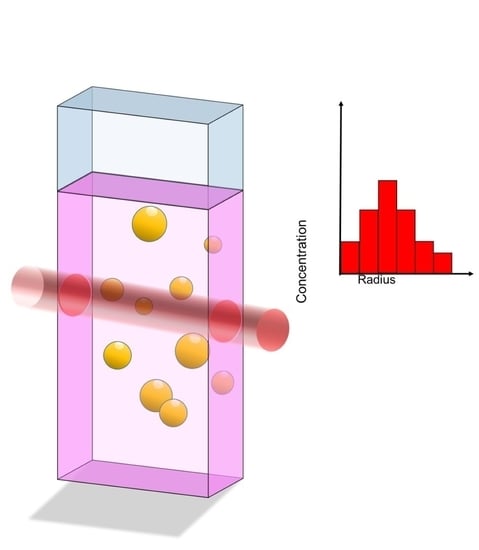
Determination of the Size Distribution of Metallic Colloids from Extinction Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Model
4. Results and Discussion
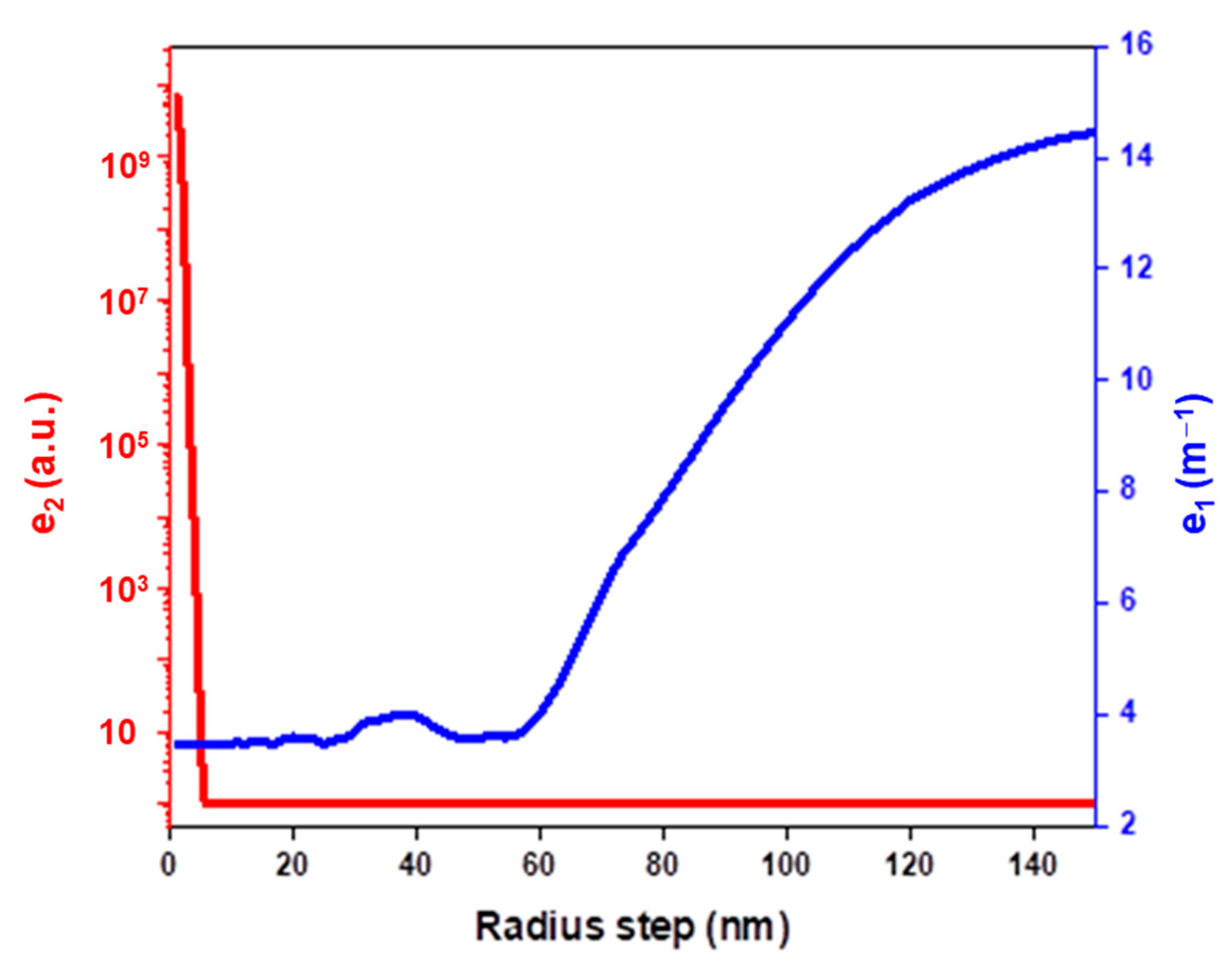
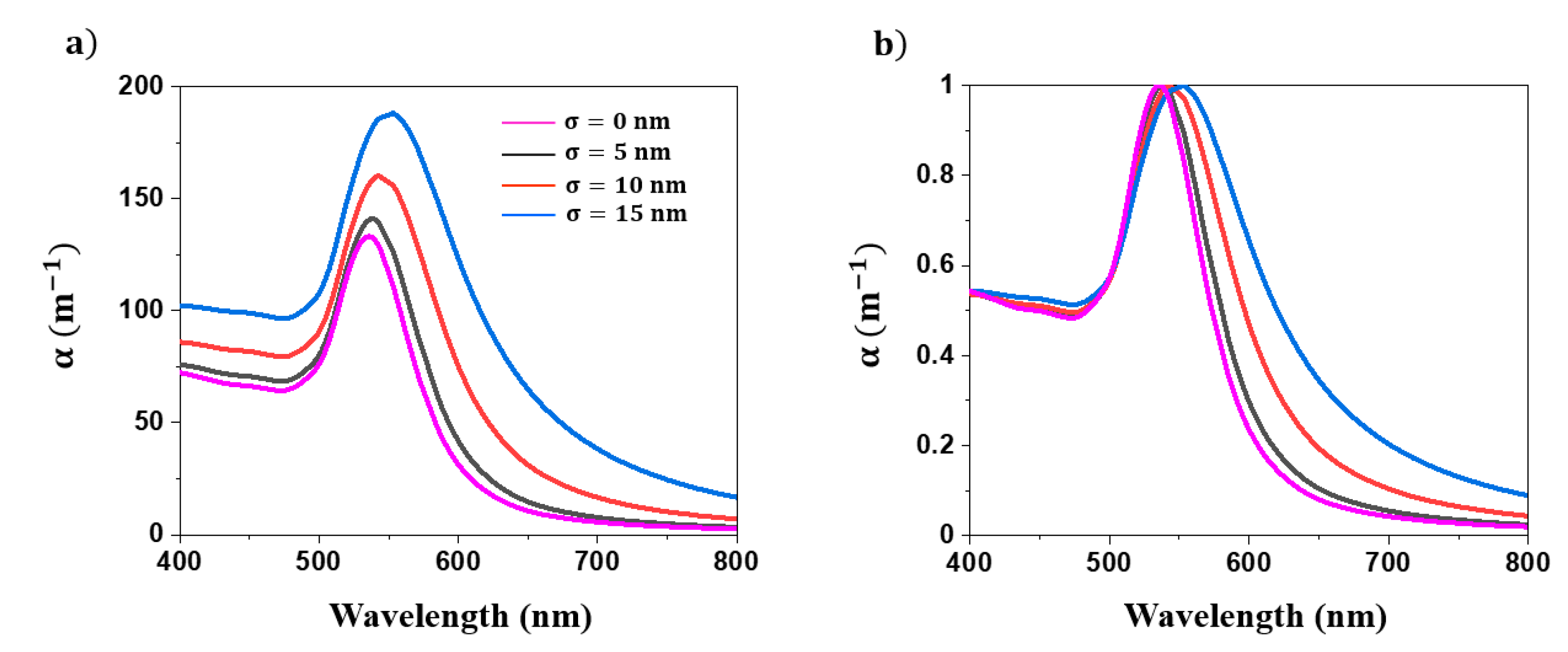
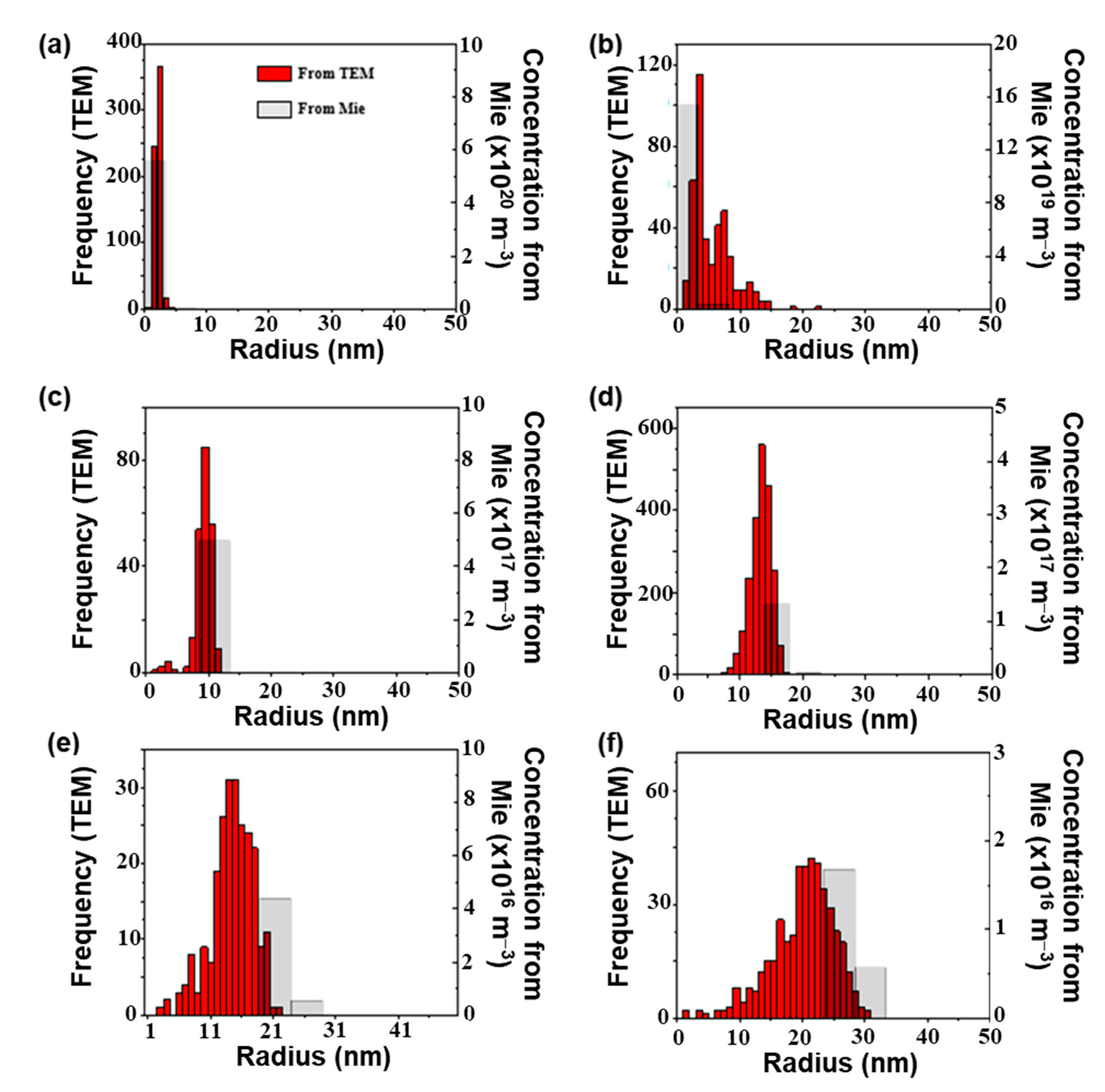
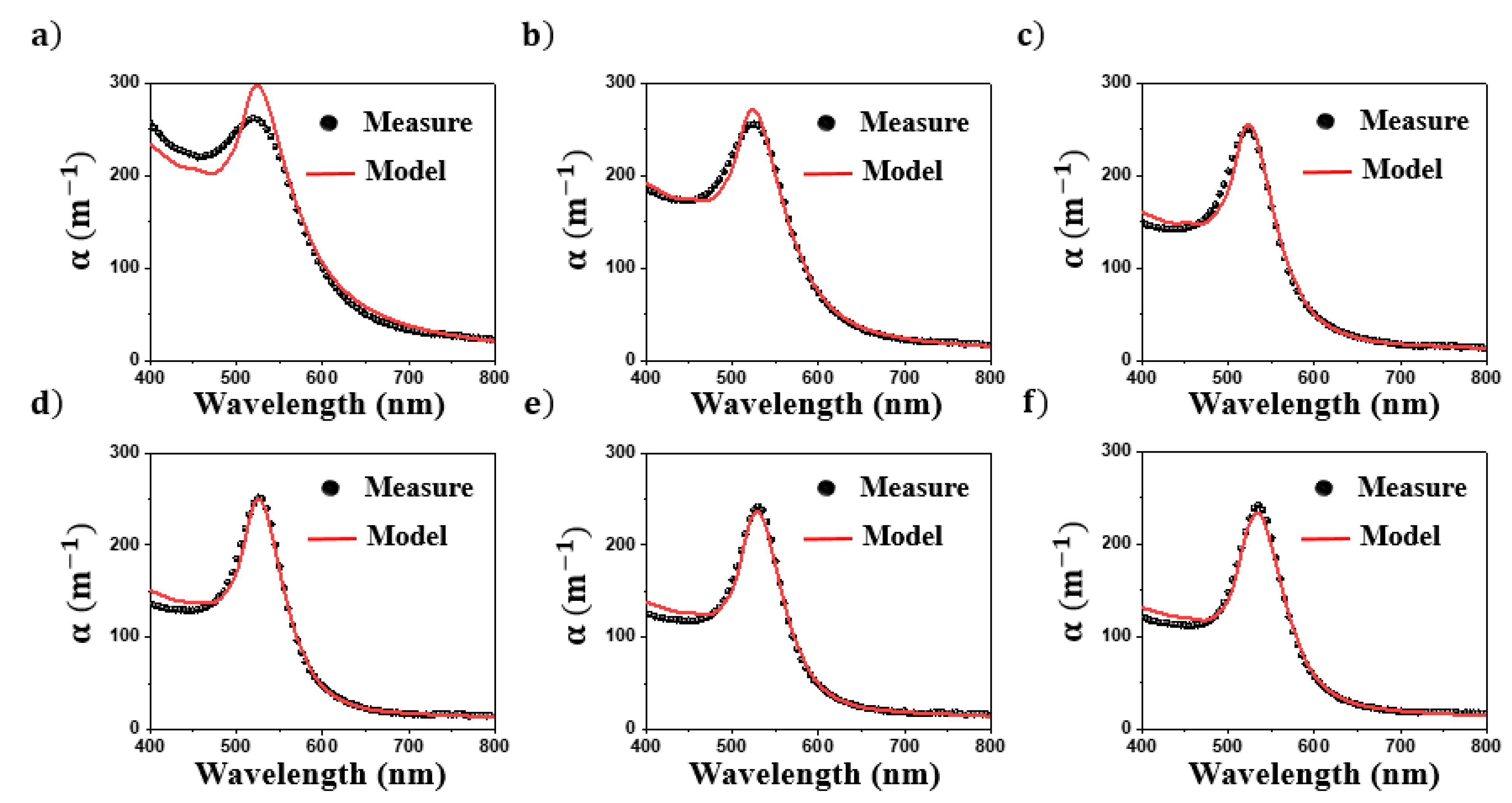
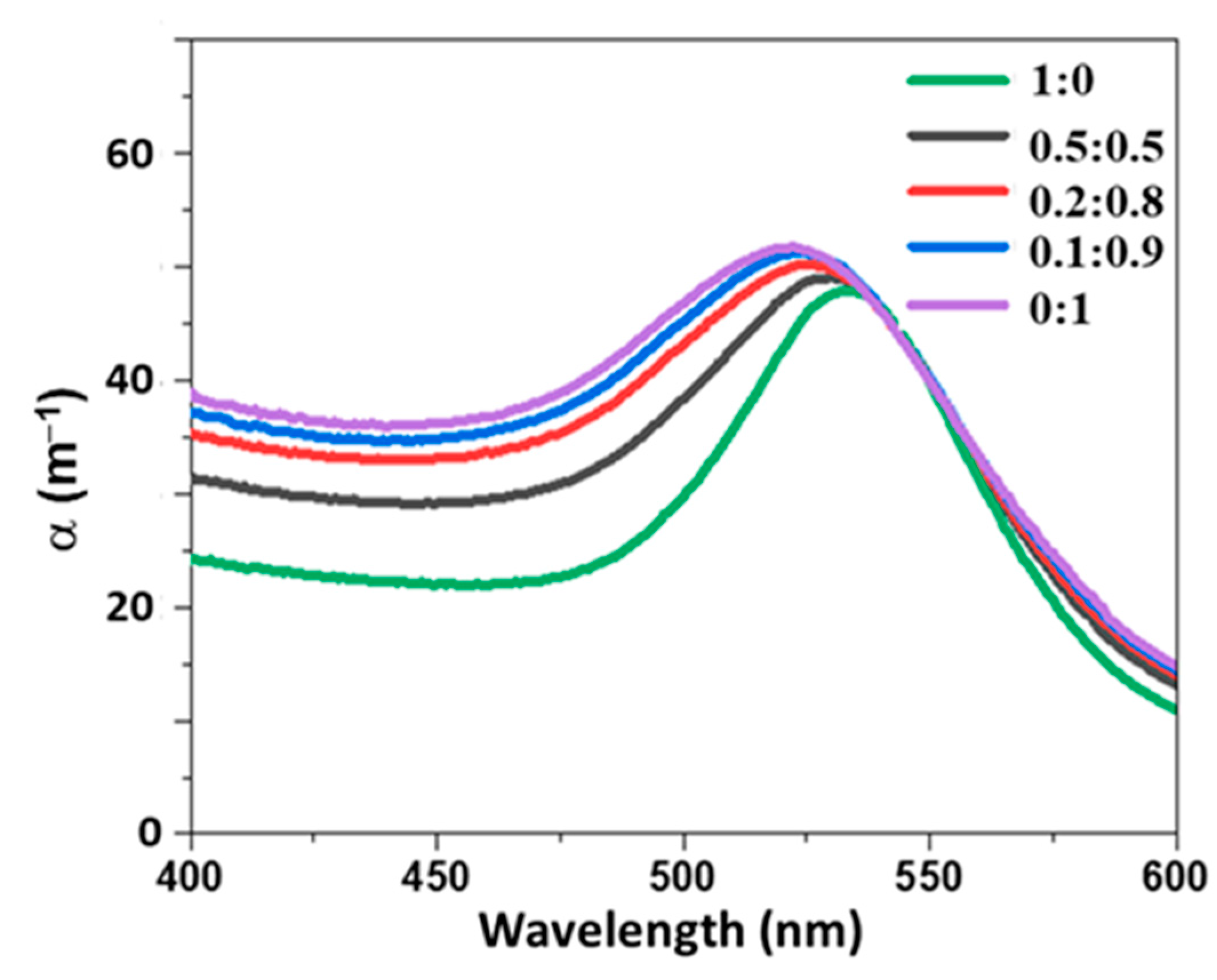
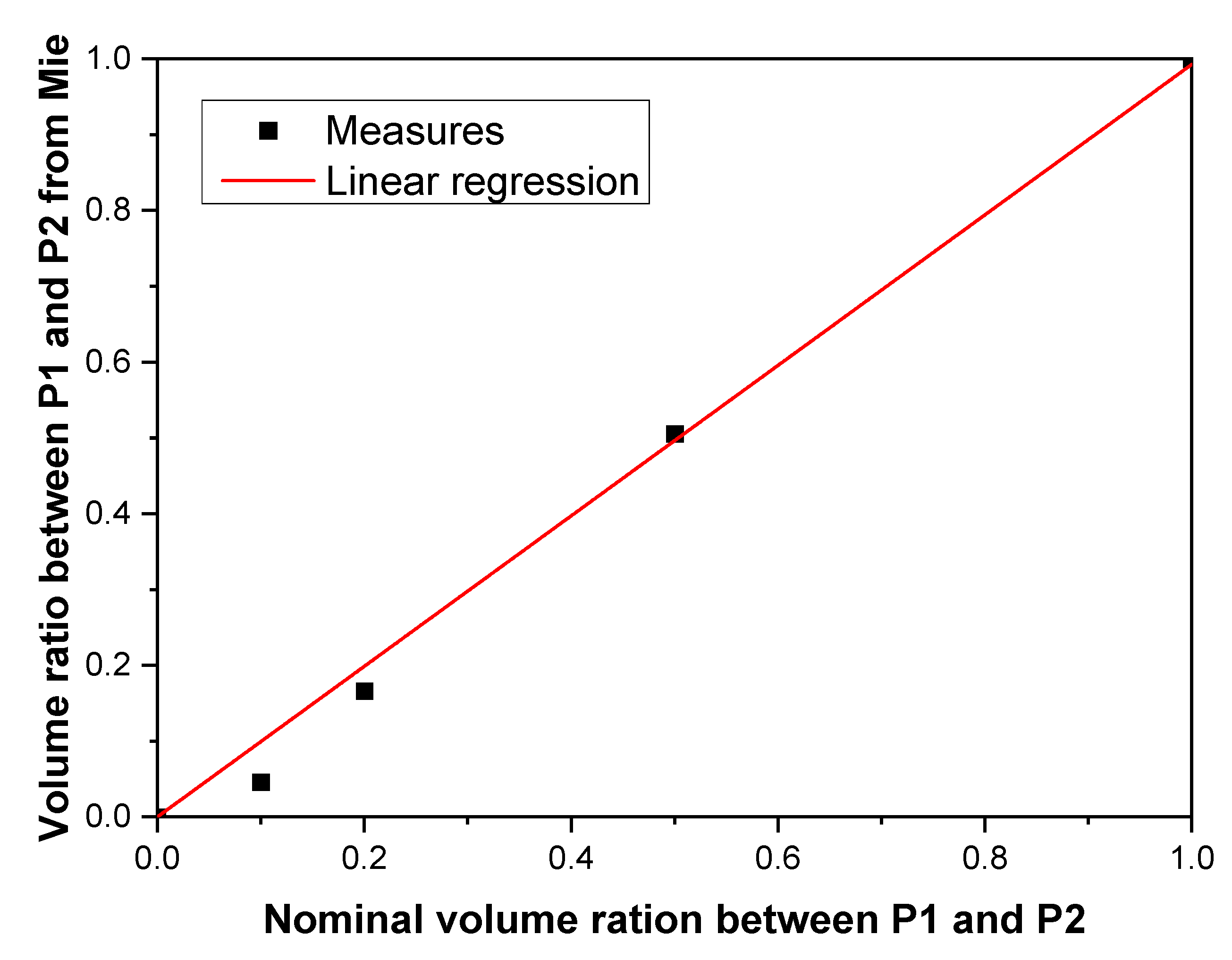
4.1. Simulations
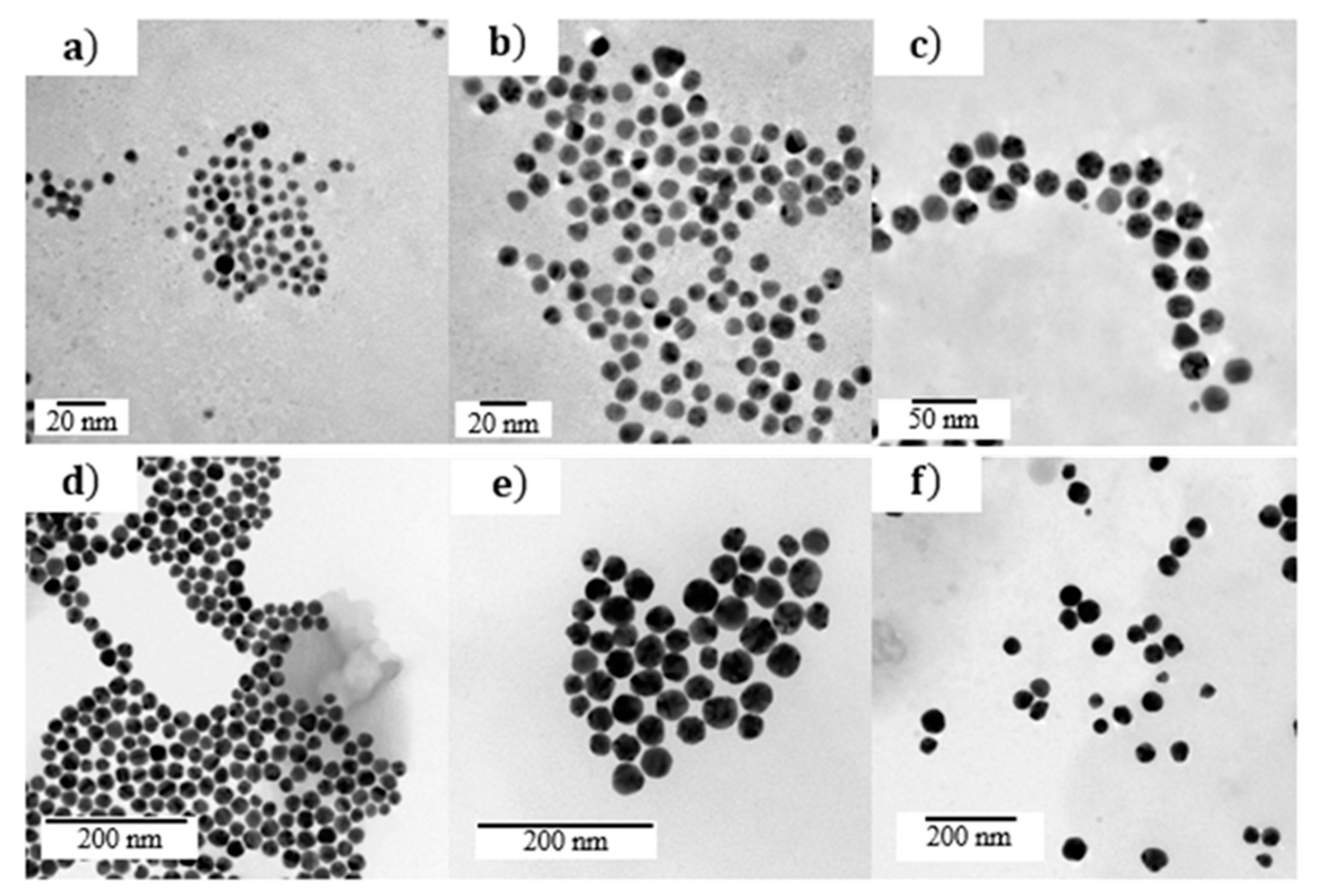
4.2. Characterization of Colloids
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric function. J. Phys. Chem. B 2003, 107, 667–668. [Google Scholar] [CrossRef]
- Myroshnychenko, V.; Rodriguez-Fernandez, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Garcia de Abajo, F.J. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [Green Version]
- Bhana, S.; O’Connor, R.; Johnson, J.; Ziebarth, J.D.; Henderson, L.; Huang, X. Photosensitizer-loaded gold nanorods for near infrared photodynamic and photothermal cancer therapy. J. Colloid Interface Sci. 2016, 469, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, C.; Zheng, H.; Xu, H. Plasmon-driven catalysis on molecules and nanomaterials. Acc. Chem. Res. 2019, 52, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Krajczewski, J.; Kolataj, K.; Kudelski, A. Plasmonic nanoparticles in chemical analysis. RSC Adv. 2017, 7, 17559–17576. [Google Scholar] [CrossRef] [Green Version]
- Ohko, Y.; Tatsuma, T.; Fujii, T.; Naoi, K.; Niwa, C.; Kubota, Y.; Fujishima, A. Multicolour photochromism of TiO2 films loaded with silver nanoparticles. Nat. Mater. 2003, 2, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Erler, R.; Thunemann, A.F.; Sokolov, S.; Ahner, T.T.; Rademann, K.; Emmerling, F.; Kraehnert, R. Nucleation and growth of gold nanoparticles studied via in situ small angle X-ray scattering at millisecond time resolution. ACS Nano 2010, 4, 1076–1082. [Google Scholar] [CrossRef]
- Abécassis, B.; Testard, F.; Spalla, O.; Barboux, P. Probing in situ the nucleation and growth of gold nanoparticles by small-angle X-ray scattering. Nano Lett. 2007, 7, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.; Rodríguez-Fernández, L.; Rodríguez-Iglesias, V.; Kellermann, G.; Crespo-Sosa, A.; Cheang-Wong, J.C.; Silva-Pereyra, H.G.; Arenas-Alatorre, J.; Oliver, A. Determination of the size distribution of metallic nanoparticles by optical extinction spectroscopy. Appl. Opt. 2009, 48, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Qazi, S.J.S.; Rennie, A.R.; Cockcroft, J.K.; Vickers, M. Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles. J. Colloid Interface Sci. 2009, 338, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Murphy, K.E.; MacCuspie, R.I.; Winchester, M.R. Capabilities of single particle inductively coupled plasma mass spectrometry for the size measurement of nanoparticles: A case study on gold nanoparticles. Anal. Chem. 2014, 86, 3405–3414. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khlebtsov, N.G. On the measurement of gold nanoparticle sizes by the dynamic light scattering method. Colloid J. 2011, 73, 118–127. [Google Scholar] [CrossRef]
- Zimbone, M.; Calcagno, L.; Messina, G.; Baeri, P.; Compagnini, G. Dynamic light scattering and UV–vis spectroscopy of gold nanoparticles solution. Mater. Lett. 2011, 65, 2906–2909. [Google Scholar] [CrossRef]
- Alexander, C.M.; Dabrowiak, J.C.; Goodisman, J. Gravitational sedimentation of gold nanoparticles. J. Colloid Interface Sci. 2013, 396, 53–62. [Google Scholar] [CrossRef]
- Alexander, C.M.; Goodisman, J. Size histograms of gold nanoparticles measured by gravitational sedimentation. J. Colloid Interface Sci. 2014, 418, 103–112. [Google Scholar] [CrossRef]
- Bowen, M.S.; Broide, M.L.; Cohen, R.J. Determination of cluster size distributions using an optical pulse particle size analyzer. J. Colloid Interface Sci. 1985, 105, 605–616. [Google Scholar] [CrossRef]
- Walter, J.; Sherwood, P.J.; Lin, W.; Segets, D.; Stafford, W.F.; Peukert, W. Simultaneous analysis of hydrodynamic and optical properties using analytical ultracentrifugation equipped with multiwavelength detection. Anal. Chem. 2015, 87, 3396–3403. [Google Scholar] [CrossRef]
- Mahl, D.; Diendorf, J.; Meyer-Zaika, W.; Epple, M. Possibilities and limitations of different analytical methods for the size determination of a bimodal dispersion of metallic nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 386–392. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles form UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Resano-Garcia, A.; Battie, Y.; En Naciri, A.; Akil, S.; Chaoui, N. Experimental and theoretical determination of the plasmonic responses and shape distribution of colloidal metallic nanoparticles. J. Chem. Phys. 2015, 142, 134108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battie, Y.; Resano-Garcia, A.; En Naciri, A.; Akil, S.; Chaoui, N. Determination of morphological characteristics of metallic nanoparticles based on modified Maxwell-Garnett fitting of optical responses. Appl. Phys. Lett. 2015, 107, 143104. [Google Scholar] [CrossRef]
- Kumar, R.; Binetti, L.; Nguyen, T.H.; Alwis, L.S.M.; Agrawal, A.; Sun, T.; Grattan, K.T.V. Determination of the aspect-ratio distribution of gold nanorods in a colloidal solution using UV-visible absorption spectroscopy. Sci. Rep. 2019, 9, 17469. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Bai, B.; Tan, Q.; Jin, G. Fast statistical measurement of aspect ratio distribution of gold nanorod ensembles by optical extinction spectroscopy. Opt. Express 2013, 21, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Mansour, Y.; Battie, Y.; En Naciri, A.; Chaoui, N. Artificial neural network for the classification of nanoparticles shape distributions. Opt. Lett. 2019, 44, 3390–3393. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M. A Determination of the aspect ratio statistical distribution of gold nanorods in solution from a theoretical fit of the observed inhomogeneously broadened longitudinal plasmon resonance absorption spectrum. J. Appl. Phys. 2006, 100, 044324. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. Determination of size and concentration of gold nanoparticles from extinction spectra. Anal. Chem. 2008, 80, 6620–6625. [Google Scholar] [CrossRef]
- Desai, R.; Mankad, V.; Gupta, S.K.; Jha, P.K. Size distribution of silver nanoparticles: UV-Visible spectroscopic assessment. Nanosci. Nanotechnol. Lett. 2012, 4, 30–34. [Google Scholar] [CrossRef]
- Torchia, G.A.; Scaffardi, L.B.; Méndez, C.; Moreno, P.; Tocho, J.O.; Roso, L. Optical extinction for determining the size distribution of gold nanoparticles fabricated by ultra-short pulsed laser ablation. Appl. Phys. A 2008, 93, 967–971. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Size evaluation of gold nanoparticles by UV-vis spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1983. [Google Scholar]
- Battie, Y.; Resano-Garcia, A.; Chaoui, N.; Zhang, Y.; En Naciri, A. Extended Maxwell-Garnett-Mie formulation applied to size dispersion of metallic nanoparticles embedded in host liquid matrix. J. Chem. Phys. 2014, 140, 044705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, C.L.; Hanson, R.J. Solving Least Squares Problems; SIAM: Philadelphia, PA, USA, 1995. [Google Scholar]
- Mansour, Y.; Battie, Y.; En Naciri, A.; Chaoui, N. Monitoring the aspect ratio distribution of colloidal gold nanoparticles under pulsed-laser exposure. Opt. Express 2020, 28, 34501–34515. [Google Scholar] [CrossRef]
- Battie, Y.; Stchakovsky, M.; En Naciri, A.; Akil, S.; Chaoui, N.; Broch, L. Ellipsometry of Colloidal solutions: New experimental setup and application to metallic colloids. Langmuir 2017, 33, 7425–7434. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Christy, R.W. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Olmon, R.L.; Slovick, B.; Johnson, T.W.; Shelton, D.; Oh, S.H.; Boreman, G.D.; Raschke, M.B. Optical dielectric function of gold. Phys. Rev. B 2012, 86, 235147. [Google Scholar] [CrossRef] [Green Version]
- Mansour, Y.; Battie, Y.; En Naciri, A.; Chaoui, N. Mechanisms and advanced photothermal modelling of laser-induced shape transformations of colloidal gold nanorods by nanosecond laser pulses. Nanoscale 2019, 11, 11679–11686. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, Y.; Battie, Y.; En Naciri, A.; Chaoui, N. Determination of the Size Distribution of Metallic Colloids from Extinction Spectroscopy. Nanomaterials 2021, 11, 2872. https://doi.org/10.3390/nano11112872
Mansour Y, Battie Y, En Naciri A, Chaoui N. Determination of the Size Distribution of Metallic Colloids from Extinction Spectroscopy. Nanomaterials. 2021; 11(11):2872. https://doi.org/10.3390/nano11112872
Chicago/Turabian StyleMansour, Yehia, Yann Battie, Aotmane En Naciri, and Nouari Chaoui. 2021. "Determination of the Size Distribution of Metallic Colloids from Extinction Spectroscopy" Nanomaterials 11, no. 11: 2872. https://doi.org/10.3390/nano11112872