McLean, A. Rational Design of Phenothiazinium Derivatives and Photoantimicrobial Drug Discovery. Dye. Pigment. 2017, 136, 590–600. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene Blue in Photodynamic Therapy: From Basic Mechanisms to Clinical Applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Freire, F.; de Barros, P.P.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O.C. Photodynamic Inactivation in the Expression of the Candida Albicans Genes ALS3, HWP1, BCR1, TEC1, CPH1, and EFG1 in Biofilms. Lasers Med. Sci. 2018, 33, 1447–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho Leonel, L.; Carvalho, M.L.; da Silva, B.M.; Zamuner, S.; Alberto-Silva, C.; Silva Costa, M. Photodynamic Antimicrobial Chemotherapy (PACT) Using Methylene Blue Inhibits the Viability of the Biofilm Produced by Candida Albicans. Photodiagn. Photodyn. Ther. 2019, 26, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Sabino, C.P.; Núñez, S.C.; Miyakawa, W.; Martin, A.A.; Ribeiro, M.S. Photodynamic Damage Predominates on Different Targets Depending on Cell Growth Phase of Candida Albicans. J. Photochem. Photobiol. B Biol. 2017, 177, 76–84. [Google Scholar] [CrossRef]
- Kato, I.T.; Prates, R.A.; Sabino, C.P.; Fuchs, B.B.; Tegos, G.P.; Mylonakis, E.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial Photodynamic Inactivation Inhibits Candida Albicans Virulence Factors and Reduces In Vivo Pathogenicity. Antimicrob. Agents Chemother. 2013, 57, 445–451. [Google Scholar] [CrossRef] [Green Version]
- da Collina, G.A.; Freire, F.; da Costa Santos, T.P.; Sobrinho, N.G.; Aquino, S.; Prates, R.A.; de Fátima Teixeira da Silva, D.; Tempestini Horliana, A.C.R.; Pavani, C. Controlling Methylene Blue Aggregation: A More Efficient Alternative to Treat Candida Albicans Infections Using Photodynamic Therapy. Photochem. Photobiol. Sci. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Torres-Hurtado, S.A.; Ramírez-Ramírez, J.; Larios-Morales, A.C.; Ramírez-San-Juan, J.C.; Ramos-García, R.; Espinosa-Texis, A.P.; Spezzia-Mazzocco, T. Efficient in Vitro Photodynamic Inactivation Using Repetitive Light Energy Density on Candida Albicans and Trichophyton Mentagrophytes. Photodiagn. Photodyn. Ther. 2019, 26, 203–209. [Google Scholar] [CrossRef]
- Černáková, L.; Dižová, S.; Bujdáková, H. Employment of Methylene Blue Irradiated with Laser Light Source in Photodynamic Inactivation of Biofilm Formed by Candida Albicans Strain Resistant to Fluconazole. Med. Mycol. 2017, 55, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Rossoni, R.D.; Barbosa, J.O.; de Oliveira, F.E.; de Oliveira, L.D.; Jorge, A.O.C.; Junqueira, J.C. Biofilms of Candida Albicans Serotypes A and B Differ in Their Sensitivity to Photodynamic Therapy. Lasers Med. Sci. 2014, 29, 1679–1684. [Google Scholar] [CrossRef]
- Carvalho, G.G.; Felipe, M.P.; Costa, M.S. The Photodynamic Effect of Methylene Blue and Toluidine Blue on Candida Albicans Is Dependent on Medium Conditions. J. Microbiol. 2009, 47, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, L.C.; Kato, I.T.; Prates, R.A.; Sabino, C.P.; Yoshimura, T.M.; Silva, T.O.; Ribeiro, M.S. Glucose Modulates Antimicrobial Photodynamic Inactivation of Candida Albicans in Biofilms. Photodiagn. Photodyn. Ther. 2017, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira-Silva, T.; Alvarenga, L.H.; Lima-Leal, C.; Godoy-Miranda, B.; Carribeiro, P.; Suzuki, L.C.; Simões Ribeiro, M.; Tiemy Kato, I.; Pavani, C.; Prates, R.A. Effect of Photodynamic Antimicrobial Chemotherapy on Candida Albicans in the Presence of Glucose. Photodiagn. Photodyn. Ther. 2019, 27, 54–58. [Google Scholar] [CrossRef]
- Fabio, C.-A.; Yolanda, M.-B.; Carmen, G.M.; Francisco, C.; Antonio Julián, B.; Leonor, P.-L.; Jesús, S. Use of Photodynamic Therapy and Chitosan for Inactivacion of Candida Albicans in a Murine Model. J. Oral Pathol. Med. 2016, 45, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.P.; Rezende, R.R.; Rabelo, M.P.; de Lima, C.J.; Moreira, L.M. Synergic Effect of Photodynamic Therapy with Methylene Blue and Surfactants in the Inhibition of Candida Albicans. Mycopathologia 2013, 175, 159–164. [Google Scholar] [CrossRef]
- Gonzales, F.P.; Da Silva, S.H.; Roberts, D.W.; Braga, G.U.L. Photodynamic Inactivation of Conidia of the Fungi Metarhizium Anisopliae and Aspergillus Nidulans with Methylene Blue and Toluidine Blue. Photochem. Photobiol. 2010, 86, 653–661. [Google Scholar] [CrossRef]
- Pinto, A.P.; Rosseti, I.B.; Carvalho, M.L.; da Silva, B.G.M.; Alberto-Silva, C.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT), Using Toluidine Blue O Inhibits the Viability of Biofilm Produced by Candida Albicans at Different Stages of Development. Photodiagn. Photodyn. Ther. 2018, 21, 182–189. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.G.M.; Carvalho, M.L.; Rosseti, I.B.; Zamuner, S.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT) Using Toluidine Blue Inhibits Both Growth and Biofilm Formation by Candida Krusei. Lasers Med. Sci. 2018, 33, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Wiench, R.; Skaba, D.; Stefanik, N.; Kępa, M.; Gilowski, Ł.; Cieślar, G.; Kawczyk-Krupka, A. Assessment of Sensitivity of Selected Candida Strains on Antimicrobial Photodynamic Therapy Using Diode Laser 635 Nm and Toluidine Blue—In Vitro Research. Photodiagn. Photodyn. Ther. 2019, 27, 241–247. [Google Scholar] [CrossRef]
- Mehra, T.; Schaller, M.; Walker, B.; Braunsdorf, C.; Mailänder-Sanchez, D.; Schynowski, F.; Hahn, R.; Röcken, M.; Köberle, M.; Borelli, C. Efficacy of Antifungal PACT in an in Vitro Model of Onychomycosis. J. Eur. Acad. Derm. Venereol. 2015, 29, 86–90. [Google Scholar] [CrossRef]
- Baltazar, L.d.M.; Soares, B.M.; Carneiro, H.C.S.; Avila, T.V.; Gouveia, L.F.; Souza, D.G.; Ferreira, M.V.L.; Pinotti, M.; Santos, D.d.A.; Cisalpino, P.S. Photodynamic Inhibition of Trichophyton Rubrum: In Vitro Activity and the Role of Oxidative and Nitrosative Bursts in Fungal Death. J. Antimicrob. Chemother. 2013, 68, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.-C.; Shen, M.; Huang, Y.-J.; Lin, H.-C.; Chen, C.-T. Photodynamic Inactivation Potentiates the Susceptibility of Antifungal Agents against the Planktonic and Biofilm Cells of Candida Albicans. Int. J. Mol. Sci. 2018, 19, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chibebe, J., Jr.; Sabino, C.P.; Tan, X.; Junqueira, J.C.; Wang, Y.; Fuchs, B.B.; Jorge, A.O.; Tegos, G.P.; Hamblin, M.R.; Mylonakis, E. Selective Photoinactivation of Candida Albicans in the Non-Vertebrate Host Infection Model Galleria Mellonella. BMC Microbiol 2013, 13, 217. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.R.; Sousa, A.S.; Alvarenga, L.H.; Deana, A.M.; de Santi, M.E.O.S.; Kato, I.T.; Leal, C.R.L.; Ribeiro, M.S.; Prates, R.A. Antimicrobial Photodynamic Therapy on Candida Albicans Pre-Treated by Fluconazole Delayed Yeast Inactivation. Photodiagn. Photodyn. Ther. 2016, 15, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jiang, S.; Sun, Y.; Deng, M.; Wu, Q.; Li, M.; Zeng, T. Evaluation of the Effects of Photodynamic Therapy Alone and Combined with Standard Antifungal Therapy on Planktonic Cells and Biofilms of Fusarium spp. and Exophiala spp. Front. Microbiol. 2016, 7, 617. [Google Scholar] [CrossRef] [PubMed]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Efficacy of Photodynamic Therapy versus Local Nystatin in the Treatment of Denture Stomatitis: A Randomized Clinical Study. Photodiagn. Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Daliri, F.; Azizi, A.; Goudarzi, M.; Lawaf, S.; Rahimi, A. In Vitro Comparison of the Effect of Photodynamic Therapy with Curcumin and Methylene Blue on Candida Albicans Colonies. Photodiagn. Photodyn. Ther. 2019, 26, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, Z.; Sun, Y.; Yang, L.; Gao, L. Inhibitory Effects of Photodynamic Inactivation on Planktonic Cells and Biofilms of Candida Auris. Mycopathologia 2019, 184, 525–531. [Google Scholar] [CrossRef]
- Alberdi, E.; Gómez, C. Efficiency of Methylene Blue-Mediated Photodynamic Therapy vs Intense Pulsed Light in the Treatment of Onychomycosis in the Toenails. Photodermatol. Photoimmunol. Photomed. 2019, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- de Senna, A.M.; Vieira, M.M.F.; Machado-de-Sena, R.M.; Bertolin, A.O.; Núñez, S.C.; Ribeiro, M.S. Photodynamic Inactivation of Candida Ssp. on Denture Stomatitis. A Clinical Trial Involving Palatal Mucosa and Prosthesis Disinfection. Photodiagn. Photodyn. Ther. 2018, 22, 212–216. [Google Scholar] [CrossRef]
- Ahangari, Z.; Mojtahed Bidabadi, M.; Asnaashari, M.; Rahmati, A.; Tabatabaei, F.S. Comparison of the Antimicrobial Efficacy of Calcium Hydroxide and Photodynamic Therapy Against Enterococcus Faecalis and Candida Albicans in Teeth With Periapical Lesions; An In Vivo Study. J. Lasers Med. Sci. 2017, 8, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Fekrazad, R.; Poorsattar Bejeh Mir, A.; Kahyaie Aghdam, M.; Ghasemi Barghi, V. Comparison of Photoinactivation of T. Rubrum by New Methylene Blue (NMB) and Indocyanine Green (EmunDo®). Photodiagn. Photodyn. Ther. 2017, 18, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Tonani, L.; Morosini, N.S.; Dantas de Menezes, H.; Nadaletto Bonifácio da Silva, M.E.; Wainwright, M.; Leite Braga, G.Ú.; Regina von Zeska Kress, M. In Vitro Susceptibilities of Neoscytalidium spp. Sequence Types to Antifungal Agents and Antimicrobial Photodynamic Treatment with Phenothiazinium Photosensitizers. Fungal Biol. 2018, 122, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Sun, Y.; Tian, D.; Xiang, S.; Gao, L. Effects of Photodynamic Therapy on the Growth and Antifungal Susceptibility of Scedosporium and Lomentospora spp. Mycopathologia 2017, 182, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Guffey, J.S.; Payne, W.; Roegge, W. In Vitro Fungicidal Effects of Methylene Blue at 625-Nm. Mycoses 2017, 60, 723–727. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Noaman, I.; El-Elsayyad, H.; El-Mashad, N.; Soliman, M. A Study of the Treatment of Cutaneous Fungal Infection in Animal Model Using Photoactivated Composite of Methylene Blue and Gold Nanoparticle. Photodiagn. Photodyn. Ther. 2016, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Amirzadeh, Z.; Rezai, M.; Lawaf, S.; Rahimi, A. Effect of Photodynamic Therapy with Two Photosensitizers on Candida Albicans. J. Photochem. Photobiol. B Biol. 2016, 158, 267–273. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, H.D.; Tonani, L.; Bachmann, L.; Wainwright, M.; Braga, G.Ú.L.; von Zeska Kress, M.R. Photodynamic Treatment with Phenothiazinium Photosensitizers Kills Both Ungerminated and Germinated Microconidia of the Pathogenic Fungi Fusarium Oxysporum, Fusarium Moniliforme and Fusarium Solani. J. Photochem. Photobiol. B Biol. 2016, 164, 1–12. [Google Scholar] [CrossRef]
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic Therapy of Oral Candida Infection in a Mouse Model. J. Photochem. Photobiol. B Biol. 2016, 159, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Černáková, L.; Chupáčová, J.; Židlíková, K.; Bujdáková, H. Effectiveness of the Photoactive Dye Methylene Blue versus Caspofungin on the Candida Parapsilosis Biofilm in Vitro and Ex Vivo. Photochem. Photobiol. 2015, 91, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, M.A.; Tufail, S.; Khan, A.A.; Owais, M. Gold Nanoparticle-Photosensitizer Conjugate Based Photodynamic Inactivation of Biofilm Producing Cells: Potential for Treatment of C. Albicans Infection in BALB/c Mice. PLoS ONE 2015, 10, e0131684. [Google Scholar] [CrossRef]
- Lopes, M.; Alves, C.T.; Rama Raju, B.; Gonçalves, M.S.T.; Coutinho, P.J.G.; Henriques, M.; Belo, I. Application of Benzo[a]Phenoxazinium Chlorides in Antimicrobial Photodynamic Therapy of Candida Albicans Biofilms. J. Photochem. Photobiol. B Biol. 2014, 141, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekrazad, R.; Ghasemi Barghi, V.; Poorsattar Bejeh Mir, A.; Shams-Ghahfarokhi, M. In Vitro Photodynamic Inactivation of Candida Albicans by Phenothiazine Dye (New Methylene Blue) and Indocyanine Green (EmunDo®). Photodiagn. Photodyn. Ther. 2015, 12, 52–57. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, H.D.; Rodrigues, G.B.; Teixeira, S.d.P.; Massola, N.S.; Bachmann, L.; Wainwright, M.; Braga, G.U.L. In Vitro Photodynamic Inactivation of Plant-Pathogenic Fungi Colletotrichum Acutatum and Colletotrichum Gloeosporioides with Novel Phenothiazinium Photosensitizers. Appl. Environ. Microbiol. 2014, 80, 1623–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado-de-Sena, R.M.; Corrêa, L.; Kato, I.T.; Prates, R.A.; Senna, A.M.; Santos, C.C.; Picanço, D.A.; Ribeiro, M.S. Photodynamic Therapy Has Antifungal Effect and Reduces Inflammatory Signals in Candida Albicans-Induced Murine Vaginitis. Photodiagn. Photodyn. Ther. 2014, 11, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.G.; Montes de Oca, M.N.; Milanesio, M.E.; Ortiz, C.S.; Durantini, E.N. Photodynamic Properties and Photoinactivation of Candida Albicans Mediated by Brominated Derivatives of Triarylmethane and Phenothiazinium Dyes. Photodiagn. Photodyn. Ther. 2014, 11, 148–155. [Google Scholar] [CrossRef]
- Figueiredo Souza, L.W.; Souza, S.V.T.; Botelho, A.C.C. Randomized Controlled Trial Comparing Photodynamic Therapy Based on Methylene Blue Dye and Fluconazole for Toenail Onychomycosis. Dermatol. Ther. 2014, 27, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.B.; Dias-Baruffi, M.; Holman, N.; Wainwright, M.; Braga, G.U.L. In Vitro Photodynamic Inactivation of Candida Species and Mouse Fibroblasts with Phenothiazinium Photosensitisers and Red Light. Photodiagn. Photodyn. Ther. 2013, 10, 141–149. [Google Scholar] [CrossRef]
- Rosseti, I.B.; Chagas, L.R.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT) Inhibits Biofilm Formation by Candida Albicans, Increasing Both ROS Production and Membrane Permeability. Lasers Med. Sci. 2014, 29, 1059–1064. [Google Scholar] [CrossRef]
- Barbério, G.S.; da Costa, S.V.; dos Santos Silva, M.; de Oliveira, T.M.; Silva, T.C.; de Andrade Moreira Machado, M.A. Photodynamic Inactivation of Candida Albicans Mediated by a Low Density of Light Energy. Lasers Med. Sci. 2013. [Google Scholar] [CrossRef]
- Paz-Cristobal, M.P.; Royo, D.; Rezusta, A.; Andrés-Ciriano, E.; Alejandre, M.C.; Meis, J.F.; Revillo, M.J.; Aspiroz, C.; Nonell, S.; Gilaberte, Y. Photodynamic Fungicidal Efficacy of Hypericin and Dimethyl Methylene Blue against Azole-Resistant Candida Albicans Strains. Mycoses 2014, 57, 35–42. [Google Scholar] [CrossRef]
- Chien, H.-F.; Chen, C.-P.; Chen, Y.-C.; Chang, P.-H.; Tsai, T.; Chen, C.-T. The Use of Chitosan to Enhance Photodynamic Inactivation against Candida Albicans and Its Drug-Resistant Clinical Isolates. Int. J. Mol. Sci. 2013, 14, 7445–7456. [Google Scholar] [CrossRef] [Green Version]
- Scwingel, A.R.; Barcessat, A.R.P.; Núñez, S.C.; Ribeiro, M.S. Antimicrobial Photodynamic Therapy in the Treatment of Oral Candidiasis in HIV-Infected Patients. Photomed. Laser Surg. 2012, 30, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Alam, F.; Azam, A.; Khan, A.U. Gold Nanoparticles Enhance Methylene Blue–Induced Photodynamic Therapy: A Novel Therapeutic Approach to Inhibit Candida Albicans Biofilm. IJN 2012, 3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, J.P.; Moreira, L.M.; Dutra de Carvalho, V.S.; dos Santos, F.V.; de Lima, C.J.; de Resende, M.A. In Vitro Photodynamic Therapy against Foncecaea Pedrosoi and Cladophialophora Carrionii: PDT against F. Pedrosoi and C. Carrionii. Mycoses 2013, 56, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.B.; Ferreira, L.K.S.; Wainwright, M.; Braga, G.U.L. Susceptibilities of the Dermatophytes Trichophyton Mentagrophytes and T. Rubrum Microconidia to Photodynamic Antimicrobial Chemotherapy with Novel Phenothiazinium Photosensitizers and Red Light. J. Photochem. Photobiol. B Biol. 2012, 116, 89–94. [Google Scholar] [CrossRef]
- Queiroga, A.S.; Trajano, V.N.; Lima, E.O.; Ferreira, A.F.M.; Queiroga, A.S.; Limeira, F.A. In Vitro Photodynamic Inactivation of Candida spp. by Different Doses of Low Power Laser Light. Photodiagn. Photodyn. Ther. 2011, 8, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.P.; de Maria Pedroso e Silva Azevedo, C.; Moreira, L.M.; de Lima, C.J.; de Resende, M.A. Photodynamic Antifungal Therapy Against Chromoblastomycosis. Mycopathologia 2011, 172, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Prates, R.A.; Kato, I.T.; Ribeiro, M.S.; Tegos, G.P.; Hamblin, M.R. Influence of Multidrug Efflux Systems on Methylene Blue-Mediated Photodynamic Inactivation of Candida Albicans. J. Antimicrob. Chemother. 2011, 66, 1525–1532. [Google Scholar] [CrossRef]
- Dai, T.; Bil de Arce, V.J.; Tegos, G.P.; Hamblin, M.R. Blue Dye and Red Light, a Dynamic Combination for Prophylaxis and Treatment of Cutaneous Candida Albicans Infections in Mice. Antimicrob. Agents Chemother. 2011, 55, 5710–5717. [Google Scholar] [CrossRef] [Green Version]
- Amorim, J.C.F.; Soares, B.M.; Alves, O.A.; Ferreira, M.V.L.; Sousa, G.R.; Silveira, L.d.B.; Piancastelli, A.C.C.; Pinotti, M. Phototoxic Action of Light Emitting Diode in the in Vitro Viability of Trichophyton Rubrum. Bras. Derm. 2012, 87, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.C.B.P.; Rasteiro, V.M.C.; Pereira, C.A.; Rossoni, R.D.; Junqueira, J.C.; Jorge, A.O.C. The Effects of Rose Bengal- and Erythrosine-Mediated Photodynamic Therapy on Candida Albicans: Photodynamic Therapy on Candida Albicans. Mycoses 2012, 55, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Junqueira, J.C.; Rossoni, R.D.; Pereira, C.A.; Munin, E.; Jorge, A.O.C. Comparison of the Photodynamic Fungicidal Efficacy of Methylene Blue, Toluidine Blue, Malachite Green and Low-Power Laser Irradiation Alone against Candida Albicans. Lasers Med. Sci. 2010, 25, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Giroldo, L.M.; Felipe, M.P.; de Oliveira, M.A.; Munin, E.; Alves, L.P.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT) with Methylene Blue Increases Membrane Permeability in Candida Albicans. Lasers Med. Sci. 2009, 24, 109–112. [Google Scholar] [CrossRef]
- Soares, B.M.; da Silva, D.L.; Sousa, G.R.; Amorim, J.C.F.; de Resende, M.A.; Pinotti, M.; Cisalpino, P.S. In Vitro Photodynamic Inactivation of Candida spp. Growth and Adhesion to Buccal Epithelial Cells. J. Photochem. Photobiol. B Biol. 2009, 94, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Munin, E.; Giroldo, L.M.; Alves, L.P.; Costa, M.S. Study of Germ Tube Formation by Candida Albicans after Photodynamic Antimicrobial Chemotherapy (PACT). J. Photochem. Photobiol. B Biol. 2007, 88, 16–20. [Google Scholar] [CrossRef]
- de Souza, S.C.; Junqueira, J.C.; Balducci, I.; Koga-Ito, C.Y.; Munin, E.; Jorge, A.O.C. Photosensitization of Different Candida Species by Low Power Laser Light. J. Photochem. Photobiol. B Biol. 2006, 83, 34–38. [Google Scholar] [CrossRef]
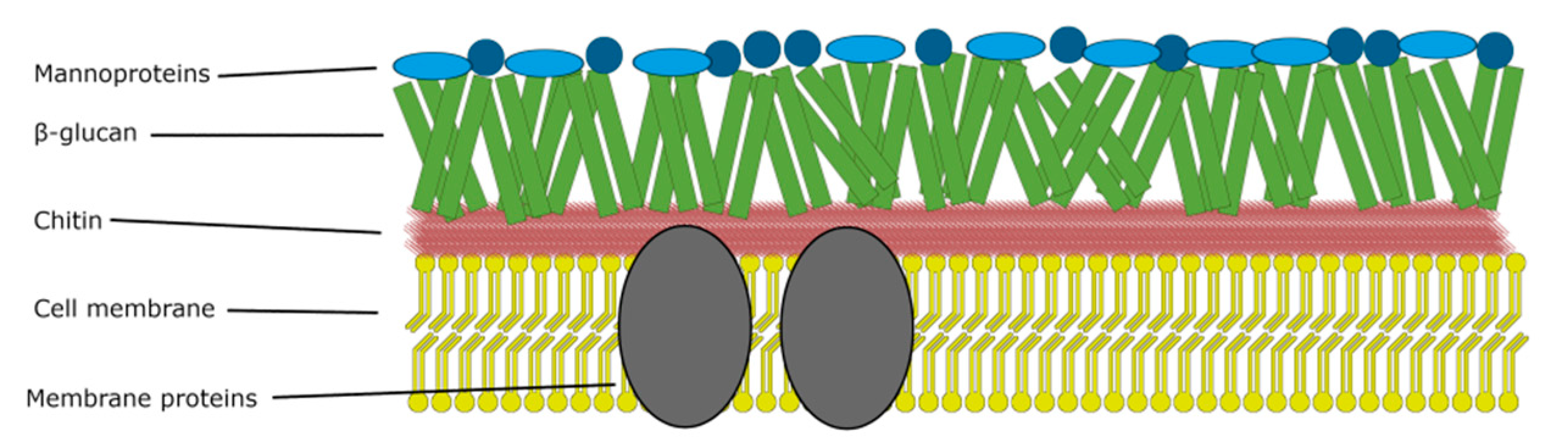
- Fuchs, B.B.; Tegos, G.P.; Hamblin, M.R.; Mylonakis, E. Susceptibility of Cryptococcus Neoformans to Photodynamic Inactivation Is Associated with Cell Wall Integrity. Antimicrob. Agents Chemother. 2007, 51, 2929–2936. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Ding, X.; Zhang, B.; Xu, N.; Jia, C.; Mao, J.; Zhang, B.; Xing, L.; Li, M. Inhibitory Effect of Verapamil on Candida Albicans Hyphal Development, Adhesion and Gastrointestinal Colonization. FEMS Yeast Res. 2014, 14, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Brown, V.; Sexton, J.A.; Johnston, M. A Glucose Sensor in Candida Albicans. Eukaryot. Cell 2006, 5, 1726–1737. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-P.; Chen, C.-T.; Tsai, T. Chitosan Nanoparticles for Antimicrobial Photodynamic Inactivation: Characterization and in Vitro Investigation. Photochem. Photobiol. 2012, 88, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.; Shankar, A.A. Toluidine Blue: A Review of Its Chemistry and Clinical Utility. J. Oral Maxillofac. Pathol. 2012, 16, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. The Role of the Methylene Blue and Toluidine Blue Monomers and Dimers in the Photoinactivation of Bacteria. J. Photochem. Photobiol. B Biol. 2003, 71, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. Comparison of the Methylene Blue and Toluidine Blue Photobactericidal Efficacy against Gram-Positive and Gram-Negative Microorganisms. Lasers Surg. Med. 2001, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Z.; Zhao, K.-Q.; Wu, Y.; Li, X.-H.; Yang, C.; Guo, L.-M.; Liu, C.-H.; Qu, D.; Zheng, C.-Q. 5-Aminolevulinic Acid-Mediated Photodynamic Therapy and Its Strain-Dependent Combined Effect with Antibiotics on Staphylococcus Aureus Biofilm. PLoS ONE 2017, 12, e0174627. [Google Scholar] [CrossRef]
- Tichaczek-Goska, D.; Wojnicz, D.; Symonowicz, K.; Ziółkowski, P.; Hendrich, A.B. Photodynamic Enhancement of the Activity of Antibiotics Used in Urinary Tract Infections. Lasers Med. Sci. 2019, 34, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial Strategies Centered around Reactive Oxygen Species—Bactericidal Antibiotics, Photodynamic Therapy, and Beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [Green Version]
- Kashef, N.; Hamblin, M.R. Can Microbial Cells Develop Resistance to Oxidative Stress in Antimicrobial Photodynamic Inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; de Oliveira Mima, E.G.; Giampaolo, E.T.; Vergani, C.E.; Bagnato, V.S. Fungicidal Effect of Photodynamic Therapy against Fluconazole-Resistant Candida Albicans and Candida Glabrata. Mycoses 2011, 54, 123–130. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Introduction to Nanoparticles. In Microwaves in Nanoparticle Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1–24. ISBN 978-3-527-64812-2. [Google Scholar]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Transition Metal Nanoparticles in Ionic Liquids: Synthesis and Stabilization. J. Mol. Liq. 2019, 276, 826–849. [Google Scholar] [CrossRef]
- Samiei, M.; Farjami, A.; Dizaj, S.M.; Lotfipour, F. Nanoparticles for Antimicrobial Purposes in Endodontics: A Systematic Review of In Vitro Studies. Mater. Sci. Eng. C 2016, 58, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Bera, S. Porphyrins and Phthalocyanines: Promising Molecules for Light-Triggered Antibacterial Nanoparticles. Adv. Nat. Sci: Nanosci. Nanotechnol. 2014, 5, 033002. [Google Scholar] [CrossRef]
- Kashef, N.; Huang, Y.-Y.; Hamblin, M.R. Advances in Antimicrobial Photodynamic Inactivation at the Nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in Photodynamic Therapy: An Emerging Paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.C.B.P.; de Campos Rasteiro, V.M.; Pereira, C.A.; da Silva Hashimoto, E.S.H.; Beltrame, M.; Junqueira, J.C.; Jorge, A.O.C. Susceptibility of Candida Albicans and Candida Dubliniensis to Erythrosine- and LED-Mediated Photodynamic Therapy. Arch. Oral Biol. 2011, 56, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracalossi, C.; Nagata, J.Y.; Pellosi, D.S.; Terada, R.S.S.; Hioka, N.; Baesso, M.L.; Sato, F.; Rosalen, P.L.; Caetano, W.; Fujimaki, M. Singlet Oxygen Production by Combining Erythrosine and Halogen Light for Photodynamic Inactivation of Streptococcus Mutans. Photodiagn. Photodyn. Ther. 2016, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Freitas, L.S.; Rossoni, R.D.; Jorge, A.O.C.; Junqueira, J.C. Repeated Applications of Photodynamic Therapy on Candida Glabrata Biofilms Formed in Acrylic Resin Polymerized. Lasers Med. Sci. 2017, 32, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.C.B.P.; Campos Rasteiro, V.M.; da Silva Hashimoto, E.S.H.; Araújo, C.F.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O.C. Effect of Erythrosine- and LED-Mediated Photodynamic Therapy on Buccal Candidiasis Infection of Immunosuppressed Mice and Candida Albicans Adherence to Buccal Epithelial Cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 67–74. [Google Scholar] [CrossRef] [Green Version]
- da Silva, N.R.; Ribeiro, D.G.; Issa, J.P.M.; Bonfá, K.; Menezes, M.S.; de Cássia Oliveira, V.; de Souza, R.F. Preclinical Study of a Cost-Effective Photodynamic Therapy Protocol for Treating Oral Candidoses. Lasers Med. Sci. 2017, 32, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Tomé, F.M.; Paula Ramos, L.D.; Freire, F.; Pereira, C.A.; de Oliveira, I.C.B.; Junqueira, J.C.; Jorge, A.O.C.; de Oliveira, L.D. Influence of Sucrose on Growth and Sensitivity of Candida Albicans Alone and in Combination with Enterococcus faecalis and Streptococcus mutans to Photodynamic Therapy. Lasers Med Sci 2017, 32, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- do Rosario Palma, A.L.; de Paula-Ramos, L.; Domingues, N.; Back-Brito, G.N.; de Oliveira, L.D.; Pereira, C.A.; Jorge, A.O.C. Biofilms of Candida albicans and Streptococcus sanguinis and Their Susceptibility to Antimicrobial Effects of Photodynamic Inactivation. Photodiagn. Photodyn. Ther. 2018, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Domingues, N.; Silva, M.P.; Costa, A.C.B.P.; Junqueira, J.C.; Jorge, A.O.C. Photodynamic Inactivation of Virulence Factors of Candida strains Isolated from Patients with Denture Stomatitis. J. Photochem. Photobiol. B Biol. 2015, 153, 82–89. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Priefer, R. Non-Porphyrin Dyes Used as Photosensitizers in Photodynamic Therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101979. [Google Scholar] [CrossRef]
- Silva, M.P.; dos Santos, T.A.; de Barros, P.P.; de Camargo Ribeiro, F.; Junqueira, J.C.; Jorge, A.O.C. Action of Antimicrobial Photodynamic Therapy on Heterotypic Biofilm: Candida albicans and Bacillus atrophaeus. Lasers Med Sci 2016, 31, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Freire, F.; Costa, A.C.B.P.; Pereira, C.A.; Beltrame, M., Jr.; Junqueira, J.C.; Jorge, A.O.C. Comparison of the Effect of Rose Bengal- and Eosin Y-Mediated Photodynamic Inactivation on Planktonic Cells and Biofilms of Candida Albicans. Lasers Med. Sci. 2014, 29, 949–955. [Google Scholar] [CrossRef]
- Cronin, L.; Moffitt, M.; Mawad, D.; Morton, O.C.; Lauto, A.; Stack, C. An in Vitro Study of the Photodynamic Effect of Rose Bengal on Trichophyton Rubrum. J. Biophotonics 2014, 7, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, A.; Miller, D.; Cabot, F.; Taneja, M.; Aguilar, M.C.; Alawa, K.; Amescua, G.; Yoo, S.H.; Parel, J.-M. Assessment of Rose Bengal versus Riboflavin Photodynamic Therapy for Inhibition of Fungal Keratitis Isolates. Am. J. Ophthalmol. 2014, 158, 64–70.e2. [Google Scholar] [CrossRef] [Green Version]
- Maliszewska, I.; Lisiak, B.; Popko, K.; Matczyszyn, K. Enhancement of the Efficacy of Photodynamic Inactivation of Candida albicans with the Use of Biogenic Gold Nanoparticles. Photochem. Photobiol. 2017, 93, 1081–1090. [Google Scholar] [CrossRef]
- Morton, C.O.; Chau, M.; Stack, C. In Vitro Combination Therapy Using Low Dose Clotrimazole and Photodynamic Therapy Leads to Enhanced Killing of the Dermatophyte Trichophyton Rubrum. BMC Microbiol. 2014, 14, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Picco, D.d.C.R.; Cavalcante, L.L.R.; Trevisan, R.L.B.; Souza-Gabriel, A.E.; Borsatto, M.C.; Corona, S.A.M. Effect of Curcumin-Mediated Photodynamic Therapy on Streptococcus mutans and Candida albicans: A Systematic Review of in Vitro Studies. Photodiagn. Photodyn. Ther. 2019, 27, 455–461. [Google Scholar] [CrossRef]
- Araújo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E.M. Photodynamic Antimicrobial Therapy of Curcumin in Biofilms and Carious Dentine. Lasers Med. Sci. 2014, 29, 629–635. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; Machado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of Clinical Isolates of Candida to Photodynamic Effects of Curcumin: PHOTODYNAMIC EFFECTS OF CURCUMIN AGAINST Candida spp. Lasers Surg. Med. 2011, 43, 927–934. [Google Scholar] [CrossRef]
- Sanitá, P.V.; Pavarina, A.C.; Dovigo, L.N.; Ribeiro, A.P.D.; Andrade, M.C.; de Oliveira Mima, E.G. Curcumin-Mediated Anti-Microbial Photodynamic Therapy against Candida Dubliniensis Biofilms. Lasers Med. Sci. 2018, 33, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.-H.; Zhang, J.-H.; Chuang, W.-C.; Yu, K.-H.; Huang, X.-B.; Lee, Y.-C.; Lee, C.-I. An in Vitro Study on the Effect of Combined Treatment with Photodynamic and Chemical Therapies on Candida albicans. IJMS 2018, 19, 337. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Shi, H.; Sun, H.; Li, J.; Bai, Y. Antifungal Effect of Photodynamic Therapy Mediated by Curcumin on Candida Albicans Biofilms in Vitro. Photodiagn. Photodyn. Ther. 2019, 27, 280–287. [Google Scholar] [CrossRef]
- Jordão, C.C.; Viana de Sousa, T.; Inêz Klein, M.; Mendonça Dias, L.; Pavarina, A.C.; Carmello, J.C. Antimicrobial Photodynamic Therapy Reduces Gene Expression of Candida Albicans in Biofilms. Photodiagn. Photodyn. Ther. 2020, 31, 101825. [Google Scholar] [CrossRef]
- Andrade, M.C.; Ribeiro, A.P.D.; Dovigo, L.N.; Brunetti, I.L.; Giampaolo, E.T.; Bagnato, V.S.; Pavarina, A.C. Effect of Different Pre-Irradiation Times on Curcumin-Mediated Photodynamic Therapy against Planktonic Cultures and Biofilms of Candida spp. Arch. Oral Biol. 2013, 58, 200–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltazar, L.M.; Krausz, A.E.; Souza, A.C.O.; Adler, B.L.; Landriscina, A.; Musaev, T.; Nosanchuk, J.D.; Friedman, A.J. Trichophyton Rubrum Is Inhibited by Free and Nanoparticle Encapsulated Curcumin by Induction of Nitrosative Stress after Photodynamic Activation. PLoS ONE 2015, 10, e0120179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmello, J.C.; Pavarina, A.C.; Oliveira, R.; Johansson, B. Genotoxic Effect of Photodynamic Therapy Mediated by Curcumin on Candida albicans. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.C.; Fernandes Rodrigues, P.L.; Santos Dantas Araújo, T.; Sousa Santos, M.; de Oliveira, J.M.; Pereira Rosa, L.; de Oliveira Santos, G.P.; de Araújo, B.P.; Bagnato, V.S. Fluorescence Spectroscopy of Candida Albicans Biofilms in Bone Cavities Treated with Photodynamic Therapy Using Blue LED (450 Nm) and Curcumin. Photodiagn. Photodyn. Ther. 2019, 26, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.d.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the Photodynamic Effects of Curcumin Against Candida Albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Carmello, J.C.; de Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-Mediated Photodynamic Inactivation of Candida Albicans in a Murine Model of Oral Candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Quishida, C.C.C.; De Oliveira Mima, E.G.; Jorge, J.H.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic Inactivation of a Multispecies Biofilm Using Curcumin and LED Light. Lasers Med. Sci. 2016, 31, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- Pellissari, C.V.G.; Pavarina, A.C.; Bagnato, V.S.; de Oliveira Mima, E.G.; Vergani, C.E.; Jorge, J.H. Cytotoxicity of Antimicrobial Photodynamic Inactivation on Epithelial Cells When Co-Cultured with Candida albicans. Photochem. Photobiol. Sci. 2016, 15, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Sakima, V.; Barbugli, P.; Cerri, P.; Chorilli, M.; Carmello, J.; Pavarina, A.; Mima, E. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules 2018, 23, 2075. [Google Scholar] [CrossRef] [Green Version]
- Bliss, J.M.; Bigelow, C.E.; Foster, T.H.; Haidaris, C.G. Susceptibility of Candida Species to Photodynamic Effects of Photofrin. Antimicrob. Agents Chemother. 2004, 48, 2000–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabrier-Roselló, Y.; Foster, T.H.; Pérez-Nazario, N.; Mitra, S.; Haidaris, C.G. Sensitivity of Candida Albicans Germ Tubes and Biofilms to Photofrin-Mediated Phototoxicity. Antimicrob. Agents Chemother. 2005, 49, 4288–4295. [Google Scholar] [CrossRef] [Green Version]
- Mang, T.S.; Mikulski, L.; Hall, R.E. Photodynamic Inactivation of Normal and Antifungal Resistant Candida Species. Photodiagn. Photodyn. Ther. 2010, 7, 98–105. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, D.G.; Adriano, C.S.; Bagnato, V.S. Photodynamic Inactivation of Four Candida Species Induced by Photogem®. Braz. J. Microbiol. 2010, 41, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Mima, E.G.d.O.; Pavarina, A.C.; Ribeiro, D.G.; Dovigo, L.N.; Vergani, C.E.; Bagnato, V.S. Effectiveness of Photodynamic Therapy for the Inactivation of Candida Spp. on Dentures: In Vitro Study. Photomed. Laser Surg. 2011, 29, 827–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Mima, E.G.; Pavarina, A.C.; Dovigo, L.N.; Vergani, C.E.; de Souza Costa, C.A.; Kurachi, C.; Bagnato, V.S. Susceptibility of Candida Albicans to Photodynamic Therapy in a Murine Model of Oral Candidosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 392–401. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Mima, E.G.; Pavarina, A.C.; Silva, M.M.; Ribeiro, D.G.; Vergani, C.E.; Kurachi, C.; Bagnato, V.S. Denture Stomatitis Treated with Photodynamic Therapy: Five Cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 602–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mima, E.G.; Vergani, C.E.; Machado, A.L.; Massucato, E.M.S.; Colombo, A.L.; Bagnato, V.S.; Pavarina, A.C. Comparison of Photodynamic Therapy versus Conventional Antifungal Therapy for the Treatment of Denture Stomatitis: A Randomized Clinical Trial. Clin. Microbiol. Infect. 2012, 18, E380–E388. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.P.; Kurachi, C.; Bagnato, V.S.; Inada, N.M. Fast Elimination of Onychomycosis by Hematoporphyrin Derivative-Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2013, 10, 328–330. [Google Scholar] [CrossRef]
- Romano, R.A.; Pratavieira, S.; Silva, A.P.D.; Kurachi, C.; Guimarães, F.E.G. Light-Driven Photosensitizer Uptake Increases Candida Albicans Photodynamic Inactivation. J. Biophotonics 2017, 10, 1538–1546. [Google Scholar] [CrossRef]
- Liu, C.; Hu, M.; Zeng, X.; Nair, S.P.; Xu, J. Photodynamic Inactivation of Candida Albicans by Hematoporphyrin Monomethyl Ether. Future Microbiol. 2016, 11, 351–362. [Google Scholar] [CrossRef]
- Liu, C.; Hu, M.; Zhang, B.; Zeng, X. Haematoporphyrin Monomethyl Ether-Mediated Photodynamic Inactivation of the Biofilms Produced by Standard and Fluconazole-Resistant Candida Albicans Strains. Clin Exp Derm. 2017, 42, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Li, R.; Ding, Y.; Fang, H. Photodynamic Therapy in the Treatment of Superficial Mycoses: An Evidence-Based Evaluation. Mycopathologia 2010, 170, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Simmons, B.J.; Griffith, R.D.; Falto-Aizpurua, L.A.; Nouri, K. An Update on Photodynamic Therapies in the Treatment of Onychomycosis. J. Eur. Acad. Derm. Venereol. 2015, 29, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Robres, P.; Aspiroz, C.; Rezusta, A.; Gilaberte, Y. Usefulness of Photodynamic Therapy in the Management of Onychomycosis. Actas Dermo-Sifiliográficas (Engl. Ed.) 2015, 106, 795–805. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Capezzera, R.; Sala, R.; Zane, C. Photodynamic Therapy of Interdigital Mycoses of the Feet with Topical Application of 5-Aminolevulinic Acid. Photoderm. Photoimm. Photomed. 2004, 20, 144–147. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, L.; Chen, J.; Yang, H.; Gao, Z.; Wang, X. Successful Sequential Treatment with Itraconazole and ALA-PDT for Cutaneous Granuloma by Candida Albicans: A Case Report and Literature Review. Mycopathologia 2018, 183, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, B.J.; Kim, M.N. Photodynamic Therapy: New Treatment for Recalcitrant Malassezia Folliculitis: PHOTODYNAMIC THERAPY. Lasers Surg. Med. 2010, 42, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Aspiroz, C.; Fortuño Cebamanos, B.; Rezusta, A.; Paz-Cristóbal, P.; Domínguez-Luzón, F.; Gené Díaz, J.; Gilaberte, Y. Terapia fotodinámica aplicada al tratamiento de las onicomicosis. Presentación de un caso y revisión de la literatura. Rev. Iberoam. Micol. 2011, 28, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Aspiroz, C.; Fortuño-Cebamanos, B.; Rezusta, A.; Gilaberte, Y. Photodynamic Therapy With Methyl-Aminolevulinate Can Be Useful in the Management of Scytalidium Infections. Actas Dermo-Sifiliográficas (Engl. Ed.) 2013, 104, 725–727. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, X.; Lu, S.; Hamblin, M.R.; Mylonakis, E.; Zhang, J.; Xi, L. Photodynamic Therapy Combined with Terbinafine Against Chromoblastomycosis and the Effect of PDT on Fonsecaea Monophora In Vitro. Mycopathologia 2015, 179, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Qi, X.; Sun, H.; Lu, Y.; Hu, Y.; Chen, X.; Liu, K.; Yang, Y.; Mao, Z.; Wu, Z.; et al. Photodynamic Therapy Combined with Antifungal Drugs against Chromoblastomycosis and the Effect of ALA-PDT on Fonsecaea In Vitro. PLoS Negl. Trop. Dis. 2019, 13, e0007849. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Fransisca, C.; He, Y.; Liu, Y.; Lu, S.; He, L.; Xi, L. Photodynamic Effects on Fonsecaea Monophora Conidia and RAW264.7 In Vitro. J. Photochem. Photobiol. B Biol. 2017, 176, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, J.; Zhang, H.; Zhang, J.; Sun, H. Effect of 5-Aminolevulinic Acid Photodynamic Therapy on Candida Albicans Biofilms: An In Vitro Study. Photodiagn. Photodyn. Ther. 2016, 15, 40–45. [Google Scholar] [CrossRef]
- de Santi, M.E.S.O.; Prates, R.A.; França, C.M.; Lopes, R.G.; Sousa, A.S.; Ferreira, L.R.; Bussadori, S.K.; Fernandes, A.U.; Deana, A.M. Antimicrobial Photodynamic Therapy as a New Approach for the Treatment of Vulvovaginal Candidiasis: Preliminary Results. Lasers Med. Sci. 2018, 33, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Chabrier-Roselló, Y.; Foster, T.H.; Mitra, S.; Haidaris, C.G. Respiratory Deficiency Enhances the Sensitivity of the Pathogenic Fungus Candida to Photodynamic Treatment. Photochem. Photobiol. 2008, 84, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Chabrier-Roselló, Y.; Giesselman, B.R.; De Jesús-Andino, F.J.; Foster, T.H.; Mitra, S.; Haidaris, C.G. Inhibition of Electron Transport Chain Assembly and Function Promotes Photodynamic Killing of Candida. J. Photochem. Photobiol. B Biol. 2010, 99, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. Susceptibility of Candida Albicans to Photodynamic Action of 5,10,15,20-Tetra(4-N-Methylpyridyl)Porphyrin in Different Media. FEMS Immunol. Med. Microbiol. 2010, 60, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Bornhütter, T.; Shamali, N.; Saltsman, I.; Mahammed, A.; Gross, Z.; Däschlein, G.; Röder, B. Singlet Oxygen Luminescence Kinetics under PDI Relevant Conditions of Pathogenic Dermatophytes and Molds. J. Photochem. Photobiol. B Biol. 2018, 178, 606–613. [Google Scholar] [CrossRef]
- Shamali, N.; Preuß, A.; Saltsman, I.; Mahammed, A.; Gross, Z.; Däschlein, G.; Röder, B. In Vitro Photodynamic Inactivation (PDI) of Pathogenic Germs Inducing Onychomycosis. Photodiagn. Photodyn. Ther. 2018, 24, 358–365. [Google Scholar] [CrossRef]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic Inactivation of Candida Albicans Sensitized by Tri- and Tetra-Cationic Porphyrin Derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef]
- Cormick, M.P.; Quiroga, E.D.; Bertolotti, S.G.; Alvarez, M.G.; Durantini, E.N. Mechanistic Insight of the Photodynamic Effect Induced by Tri- and Tetra-Cationic Porphyrins on Candida Albicans Cells. Photochem. Photobiol. Sci. 2011, 10, 1556. [Google Scholar] [CrossRef]
- Quiroga, E.D.; Cormick, M.P.; Pons, P.; Alvarez, M.G.; Durantini, E.N. Mechanistic Aspects of the Photodynamic Inactivation of Candida Albicans Induced by Cationic Porphyrin Derivatives. Eur. J. Med. Chem. 2012, 58, 332–339. [Google Scholar] [CrossRef]
- Davies, A.; Gebremedhin, S.; Yee, M.; Padilla, R.J.; Duzgunes, N.; Konopka, K.; Dorocka-Bobkowska, B. Cationic Porphyrin-Mediated Photodynamic Inactivation of Candida Biofilms and the Effect of Miconazole. J. Physiol. Pharmacol. 2016, 67, 777–783. [Google Scholar]
- Snell, S.B.; Foster, T.H.; Haidaris, C.G. Miconazole Induces Fungistasis and Increases Killing of Candida Albicans Subjected to Photodynamic Therapy. Photochem. Photobiol. 2012, 88, 596–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiroga, E.D.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Photodynamic Inactivation of Candida Albicans by a Tetracationic Tentacle Porphyrin and Its Analogue without Intrinsic Charges in Presence of Fluconazole. Photodiagn. Photodyn. Ther. 2016, 13, 334–340. [Google Scholar] [CrossRef]
- Gomes, M.C.; Woranovicz-Barreira, S.M.; Faustino, M.A.F.; Fernandes, R.; Neves, M.G.P.M.S.; Tomé, A.C.; Gomes, N.C.M.; Almeida, A.; Cavaleiro, J.A.S.; Cunha, Â.; et al. Photodynamic Inactivation of Penicillium Chrysogenum Conidia by Cationic Porphyrins. Photochem. Photobiol. Sci. 2011, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Haidaris, C.G.; Snell, S.B.; Giesselman, B.R.; Hupcher, S.M.; Foster, T.H. Effective Photosensitization and Selectivity In Vivo of Candida Albicans by Meso-Tetra(N-Methyl-4-Pyridyl) Porphine Tetratosylate: Photodynamic Treatment of Candidiasis. Lasers Surg. Med. 2011, 43, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Lambrechts, S.A.G.; Aalders, M.C.G.; Van Marle, J. Mechanistic Study of the Photodynamic Inactivation of Candida Albicans by a Cationic Porphyrin. Antimicrob. Agents Chemother. 2005, 49, 2026–2034. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, F.P.; Felgenträger, A.; Bäumler, W.; Maisch, T. Fungicidal Photodynamic Effect of a Twofold Positively Charged Porphyrin against Candida Albicans Planktonic Cells and Biofilms. Future Microbiol. 2013, 8, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Vandresen, C.C.; Gonçalves, A.G.; Ducatti, D.R.B.; Murakami, F.S.; Noseda, M.D.; Duarte, M.E.R.; Barreira, S.M.W. In Vitro Photodynamic Inactivation of Conidia of the Phytopathogenic Fungus Colletotrichum Graminicola with Cationic Porphyrins. Photochem. Photobiol. Sci. 2016, 15, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.M.; Schuitmaker, H.J. Photodynamic Inactivation of the Dermatophyte. Trichophyton Rubrum 2003, 77, 555–560. [Google Scholar] [CrossRef]
- Smijs, T.G.M.; Bouwstra, J.A.; Schuitmaker, H.J.; Talebi, M.; Pavel, S. A Novel Ex Vivo Skin Model to Study the Susceptibility of the Dermatophyte Trichophyton Rubrum to Photodynamic Treatment in Different Growth Phases. J. Antimicrob. Chemother. 2007, 59, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Smijs, T.G.M.; Bouwstra, J.A.; Talebi, M.; Pavel, S. Investigation of Conditions Involved in the Susceptibility of the Dermatophyte Trichophyton Rubrum to Photodynamic Treatment. J. Antimicrob. Chemother. 2007, 60, 750–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smijs, T.G.M.; Pavel, S.; Talebi, M.; Bouwstra, J.A. Preclinical Studies with 5,10,15-Tris(4-Methylpyridinium)-20-Phenyl-[21H,23H]-Porphine Trichloride for the Photodynamic Treatment of Superficial Mycoses Caused by Trichophyton Rubrum. Photochem. Photobiol. 2009, 85, 733–739. [Google Scholar] [CrossRef]
- Smijs, T.; Dame, Z.; de Haas, E.; Aans, J.-B.; Pavel, S.; Sterenborg, H. Photodynamic and Nail Penetration Enhancing Effects of Novel Multifunctional Photosensitizers Designed for The Treatment of Onychomycosis. Photochem. Photobiol. 2014, 90, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hollander, C.; Visser, J.; de Haas, E.; Incrocci, L.; Smijs, T. Effective Single Photodynamic Treatment of Ex Vivo Onychomycosis Using a Multifunctional Porphyrin Photosensitizer and Green Light. JoF 2015, 1, 138–153. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Gómez, M.L.; Mora, S.J.; Milanesio, M.E.; Durantini, E.N. Photodynamic Inactivation of Candida Albicans Using Bridged Polysilsesquioxane Films Doped with Porphyrin. Bioorg. Med. Chem. 2012, 20, 4032–4039. [Google Scholar] [CrossRef] [PubMed]
- Viana, O.; Ribeiro, M.; Rodas, A.; Rebouças, J.; Fontes, A.; Santos, B. Comparative Study on the Efficiency of the Photodynamic Inactivation of Candida Albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers. Molecules 2015, 20, 8893–8912. [Google Scholar] [CrossRef] [Green Version]
- Wijesiri, N.; Yu, Z.; Tang, H.; Zhang, P. Antifungal Photodynamic Inactivation against Dermatophyte Trichophyton Rubrum Using Nanoparticle-Based Hybrid Photosensitizers. Photodiagn. Photodyn. Ther. 2018, 23, 202–208. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Rech, G.; Tosti, A. Photodynamic Therapy of Onychomycosis Caused by Trichophyton Rubrum. J. Am. Acad. Dermatol. 2008, 59, S75–S76. [Google Scholar] [CrossRef]
- Amirshaghaghi, A.; Yan, L.; Miller, J.; Daniel, Y.; Stein, J.M.; Busch, T.M.; Cheng, Z.; Tsourkas, A. Chlorin E6-Coated Superparamagnetic Iron Oxide Nanoparticle (SPION) Nanoclusters as a Theranostic Agent for Dual-Mode Imaging and Photodynamic Therapy. Sci. Rep. 2019, 9, 2613. [Google Scholar] [CrossRef] [Green Version]
- Sueoka, K.; Chikama, T.; Pertiwi, Y.D.; Ko, J.-A.; Kiuchi, Y.; Sakaguchi, T.; Obana, A. Antifungal Efficacy of Photodynamic Therapy with TONS 504 for Pathogenic Filamentous Fungi. Lasers Med. Sci. 2019, 34, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.L.; Pinto, A.P.; Raniero, L.J.; Costa, M.S. Biofilm Formation by Candida Albicans Is Inhibited by Photodynamic Antimicrobial Chemotherapy (PACT), Using Chlorin E6: Increase in both ROS Production and Membrane Permeability. Lasers Med. Sci. 2018, 33, 647–653. [Google Scholar] [CrossRef]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.a.F.; Neves, M.G.P.M.S.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial Photodynamic Therapy against Endodontic Enterococcus Faecalis and Candida Albicans Mono and Mixed Biofilms in the Presence of Photosensitizers: A Comparative Study with Classical Endodontic Irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.-T.; Chien, H.-F.; Chang, P.-H.; Chen, Y.-C.; Jay, M.; Tsai, T.; Chen, C.-T. Photodynamic Inactivation of Chlorin E6-Loaded CTAB-Liposomes against Candida Albicans. Lasers Surg. Med. 2013, 45, 175–185. [Google Scholar] [CrossRef]
- Tegos, G.P.; Anbe, M.; Yang, C.; Demidova, T.N.; Satti, M.; Mroz, P.; Janjua, S.; Gad, F.; Hamblin, M.R. Protease-Stable Polycationic Photosensitizer Conjugates between Polyethyleneimine and Chlorin(E6) for Broad-Spectrum Antimicrobial Photoinactivation. Antimicrob. Agents Chemother. 2006, 50, 1402–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astuty, S.D.; Suhariningsih, A.B.; Baktir, A.; Astuti, S.D. The Efficacy of Photodynamic Inactivation of the Diode Laser in Inactivation of the Candida Albicans Biofilms with Exogenous Photosensitizer of Papaya Leaf Chlorophyll. J. Lasers Med. Sci. 2019, 10, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, M.; Huang, Y.-Y.; El-Hussein, A.; Wolf, L.M.; Chiang, L.Y.; Hamblin, M.R. Progressive Cationic Functionalization of Chlorin Derivatives for Antimicrobial Photodynamic Inactivation and Related Vancomycin Conjugates. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Pavarina, A.C.; Mima, E.G.d.O.; McHale, A.P.; Callan, J.F. Antimicrobial Sonodynamic and Photodynamic Therapies against Candida Albicans. Biofouling 2018, 34, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeth Rimachi Hidalgo, K.; Carmello, J.C.; Carolina Jordão, C.; Aboud Barbugli, P.; de Sousa Costa, C.A.; de Oliveira Mima, E.G.; Pavarina, A.C. Antimicrobial Photodynamic Therapy in Combination with Nystatin in the Treatment of Experimental Oral Candidiasis Induced by Candida Albicans Resistant to Fluconazole. Pharmaceuticals 2019, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Alves, F.; Carmello, J.C.; de Oliveira Mima, E.G.; de Souza Costa, C.A.; Bagnato, V.S.; Pavarina, A.C. Photodithazine-Mediated Antimicrobial Photodynamic Therapy against Fluconazole-Resistant Candida Albicans in Vivo. Med. Mycol. 2019, 57, 609–617. [Google Scholar] [CrossRef]
- Quishida, C.C.C.; Carmello, J.C.; de Oliveira Mima, E.G.; Bagnato, V.S.; Machado, A.L.; Pavarina, A.C. Susceptibility of Multispecies Biofilm to Photodynamic Therapy Using Photodithazine®. Lasers Med. Sci. 2015, 30, 685–694. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Carmello, J.C.; Carvalho, M.T.; Mima, E.G.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic Inactivation of Clinical Isolates of Candida Using Photodithazine®. Biofouling 2013, 29, 1057–1067. [Google Scholar] [CrossRef]
- Miller, J.; Baron, E.; Scull, H.; Hsia, A.; Berlin, J.; Mccormick, T.; Colussi, V.; Kenney, M.; Cooper, K.; Oleinick, N. Photodynamic Therapy with the Phthalocyanine Photosensitizer Pc 4: The Case Experience with Preclinical Mechanistic and Early Clinical–Translational Studies. Toxicol. Appl. Pharmacol. 2007, 224, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Wierzchowski, M.; Sobotta, L.; Łażewski, D.; Kasprzycki, P.; Fita, P.; Goslinski, T. Spectroscopic and Quantum Chemical Study of Phthalocyanines with 1,4,7-Trioxanonyl Moieties. J. Mol. Struct. 2020, 1203, 127371. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Chuang, W.-C.; Yu, K.-H.; Jheng, C.-P.; Lee, C.-I. Sequential Photodynamic Therapy with Phthalocyanine Encapsulated Chitosan-Tripolyphosphate Nanoparticles and Flucytosine Treatment against Candida Tropicalis. Pharmaceutics 2019, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Mantareva, V.; Angelov, I.; Kussovski, V.; Dimitrov, R.; Lapok, L.; Wöhrle, D. Photodynamic Efficacy of Water-Soluble Si(IV) and Ge(IV) Phthalocyanines towards Candida Albicans Planktonic and Biofilm Cultures. Eur. J. Med. Chem. 2011, 46, 4430–4440. [Google Scholar] [CrossRef]
- Mantareva, V.; Kussovski, V.; Durmuş, M.; Borisova, E.; Angelov, I. Photodynamic Inactivation of Pathogenic Species Pseudomonas Aeruginosa and Candida Albicans with Lutetium (III) Acetate Phthalocyanines and Specific Light Irradiation. Lasers Med. Sci. 2016, 31, 1591–1598. [Google Scholar] [CrossRef]
- Ribeiro, A.P.D.; Andrade, M.C.; de Fátima da Silva, J.; Jorge, J.H.; Primo, F.L.; Tedesco, A.C.; Pavarina, A.C. Photodynamic Inactivation of Planktonic Cultures and Biofilms of Candida Albicans Mediated by Aluminum-Chloride-Phthalocyanine Entrapped in Nanoemulsions: Photochemistry and Photobiology. Photochem. Photobiol. 2013, 89, 111–119. [Google Scholar] [CrossRef]
- Morgado, L.F.; Trávolo, A.R.F.; Muehlmann, L.A.; Narcizo, P.S.; Nunes, R.B.; Pereira, P.A.G.; Py-Daniel, K.R.; Jiang, C.-S.; Gu, J.; Azevedo, R.B.; et al. Photodynamic Therapy Treatment of Onychomycosis with Aluminium-Phthalocyanine Chloride Nanoemulsions: A Proof of Concept Clinical Trial. J. Photochem. Photobiol. B Biol. 2017, 173, 266–270. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Primo, F.L.; Tedesco, A.C.; Braga, G.U.L. In Vitro Photodynamic Inactivation of Cryptococcus Neoformans Melanized Cells with Chloroaluminum Phthalocyanine Nanoemulsion. Photochem. Photobiol. 2012, 88, 440–447. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Brancini, G.T.P.; Pinto, M.R.; Primo, F.L.; Wainwright, M.; Tedesco, A.C.; Braga, G.Ú.L. Photodynamic Inactivation of Candida Albicans and Candida Tropicalis with Aluminum Phthalocyanine Chloride Nanoemulsion. Fungal Biol. 2020, 124, 297–303. [Google Scholar] [CrossRef]
- Lam, M.; Jou, P.C.; Lattif, A.A.; Lee, Y.; Malbasa, C.L.; Mukherjee, P.K.; Oleinick, N.L.; Ghannoum, M.A.; Cooper, K.D.; Baron, E.D. Photodynamic Therapy with Pc 4 Induces Apoptosis of Candida Albicans. Photochem. Photobiol. 2011, 87, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Carmello, J.; Alves, F.; Ribeiro, A.; Basso, F.; de Souza Costa, C.; Tedesco, A.; Primo, F.; Mima, E.; Pavarina, A. In Vivo Photodynamic Inactivation of Candida Albicans Using Chloro-Aluminum Phthalocyanine. Oral Dis. 2016, 22, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, M.A.; Alvarez, M.G.; Ochoa, A.L.; Milanesio, M.E.; Durantini, E.N. Optimization of Cellular Uptake of Zinc(II) 2,9,16,23-Tetrakis[4-(N-Methylpyridyloxy)]Phthalocyanine for Maximal Photoinactivation of Candida Albicans. Fungal Biol. 2013, 117, 744–751. [Google Scholar] [CrossRef]
- Di Palma, M.A.; Alvarez, M.G.; Durantini, E.N. Photodynamic Action Mechanism Mediated by Zinc(II) 2,9,16,23-Tetrakis[4-(N-Methylpyridyloxy)]Phthalocyanine in Candida Albicans Cells. Photochem. Photobiol. 2015, 91, 1203–1209. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Jorge, A.O.C.; Barbosa, J.O.; Rossoni, R.D.; Vilela, S.F.G.; Costa, A.C.B.P.; Primo, F.L.; Gonçalves, J.M.; Tedesco, A.C.; Suleiman, J.M.A.H. Photodynamic Inactivation of Biofilms Formed by Candida Spp., Trichosporon Mucoides, and Kodamaea Ohmeri by Cationic Nanoemulsion of Zinc 2,9,16,23-Tetrakis(Phenylthio)-29H, 31H-Phthalocyanine (ZnPc). Lasers Med. Sci. 2012, 27, 1205–1212. [Google Scholar] [CrossRef]
- Lam, M.; Dimaano, M.L.; Oyetakin-White, P.; Retuerto, M.A.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A.; Cooper, K.D.; Baron, E.D. Silicon Phthalocyanine 4 Phototoxicity in Trichophyton Rubrum. Antimicrob. Agents Chemother. 2014, 58, 3029–3034. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-S.; Guo, J.; Zhuang, J.-J.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. Highly Positive-Charged Zinc(II) Phthalocyanine as Non-Aggregated and Efficient Antifungal Photosensitizer. Bioorg. Med. Chem. Lett. 2015, 25, 2386–2389. [Google Scholar] [CrossRef]
- Mantareva, V.; Kussovski, V.; Angelov, I.; Borisova, E.; Avramov, L.; Schnurpfeil, G.; Wöhrle, D. Photodynamic Activity of Water-Soluble Phthalocyanine Zinc(II) Complexes against Pathogenic Microorganisms. Bioorg. Med. Chem. 2007, 15, 4829–4835. [Google Scholar] [CrossRef]
- Tang, F.; Gao, F.; Xie, W.; Li, S.; Zheng, B.; Ke, M.; Huang, J. Carboxymethyl Chitosan-Zinc(II) Phthalocyanine Conjugates: Synthesis, Characterization and Photodynamic Antifungal Therapy. Carbohydr. Polym. 2020, 235, 115949. [Google Scholar] [CrossRef]
- Ozturk, I.; Tunçel, A.; Yurt, F.; Biyiklioglu, Z.; Ince, M.; Ocakoglu, K. Antifungal Photodynamic Activities of Phthalocyanine Derivatives on Candida Albicans. Photodiagn. Photodyn. Ther. 2020, 30, 101715. [Google Scholar] [CrossRef]
- So, C.-W.; Tsang, P.W.K.; Lo, P.-C.; Seneviratne, C.J.; Samaranayake, L.P.; Fong, W.-P. Photodynamic Inactivation of Candida Albicans by BAM-SiPc. Mycoses 2010, 53, 215–220. [Google Scholar] [CrossRef]
- Thyzel, R. Active Ingredient Comprising Indocyanne Green and/or Infracyanine Green. U.S. Patent 13/181,295, 5 July 2012. [Google Scholar]
- Fekrazad, R.; Poorsattar Bejeh Mir, A.; Ghasemi Barghi, V.; Shams-Ghahfarokhi, M. Eradication of C. Albicans and T. Rubrum with Photoactivated Indocyanine Green, Citrus Aurantifolia Essential Oil and Fluconazole. Photodiagn. Photodyn. Ther. 2015, 12, 289–297. [Google Scholar] [CrossRef]
- Afroozi, B.; Zomorodian, K.; Lavaee, F.; Zare Shahrabadi, Z.; Mardani, M. Comparison of the Efficacy of Indocyanine Green-Mediated Photodynamic Therapy and Nystatin Therapy in Treatment of Denture Stomatitis. Photodiagn. Photodyn. Ther. 2019, 27, 193–197. [Google Scholar] [CrossRef]
- He, Y.-Y.; Jiang, L.-J. Synthesis and EPR Investigations of New Aminated Hypocrellin Derivatives. Free Radic. Biol. Med. 2000, 28, 1642–1651. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Zhuge, Y.; Zhang, J.; Xu, K.; Zhang, Q.; Zhang, H.; Chen, H.; Chu, M.; Jia, C. Photodynamic Antifungal Activity of Hypocrellin A against Candida Albicans. Front. Microbiol. 2019, 10, 1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, A.; Liu, C.; Deng, H.; Li, J.; Ma, W.; Zeng, X.; Ji, Y. In Vitro Photodynamic Inactivation Effects of Hypocrellin B on Azole-Sensitive and Resistant Candida Albicans. Photodiagn. Photodyn. Ther. 2019, 27, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; de Witte, P.A.M. Hypericin in Cancer Treatment: More Light on the Way. Int. J. Biochem. Cell Biol. 2002, 34, 221–241. [Google Scholar] [CrossRef]
- Rezusta, A.; López-Chicón, P.; Paz-Cristobal, M.P.; Alemany-Ribes, M.; Royo-Díez, D.; Agut, M.; Semino, C.; Nonell, S.; Revillo, M.J.; Aspiroz, C.; et al. In Vitro Fungicidal Photodynamic Effect of Hypericin on Candida Species†. Photochem. Photobiol. 2012, 88, 613–619. [Google Scholar] [CrossRef]
- Paz-Cristobal, M.P.; Gilaberte, Y.; Alejandre, C.; Pardo, J.; Revillo, M.J.; Rezusta, A. In Vitro Fungicidal Photodynamic Effect of Hypericin on Trichophyton spp. Mycopathologia 2014, 178, 221–225. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, Z.; Ye, Z.; Wang, Y.; Wang, L.; Xing, L.; Qiu, H.; Huang, N.; Luo, Y.; Zhao, Y.; et al. In Vitro Photodynamic Inactivation Effects of Benzylidene Cyclopentanone Photosensitizers on Clinical Fluconazole-Resistant Candida Albicans. Photodiagn. Photodyn. Ther. 2018, 22, 178–186. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical Potential of the Reactive Oxygen Species Generation and Quenching by Fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Milanesio, M.E.; Spesia, M.B.; Cormick, M.P.; Durantini, E.N. Mechanistic Studies on the Photodynamic Effect Induced by a Dicationic Fullerene C60 Derivative on Escherichia Coli and Candida Albicans Cells. Photodiagn. Photodyn. Ther. 2013, 10, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Postigo, A.; Bulacio, L.; Sortino, M. Photodynamic Inactivation of Oropharyngeal Candida Strains. Phytomedicine 2014, 21, 1424–1431. [Google Scholar] [CrossRef]
- Taraszkiewicz, A.; Szewczyk, G.; Sarna, T.; Bielawski, K.P.; Nakonieczna, J. Photodynamic Inactivation of Candida Albicans with Imidazoacridinones: Influence of Irradiance, Photosensitizer Uptake and Reactive Oxygen Species Generation. PLoS ONE 2015, 10, e0129301. [Google Scholar] [CrossRef] [Green Version]
- de Menezes, H.D.; Pereira, A.C.; Brancini, G.T.P.; de Leão, H.C.; Massola Júnior, N.S.; Bachmann, L.; Wainwright, M.; Bastos, J.K.; Braga, G.U.L. Furocoumarins and Coumarins Photoinactivate Colletotrichum Acutatum and Aspergillus Nidulans Fungi under Solar Radiation. J. Photochem. Photobiol. B Biol. 2014, 131, 74–83. [Google Scholar] [CrossRef]
- Fracarolli, L.; Rodrigues, G.B.; Pereira, A.C.; Massola, N.S., Jr.; Silva, G.J., Jr.; Bachmann, L.; Wainwright, M.; Bastos, J.K.; Braga, G.U.L. Inactivation of Plant-Pathogenic Fungus Colletotrichum Acutatum with Natural Plant-Produced Photosensitizers under Solar Radiation. J. Photochem. Photobiol. B Biol. 2016, 162, 402–411. [Google Scholar] [CrossRef]
- Sobotta, L.; Wierzchowski, M.; Mierzwicki, M.; Gdaniec, Z.; Mielcarek, J.; Persoons, L.; Goslinski, T.; Balzarini, J. Photochemical Studies and Nanomolar Photodynamic Activities of Phthalocyanines Functionalized with 1,4,7-Trioxanonyl Moieties at Their Non-Peripheral Positions. J. Inorg. Biochem. 2016, 155, 76–81. [Google Scholar] [CrossRef]


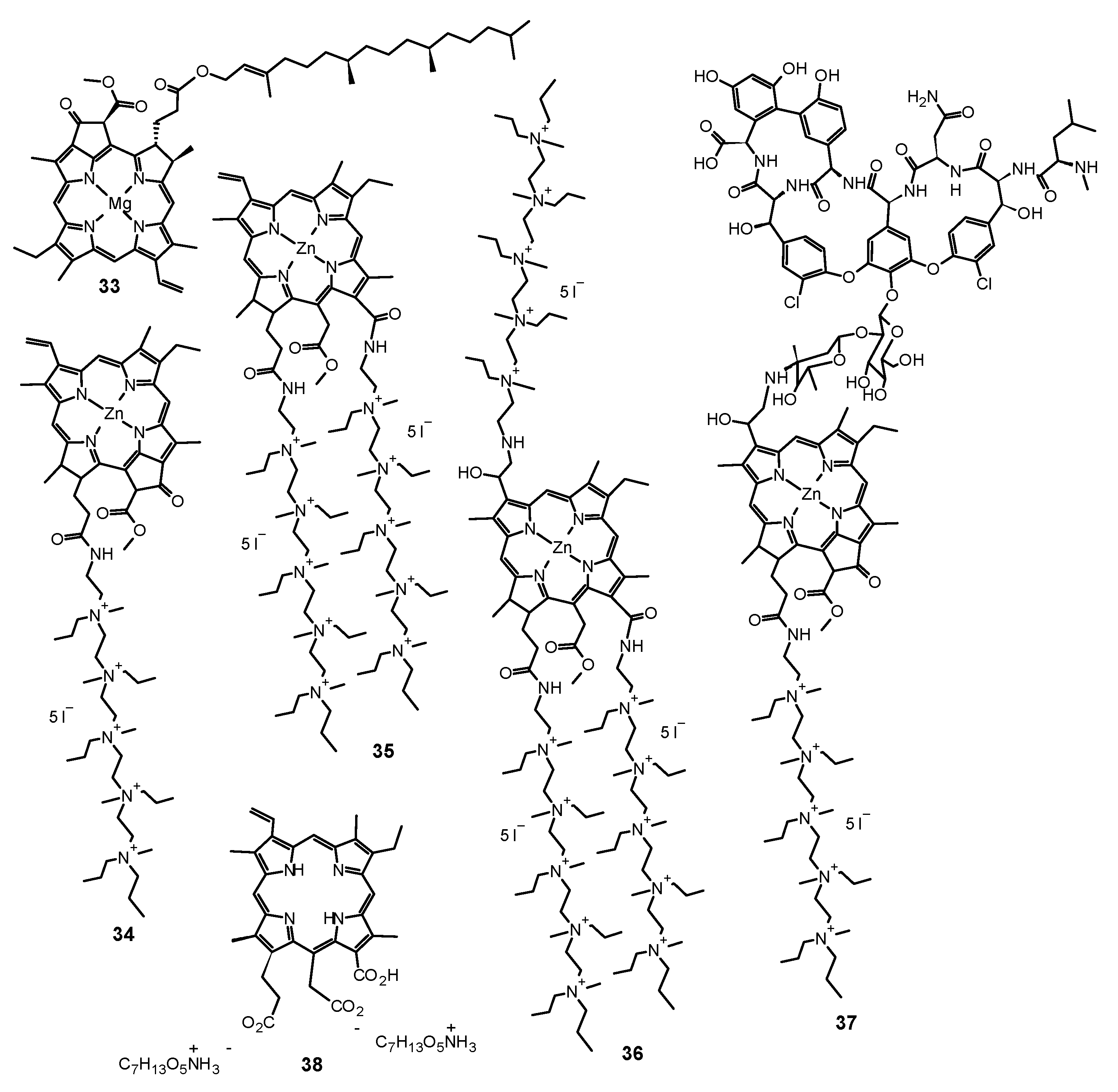
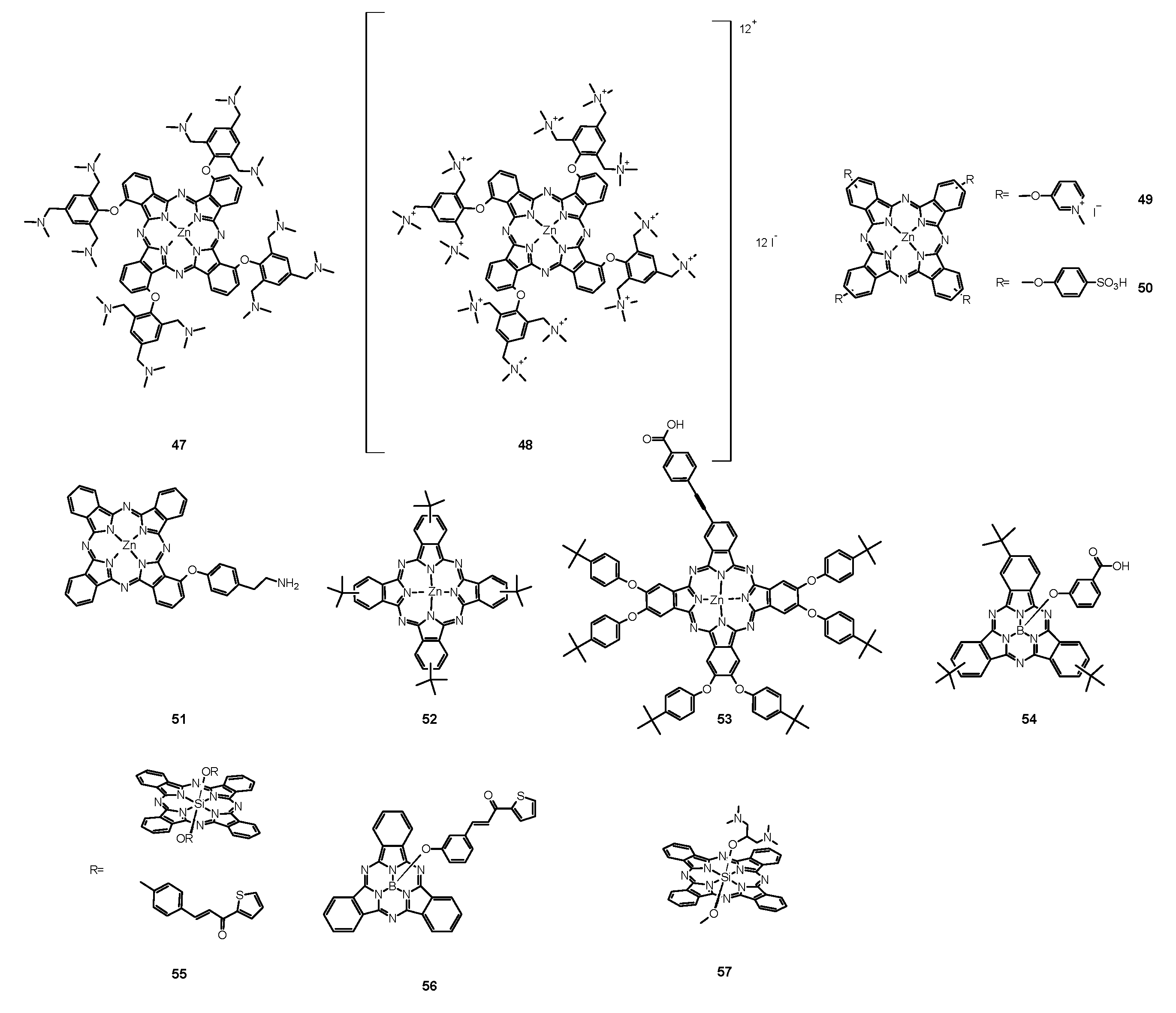
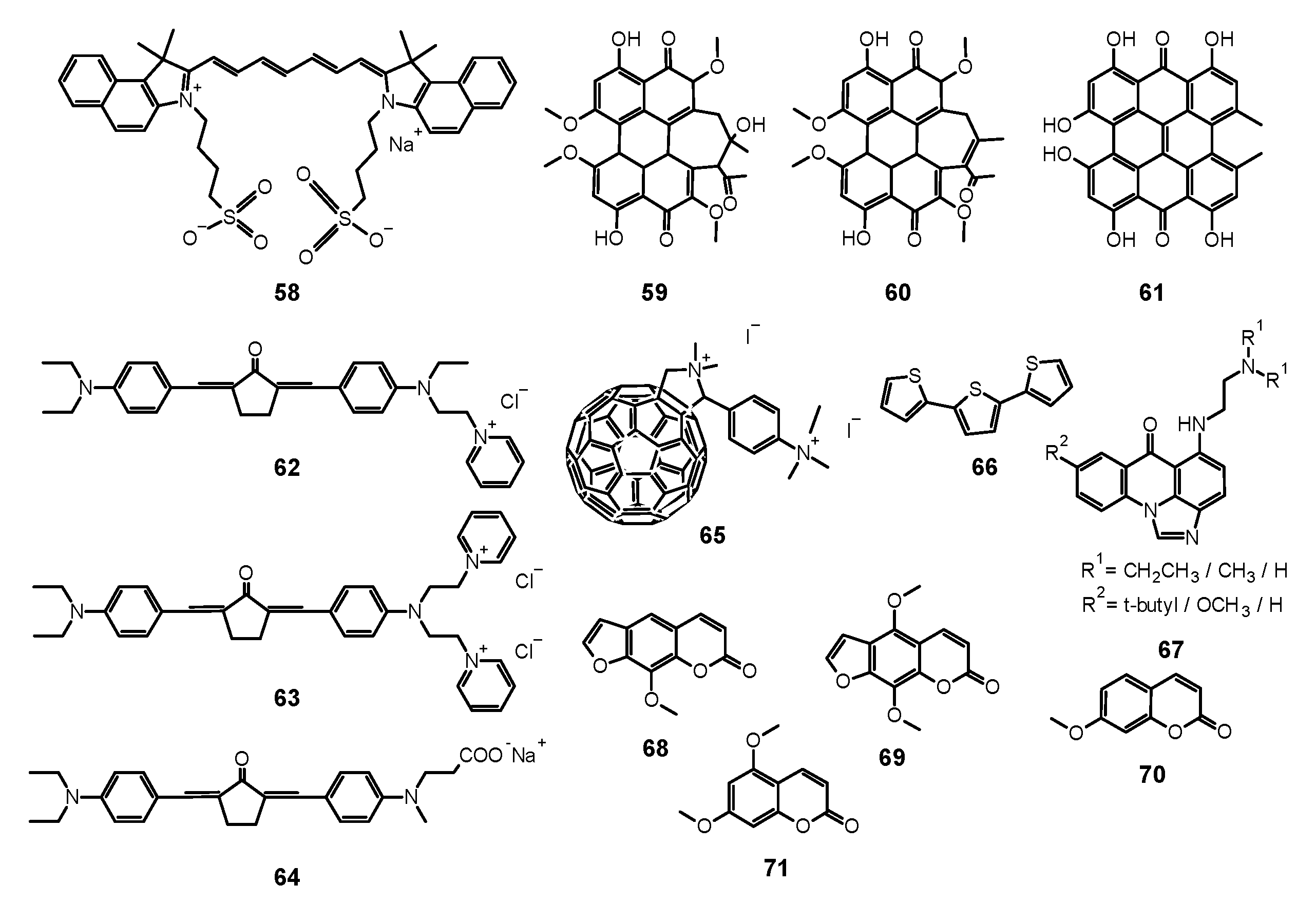
| Reference | Photosensitizer | Incubation Time | Irradiation Wavelength (nm) | Light Dose (J/cm2) | Antimicrobial Activity Against | Reported Fungal Growth Inhibition |
|---|---|---|---|---|---|---|
| [28] | 1 | 1 min | 660 | 245 | C. albicans | No decrease in number of CFUs; proteinase activity lowered |
| [35] | 1 | 30 min | 660 | 6; 15 | C. albicans (in G. mellonella) | 1 PACT significantly increased the survival rate |
| [36] | 1, 2 and 5 | - | 570–670 | 15 | F. keratoplasticum, F. moniliforme | 1, 2 and 5 in G. mellonella-infected larvae reduced the survival of F.k. microconidia to 38, 57, and 2%, respectively. The survival levels of F.m. microconidia in infected larvae were 69, 37, and 21%, respectively. |
| [45] | 1, 9 | - | 660; 532 | 21.42 | C. albicans | max ca. 6 log red. (RB)* max ca. 6 log red. (PD) * *estimated data Gene expression studies |
| [46] | 1 | 10 min | 660 | 10; 20; 30 | C. albicans | max. 53% red. (plankton) max. 74% red. (biofilm) |
| [47] | 1 | 10 min | 662 | 129.6; 162; 194.4 | C. albicans | Complete eradication (lag phase cells) max. 2 log red. (stationary phase cells) |
| [48] | 1 | 10 min | 660 | 9; 27 | C. albicans | In vivo experiment (clinical trial) Slightly increased sensitivity to fluconazole in in vitro tests |
| [49] | 1 | 1–20 min | 640 | 4.68 | C. albicans | max ca. 1.5 log red.* *estimated data |
| [50] | 1 and 10 | 30 min | 600–650 (1); 490–540 (10) | 10 to 60 | C. albicans; T. mentagrophytes | C.a./1 max. >95% of inhibition C.a./10 max. >98% of inhibition T.m./10 max. > 98% of inhibition |
| [51] | 1 | 1 h | 660 | 15; 23; 57 | C. albicans | max. 40.28% red. (biofilm) |
| [52] | 1 | 5 min | 660 | 26.3 | C. albicans | 0.49 log red. (serotype A) 2.34 log red. (serotype B) |
| [53] | 3, 3 with verapamil or sodium azide | 5 min | 684 (1), 660 (3) | 28 | C. albicans | increasing calcium levels decreased the 3-mediated PACT efficiency; Verapamil decreased 1-mediated PACT effectiveness. |
| [54] | 1 (with glucose) | 30 min | 660 | 23; 46; 69; 92; 115; 138 | C. albicans | max ca. 85% red. (biofilm) |
| [55] | 1 (with glucose) | 10 min | 660 | 10; 30; 60 | C. albicans | max. 6-log reduction |
| [56] | 1 (with chitosan) | 3 min | 660 | - | C. albicans | In vivo experiment (mouse model) |
| [57] | 1 (with CTAC, HPS, SDS, and Triton X-100 as surfactant) | 1 h | - | - | C. albicans | max. ca. 47% red. PDT max. 100% red. SDS + PDT max. 100% red. CTAC + PDT max. 100% red. HPS + PDT max. ca. 39% Triton + X−100 |
| [58] | 1, 3 | 30 min | Visible light; 675 (laser light) | 20 (laser light) | M. anisopliae, A. nidulans, | Significant decrease of germination of conidia. |
| [59] | 3 | 5 min | 630 | 21.7 | C. albicans | max. 50% of inhibition (biofilm) |
| [60] | 3 | 5 min | 630 | 20; 30; 40 | C. krusei | max. 70% growth inhibition (plankton) max. 90% growth inhibition (biofilm) |
| [61] | 3 | 60 s | 635 | 24;18; 12 | C. albicans, C. glabrata, C. krusei | C.a. max. 100% red. C. g. max. 100% red. C. k. max. 100% red. |
| [62] | 3 | 1 min | 635 | 200 | T. rubrum | no fungal growth observed after PACT in vitro, and in an onychomycosis model Patient cured of onychomycosis confirmed in up to 6 months follow-up |
| [63] | 3 | 5 min | 630 | 18; 48;72 | T. rubrum | >98% inhibition of fungal growth |
| [64] | 3 (with posaconazole or fluconazole) | 30 min | 630 | 50 | C. albicans | 3-log reduction |
| [65] | 1 (with fluconazole) | 30 min | 660 | 1.8; 3.6; 5.4; 7.2; 10.8; 14.4; 18 | C. albicans (in G. mellonella) | 1 PACT alone and combined with fluconazole prolonged the larvae survival |
| [66] | 1 (fluconazole pre-treatment) | 30 min-with PS alone; 2 h-with fluconazole | 660 | 30; 60; 120 | C. albicans | complete eradication |
| [67] | 1 (in combination with itraconazole, voriconazole, posaconazole, amphotericin B) | 2 h | 635 | 12; 24 | Fusarium spp., Exophiala spp. | Reductions of up to 3.8 log and 6.4 log against planktonic Exophiala spp. and Fusarium spp Reductions of up to 4.2 log and 5.6 log against biofilms formed by Exophiala spp. and Fusarium spp. |
| [68] | 1 | 10 min | 660 | 28 | Candida spp. (C. albicans, C. tropicalis, C. glabrata)-(clinical trial) | Clinical trial; PACT with 1 as effective as nystatin therapy |
| [69] | 1 | - | 660 | - | C. albicans | max. 73% red. |
| [70] | 1 | 1 h | 635 | 12, 24 | C. auris | eradication of planktonic forms, ca. 2,7 log red. of biofilms, C.a. AR385 biofilm > 7 log red. |
| [71] | 1 | 3 min | 670 | - | Trichophyton sp., T. rubrum, T. mentagrophytes, C. parapsilosis, C. famata, A. fumigatus, Rhodotula, Microsporum, Microsporum + Epidermophyton, Fusarium (clinical trial) | Clinical trial; 70% of patients cured |
| [72] | 1 | 10 min | 660 | 28 | Candida ssp. (clinical trial) | Clinical trial |
| [73] | 1 | 5 min | 808 | - | C. albicans (Clinical trial) | Clinical trial; >6 log red. |
| [74] | 2 and 58 | 5 min (58); 30 min (2) | 810 (58); 630 (2) | 52 (58); 5 (2) | T. rubrum | 0.64 log red. (58) 0.4 log red. (2) |
| [75] | 1, 2, 3 and 5 | 30 min | 400–700 (max. 631) | 3; 6; 11 | N. dimidiatum, N. dimidiatum var. hyalinum | max. 4.5 log red. (1/3; Neoscytalidium dimidiatum) max. 5 log red. (1/3; Neoscytalidium dimidiatum var. Hyalinum) max. 4–5 log red. (2; Neoscytalidium dimidiatum) max. 5 log red. (2; Neoscytalidium dimidiatum var. Hyalinum) max. 5 log red. (5; Neoscytalidium dimidiatum) max. 3.5 log red. (5; Neoscytalidium dimidiatum var. Hyalinum) |
| [76] | 1 | 2 h | 635 | 12 | Scedosporium and Lomentospora spp. | 3.3 log red. (L. prolificans) 2.8 log red. (S. boydii) 3 log red. (S. aurantiacum) 3 log red. (S. dehoogii) 5.2 log red. (S. apiospermum) 2.7 log red. (S. minutispora) |
| [77] | 1 | 10 min | 625 | 3; 12; 24; 40; 60 | T. rubrum | up to 100% |
| [78] | 1@AuNPs | - | 650; 530 | 80; 100 | T. mentagrophytes | In vivo experiment (rabbit model) |
| [79] | 1, 58 in compilation with nystatin and chlorhexidine) | - | 660; 808 | 10 | C. albicans | 1.04 log red. (1) 1.2 log red. (ICG) |
| [80] | 1, 3, 2 and 5 | 30 min | 635 | 0; 10; 15; 20 | F. oxysporum, F. moniliforme, and F. solani | F. oxysporium (MIC): 1 12.5–25 µM 3 25–50 µM 2 2.5 µM 5 5 µM F. moniliforme (MIC): 1 25 µM 3 10–12.5 µM 2 10 µM 5 5 µM F. solani (MIC): 1 25–75 µM 3 12.5–25 µM 2 2.5–5 µM 5 5 µM |
| [81] | 1, 2 (combined or not with potassium iodide) | 10 min | 660 | 3.18; 6.36; 12.73; 19.10 in vitro 105.26; 157.89 in vivo | Candida albicans. | In vitro (C.a.biofilm): 1 + KI 2.31 log red. 2 1.77 log red. In vivo, almost complete eradication of C.a. |
| [82] | 1 | 24 h | 576–672 | 15 | C. albicans, C. parapsilosis | C. albicans biofilm MIC 30 µM C. parapsilosis biofilm MIC up to 7.5 µM |
| [83] | AuNPs@1; AuNPs@3 | 30 min | 662 (1); 635 (3) | 21.6 | C. albicans | max. 80% red. in biofilm formation * * Estimated data in case of both types of nanoparticles used |
| [84] | 7 and 8 | 3 or 18 h | 600 | 12; 36 | C. albicans biofilms | up to total cell inactivation with FSc |
| [85] | 2, 58 (EmunDo®) | 30 min | 810; 630 | 15 (630 nm); 55 (810 nm) | C. albicans | max. 1.9 log (58) max. 3.37 log red. (2) |
| [86] | 1, 2, 3, 5 | 30 min | 400–790 | 5; 10; 15; 20; 25; 30 | C. acutatum, C. gloeosporioides | max. antifungal activity ca. 5 log red. (2 and 5 against conidia) |
| [87] | 1 | 10 min | 660 | 18 (one session); 36 (two sessions) | C. albicans | In vivo experiment (mouse model) |
| [88] | Crystal violet, New Fuchsin; Azure B | 30 min | 455–800 | - | C. albicans | max. ca. 5 log red. (CV) max. ca. 2.7 log red. (AzB) max. ca. 1 log red. (NF) |
| [89] | 1 | - | 630 | 18 | various species of fungimainly T. rubrum, and T. mentagrophytes (Clinical trial) | In vivo experiment (clinical trial) |
| [90] | 1, 2, 3, 5 | 30 min | 634; 631 | 5; 10; 15; 25 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis | MICs up to 1 µM |
| [91] | 3 | 10 min | 630 | 21.47 | C. albicans | max. ca. 62% of inhibition (biofilm formation) |
| [92] | 3 | 5 min | 637 | 18 | C. albicans | 0.46 log |
| [93] | 6 | 0, 15, 30, 60 min, 3, 5 and 24 h | 639.8 | 18; 37 | C. albicans | max. 6 log reduction |
| [94] | 3 (with chitosan) | 10, 30 or 60 min | 630 | 50 | C. albicans | Complete eradication (ca. 7 log10) |
| [95] | 1 | 1 min | 660 | 7.5 | Candida spp. (clinical trial) | Clinical trial |
| [96] | 1@AuNP | 12 and 24 h | 660 | 38.2 | C. albicans | 1: 63.2% (crystal violet assay) or 81.9% (XTT assay) biofilm inhibition 1@AuNP: 82.2% (crystal violet) or 95.4% (XTT assay) biofilm inhibition |
| [97] | 1 | 1 h | - | - | F. pedrosoi, C. carrionii | Ca. 4 log red. |
| [98] | 1, 2, 3 and 5 | 30 min | 634/631 | 5; 10; 15; 20; 25; 30 | T. mentagrophytes, T. rubrum | T.m. MIC: 1 up to 5 µM 3 up to 2.5 µM 2 up to 2.5 µM 5 up to 0.1 µM T.r. MIC: 1 up to 5 µM 3 up to 1 µM 2 up to 2.5 µM 5 up to 0.5 µM |
| [99] | 1 | 5 min | 660 | 60; 120; 180 | C. albicans, C. tropicalis, C. krusei, C. guillermondii | up to 78% reduction |
| [100] | 1 (ointment) | 4 h | 660 | 28 | Fungi causing chromoblastomycosis (Clinical trial) | 80–90% volume reduction of the lesion in 10 patients |
| [101] | 1 (with verapamil) | 30 min | 660 | 60 | C. albicans | ABC pump decreased the effectiveness of 1 more than MFS pump |
| [102] | 3, 1, and 2 | 30 min | 635; 660 | in vitro: 0; 1.95; 3.90; 5.85; 7.80; 9.75 in vivo: 78; 120 | C. albicans | in vitro: 2 4.43 log red 1, 3 < 1 log red |
| [103] | 3 | 5 min | 630 | 18–90 | T. rubrum | max. 1.72 log red. |
| [104] | 9, 10 | 5 min | 455 | 95 | C. albicans | 9 max. 3.45 log red. (plankton) 10 max. 1.97 log red. (plankton) biofilms < 1 log red. |
| [105] | 1, 3, malachite green | 5 min | 660 | 15.8; 26.3; 39.5 | C. albicans | 1 max. 2.71 log red. 3 max. 3.07 log red. malachite green max. 2.25 log red. |
| [106] | 1 | 5 min | 684 | 28 | C. albicans | max. ca. 2.5 log red |
| [107] | 3 | 5 min | 630 | 36–180 | Candida spp. (Clinical isolates) | max. 3.54 log red. single irradiation; complete eradication after second irradiation 55% inhibition of adhesion to buccal cells |
| [108] | 1 | 5 min | 683 | 28 | C. albicans | <10% cel viability (estimated data) |
| [109] | 1 | - | 685 | 28 | C. albicans, C. dubliniensis, C. krusei, and C. tropicalis | C.a. 88.6% CFU number reduction C.d. 84.8% CFU number reduction C.k. 91.6% CFU number reduction C.t. 82.3% CFU number reduction |
| Reference | Photosensitizer | Incubation Time (Min.) | Irradiation Wavelength (nm) | Light Dose (J/cm2) | Antimicrobial Activity | Reported Inhibition in Bacterial Growth |
|---|---|---|---|---|---|---|
| [130] | 9 | 5 | 532 | 42.63 | C. albicans, C. dubliniensis | Complete eradication for both (plankton) Biofilm: C.a. 0.74 log red. C.d. 0.21 log red. |
| [132] | 9 | 10 | 532 | 42.63 | C. glabrata | One application: 4.64 log red. Four applications: 5.94 log red. |
| [133] | 9 | 1 | 532 | 14.34 | C. albicans | In vivo experiment (mouse model) |
| [134] | 9 | 0.5 or 1 | 450 | 28.8 | C. albicans | In vitro: Complete eradication for plankton and biofilm In vivo: No significant red. |
| [136] | 9 | 5 | 532 | 42.63 | C. albicans, S. sanguinis | 1.07 log red. (C.a. biofilm) 0.39 log red. (C.a. and S.s. mixed biofilm) |
| [137] | 9 | 5 | 532 | 42.63 | C. albicans, C. glabrata, C. tropicalis, C. dubliniensis | C.a. 4.62/1.01 log red.* C.g. 2.58/1.17 log red.* C.t. 2.81/1.17 log red.* C.d. 3.99/1.13 log red.** (plankton/biofilm) |
| [140] | 10 and 12 | 5 | 532 | 42.63 | C. albicans | 0.22 log red. (10) 0.45 log red. (12) |
| [141] | 10 | 30 | 532 | 68; 133; 228 | T. rubrum | max. ca. 85% red. |
| [142] | 10 and 11 | - | 518 (RB); 375 (riboflavin) | 5.4 | F. solani, A. fumigatus, C. albicans | C.a. 95.6% growth inhibition A.f. 79.8% growth inhibition F.s. 78.2% growth inhibition |
| [143] | 10 with gold nanoparticles | 120 | 500–780 | 3.18; 15.9; 31.8; 63.6; 95.4 | C. albicans | max. 4.89 log red. (plankton) max. 1.53 log red. (biofilm) |
| [144] | 10 with clotrimazolum | 30 | 530 | 4; 8; 12; 24 | T. rubrum | max. 100% inhibition |
| Reference | Photosensitizer-13 Concentration | Incubation Time (Min.) | Irradiation Wavelength (nm) | Light Dose (J/cm2) | Antimicrobial Activity Against | Reported Inhibition in Bacterial Growth |
|---|---|---|---|---|---|---|
| [149] | 20 µM, 30 µM and 40 µM | 20 | - | 5.28 and 18 | C. albicans, C. tropicalis, and C. glabrata | 40 µM of CUR at 18 J/cm2 reduced the metabolic activity of C. albicans biofilm (by 85%), C. glabrata biofilm (by 85%), and C. tropicalis biofilm (by 73%). |
| [150] | 20 µM, 30 µM and 40 µM | 20 | 455 | 5.28 | C. dubliniensis | The combination of Cur and LED light led to a reduction in CFU values of fungal planktonic cultures from 106 to 103 (CD6 strain) and 102 (CD7 and CD8 strains). The mean percentage reduction in biofilm cultures ranged from 57.70 to 82.05% |
| [151] | 1 µM, 5 µM, 10 µM, 20 µM, 40 µM and 80 µM Also in combination of 208 µM fluconazole | 20 | 430 | 9 | C. albicans | PDT utilizing 1 μM curcumin and 9 J/cm2 of light decreased the number of planktonic C. albicans colonies by three orders of magnitude and at curcumin concentrations of 5 μM or higher, PDT eliminated all C. albicans colonies. Combined treatment of fluconazole with PDT and curcumin led to decrease of C. albicans cell viability to 5%. albicans in adherent culture. |
| [152] | 60 µM | 20 | 455 | 2.64, 5.28, 7.92, 10.56, 13.2 | C. albicans | The inhibition rates after CUR-PDT in three biofilms (ATCC 90028, CCA1, and CCA2) were 90.87%, 66.44%, and 86.74%, respectively (p < 0.05). |
| [153] | 40 µM and 80 µM | 20 | 450 | 37.5 or 50 | C. albicans | CUR-mediated aPDT promoted a downregulation of gene expression of fungal genes related to adhesion and biofilm formation (ALS1 and HWP1) and the genes responsible for the oxidative stress response (CAP1, CAT1, and SOD1). |
| [154] | 5, 10, 20, 30 and 40 µM | 1, 5, 10 and 20 | 455 | 5.28 | C. albicans, C. glabrata, and C. dubliniensis | For the planktonic cultures, photoinactivation depended on Cur concentration and complete inactivation of the suspensions was achieved. Cur-mediated PDT was effective against Candida biofilms, with reductions of 94%, 89%, and 85% in the cell viabilities of C. albicans, C. glabrata, and C. dubliniensis, respectively. The highest decrease in cell viability for the biofilms was observed for the combination of 40 µM Cur with 20 min of PIT. |
| [155] | 0.1 μg/mL; 1 μg/mL; 10 μg/mL curcumin and curcumin incorporated in nanoparticles | 10 | 417 | 10, 20, and 40 | Trichophyton rubrum | Antimicrobial photodynamic inhibition utilizing curcumin (10 μg/mL) with 10 J/cm2 of blue light completely inhibited fungal growth via induction of ROS and nitrogen species (RNS). Curcumin in nanoparticles induced greater NO• expression and increased apoptosis of fungal cells. |
| [156] | 2.5 µM | 20 | 440–460 | 37.5 | C. albicans | The DNA damage was analyzed by the comet assay. DNA damage was significantly increased for longer comet tails observed after application of Cur mediated PDT. |
| [157] | 1.5 g/L | - | 450 | 20.1 | C. albicans | PDT reduced 1.46 C. albicans log10 CFU/mL, in contrast to 450 nm blue LED or curcumin for 5 min. |
| [158] | 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, and 20 µM for planktonic culture 5, 10, 20, 30 and 40 µM for biofilms | 20 (planktonic) 5 or 20 (biofilm) | 455 | 5.28 and 37.5 | C. albicans | In the conditions of complete inactivation of the fungal planktonic form (20 µM/20 min preirradiation time/5.28 J cm−2), the metabolic activity of biofilms was reduced by 70%. |
| [159] | 20, 40, and 80 µM | 20 | 455 | 37.5 | C. albicans | All curcumin and LED light treatments resulted in a significant reduction of C. albicans viability after PDT. The curcumin concentration of 80 µM combined with LED light application promoted the highest log10 reduction in colony counts (over 4 logs). |
| [160] | 80, 100, and 120 μM | 20 | from 440 to 460 | 37.5 | C. albicans, C. glabrata, and Streptococcus mutans | For the biofilms of both ages (24 and 48 h), the tested concentrations of Cur with light promoted a reduction in cell metabolism, with the highest reduction 40.62% achieved with the concentration of 120 μM of Cur. |
| [161] | 40 μM | 20 | 455 | 5.28 | C. albicans co-cultured with human keratinocytes | After applying aPDI the reduction in CFU/mL equivalent to 1.7 log10 was observed. |
| [162] | 260 μM Free curcumin and curcumin encapsulated in nanoparticles NP | 20 | 455 | 37.5 | C. albicans | aPDT with free CUR topically applied on the tongue of mice with oral candidiasis led to a reduction of 1.11 log10 in the viability of fungus, while aPDT mediated by anionic CUR-NP demonstrated no antifungal effect, and cationic CUR-NP reduced the fungal cells (0.44–0.74 log10) even in the absence of light. |
| Reference | Photosensitizer | Incubation Time | Irradiation Wavelength (nm) | Light Dose (J/cm2) | Antimicrobial Activity Against | Reported Fungal Growth Inhibition |
|---|---|---|---|---|---|---|
| [121] | Photogem® | 30 min | 455 (440–460) | 10.5, 18.0, 25.5, 37.5 | C. albicans, C. glabrata | plaktonic culture—up to complete eradication; biofilms < 1.0 log red. |
| [163] | Photofrin | 30 min | n.d. (white light) | 9 | C. albicans, C. krusei C. glabrata | significant reductions of C. albicans at 1 µg/mL, C. krusei at 3 µg/mL, C. glabrata at 10 µg/mL |
| [164] | Photofrin | 1 or 5 min | 400–700 | 9 | C. albicans (germ tubes and biofilm) | optimization of PDI conditions using Photofrin; significant reduction of germ tubes; higher activity towards biofilm than amphotericin B |
| [165] | Photofrin | 1 or 24 h | 630 | 45–135 | C. albicans, C. glabrata, C. parapsilosis, C. Krusei, C. tropicalis | Optimization of conditions, up to eradication of fungi apart from C. krusei which was reduced by up to 90%. Amphotericin B and azole-resistant strains were equally susceptible to PDI. |
| [166] | Photogem® | 30 min | 455 | 18.0, 25.5, 37.5 | C. albicans, C. dubliniensis, C. tropicalis, C. krusei | Complete eradication of C. albicans, C. dubliniensis, and C. tropicalis; C. krusei could not be eradicated |
| [167] | Photogem® | 30 min | 455 (440–460) | 37.5 | ex vivo cultures on dentures: C. albicans, C. glabrata, C. tropicalis, C. dubliniensis, C. krusei | up to 3.99 log red. |
| [168] | Photogem® | 30 min | 455 or 630 | 305 | C. albicans in vivo oral candidiasis in mice | 1–1.4 log red. |
| [169] | Photogem® | 30 min | 455 (440–460) | 122 | case reports of denture stomatitis: C. Albicans, C. Glabrata, C. tropicalis | two patients cured after the applied PDI |
| [170] | Photogem® | 30 min | 455 (440–460) | 37.5 122 | denture stomatitis: C. albicans, C. tropicalis, C. Glabrata, C. Lusitaniae, C. Rugosa, C. guillermondii, Cryptococcus humicola, Cryptococcus albidus Kloeckera apis/apiculata | clinical study, 45% success rate |
| [171] | Photogem® | 1 h | 630 | 54 | onychomycosis | case report; complete recovery of one patient |
| [172] | Photogem® | 0 5 10 20 | 410–440 (incubation) 610–640 (PDI) | 4 8 17 | C. albicans | <1 log red. when incubation in the dark 7 log red. when incubation with low intensity light (4 J/cm2) |
| [173] | 15 | 30 min | 400–780 | 72 | C. albicans | 7 log red. |
| [174] | 15 | 30 min | 400–800 | 72 | C. albicans biofilm | up to 3.26 log red. |
| [178] | 5-ALA | 4 h | broadband red light | 75 | case report of interdigital mycoses treatment (T. mentagrophytes, C. albicans, T. rubrum) | after 4 weeks follow-up only two out of 9 patients were recovered |
| [179] | ALA, itraconazole | 4 h | 630 | 360 | C. albicans (cutaneous granuloma) | case report—full recovery of one treated patient |
| [180] | methyl 5-aminolevulinate | 3 h | 630 | 37 | Malassezia | six case studies; improvement observed in five patients |
| [181] | methyl 5-aminolevulinate | 3–5 h | 630 | 37 | case report of Acremonium sclerotigenum onychomycosis | clinical cure in 12-month follow-up |
| [182] | methyl 5-aminolevulinate and bifonazole | 3 h | 635 | 37 | Neoscytalidium dimidiatum onychomycosis | case report; reinfection with the pathogen without the clinical symptoms |
| [183] | ALA | n.d. | 635 | 10 | Fonsecaea monophora | estimated 4 log red. |
| [184] | ALA combined with terbinafine, itraconazole, or voriconazole | n.d. | 635 | n.d. | chromoblastomycosis: F. nubica, F. pedrosoi, F. monophora | case report, full recovery of three out of five patients |
| [185] | ALA | 12 h | 635 | 30 60 90 | Fonsecaea monophora conidia | up to almost 6 log red. |
| [186] | ALA | 5 h | 635 | 50 100 200 300 | C. albicans biofilm | up to 75% cell viability decrease |
| [187] | 1, 16 | 10 min | 630 (16) 660 (1) | 85 (16) 6048 (1) | in vivo vaginal candidiasis (C. albicans) in mice | up to 1 log red. 16 used in 10-fold lower concentration to yield the same effect |
| [188] | 5,10,15,20-tetrakis (N-methylpyrid−4-yl) porphyrin tetra (4-toluenesulfonate), Photofrin | 10 min | 75% in range of 575–700 | n.d. | C. albicans, C. glabrata | up to 3 log red; increased susceptibility to PDI when azole-resistance expressed |
| [189] | 17 | 10 min | 67% in range 575–700 | 2.4 | C. albicans, C. glabrata S. cerevisiae | mechanistic studies |
| [190] | 17 | 30 min | 350–800 | n.d. | C. albicans | 5 log red. |
| [191] | 17, 18 | - | - | - | T. rubrum Scopulariopsis brevicaulis | localization and stability study |
| [192] | 17, 18, Eosin Y | 30 min | white light | 21.6 43.2 64.8 1382.4 | T. rubrum Trichophyton interdigitale S. brevicaulis | up to complete eradication by each of the tested PSs |
| [193] | 19, 20, 21 | 30 min | n.d. (visible light) | n.d. | C. albicans | 4 log red. for 19 and 20, almost no effect at the same concentrations of 21 |
| [194] | 17, 19, 20 | 30 min | 350–800 | n.d. | C. albicans | mechanistic study, up to 5 log red. |
| [195] | 17, 19, 20 | 30 min | 350–800 | 162 | C. albicans | 3.5 log red. |
| [196] | 17, miconazole | 30 min | 350–800 | 58.5 | biofilms: C. albicans, C. glabrata, C. tropicalis, C. parapsilosis | C. glabrata—resistant to PDI Other strains cell viability reduced to 64%. All strains susceptible to combined PDI and miconazole therapy (additive or synergistic) |
| [197] | 17 | n.d. | 67% in range 575–700 | 1.0 | C. albicans | >1.5 log red. of PDI > 2.0 log red. when PDI combined with miconazole therapy |
| [198] | 5,10,15,20-tetrakis [4-(3-N,N-dimethylaminopropoxy) phenyl]-porphyrin; 5,10,15,20-tetrakis [4-(3-N,N,N-trimethylammoniumpropoxy) phenyl] porphyrin tetraiodide | 30 min | 350–800 | 162 | C. albicans | up to 4.8 log red. |
| [199] | 17; 5,10,15,20-tetrakis (N-pentylpyrid−4-yl) porphyrin tetraiodide | 3 h | 400–800 | 240 | Penicillium chrysogenum conidia | 17: 4.1 log red 5,10,15,20-tetrakis (N-pentylpyrid−4-yl) porphyrin tetraiodide: 3.4 log red. |
| [200] | 17 | 30 min | 67% in range 575–700 (in vitro) 514 (in vivo) | 2.4 (in vitro) 90 (in vivo) | C. albicans (in vitro and in vivo in mice) | in vitro: nearly 4 log red. in vivo: 50-fold reduction of CFUs |
| [201] | 5-phenyl−10,15,20-tris (N-methyl−4-pyridyl) porphyrin chloride | 1, 15, 30 | n.d. (white light) | 0–9 | C. albicans | mechanistic study; up to 6 log reduction |
| [202] | 17, 22 | 15 min (plankton) 4 h (biofilm) | 418 | 12.1 (plakton) 48.2 (biofilm) | C. albicans | planktonic: up to 6 log red biofilm: up to 5 log red. |
| [203] | 5-(N-methylpyrid−4-yl)−10,15,20-triphenylporphyrin iodide 5,10-bis (N-methylpyrid−4-yl)−15,20-diphenylporphyrin diiodide 5,15-bis (N-methylpyrid−4-yl)−10,20-diphenylporphyrin diiodide 5,10,15-tris (N-methylpyrid−4-yl)−20-phenylporphyrin triiodide 5,10,15,20- tetrakis (N-methylpyrid−4-yl) porphyrin tetraiodide | n.d. | 400–800 | 30 60 90 120 | Colletotrichum graminicola | up to over 4.5 log red.—eradication |
| [204] | deuteroporphyrin monomethyl ester (DP mme), 23, deuteroporphyrin (DP), hematoporphyrin (HP), Photofrin, zinc(II) phthalocyanine (ZnPc), phthalocyanine tetrasulfonate (PcS4), aluminum(III) phthalocyanine chloride tetrasulfonate (AlPcS4). | 30 min at 28 °C | n.d. | 108 | T. rubrum | DP mme, Sylsens B: fungicidal effect at 3 µg/mL Photofrin, ZnPc, PcS4, AlPcS4: delay the growth, no difference from the control after 7 days of culture |
| [205] | 23, DP mme | 2 h | 580–600 | 108 | T. rubrum in ex vivo skin model | up to 100% decrease of cell viability |
| [206] | 23, DP mme | 2 or 3 h | 580–600 | 108 | T. rubrum | PDI conditions optimization; up to complete eradication |
| [207] | 23 | 2 | 340–550 | 18 | T. rubrum | Optimization of the PDI conditions, up to complete eradication at 1 µM |
| [208] | 5,10,15-tris(N-methylpyrid−4-yl)−20-(4-sulfanylphenyl)porphyrin trichloride, 23, 25 | 1 h | 532 | 0.15 0.6 | Trichophyton mentagrophytus conidia | 5,10,15-tris(N-methylpyrid−4-yl)−20-(4-sulfanylphenyl) porphyrin trichloride: 4.6 log red. 23: 4.4 log red. 25: up to 4.1 log red. |
| [209] | 25 | 1 h | 27 28 81 | Ex vivo onychomycosis: T. rubrum, T. mentagrophytes, T. tonsurans | 25: single irradiation—≥70% cell viability decrease, second irradiation—eradication | |
| [210] | 26 in poly (silesesquioxane) thin films | n.d. | 350–800 | n.d. | C. albicans | 2.5 log red. as compared to 0.5 log red for free 26 |
| [211] | CdTe quantum dots, Zn (II) 5,10,15,20-tetrakis (N-ethylpyrid−2-yl)porphyrin and their combination | 10 min | 460 | 0–81 | C. albicans | combination 1 log red. free porphyrin 3 log red. |
| [212] | silver core-silica shell nanoparticles, hematoporphyrin IX, material based on both | 30 min | 400 | n.d. | T. rubrum | nanoparticles alone < 1 log red. hematoporphyrin IX alone < 1 log red. material 3 log red. |
| [213] | methyl 5-aminolevulinate | 3 h | 630 | 37 | T. rubrum (clinical case of onychomycosis) | three sessions of irradiation resulted in clinical cure at 24-month follow-up |
| Reference | Photosensitizer | Incubation Time (Min.) | Irradiation Wavelength (nm) | Light Dose (J/cm2) | Antimicrobial Activity Against | Reported Inhibition in Bacterial Growth |
|---|---|---|---|---|---|---|
| [215] | 28 | - | 660 | 10; 20; 30 | F. solani, A. fumigatus | F.s. max. Complete eradication A.f. max. ca. 50% red. |
| [216] | 27 | 20 | 660 | 40 | C. albicans | max. ca. 90% red. for plankton and biofilm |
| [217] | 29 | 15 | 627 | 31.5 | C. albicans, E. faecalis | 58.98% red. (C.a. and E.f. mixed biofilm) |
| [218] | 27 (encapsulated in CTAB-liposomes) | 15 | 662 | 50 | C. albicans, C. krusei, C. tropicals, | In vitro: Max. Complete eradication In vivo: max. 1.75 log red. |
| [219] | PEI−27 (linear, LMW, HMW) | 10 | 665 | 0–100 | C. albicans, S. aureus, S. pyogenes, E. coli, P. aeruginosa | C. albicans: 27 2 log red. 27-LMW 2 log red. 27-HMW; 27-lin 4–6 log red. |
| [221] | 34–37 | 30 | 415; 660 | 10; 20 | C. albicans, MRSA, E. faecium, E. coli | C. albicans: 34–36—max. complete eradication 37 No significant red. |
| [222] | 38 | 20 | 660 | 25 | C. albicans | Plankton: max. Complete eradication Biofilm: PACT No significant red. PACT + SDT 3.39 log red. |
| [223] | 38 (pre-treatment with fluconazole) | 20 | 660 | 50 | C. albicans | 2.6 log |
| [224] | 38 | 20 | 660 | 37.5 | Candida spp. (clinical trial) | max 1.96 log red. without formulation > 5 log red. for formulations |
| [225] | 38 | 20 | 660 | 37.5 | C. albicans, C. glabrata, and S. mutans multispecies biofilm | C.a. ca. 1 log red. C.g. ca. 1.5 log red. estimated data |
| [226] | 38 | 20 (planktonic) | 660 | 18.0; 25.5; 37.5 | C. albicans, C. glabrata, and C. tropicalis (clinical isolates) | max. 0.9 log red. (C.a.) max. 1.4 log red. (C.t.) max. 1.5 log red. (C.g.) |
| Reference | Photosensitizer | Incubation Time | Irradiation Wavelength (nm) | Light Dose (J/cm2) | Antimicrobial Activity Against | Reported Inhibition in Bacterial Growth |
|---|---|---|---|---|---|---|
| [229] | phthalocyanine encapsulated chitosan/TPP nanoparticles (FNP) from chitosan, tripolyphosphate (TPP), and phthalocyanine-4,4′,4″,4‴-tetrasulfonic acid (FePC). In experiments combining PDT with chemical therapy, C. tropicalis was treated with 128 µM flucytosine prior to or after the PDT experiments | 4 h | 630 | 20 | C. tropicalis | After PDT with FePC (70% cell viability) or FNP (50% cell viability) Prior to and after the flucytosine therapy, FNP-PDT largely decreased the viability of C. tropicalis cells. Cell viability was ~25% for the treatment with flucytosine and subsequent PDT with 80 μM FNP. |
| [230] | 39, 40 | 1.5 h | 635 | 50 | C. albicans | The fungal biofilm inactivation with 3 log (39) was observed only after fractionated LEDs irradiation. C. albicans in suspension was completely inactivated after 39 (1.8 mM) at soft light radiation. |
| [231] | 41, 42 | 15 min. | 665 | 50 | C. albicans, Pseudomonas aeruginosa | Sufficient efficacy of PACT for planktonic cultures, but low PACT efficacy (<3 logs) of the photoinactivation of the 48 h bacterial and fungal biofilms in comparison to effect in suspension. |
| [232] | 43 entrapped in cationic and anionic nanoemulsions (NE) | 30 min. | 660 | 50, 100 | C. albicans | In case of planktonic cultures, cationic NE-43 reduced significantly both colony counts and cell metabolism. In addition, cationic NE-43 and free 34 caused significant damage to the cell membrane. In case of the biofilms, cationic NE-43 reduced cell metabolism by 70%, whereas anionic NE-43 was inactive. |
| [233] | 43-NE | - | 660 | 30.9 | onychomycosis | Clinical cure of 60% of treated lesions |
| [234] | 43-NE | 30 min. | 675 | 5, 10 | Cryptococcus neoformans | Treatment with 43-NE, using selected PS concentrations (e.g., 4.5 µM) and light doses (e.g., 10 J cm−2) resulted in a reduction of up to 6 logs in survival of fungi. |
| [235] | 43-NE | 30 min. | 675 | 25, 50 and 100 | C. albicans and C. tropicalis | APDT with 43-NE led to a reduction of five orders of magnitude in viability for C. albicans and between four and five orders of magnitude for C. tropicalis. |
| [236] | 44 | at least 1 h | 670–675 | 1–2 | C. albicans | Application of 1.0 μM Pc 4 and irradiation at 2.0 J/cm2 caused cell survival reduction by 4 logs. |
| [237] | 43 encapsulated in cationic nanoemulsions (NE) or 43 dissolved in DMSO | - | 660 | 100 | C. albicans | 43-NE-mediated PDT reduced 2.26 log 10 of C. albicans recovered from the oral lesions of immunocompromised mice when compared with the control group. |
| [238] | 45 | 2.5, 15, and 30 min | 350–800 | 54 | C. albicans | After 15 min irradiation—a 5 log decrease in the cell viability (treated with 10 mM 45). After 30 min irradiation—a 4 log decrease in the cell viability (treated with 1 mM 45). After over 30 min irradiation—a 2.5 log decrease in the cell viability (cell suspension of 107 cells mL−1 incubated with 45) |
| [239] | 45 | - | 350–800 | 54 | C. albicans | After 30 min irradiation, 5 µM 45 produced ~5 log decrease in cell viability |
| [240] | 46 | 5 min (pre-irradiation time) | 660 | 26.3 | Candida spp., Trichosporon mucoides, Kodamaea ohmeri | A mean reduction of 0.45 log in case of Candida spp. biofilms, and a reduction of 0.85 and 0.84 for biofilms formed by T. mucoides and K. ohmeri, respectively. |
| [241] | 44 | 2–16 h | 670–675 | 2 | Trichophyton rubrum | Reduction of metabolic activity by 50% within 4 h compared to controls following irradiation of Pc 4-loaded terbinafine-sensitive (24602) and terbinafine-resistant (MRL666) microconidia or hyphae. |
| [243] | 49, 50 | 10 min. | 675 | 12,30 and 60 | Staphylococcus aureus, Pseudomonas aeruginosa, C. albicans | Complete inactivation of S. aureus and C. albicans by the cationic photosensitizer. P. aeruginosa inactivated with 4 log |
| [244] | Carboxymethyl chitosan-zinc (II) phthalocyanine conjugates | 4 h | - | - | C. albicans | The highest photocytotoxicity (with an IC90 value down to 0.72 μM) observed for conjugate of ZnPcN and CMC1 (CMC1, MW = 50 kDa, a low molecular-weight CMC, N,O-carboxymethyl chitosan) |
| [245] | phthalocyanine derivatives | 30 min. | - | 30, 60, 90 | C. albicans | ZnPc reduced the mean cell viability values of C. albicans to 5 (at 30 J/cm2 light doses) and 2 CFU/mL (at 60 J/cm2 light doses). 54 and 55 reduced the mean CFU/mL values to 5 CFU/mL after irradiation with 60 J/cm2. Studied compounds had inhibitory effects on fungus after irradiation and reduced the mean CFU/mL levels to ≤100 CFU/mL. |
| [246] | 57 | 1 h | 675 | 12 | C. albicans | 57 inactivated C. albicans at sub-micromolar level. The IC50 value was 0.013 µmol−1 (for a cell density of 107 cells mL−1) |
| [242] | 47, 48 | 3 h | >610 nm | 27 | C. albicans | High PACT of the dodeca-cationic phthalocyanine against C. albicans with an IC90 value down to 1.46 µM |
| Reference | Photosensitizer | Incubation Time (Min.) | Irradiation Wavelength (nm) | Light Dose (J/cm2) | Antimicrobial Activity Against | Reported Inhibition in Bacterial Growth |
|---|---|---|---|---|---|---|
| [74] | 58 (EmunDo®) | 5 | 800 | 5 | T. rubrum | 0.64 |
| [247] | 58 (EmunDo®) | 5 | 810 | 55 | C. albicans | 1.90 |
| [252] | 60 | 30 | 400–780 | 72 | C. albicans | Dependently on strain up to 7.00 |
| [254] | 61 | <1 | 602 ± 10 | 18 | C. albicans, C. parapsilosis, C. krusei | ca. 3 |
| [256] | 62, 63, 64 | 30 | 532 | 24 | C. albicans cal-1 | 3.76, 3.08, and 3.72, respectively |
| [258] | 65 | 30 | 350–800 | 162 | C. albicans | 5.00 |
| [260] | 67 | 30 | 405 | 20 | C. albicans | Dependently on derivative up to 5.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziental, D.; Mlynarczyk, D.T.; Czarczynska-Goslinska, B.; Lewandowski, K.; Sobotta, L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials 2021, 11, 2883. https://doi.org/10.3390/nano11112883
Ziental D, Mlynarczyk DT, Czarczynska-Goslinska B, Lewandowski K, Sobotta L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials. 2021; 11(11):2883. https://doi.org/10.3390/nano11112883
Chicago/Turabian StyleZiental, Daniel, Dariusz T. Mlynarczyk, Beata Czarczynska-Goslinska, Konrad Lewandowski, and Lukasz Sobotta. 2021. "Photosensitizers Mediated Photodynamic Inactivation against Fungi" Nanomaterials 11, no. 11: 2883. https://doi.org/10.3390/nano11112883
APA StyleZiental, D., Mlynarczyk, D. T., Czarczynska-Goslinska, B., Lewandowski, K., & Sobotta, L. (2021). Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials, 11(11), 2883. https://doi.org/10.3390/nano11112883







