Green Synthesis of Iron-Doped Cobalt Oxide Nanoparticles from Palm Kernel Oil via Co-Precipitation and Structural Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Palm Kernel Oil
2.2.2. Carboxylate Synthesis
2.2.3. Titration of Carboxylate Solution
2.2.4. Synthesis of the Cobalt Iron Carboxylate Precursors
2.2.5. Thermal Decomposition of the Precursors
2.2.6. Characterization of the Precursors and Final Products
3. Results and Discussion
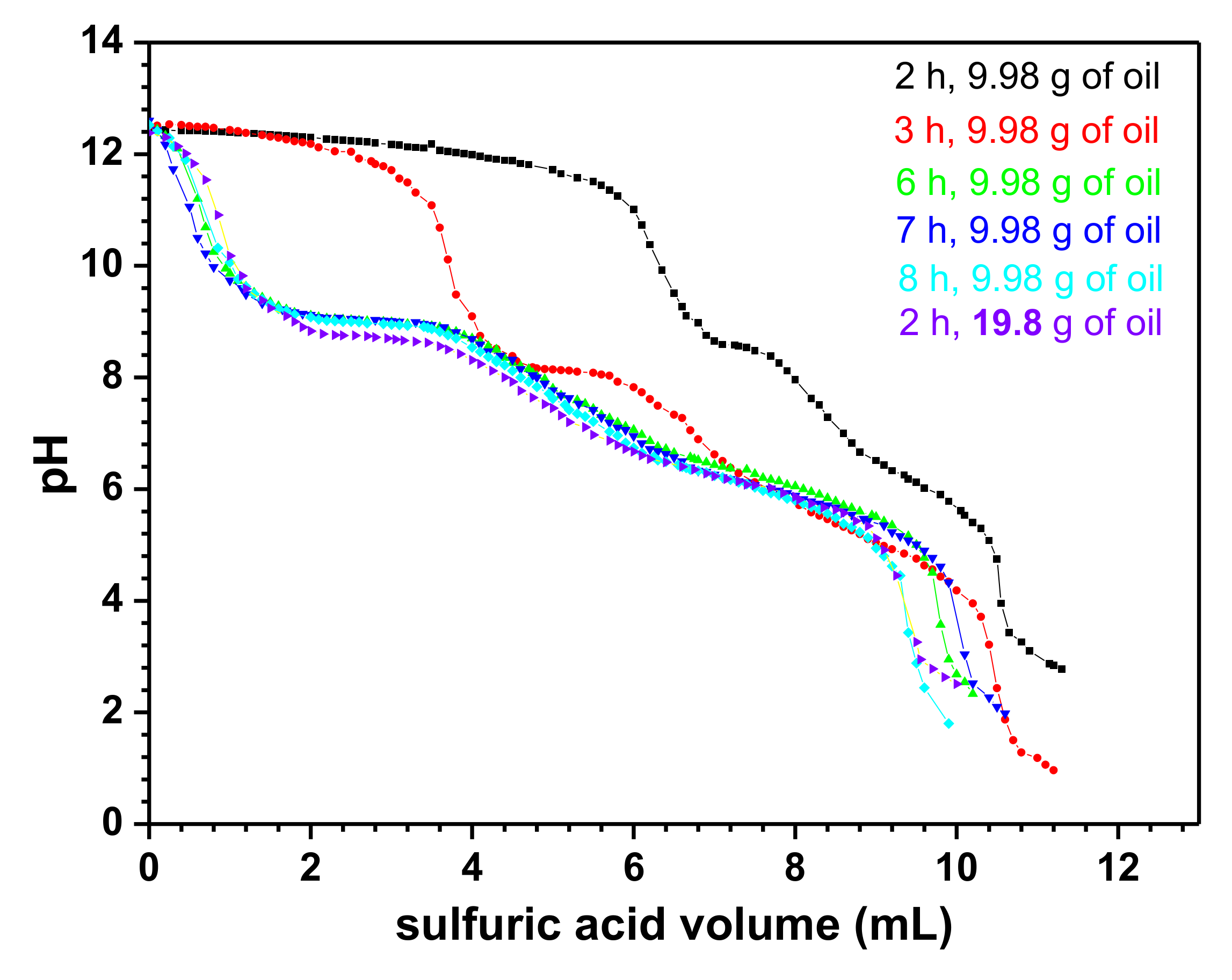
3.1. Titration Curves of the Carboxylates Solutions
3.2. ICP-AES Analysis of the Precursors
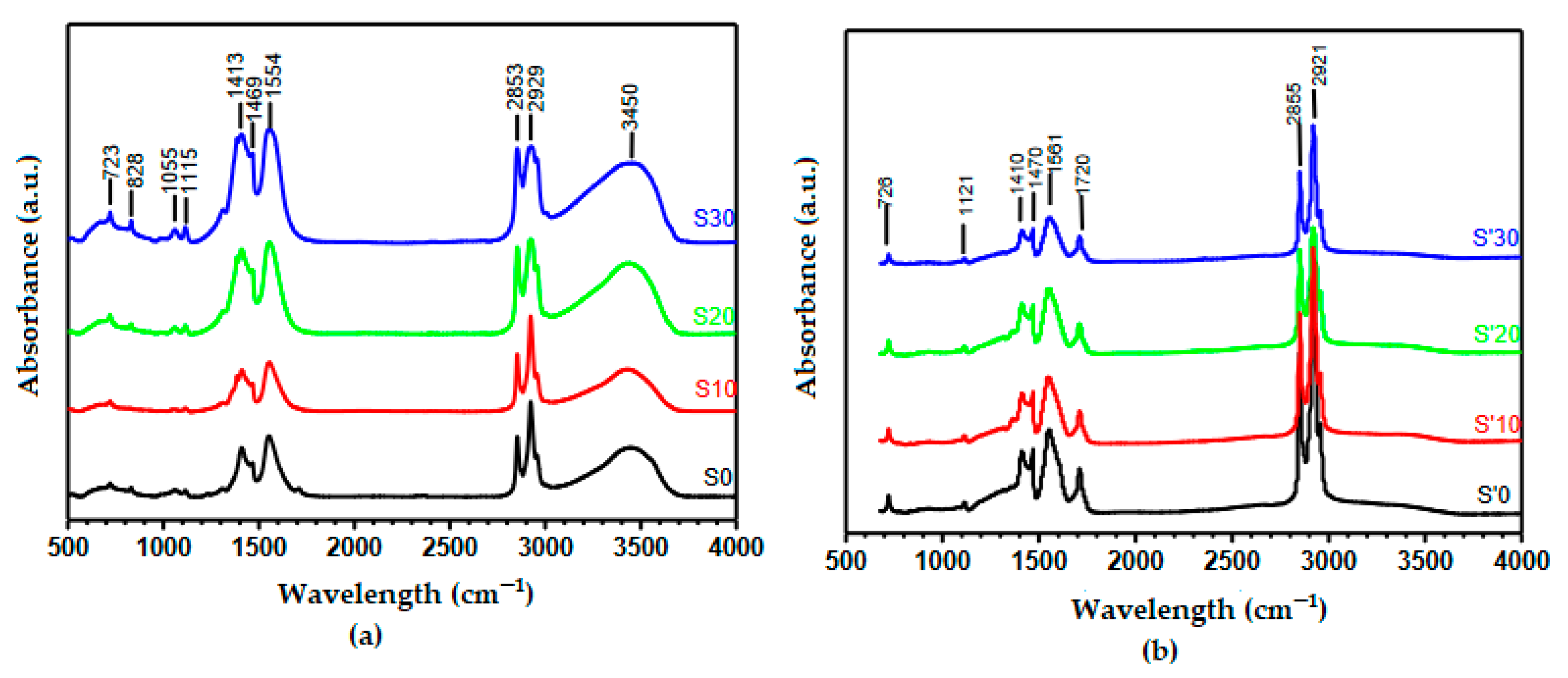
3.3. Fourier Transform Infrared (FTIR) Spectral Characterization of the Precursor
3.4. Gas Chromatography (GC) Analysis
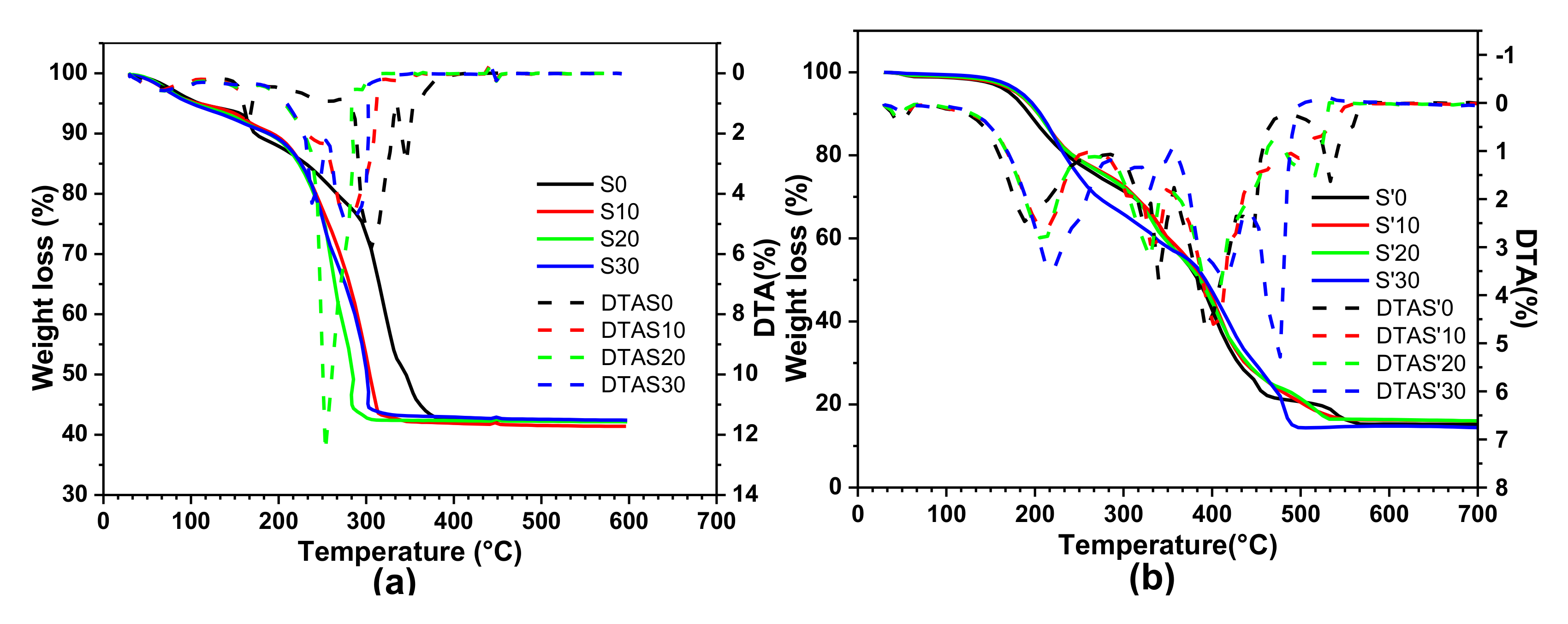
3.5. Thermogravimetry Analysis of the Precursors
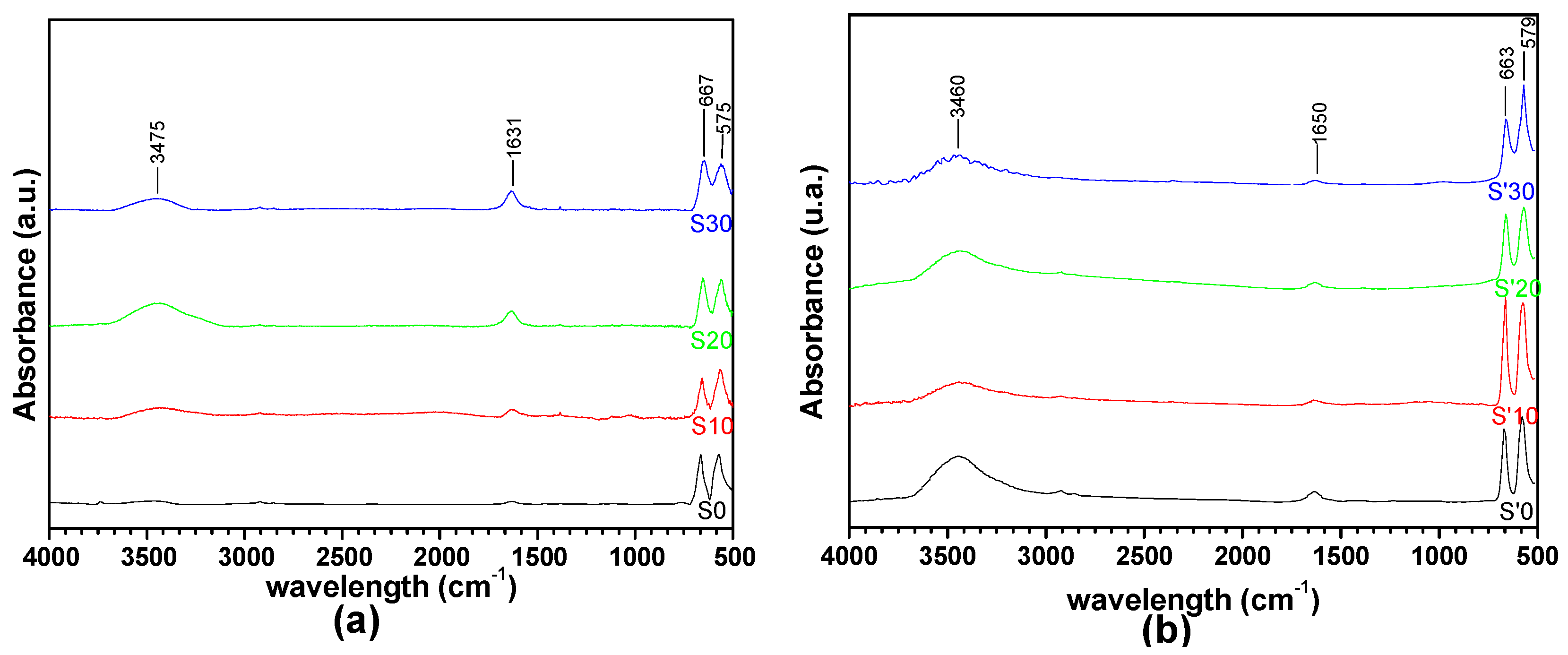
3.6. FTIR Spectral Characterization of the Decomposition Products
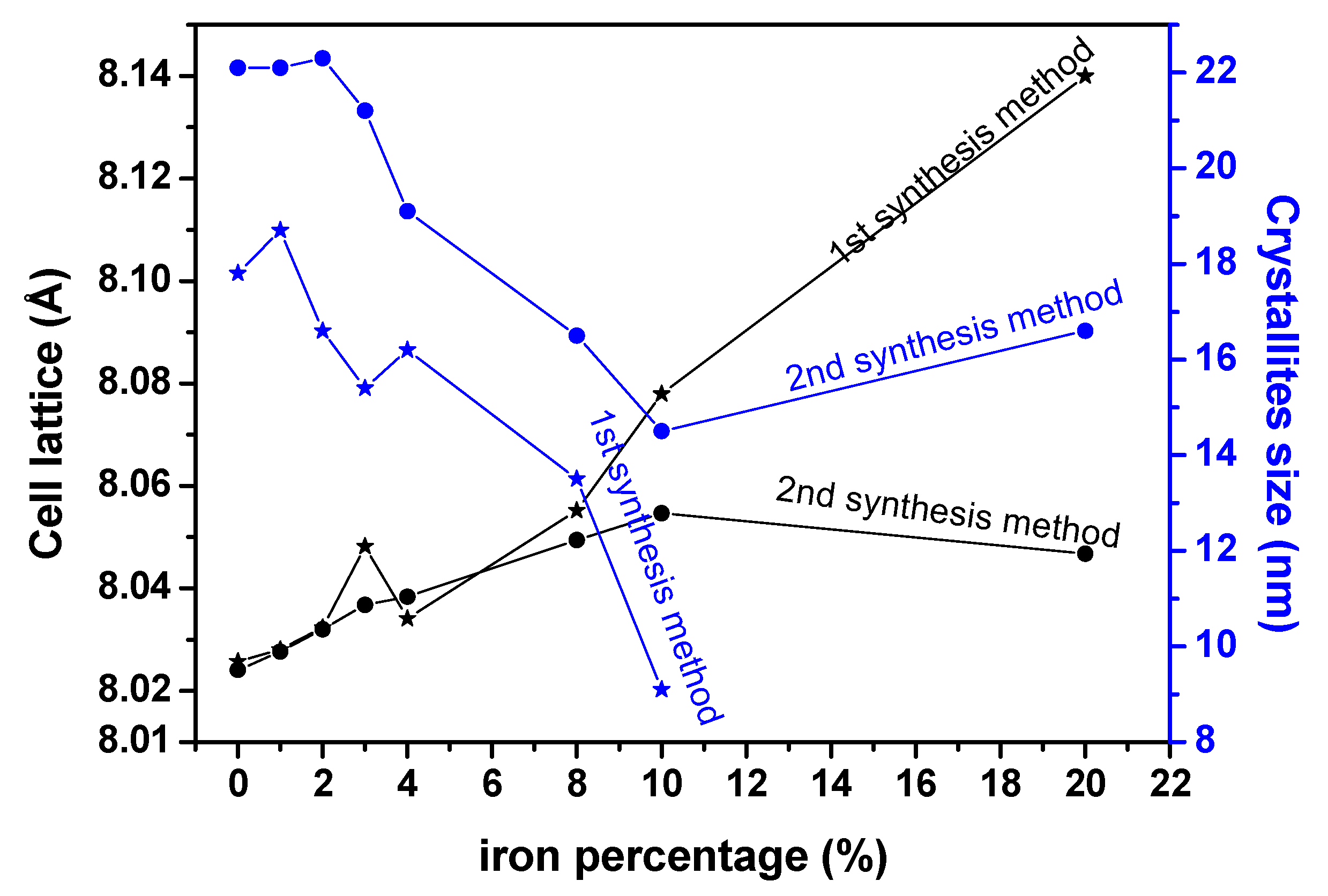
3.7. X-ray Diffraction (XRD) Analysis of the Decomposition Products
3.8. Raman Analysis of the Thermal Decomposition Products
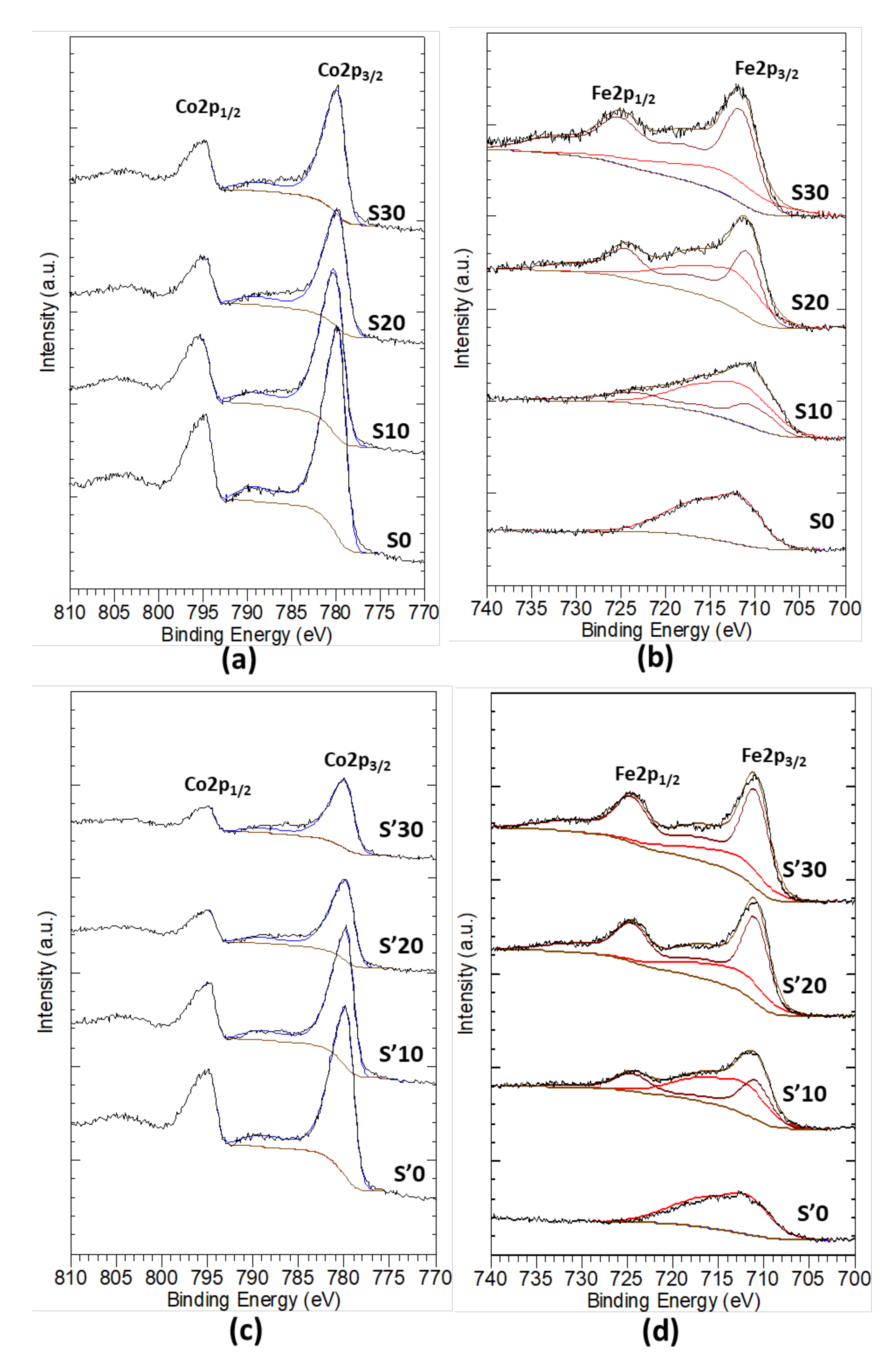
3.9. X-ray Photoelectron Spectroscopy (XPS) Analysis of the Thermal Decomposition Products
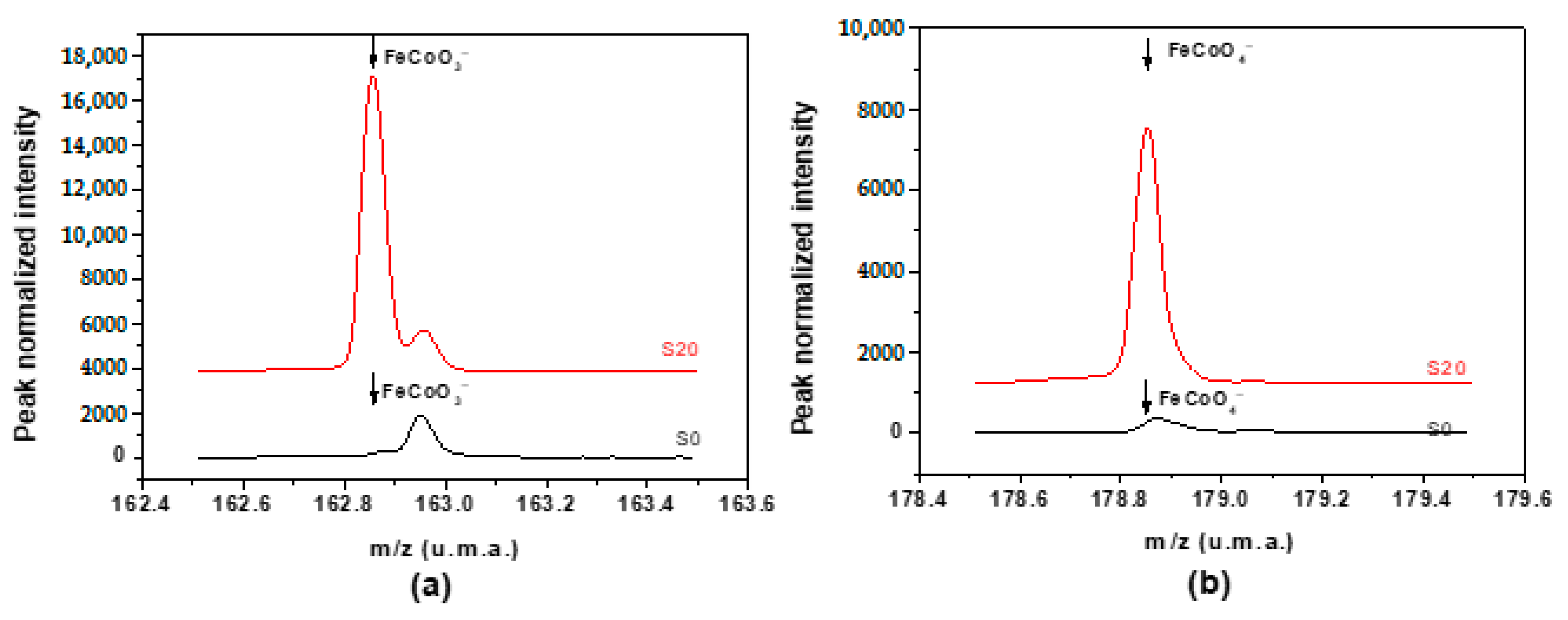
3.10. ToF-SIMS Analysis of the Thermal Decomposition Products
3.11. Transmission Electron Microscopy (TEM) Analysis of the Decomposition Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Shahid, R. Green Chemical Synthesis of II-VI Semiconductor Quantum Dots. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2012. [Google Scholar]
- Sahoo, P.; Djieutedjeu, H.; Poudeu, P.F.P. Co3O4 nanostructures: The effect of synthesis conditions on particles size, magnetism and transport properties. J. Mater. Chem. A 2013, 1, 15022–15030. [Google Scholar] [CrossRef]
- Lou, X.; Han, J.; Chu, W.; Wang, X.; Cheng, Q. Synthesis and photocatalytic property of Co3O4 nanorods. Mater. Sci. Eng. B 2007, 137, 268–271. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Stanek, J.J.; Kotarba, A.; Sojka, Z. Catalytic properties in N2O decomposition of mixed cobalt–iron spinels. Catal. Commun. 2011, 15, 127–131. [Google Scholar] [CrossRef]
- Yamaura, H.; Tamaki, J.; Moriya, K.; Miura, N.; Yamazoe, N. Highly Selective CO Sensor Using Indium Oxide Doubly Promoted by Cobalt Oxide and Gold. J. Electrochem. Soc. 1997, 144, L158–L160. [Google Scholar] [CrossRef]
- Chidambaram, K.; Malhotra, L.K.; Chopra, K.L. Spray-pyrolysed cobalt black as a high temperature selective absorber. Thin Solid Films 1982, 87, 365–371. [Google Scholar] [CrossRef]
- Kim, H.-K.; Seong, T.-Y.; Lim, J.-H.; Ii Cho, W.; Soo Yoon, Y. Electrochemical and structural properties of radio frequency sputtered cobalt oxide electrodes for thin-film supercapacitors. J. Power Sources 2001, 102, 167–171. [Google Scholar] [CrossRef]
- Makhlouf, S.A. Magnetic properties of Co3O4 nanoparticles. J. Magn. Magn. Mater. 2002, 246, 184–190. [Google Scholar] [CrossRef]
- Maruyama, T.; Arai, S. Electrochromic Properties of Cobalt Oxide Thin Films Prepared by Chemical Vapor Deposition. J. Electrochem. Soc. 1996, 143, 1383–1386. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Mir, N.; Davar, F. Synthesis and characterization of Co3O4 nanorods by thermal decomposition of cobalt oxalate. J. Phys. Chem. Solids 2009, 70, 847–852. [Google Scholar] [CrossRef]
- Omata, K.; Takada, T.; Kasahara, S.; Yamada, M. Active site of substituted cobalt spinel oxide for selective oxidation of COH2. Part II. Appl. Catal. A Gen. 1996, 146, 255–267. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Bawaked, S.M.; Kosa, S.A.; Schwieger, W. Effect of Pr, Sm, and Tb Doping on the Morphology, Crystallite Size, and N2O Decomposition Activity of Co3O4 Nanorods. J. Nanomater. 2015, 2015, 580582. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Ren, T.; Wang, X.; Ji, D.; Suo, J. Catalytic decomposition of N2O over MxCo1−xCo2O4 (M = Ni, Mg) spinel oxides. Appl. Catal. B Environ. 2003, 45, 85–90. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Li, Y.; Shi, J. Highly Moisture-resistant Fe-doped Mesoporous Co3O4 Catalyst for Efficient Low-temperature CO Oxidation. New J. Chem. 2014, 39. [Google Scholar] [CrossRef]
- Manickam, M.; Ponnuswamy, V.; Sankar, C.; Suresh, R.; Mariappan, R.; Chandrasekaran, J. Structural, optical, electrical and electrochemical properties of Fe: Co3O4 thin films for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 18951–18965. [Google Scholar] [CrossRef]
- Stella, C.; Soundararajan, N.; Ramachandran, K. Structural, optical, and magnetic properties of Mn and Fe-doped Co3O4 nanoparticles. AIP Adv. 2015, 4, 087104. [Google Scholar] [CrossRef]
- Li, G.; Chen, M.; Ouyang, Y.; Yao, D.; Lu, L.; Wang, L.; Xia, X.; Lei, W.; Chen, S.-M.; Mandler, D.; et al. Manganese doped Co3O4 mesoporous nanoneedle array for long cycle-stable supercapacitors. Appl. Surf. Sci. 2019, 469, 941–950. [Google Scholar] [CrossRef]
- Deng, S.; Xiao, X.; Chen, G.; Wang, L.; Wang, Y. Cd doped porous Co3O4 nanosheets as electrode material for high performance supercapacitor application. Electrochim. Acta 2016, 196, 316–327. [Google Scholar] [CrossRef]
- UmaSudharshini, A.; Bououdina, M.; Venkateshwarlu, M.; Dhamodharan, P.; Manoharan, C. Solvothermal synthesis of Cu-doped Co3O4 nanosheets at low reaction temperature for potential supercapacitor applications. Appl. Phys. A 2021, 127, 353. [Google Scholar] [CrossRef]
- Hao, J.; Peng, S.; Li, H.; Dang, S.; Qin, T.; Wen, Y.; Huang, J.; Ma, F.; Gao, D. A low crystallinity oxygen-vacancy-rich Co3O4 cathode for high-performance flexible asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 16094–16100. [Google Scholar] [CrossRef]
- Xiong, S.; Weng, S.; Tang, Y.; Qian, L.; Xu, Y.; Li, X.; Lin, H.; Xu, Y.; Jiao, Y.; Chen, J. Mo-doped Co3O4 ultrathin nanosheet arrays anchored on nickel foam as a bi-functional electrode for supercapacitor and overall water splitting. J. Colloid Interface Sci. 2021, 602, 355–366. [Google Scholar] [CrossRef]
- Niu, M.; Wang, Y.; Cheng, Y.; Chen, G.; Cui, L. Fabrication of Co3O4 cubic nanoframes: Facet-preferential chemical etching of Fe3+ ions to Co3O4 nanocubes. Mater. Lett. 2009, 63, 837–839. [Google Scholar] [CrossRef]
- Thota, S.; Kumar, A.; Kumar, J. Optical, electrical and magnetic properties of Co3O4 nanocrystallites obtained by thermal decomposition of sol–gel derived oxalates. Mater. Sci. Eng. B 2009, 164, 30–37. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Liu, R.-S.; Huang, K.-L.; Wang, L.-P.; Liu, S.-Q.; Zeng, W.-W. Preparation and electrochemical performance of nanosized Co3O4 via hydrothermal method. Trans. Nonferrous Met. Soc. China 2007, 17, 1334–1338. [Google Scholar] [CrossRef]
- Gu, F.; Li, C.; Hu, Y.; Zhang, L. Synthesis and optical characterization of Co3O4 nanocrystals. J. Cryst. Growth 2007, 304, 369–373. [Google Scholar] [CrossRef]
- Fomekong, R.L.; Tsobnang, P.K.; Magnin, D.; Hermans, S.; Delcorte, A.; Ngolui, J.L. Coprecipitation of nickel zinc malonate: A facile and reproducible synthesis route for Ni1− xZnxO nanoparticles and Ni1− xZnxO/ZnO nanocomposites via pyrolysis. J. Solid State Chem. 2015, 230, 381–389. [Google Scholar] [CrossRef]
- Ansari, F.; Soofivand, F.; Salavati-Niasari, M. Utilizing maleic acid as a novel fuel for synthesis of PbFe12O19 nanoceramics via sol–gel auto-combustion route. Mater. Charact. 2015, 103, 11–17. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, V. Synthesis and characterization of copper succinate and copper oxide nanoparticles by electrochemical treatment: Optimization by Taguchi robust analysis. Can J. Chem. Eng. 2016, 94, 1322–1327. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Jović Orsini, N.; Babić-Stojić, B.; Spasojević, V.; Calatayud, M.P.; Cvjetićanin, N.; Goya, G.F. Magnetic and power absorption measurements on iron oxide nanoparticles synthesized by thermal decomposition of Fe(acac)3. J. Magn. Magn. Mater. 2018, 449, 286–296. [Google Scholar] [CrossRef]
- Kamta, H.; Kenfack, T.P.; Lontio, F.R.; Etape, E.; Joy, P.; Delcorte, A.; Lambi, J. Structural characterization and magnetic properties of undoped and copper-doped cobalt ferrite nanoparticles prepared by the octanoate coprecipitation route at very low dopant concentrations. RSC Adv. 2018, 8, 38621–38630. [Google Scholar] [CrossRef] [Green Version]
- Borel, N.N.M.; Foba-Tendo, J.; Yufanyi, D.M.; Etape, E.P.; Eko, J.N.; Ngolui, L.J. Averrhoa carambola: A renewable source of oxalic acid for the facile and green synthesis of divalent metal (Fe, Co, Ni, Zn, and Cu) oxalates and oxide nanoparticles. J. Appl. Chem. 2014, 2014, 9. [Google Scholar]
- Sharma, J.K.; Srivastava, P.; Singh, G.; Akhtar, M.S.; Ameen, S. Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater. Sci. Eng. B 2015, 193, 181–188. [Google Scholar] [CrossRef]
- Vani, P.; Manikandan, N.; Vinitha, G. A green strategy to synthesize environment friendly metal oxide nanoparticles for potential applications: A review. Asian J. Pharm. Clin. Res. 2017, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Das, R.K.; Golder, A.K. Co3O4 spinel nanoparticles decorated graphite electrode: Bio-mediated synthesis and electrochemical H2O2 sensing. Electrochim. Acta 2017, 251, 415–426. [Google Scholar] [CrossRef]
- Saeed, M.; Akram, N.; Naqvi, S.A.R.; Usman, M.; Abbas, M.A.; Adeel, M.; Nisar, A. Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity. Green Process. Synth. 2019, 8, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Kaviyarasu, K.; Ramalingam, R.J.; Al-Lohedan, H.A. Green synthesis of Co3O4 nanorods for highly efficient catalytic, photocatalytic, and antibacterial activities. Int. J. Nanosci. Nanotechnol. 2019, 19, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Bilal, M.; Iqbal, H. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal. Agric. Biotechnol. 2019, 19, 101154. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Huang, Y.-H.; Chen, H.-J.; Lee, W.-C.; Chiu, H.-W.; Maity, J.P.; Chen, C.-C.; Kuo, Y.-H.; Chen, C.-Y. Green synthesis of nano-Co3O4 by Microbial Induced Precipitation (MIP) process using Bacillus pasteurii and its application as supercapacitor. Mater. Today Commun. 2018, 14, 302–311. [Google Scholar] [CrossRef]
- Koyyati, R.; Kudle, K.R.; Padigya, P.R.M. Evaluation of antibacterial and cytotoxic activity of green synthesized cobalt nanoparticles using Raphanus sativus var. longipinnatus leaf extract. Int. J. Pharmtech Res. 2016, 9, 466–472. [Google Scholar]
- Berger, K.G. Palm kernel oil. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4322–4324. [Google Scholar]
- Kapseu, C. Production, analyse et applications des huiles végétales en Afrique. Oléagineux Corps Gras Lipides 2009, 16, 215–229. [Google Scholar] [CrossRef]
- Pantzaris, T.; Ahmad, M.J. Properties and utilization of palm kernel oil. Palm Oil Dev. 2001, 35, 19–23. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hassim, N.A.M.; Dian, L. Usage of palm oil, palm kernel oil and their fractions as confectionery fats. J. Oil Palm Res. 2017, 29, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-H.; Dai, L.-Z.; Lu, D.-S.; Peng, S.-Y. Characterization of copper cobalt mixed oxide. J. Solid State Chem. 1990, 89, 167–173. [Google Scholar] [CrossRef]
- Pan, K.-L.; Overstreet, W.C.; Robinson, K.; Hubert, A.E.; Crenshaw, G.L. Equivalent Uranium and Selected Minor Elements in Magnetic Concentrates from the Candle Quadrangle, Solomon Quadrangle, and Elsewhere in Alaska. 1980. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCALGEODEBRGM8220230354 (accessed on 9 October 2021).
- Angelov, S.; Zhecheva, E.; Petrov, K.; Menandjiev, D. The properties of a spinel copper cobaltite prepared at low temperatures and normal pressure. Mater. Res. Bull. 1982, 17, 235–240. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, H.K.; Park, Y.R.; Ahn, G.Y.; Kim, C.S.; Park, J.Y. Magnetic and optical properties of spinel FexCo3−xO4 thin films. J. Magn. Magn. Mater. 2006, 300, 300–305. [Google Scholar] [CrossRef]
- Gawali, S.R.; Gandhi, A.C.; Gaikwad, S.S.; Pant, J.; Chan, T.S.; Cheng, C.L.; Ma, Y.R.; Wu, S.Y. Role of cobalt cations in short range antiferromagnetic Co3O4 nanoparticles: A thermal treatment approach to affecting phonon and magnetic properties. Sci. Rep. 2018, 8, 249. [Google Scholar] [CrossRef]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 Nanoparticles: Synthesis, Characterizations, and Raman Spectroscopy. J. Nanomater. 2013, 2013, 714853. [Google Scholar] [CrossRef] [Green Version]
- Blakemore, J.D.; Gray, H.B.; Winkler, J.R.; Müller, A.M. Co3O4 Nanoparticle Water-Oxidation Catalysts Made by Pulsed-Laser Ablation in Liquids. ACS Catal. 2013, 3, 2497–2500. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Artyushkova, K.; Levendosky, S.; Atanassov, P.; Fulghum, J. XPS Structural Studies of Nano-composite Non-platinum Electrocatalysts for Polymer Electrolyte Fuel Cells. Top. Catal. 2007, 46, 263–275. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Cook, M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Casella, I.G.; Guascito, M.R. Anodic electrodeposition of conducting cobalt oxyhydroxide films on a gold surface. XPS study and electrochemical behaviour in neutral and alkaline solution. J. Electroanal. Chem. 1999, 476, 54–63. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Maltha, A.; Reintjes, J.G.H.; Drimal, J.; Ponec, V.; Brongersma, H.H. The surface of catalytically active spinels. J. Catal. 1994, 147, 294–300. [Google Scholar] [CrossRef]
- Shelef, M.; Wheeler, M.A.Z.; Yao, H.C. Ion scattering spectra from spinel surfaces. Surf. Sci. 1975, 47, 697–703. [Google Scholar] [CrossRef]
- Brundle, C.; Chuang, T.; Wandelt, K. Core and valence level photoemission studies of iron oxide surfaces and the oxidation of iron. Surf. Sci. 1977, 68, 459–468. [Google Scholar] [CrossRef]
- Graat, P.C.; Somers, M.A. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra. Appl. Surf. Sci. 1996, 100, 36–40. [Google Scholar] [CrossRef]
- Delcorte, A.; Leblanc, C.; Poleunis, C.; Hamraoui, K. Computer Simulations of the Sputtering of Metallic, Organic, and Metal–Organic Surfaces with Bin and C60 Projectiles. J. Phys. Chem. C 2013, 117, 2740–2752. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Y.; Zhai, T.; Guo, Y.; Chen, S.; Ma, Y.; Yao, J.; Bando, Y.; Golberg, D. Multishelled Co3O4-Fe3O4 hollow spheres with even magnetic phase distribution: Synthesis, magnetic properties and their application in water treatment. J. Mater. Chem. 2011, 21, 17680–17687. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Zhao, H.; Chen, D.; Liu, F.; Xiang, J.; Hu, Z.; Li, Y. Facile shape control of Co3O4 and the effect of the crystal plane on electrochemical performance. Adv. Mater. 2012, 24, 5762–5766. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-P.; Miao, H.-J.; Zhao, H.; Xu, Z. Synthesis, characterization and magnetic properties of Co3O4 nanotubes. Appl. Phys. A 2008, 91, 47–51. [Google Scholar] [CrossRef]
- Wang, R.; Liu, C.; Zhang, H.; Chen, C.; Guo, L.; Xu, H.; Yang, S. Porous nanotubes of Co3O4: Synthesis, characterization, and magnetic properties. Appl. Phys. Lett. 2004, 85, 2080–2082. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhou, J.F.; Mullarkey, D.; O’Connell, R.; Schmitt, W.; Venkatesan, M.; Coey, M.; Zhang, H.Z. Synthesis, characterization and magnetic properties of ultrafine Co3O4 octahedra. AIP Adv. 2015, 5, 087122. [Google Scholar] [CrossRef] [Green Version]




| Reagents (g) | S0 | S1 | S2 | S3 | S4 | S10 | S20 | S30 |
|---|---|---|---|---|---|---|---|---|
| CoCl2.6H2O | 0.602 | 0.593 | 0.584 | 0.576 | 0.567 | 0.516 | 0.438 | 0.367 |
| Fe(NO3)3·9H2O | 0 | 0.010 | 0.020 | 0.030 | 0.040 | 0.097 | 0.186 | 0.267 |
| S’0 | S’1 | S’2 | S’3 | S’4 | S’10 | S’20 | S’30 | |
| Co(NO3)2·6H2O | 0.243 | 0.241 | 0.238 | 0.236 | 0.233 | 0.219 | 0.194 | 0.170 |
| Fe(NO3)3.9H2O | 0.000 | 0.003 | 0.007 | 0.010 | 0.013 | 0.034 | 0.067 | 0.101 |
| Elements | S0/S’0 | S1/S’1 | S2/S’2 | S3/S’3 | S4/S’4 | S10/S’10 | S20/S’20 | S30/S’30 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Found experimentally | First synthesis route | Fe/Co | 0.000 | 0.0103 | 0.0210 | 0.0319 | 0.0428 | 0.113 | 0.247 | 0.414 |
| Second synthesis route | Fe/Co | 0.000 | 0.0116 | 0.0200 | 0.0315 | 0.0396 | 0.0974 | 0.219 | 0.440 | |
| calculated | Fe/Co | 0.000 | 0.0101 | 0.0204 | 0.0309 | 0.0417 | 0.111 | 0.250 | 0.429 | |
| Acids | Palm Kernel Oil (%) | Metallo-Organic Precursors (%) |
|---|---|---|
| Lauric acid, C12:0 | 47.16 | 52.75 |
| Myristic acid, C14:0 | 16.89 | 18.60 |
| Oleic acid, C18:1; cis9 | 13.90 | 11.05 |
| Palmitic acid, C16:0 | 8.48 | 9.35 |
| Caprilic acid, C8:0 | 4.11 | 1.34 |
| Capric acid, C10:0 | 3.59 | 2.85 |
| Linoleic acid, C18:2; C9C12 | 2.29 | 0.22 |
| Stearic acid, C18:0 | 2.79 | 2.91 |
| Caproic acid, C6:0 | 0.36 | 0.26 |
| Arachidic acid, C20:0 | 0.12 | 0.12 |
| Oleic acid C18:1; cis11 | 0.11 | 0.09 |
| Tridecylic acid C13:0 | 0.10 | 0.12 |
| Arachidic acid C20:1; C11 | 0.08 | 0.05 |
| Pentadecylic acid C15:0 | 0.02 | 0.03 |
| Oleic acid C18:1; trans9 | 0.00 | 0.10 |
| Arachidic acid C20:2; C11, C14 | 0.00 | 0.10 |
| 1st Synthesis Method | 2nd Synthesis Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Elements | S0 | S10 | S20 | S30 | S’0 | S’10 | S’20 | S’30 | |
| Calculated | Fe/Co | 0 | 0.11 | 0.25 | 0.43 | 0 | 0.11 | 0.25 | 0.43 |
| ICP results | Fe/Co | 0 | 0.11 | 0.25 | 0.41 | 0 | 0.12 | 0.24 | 0.48 |
| XPS results | Fe/Co | 0 | 0.12 | 0.27 | 0.65 | 0 | 0.18 | 0.64 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngnintedem Yonti, C.; Kenfack Tsobnang, P.; Lontio Fomekong, R.; Devred, F.; Mignolet, E.; Larondelle, Y.; Hermans, S.; Delcorte, A.; Lambi Ngolui, J. Green Synthesis of Iron-Doped Cobalt Oxide Nanoparticles from Palm Kernel Oil via Co-Precipitation and Structural Characterization. Nanomaterials 2021, 11, 2833. https://doi.org/10.3390/nano11112833
Ngnintedem Yonti C, Kenfack Tsobnang P, Lontio Fomekong R, Devred F, Mignolet E, Larondelle Y, Hermans S, Delcorte A, Lambi Ngolui J. Green Synthesis of Iron-Doped Cobalt Oxide Nanoparticles from Palm Kernel Oil via Co-Precipitation and Structural Characterization. Nanomaterials. 2021; 11(11):2833. https://doi.org/10.3390/nano11112833
Chicago/Turabian StyleNgnintedem Yonti, Cedrik, Patrice Kenfack Tsobnang, Roussin Lontio Fomekong, Francois Devred, Eric Mignolet, Yvan Larondelle, Sophie Hermans, Arnaud Delcorte, and John Lambi Ngolui. 2021. "Green Synthesis of Iron-Doped Cobalt Oxide Nanoparticles from Palm Kernel Oil via Co-Precipitation and Structural Characterization" Nanomaterials 11, no. 11: 2833. https://doi.org/10.3390/nano11112833
APA StyleNgnintedem Yonti, C., Kenfack Tsobnang, P., Lontio Fomekong, R., Devred, F., Mignolet, E., Larondelle, Y., Hermans, S., Delcorte, A., & Lambi Ngolui, J. (2021). Green Synthesis of Iron-Doped Cobalt Oxide Nanoparticles from Palm Kernel Oil via Co-Precipitation and Structural Characterization. Nanomaterials, 11(11), 2833. https://doi.org/10.3390/nano11112833








