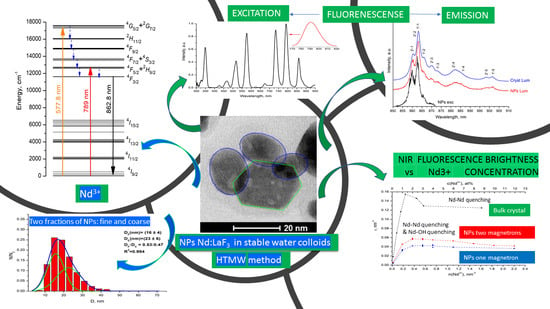
Stable Aqueous Colloidal Solutions of Nd3+: LaF3 Nanoparticles, Promising for Luminescent Bioimaging in the Near-Infrared Spectral Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Nonoptical Characterization
2.3. Optical Characterization
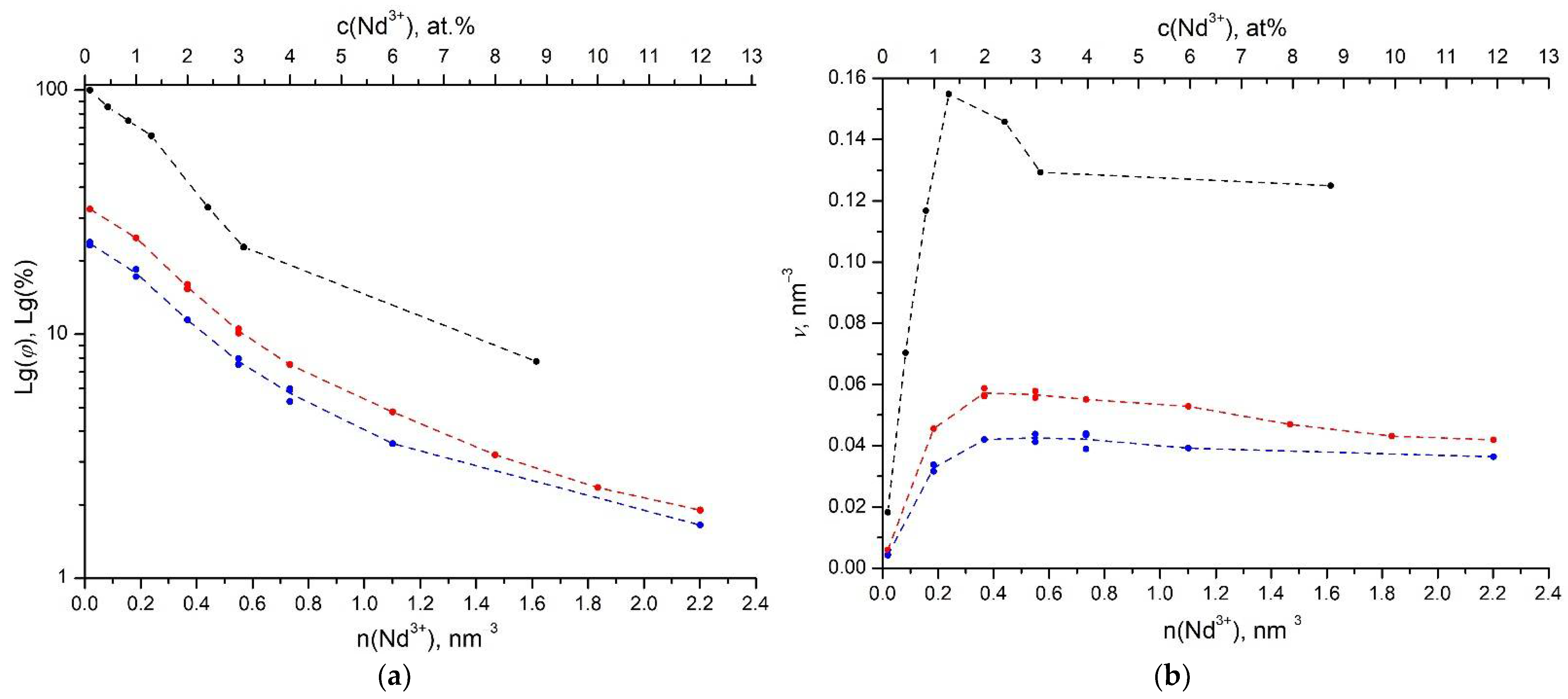
3. Results and Discussion
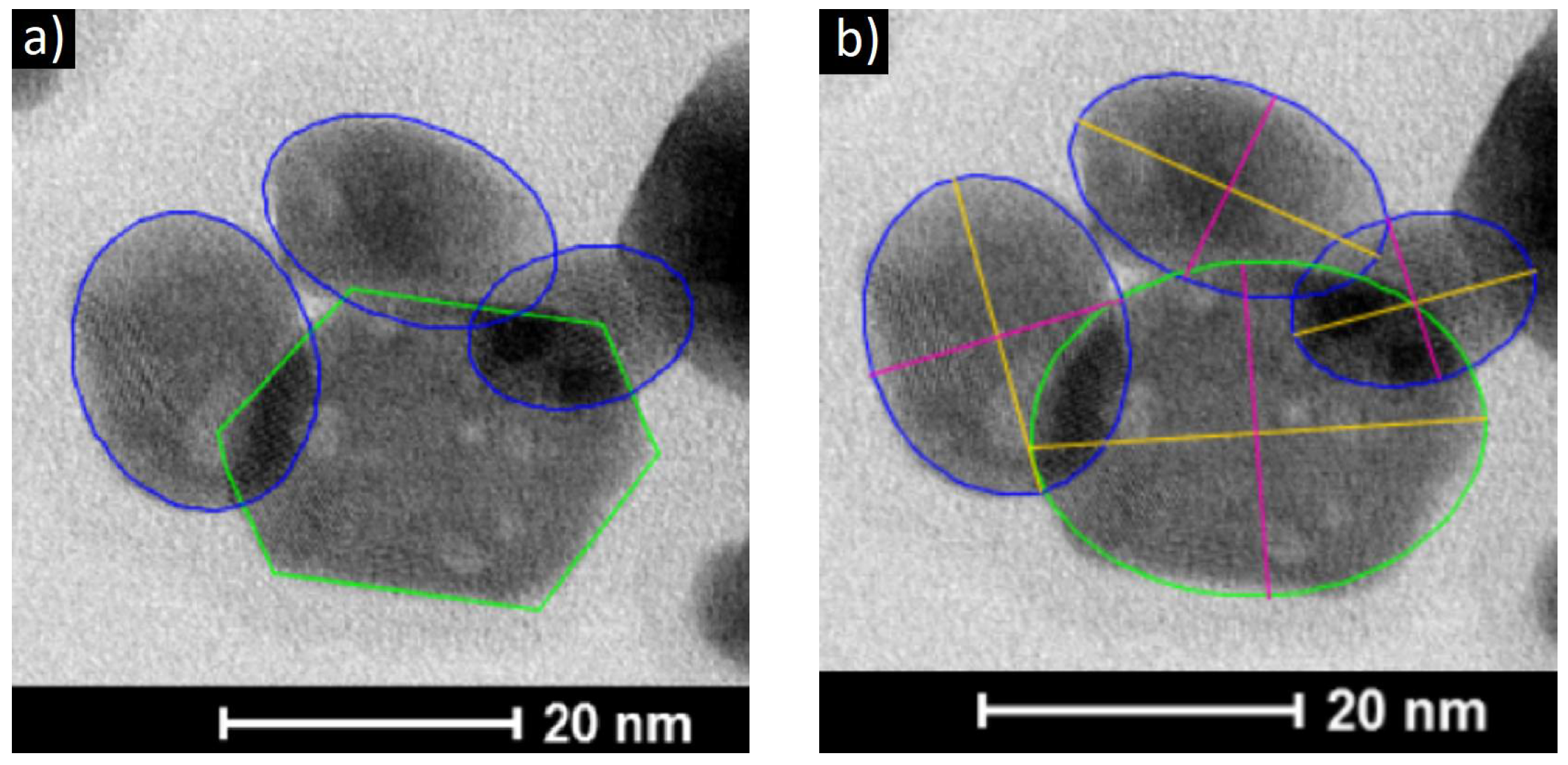
3.1. Structure of the Nanoaprticles
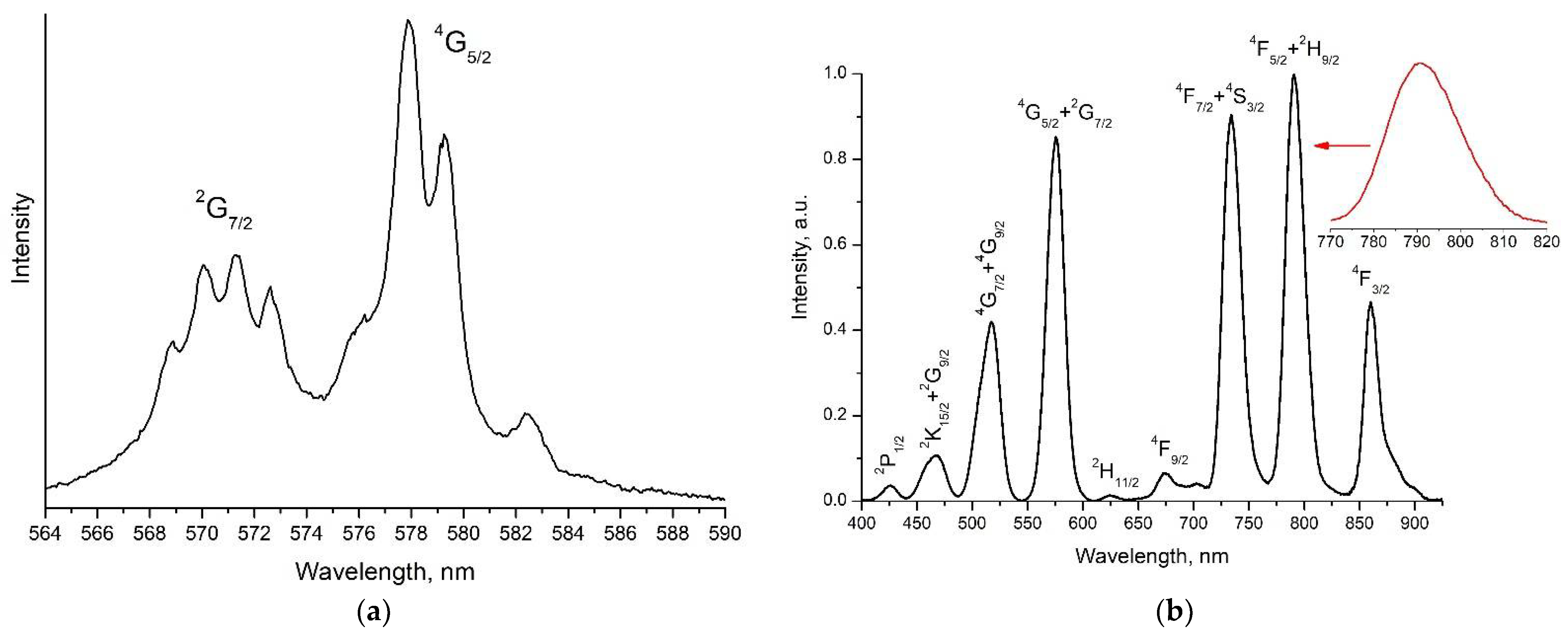
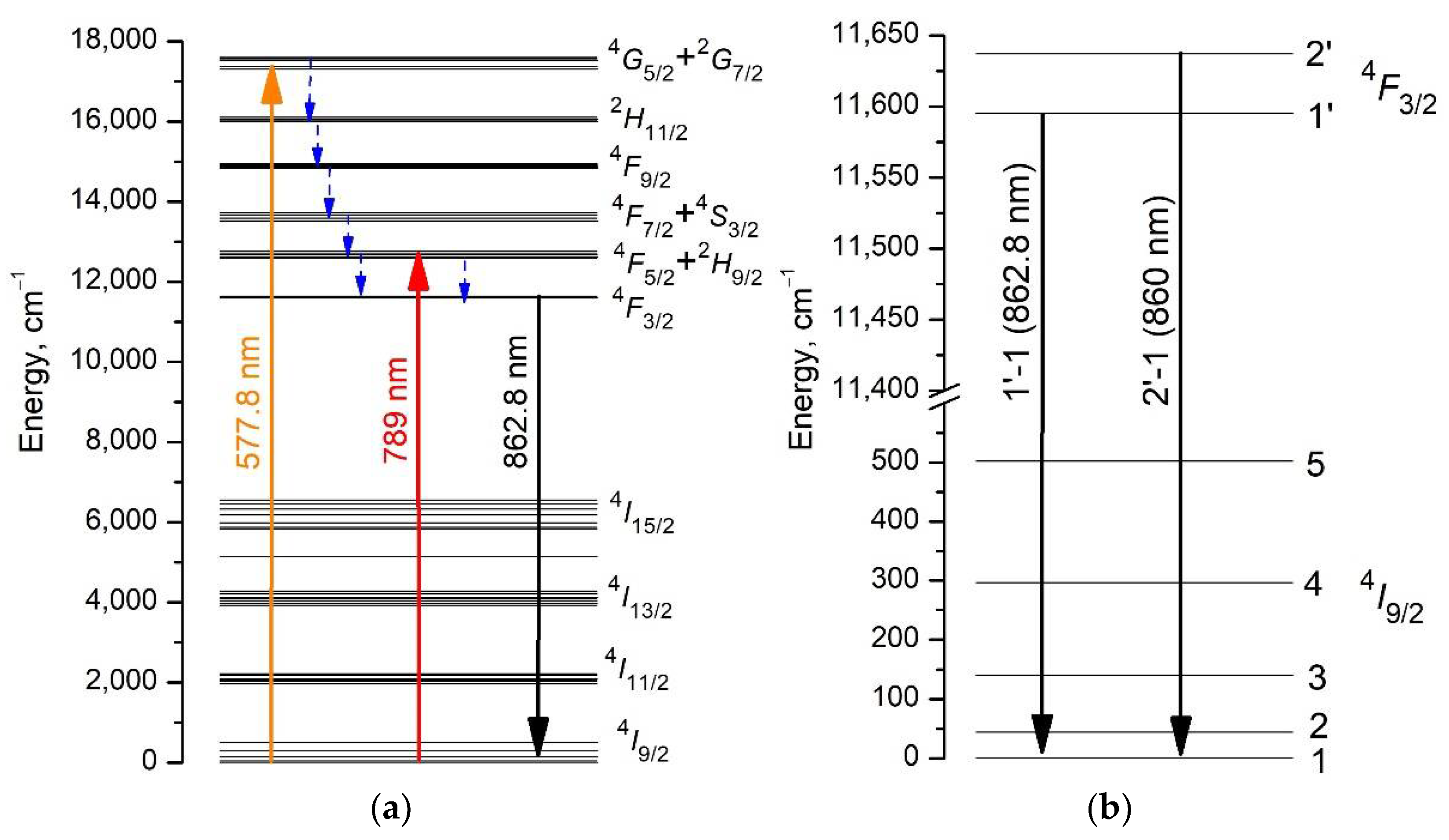
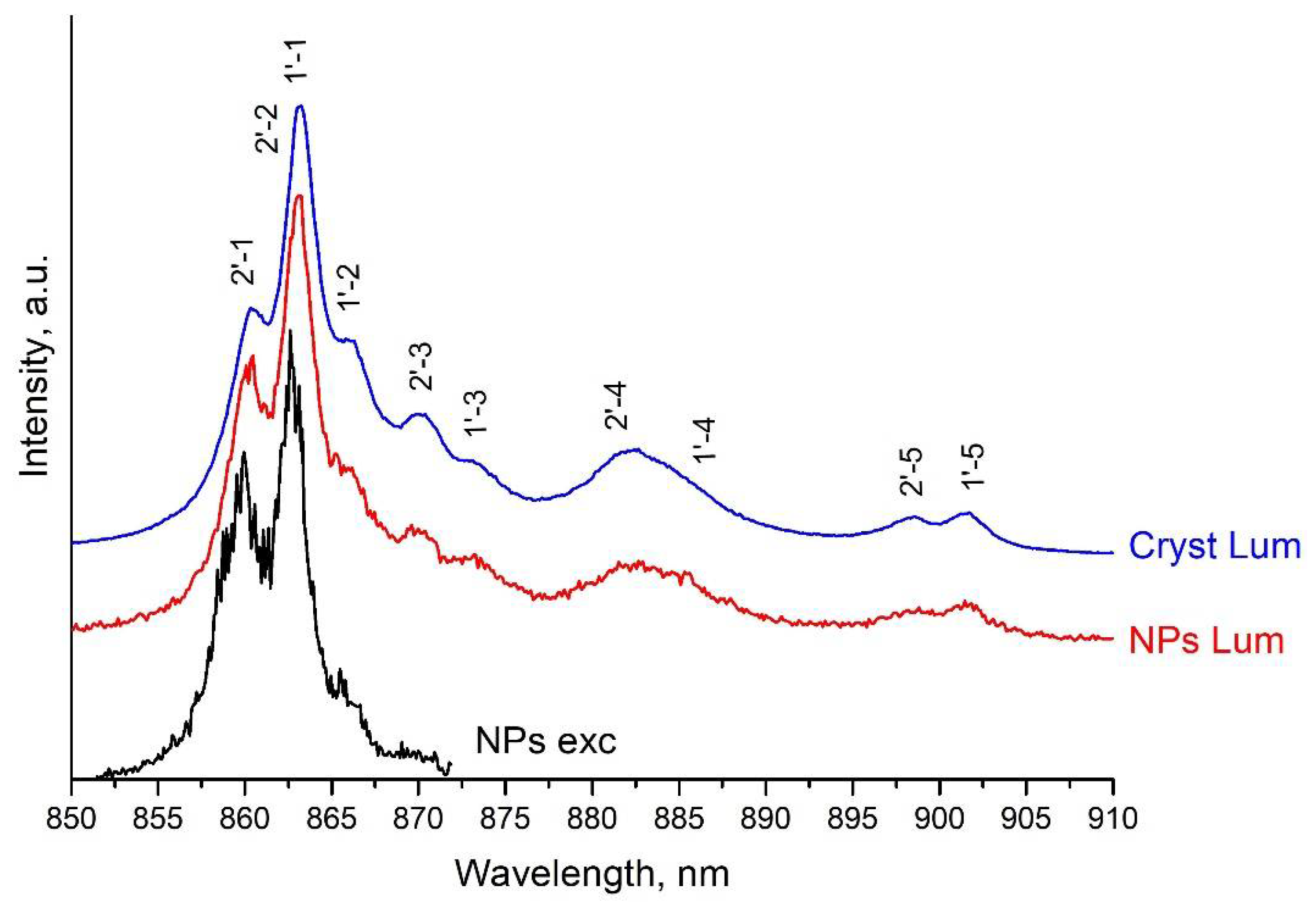
3.2. Excitation and Fluorescence Spectra
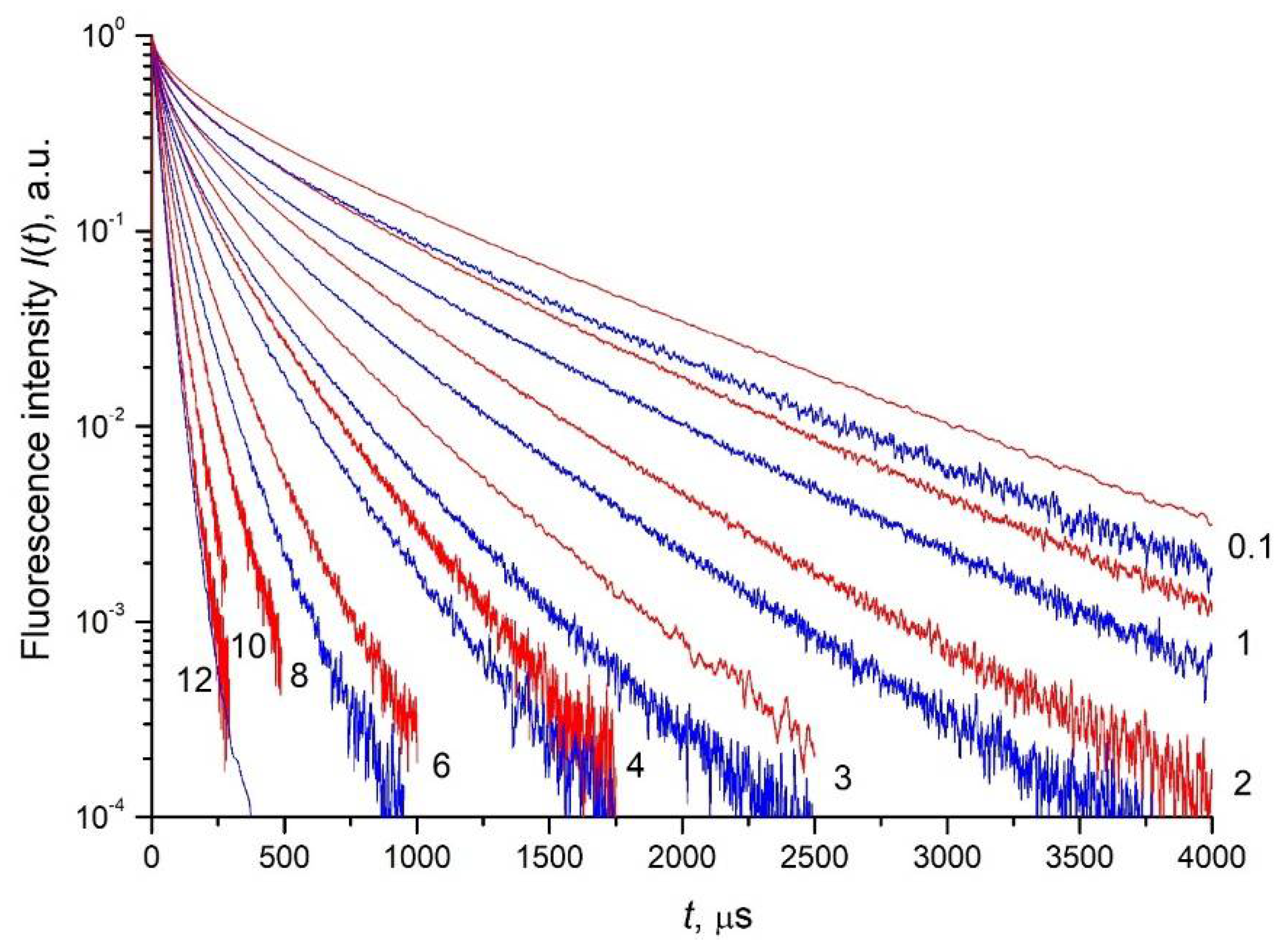
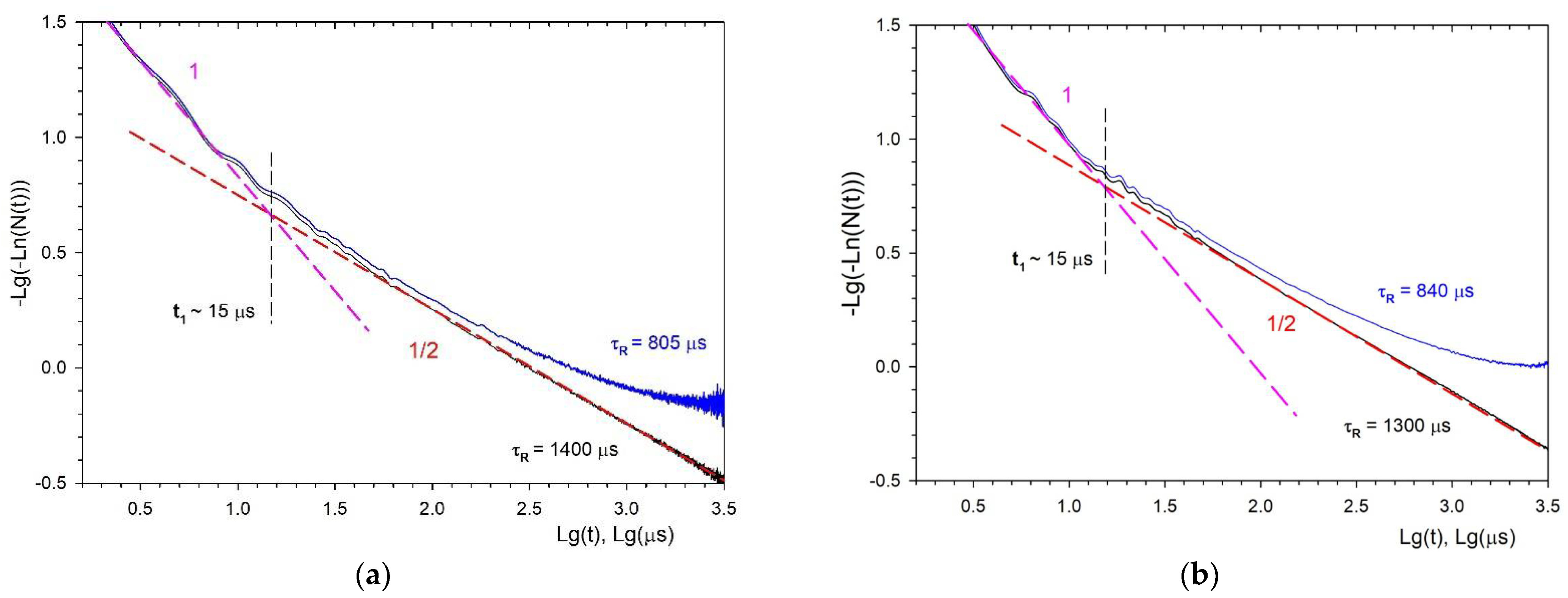
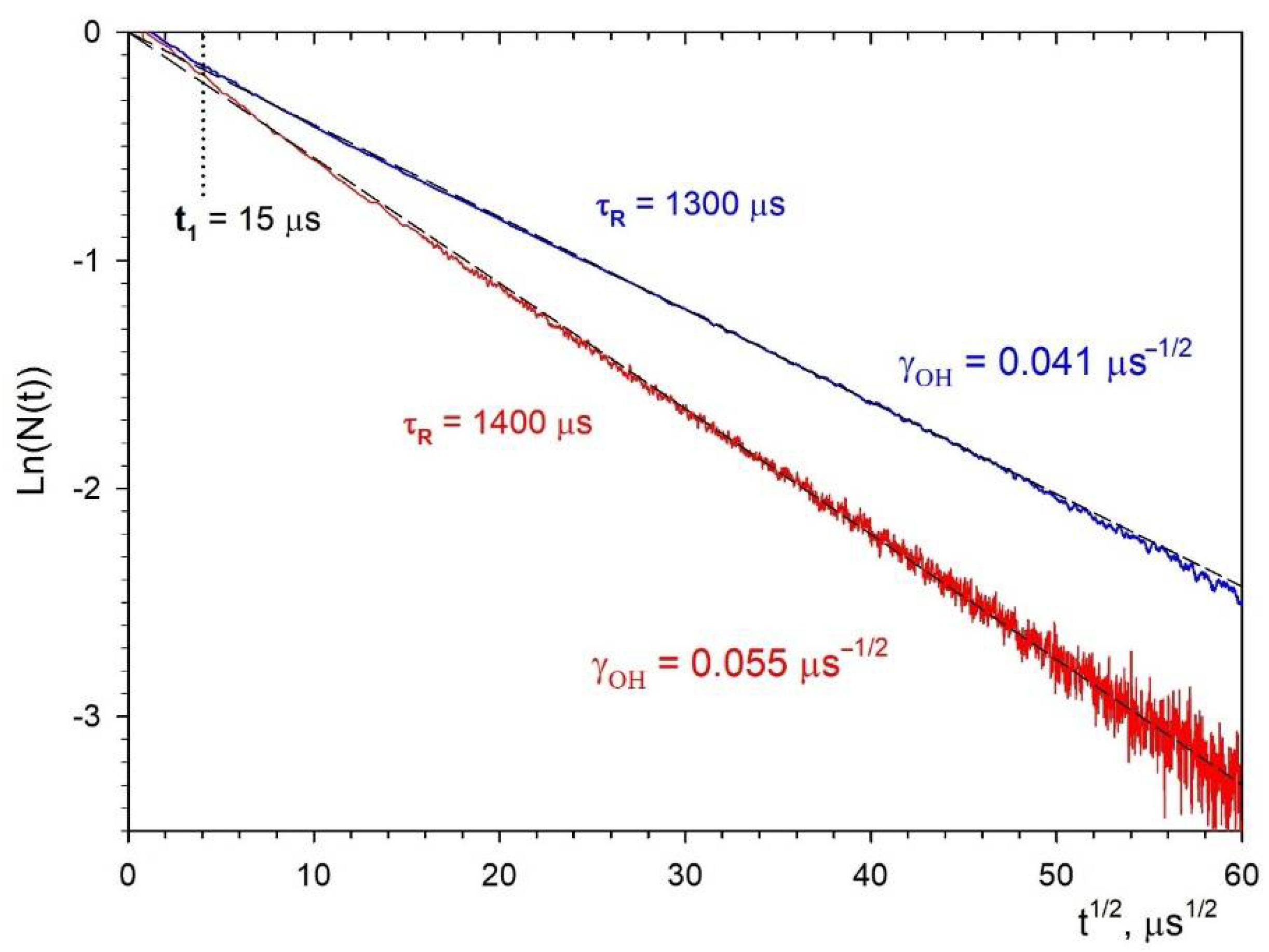
3.3. Fluorescence Decay Kinetics, Relative Fluorescence Quantum Yield, and Brightness of Aqueous Colloidal Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zhu, X.; Su, Q.; Feng, W.; Li, F. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Páez, L.; Ximendes, E.C.; Rodríguez-Sevilla, P.; Ortgies, D.H.; Rocha, U.; Jacinto, C.; Rodríguez, E.M.; Haro-González, P.; Jaque, D. Core–shell rare-earth-doped nanostructures in biomedicine. Nanoscale 2018, 10, 12935–12956. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Bunzli, J.-C. Lanthanide light for biology and medical diagnosis. J. Lumin. 2016, 170, 866–878. [Google Scholar] [CrossRef]
- Pokhrel, M.; Mimun, L.C.; Yust, B.; Kumar, G.A.; Dhanale, A.; Tang, L.; Sardar, D.K. Stokes emission in GdF3:Nd3+ nanoparticles for bioimaging probes. Nanoscale 2014, 6, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aw, J.; Xing, B. Nanostructures for NIR light-controlled therapies. Nanoscale 2017, 9, 3698–3718. [Google Scholar] [CrossRef]
- Villa, I.; Vedda, A.; Cantarelli, I.X.; Pedroni, M.; Piccinelli, F.; Bettinelli, M.; Speghini, A.; Quintanilla, M.; Vetrone, F.; Rocha, U.; et al. 1.3 μm emitting SrF2:Nd3+ nanoparticles for high contrast in vivo imaging in the second biological window. Nano Res. 2015, 8, 649–665. [Google Scholar] [CrossRef]
- Fedorenko, S.; Gilmanova, D.; Mukhametshina, A.; Nizameev, I.; Kholin, K.; Akhmadeev, B.; Voloshina, A.; Sapunova, A.; Kuznetsova, S.; Daminova, A.; et al. Silica nanoparticles with dual visible–NIR luminescence affected by silica confinement of Tb(III) and Yb(III) complexes for cellular imaging application. J. Mater. Sci. 2019, 54, 9140–9154. [Google Scholar] [CrossRef]
- Zairov, R.R.; Dovzhenko, A.P.; Sapunova, A.S.; Voloshina, A.D.; Tatarinov, D.A.; Nizameev, I.R.; Gubaidullin, A.T.; Petrov, K.A.; Enrichi, F.; Vomiero, A.; et al. Dual red-NIR luminescent Eu Yb heterolanthanide nanoparticles as promising basis for cellular imaging and sensing. Mater. Sci. Eng. C 2019, 105, 110057. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.-S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef]
- Cui, S.; Yin, D.; Chen, Y.; Di, Y.; Chen, H.; Ma, Y.; Achilefu, S.; Gu, Y. In Vivo Targeted Deep-Tissue Photodynamic Therapy Based on Near-Infrared Light Triggered Upconversion Nanoconstruct. ACS Nano 2013, 7, 676–688. [Google Scholar] [CrossRef]
- Jalani, G.; Naccache, R.; Rosenzweig, D.H.; Haglund, L.; Vetrone, F.; Cerruti, M. Photocleavable Hydrogel-Coated Upconverting Nanoparticles: A Multifunctional Theranostic Platform for NIR Imaging and On-Demand Macromolecular Delivery. J. Am. Chem. Soc. 2016, 138, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Del Rosal, B.; Ortgies, D.; Fernández, N.; Sanz-Rodríguez, F.; Jaque, D.; Rodríguez, E.M. Overcoming Autofluorescence: Long-Lifetime Infrared Nanoparticles for Time-Gated In Vivo Imaging. Adv. Mater. 2016, 28, 10188–10193. [Google Scholar] [CrossRef]
- Kim, D.; Lee, N.; Park, Y.I.; Hyeon, T. Recent Advances in Inorganic Nanoparticle-Based NIR Luminescence Imaging: Semiconductor Nanoparticles and Lanthanide Nanoparticles. Bioconjugate Chem. 2017, 28, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Htun, N.M.; Chen, Y.C.; Lim, B.; Schiller, T.; Maghzal, G.J.; Huang, A.L.; Elgass, K.D.; Rivera, J.; Schneider, H.G.; Wood, B.R.; et al. Near-infrared autofluorescence induced by intraplaque hemorrhage and heme degradation as marker for high-risk atherosclerotic plaques. Nat. Commun. 2017, 8, 75. [Google Scholar] [CrossRef]
- Voron’ko, Y.K.; Mamedov, T.G.; Osiko, V.V.; Prokhorov, A.M.; Sakun, V.P.; Shcherbakov, I.A. Nature of nonradiative excitation-energy relaxation in condensed media with high activator concentrations. Sov. J. Exp. Theor. Phys. 1976, 44, 251–261. [Google Scholar]
- Fedorenko, S.; Popov, A.; Vagapova, E.; Baranchikov, A.; Orlovskii, Y. Concentration self-quenching of luminescence in crystal matrices activated by Nd3+ ions: Theory and experiment. J. Lumin. 2018, 198, 138–145. [Google Scholar] [CrossRef]
- Rocha, U.; Kumar, K.U.; Jacinto, C.; Villa, I.; Sanz-Rodríguez, F.; Cruz, M.C.I.; Juarranz, A.; Carrasco, E.; Van Veggel, F.C.J.M.; Bovero, E.; et al. Neodymium-Doped LaF3 Nanoparticles for Fluorescence Bioimaging in the Second Biological Window. Small 2014, 10, 1141–1154. [Google Scholar] [CrossRef]
- Orlovskii, Y.; Popov, A.; Orlovskaya, E.; Vanetsev, A.; Vagapova, E.; Rähn, M.; Sammelselg, V.; Sildos, I.; Baranchikov, A.; Grachev, P.; et al. Comparison of concentration dependence of relative fluorescence quantum yield and brightness in first biological window of wavelengths for aqueous colloidal solutions of Nd3+: LaF3 and Nd3+: KY3F10 nanocrystals synthesized by microwave-hydrothermal treatment. J. Alloy Compd. 2018, 756, 182–192. [Google Scholar] [CrossRef]
- Del Rosal, B.; Pérez-Delgado, A.; Misiak, M.; Bednarkiewicz, A.; Vanetsev, A.S.; Orlovskii, Y.; Jovanović, D.J.; Dramićanin, M.D.; Rocha, U.; Kumar, K.U.; et al. Neodymium-doped nanoparticles for infrared fluorescence bioimaging: The role of the host. J. Appl. Phys. 2015, 118, 143104. [Google Scholar] [CrossRef]
- Ryabova, A.V.; Keevend, K.; Tsolaki, E.; Bertazzo, S.; Pominova, D.V.; Romanishkin, I.D.; Grachev, P.V.; Makarov, V.I.; Burmistrov, I.A.; Vanetsev, A.; et al. Visualization of Nd3+-doped LaF3 nanoparticles for near infrared bioimaging via upconversion luminescence at multiphoton excitation microscopy. Biomed. Photon. 2018, 7, 4–12. [Google Scholar] [CrossRef]
- Rocha, U.; Hu, J.; Rodríguez, E.M.; Vanetsev, A.; Rähn, M.; Sammelselg, V.; Orlovskii, Y.; Solé, J.G.; Jaque, D.; Ortgies, D.H. Subtissue Imaging and Thermal Monitoring of Gold Nanorods through Joined Encapsulation with Nd-Doped Infrared-Emitting Nanoparticles. Small 2016, 12, 5394–5400. [Google Scholar] [CrossRef]
- Ortgies, D.H.; Teran, F.J.; Rocha, U.; De La Cueva, L.; Salas, G.; Cabrera, D.; Vanetsev, A.S.; Rähn, M.; Sammelselg, V.; Orlovskii, Y.V.; et al. Optomagnetic Nanoplatforms for In Situ Controlled Hyperthermia. Adv. Funct. Mater. 2018, 28, 1704434. [Google Scholar] [CrossRef]
- Rocha, U.; Kumar, K.U.; Jacinto, C.; Ramiro, J.; Caamano, A.J.; Solé, J.A.G.; Jaque, D. Nd3+ doped LaF3 nanoparticles as self-monitored photo-thermal agents. Appl. Phys. Lett. 2014, 104, 053703. [Google Scholar] [CrossRef]
- Rocha, U.; Da Silva, C.J.; Silva, W.F.; Guedes, I.; Benayas, A.; Maestro, L.M.; Elias, M.A.A.; Bovero, E.; Van Veggel, F.C.J.M.; Solé, J.A.G.; et al. Subtissue Thermal Sensing Based on Neodymium-Doped LaF3 Nanoparticles. ACS Nano 2013, 7, 1188–1199. [Google Scholar] [CrossRef]
- Kaldvee, K.; Nefedova, A.; Fedorenko, S.; Vanetsev, A.; Orlovskaya, E.; Puust, L.; Pärs, M.; Sildos, I.; Ryabova, A.; Orlovskii, Y. Approaches to contactless optical thermometer in the NIR spectral range based on Nd3+ doped crystalline nanoparticles. J. Lumin. 2017, 183, 478–485. [Google Scholar] [CrossRef]
- Muhr, V.; Wilhelm, S.; Hirsch, T.; Wolfbeis, O.S. Upconversion Nanoparticles: From Hydrophobic to Hydrophilic Surfaces. Accounts Chem. Res. 2014, 47, 3481–3493. [Google Scholar] [CrossRef]
- Agranovich, V.M.; Galanin, M.D. Electron Excitation Energy Transfer in Condensed Matter. In Modern Problems in Condensed Matter Sciences; Agranovich, V.M., Maradudin, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1982; Volume 3. [Google Scholar]
- Ozawa, L.; Forest, H.; Jaffe, P.M.; Ban, G. The Effect of Exciting Wavelength on Optimum Activator Concentration. J. Electrochem. Soc. 1971, 118, 482–486. [Google Scholar] [CrossRef]
- Tanner, P.A.; Zhou, L.; Duan, C.; Wong, K.-L. Misconceptions in electronic energy transfer: Bridging the gap between chemistry and physics. Chem. Soc. Rev. 2018, 47, 5234–5265. [Google Scholar] [CrossRef]
- Sun, W.; Cui, X.; Wang, Z.; Wei, W.; Peng, B. Luminescence properties of Nd3+-doped LaF3 nanocrystals with a long lifetime in organic solvents. J. Mater. Chem. 2012, 22, 6990–6993. [Google Scholar] [CrossRef]
- Kumar, G.A.; Chen, C.W.; Ballato, J.; Riman, R.E. Optical Characterization of Infrared Emitting Rare-Earth-Doped Fluoride Nanocrystals and Their Transparent Nanocomposites. Chem. Mater. 2007, 19, 1523–1528. [Google Scholar] [CrossRef]
- Shen, J.; Lowe, R.D.; Snook, R.D. A model for cw laser induced mode-mismatched dual-beam thermal lens spectrometry. Chem. Phys. 1992, 165, 385–396. [Google Scholar] [CrossRef]
- Vanetsev, A.; Kaldvee, K.; Puust, L.; Keevend, K.; Nefedova, A.; Fedorenko, S.; Baranchikov, A.; Sildos, I.; Rähn, M.; Sammelselg, V.; et al. Relation of Crystallinity and Fluorescent Properties of LaF3:Nd3+ Nanoparticles Synthesized with Different Water-Based Techniques. ChemistrySelect 2017, 2, 4874–4881. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Laser Crystals; Springer International Publishing: New York, NY, USA, 1981; p. 456. [Google Scholar]
- Ermolaev, V.L.; Sveshnikova, E.B.; Bodunov, E.N. Inductive-resonant mechanism of nonradiative transitions in ions and molecules in condensed phase. Physics-Uspekhi 1996, 39, 261–282. [Google Scholar] [CrossRef]
- Stouwdam, J.W.; van Veggel, F.C.J.M. Near-infrared Emission of Redispersible Er3+, Nd3+, and Ho3+ Doped LaF3 Nanoparticles. Nano Lett. 2002, 2, 733–737. [Google Scholar] [CrossRef]
- Rocha, U.; Jacinto, C.; Kumar, K.U.; López, F.J.; Bravo, D.; Solé, J.G.; Jaque, D. Real-time deep-tissue thermal sensing with sub-degree resolution by thermally improved Nd3+:LaF3 multifunctional nanoparticles. J. Lumin. 2016, 175, 149–157. [Google Scholar] [CrossRef]
- Gai, S.; Li, C.; Yang, P.; Lin, J. Recent Progress in Rare Earth Micro/Nanocrystals: Soft Chemical Synthesis, Luminescent Properties, and Biomedical Applications. Chem. Rev. 2014, 114, 2343–2389. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Cui, X.; Wei, W.; Peng, B.; Fan, D. Effects of different ligands on luminescence properties of LaF3: Nd nanoparticles. J. Rare Earths 2013, 31, 645–649. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D. Controlled synthesis of hexagon shaped lanthanide-doped LaF3 nanoplates with multicolor upconversion fluorescence. J. Mater. Chem. 2007, 17, 3875–3880. [Google Scholar] [CrossRef]
- Cross, A.M.; May, P.S.; Van Veggel, F.C.J.M.; Berry, M.T. Dipicolinate Sensitization of Europium Luminescence in Dispersible 5%Eu:LaF3 Nanoparticles. J. Phys. Chem. C 2010, 114, 14740–14747. [Google Scholar] [CrossRef]
- Cao, T.; Yang, Y.; Gao, Y.; Zhou, J.; Li, Z.; Li, F. High-quality water-soluble and surface-functionalized upconversion nanocrystals as luminescent probes for bioimaging. Biomaterials 2011, 32, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Tian, D.; Chong, B.; Zhang, X.; Gao, W. Rare-earth doped LaF3 hollow hexagonal nanoplates: Hydrothermal synthesis and photoluminescence properties. CrystEngComm 2014, 16, 7106–7114. [Google Scholar] [CrossRef]
- Eckert, J.O.; Hung-Houston, C.C.; Gersten, B.L.; Lencka, M.M.; Riman, R.E. Kinetics and Mechanisms of Hydrothermal Synthesis of Barium Titanate. J. Am. Ceram. Soc. 1996, 79, 2929–2939. [Google Scholar] [CrossRef]
- Vanetsev, A.S.; Gaitko, O.M.; Chuvashova, I.; Sokolov, M.N.; Tret’yakov, Y.D. Microwave hydrothermal synthesis of nanodispersed YV1 − xPxO4: Eu powders. Dokl. Chem. 2011, 441, 325–329. [Google Scholar] [CrossRef]
- Baranchikov, A.E.; Ivanov, V.K.; Tret’yakov, Y.D. Hydrothermal microwave synthesis of nanocrystalline anatase. Dokl. Chem. 2012, 447, 241–243. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Reddy, B.R.; Venkateswarlu, P. Energy up-conversion in LaF3:Nd3+. J. Chem. Phys. 1983, 79, 5845. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Pukhov, K.K.; Basiev, T.T.; Orlovskii, Y. Spontaneous emission in dielectric nanoparticles. JETP Lett. 2008, 88, 12–18. [Google Scholar] [CrossRef]
- Wirick, M.P. The Near Ultraviolet Optical Constants of Lanthanum Fluoride. Appl. Opt. 1966, 5, 1966–1967. [Google Scholar] [CrossRef]
- Daimon, M.; Masumura, A. Measurement of the refractive index of distilled water from the near-infrared region to the ultraviolet region. Appl. Opt. 2007, 46, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Feofilov, P.P.; Kaplyanskii, A.A. Latent Optical Anisotropy of Cubic Crystals Containing Local Centers and Methods for Its Investigation. Sov. Phys. Uspekhi 1962, 5, 79. [Google Scholar] [CrossRef]
- Voron’ko, Y.K.; Mamedov, T.G.; Osiko, V.V.; Timoshechkin, M.I.; Shcherbakov, I.A. Effect of donor-donor and do-nor-acceptor interactions on the decay kinetics of the metastable state of Nd3+ in crystals. Sov. J. Exp. Theor. Phys. 1974, 38, 565–572. [Google Scholar]
- Avanesov, A.G.; Basiev, T.T.; Voron’ko, Y.K.; Denker, B.I.; Karasik, A.Y.; Maksimova, G.V.; Osiko, V.V.; Pisarenko, V.F.; Prokhorov, A.M. Neodymium electron energy deactivation and transfer in highly concentrated phosphate glasses. Sov. J. Exp. Theor. Phys. 1979, 50, 886–895. [Google Scholar]
- Fedorenko, S.; Orlovskii, Y.; Samsonova, E. Fluctuation kinetics of the hopping fluorescence quenching in disordered solid solutions: A theoretical model and experimental evidence. J. Lumin. 2011, 131, 2409–2413. [Google Scholar] [CrossRef]
- Bondar, I.A.; Burshtein, A.I.; Krutikov, A.V.; Mezentseva, L.P.; Osiko, V.V.; Sakun, V.P.; Smirnov, V.A.; Shcherbakov, I.A. Investigation of the processes of relaxation of electron excitation in crystals- for arbitrary relationships between interaction microparameters and concentrations of energy donors and acceptors. Sov. J. Exp. Theor. Phys. 1981, 54, 45. [Google Scholar]
- Förster, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948, 437, 55–75. [Google Scholar] [CrossRef]
- Förster, T. Experimentelle und theoretische Untersuchung des zwischenmolekularen Übergangs von Elektronenanregungsenergie. Z. Nat. A 1949, 4, 321–327. [Google Scholar] [CrossRef]
- Levitz, P.; Drake, J.M.; Klafter, J. Critical evaluation of the application of direct energy transfer in probing the morphology of porous solids. J. Chem. Phys. 1988, 89, 5224–5236. [Google Scholar] [CrossRef]
- Mansmann, M. Die Kristallstruktur von Lanthantrifluorid. Z. Krist. 1965, 122, 375–398. [Google Scholar] [CrossRef]
- Popov, A.; Fedorenko, S.; Krut’ko, V.; Iskhakova, L.; Komova, M.; Timofeeva, E.; Kononkova, N.; Orlovskii, Y. Impurity fluorescence self-quenching in Nd3+: Gd3BWO9 crystalline powders: Experiment and analysis. J. Alloy Compd. 2020, 822, 153654. [Google Scholar] [CrossRef]
- Fedorenko, S.; Burshtein, A. Binary theory of impurity quenching accelerated by resonant excitation migration in a disordered system. Chem. Phys. 1988, 128, 185–198. [Google Scholar] [CrossRef]
- Fedorenko, S.G.; Burshtein, A.I. Deviations from linear Stern–Volmer law in hopping quenching theory. J. Chem. Phys. 1992, 97, 8223–8232. [Google Scholar] [CrossRef]
- Pudovkin, M.S.; Zelenikhin, P.V.; Shtyreva, V.V.; Evtugyn, V.G.; Salnikov, V.V.; Nizamutdinov, A.S.; Semashko, V.V. Cellular uptake and cytotoxicity of unmodified Pr3+:LaF3 nanoparticles. J. Nanoparticle Res. 2019, 21, 1–13. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, A.; Orlovskaya, E.; Shaidulin, A.; Vagapova, E.; Timofeeva, E.; Dolgov, L.; Iskhakova, L.; Uvarov, O.; Novikov, G.; Rähn, M.; et al. Stable Aqueous Colloidal Solutions of Nd3+: LaF3 Nanoparticles, Promising for Luminescent Bioimaging in the Near-Infrared Spectral Range. Nanomaterials 2021, 11, 2847. https://doi.org/10.3390/nano11112847
Popov A, Orlovskaya E, Shaidulin A, Vagapova E, Timofeeva E, Dolgov L, Iskhakova L, Uvarov O, Novikov G, Rähn M, et al. Stable Aqueous Colloidal Solutions of Nd3+: LaF3 Nanoparticles, Promising for Luminescent Bioimaging in the Near-Infrared Spectral Range. Nanomaterials. 2021; 11(11):2847. https://doi.org/10.3390/nano11112847
Chicago/Turabian StylePopov, Alexandr, Elena Orlovskaya, Artem Shaidulin, Ekaterina Vagapova, Elena Timofeeva, Leonid Dolgov, Lyudmila Iskhakova, Oleg Uvarov, Grigoriy Novikov, Mihkel Rähn, and et al. 2021. "Stable Aqueous Colloidal Solutions of Nd3+: LaF3 Nanoparticles, Promising for Luminescent Bioimaging in the Near-Infrared Spectral Range" Nanomaterials 11, no. 11: 2847. https://doi.org/10.3390/nano11112847
APA StylePopov, A., Orlovskaya, E., Shaidulin, A., Vagapova, E., Timofeeva, E., Dolgov, L., Iskhakova, L., Uvarov, O., Novikov, G., Rähn, M., Tamm, A., Vanetsev, A., Fedorenko, S., Eliseeva, S., Petoud, S., & Orlovskii, Y. (2021). Stable Aqueous Colloidal Solutions of Nd3+: LaF3 Nanoparticles, Promising for Luminescent Bioimaging in the Near-Infrared Spectral Range. Nanomaterials, 11(11), 2847. https://doi.org/10.3390/nano11112847