A Novel and Effective Recyclable BiOCl/BiOBr Photocatalysis for Lignin Removal from Pre-Hydrolysis Liquor
Abstract
:1. Introduction
2. Methods
2.1. Materials
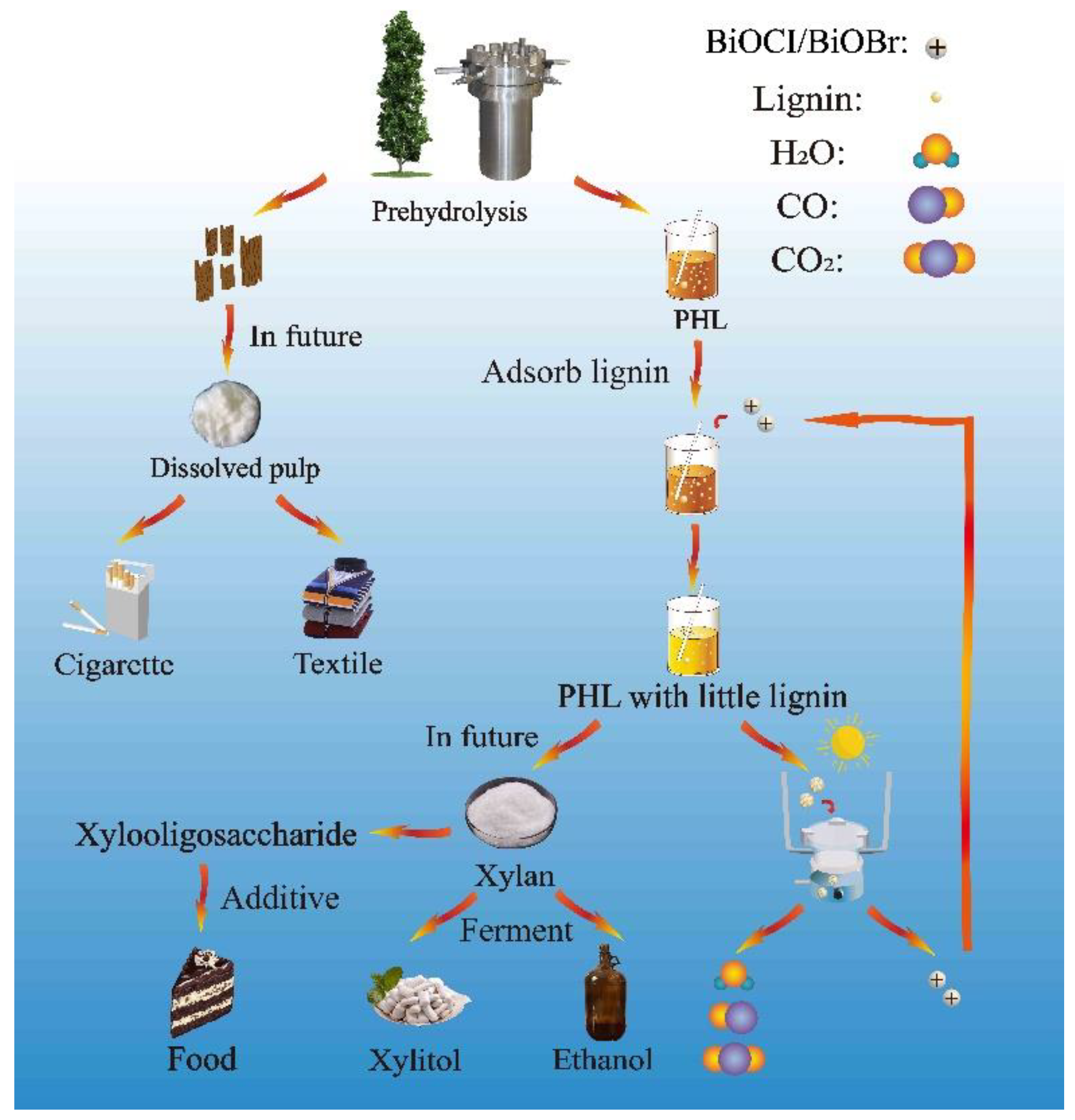
2.1.1. PHL Preparation
2.1.2. Synthesis of BiOCl/BiOBr
2.1.3. BiOCl/BiOBr Treatment of PHL
2.1.4. Photocatalytic Degradation of Lignin
2.1.5. Cycle Experiments of BiOCl/BiOBr
2.1.6. Characterization of BiOCl/BiOBr
2.1.7. Zeta Potentials and pH Values
2.1.8. Analysis of PHL
3. Results and Discussion
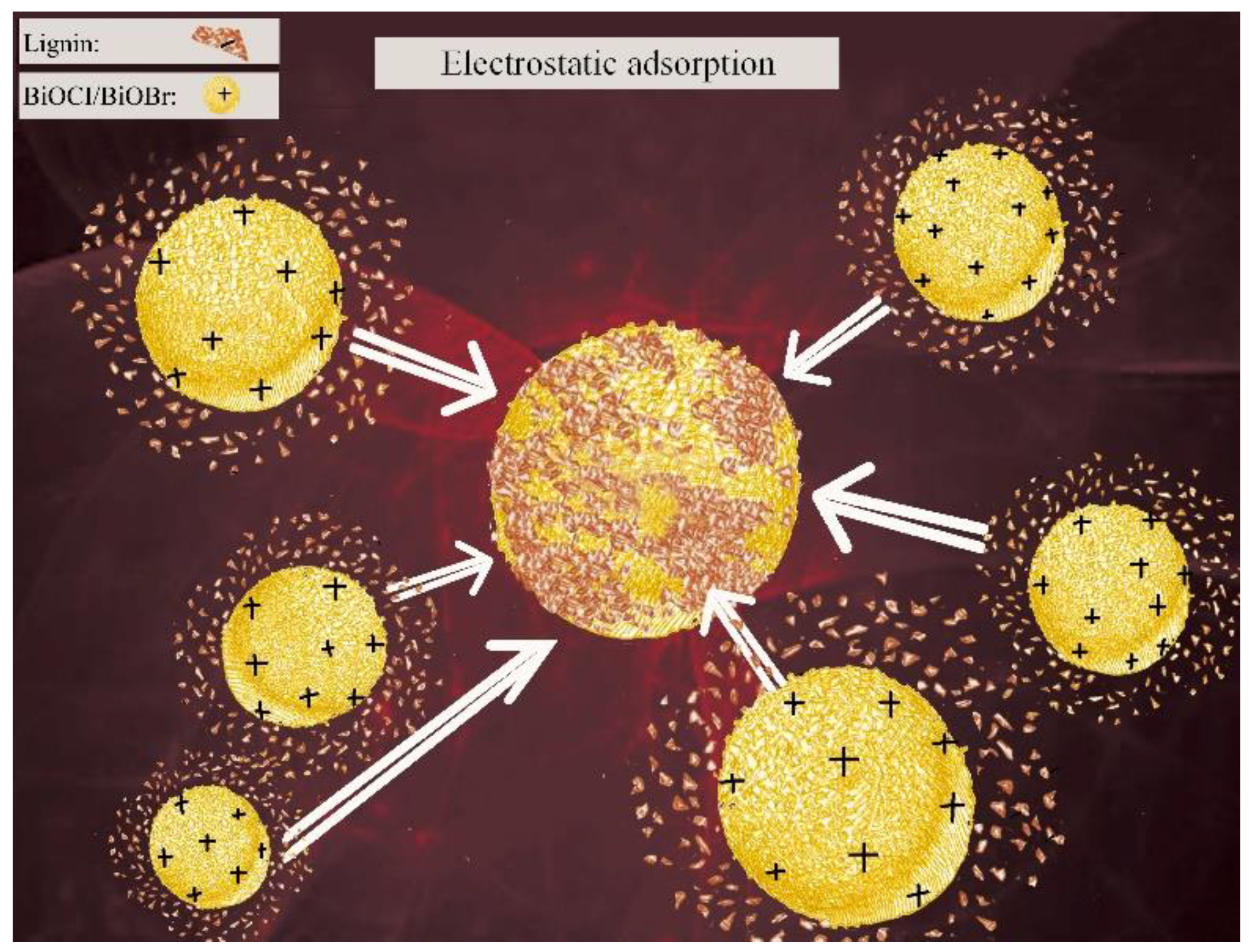
3.1. A Novel Lignin Removal Method by Electrostatic Adsorption
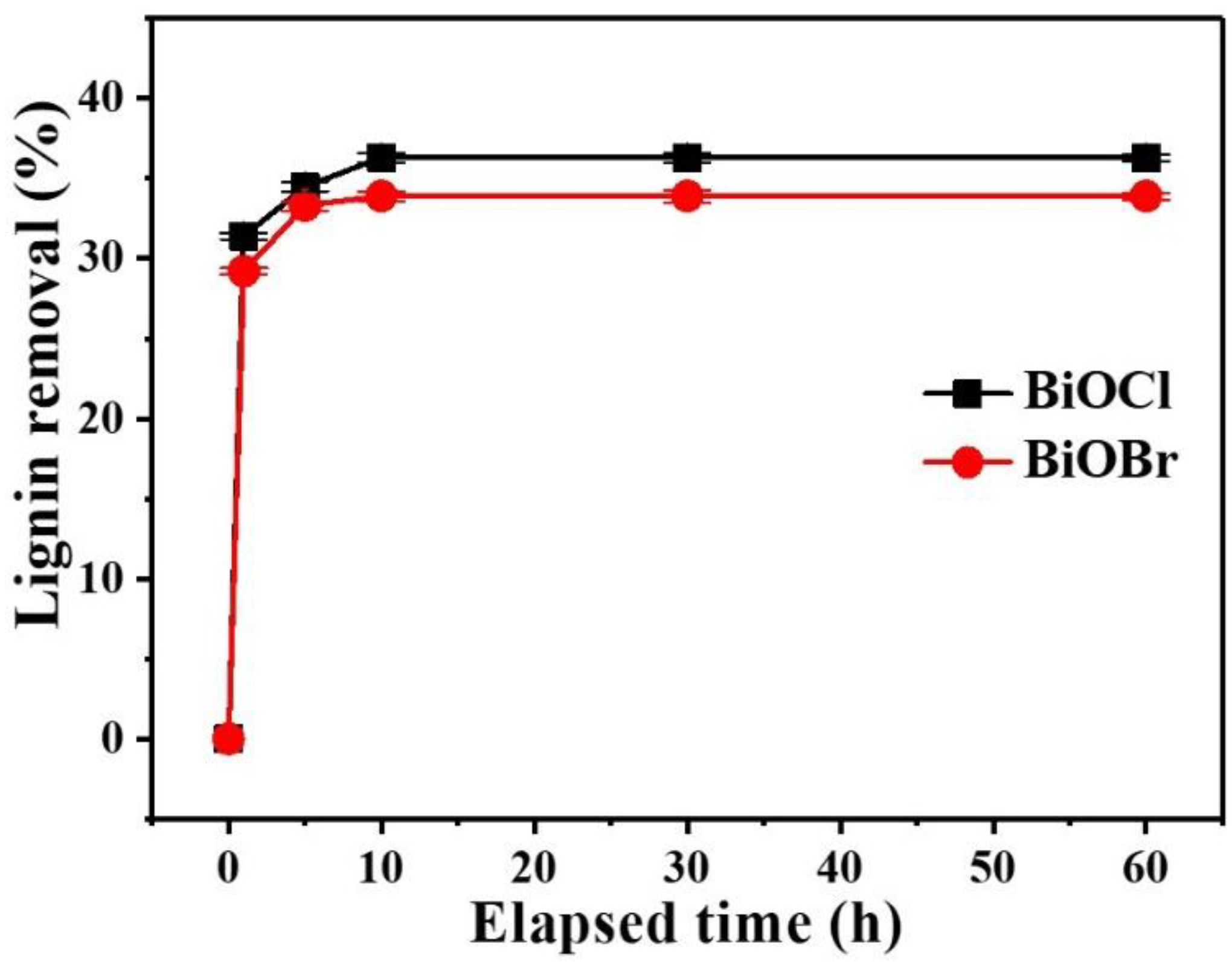
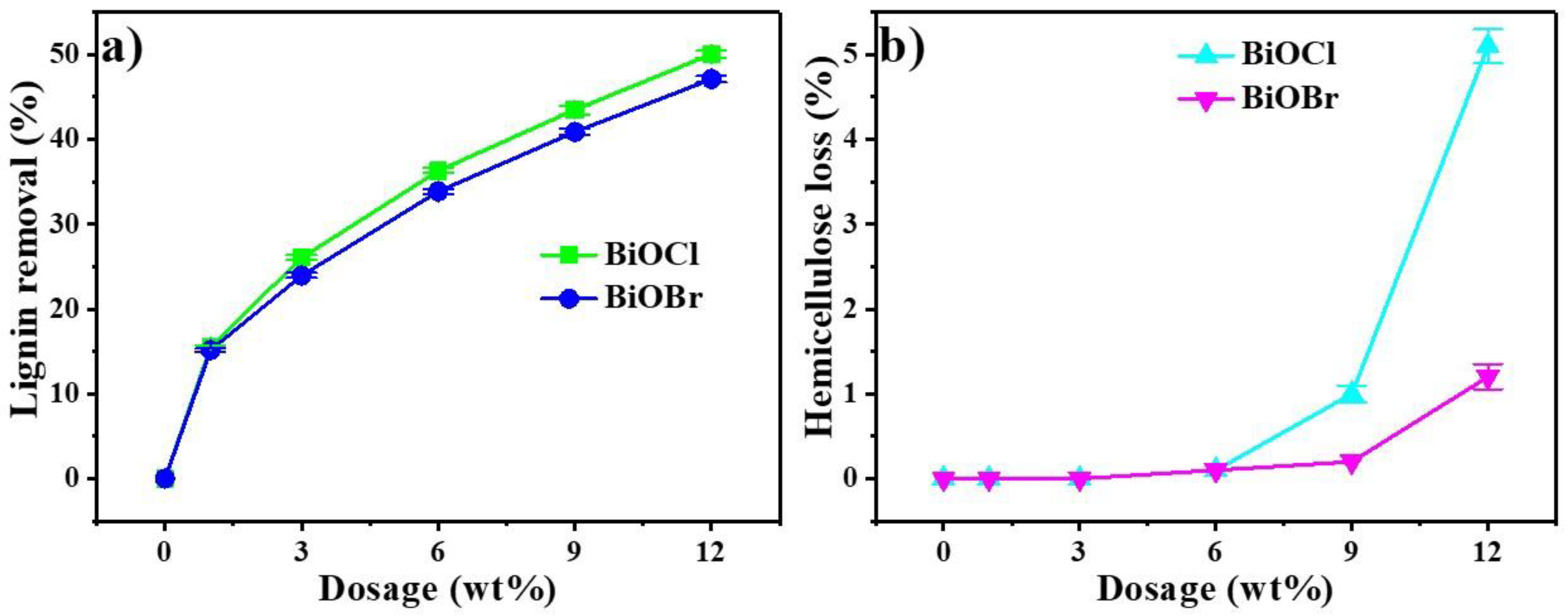
3.2. Optimization of BiOCl/BiOBr Treatment Conditions
3.3. Affinity of Lignin to BiOCl/BiOBr
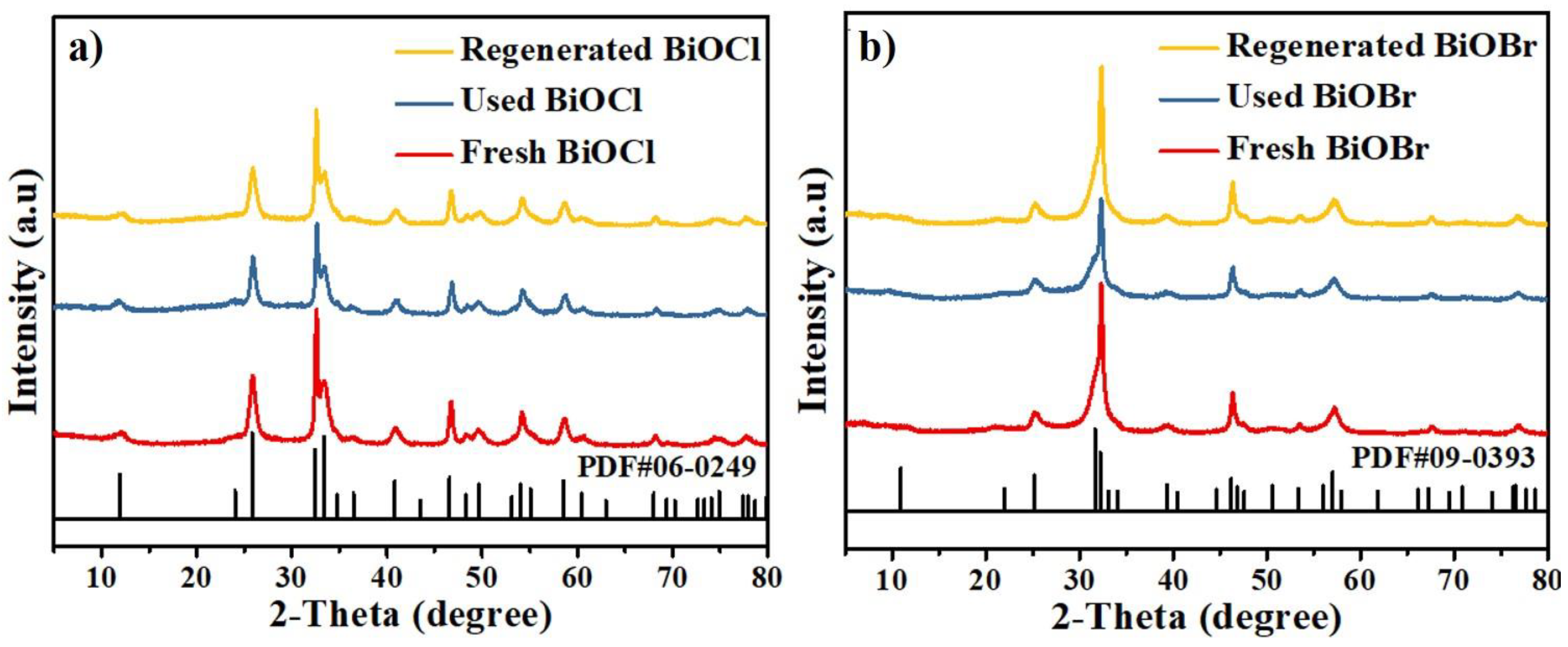
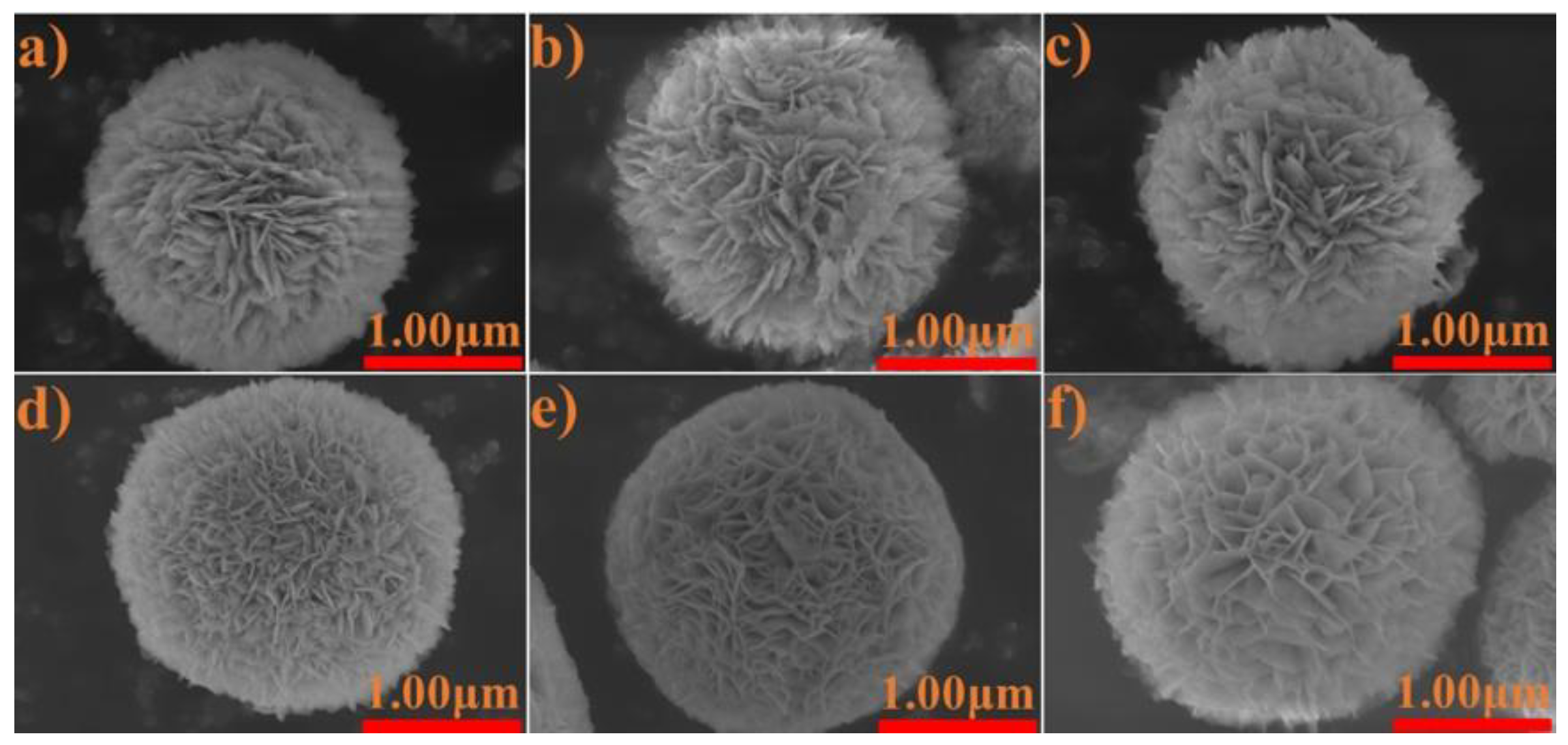
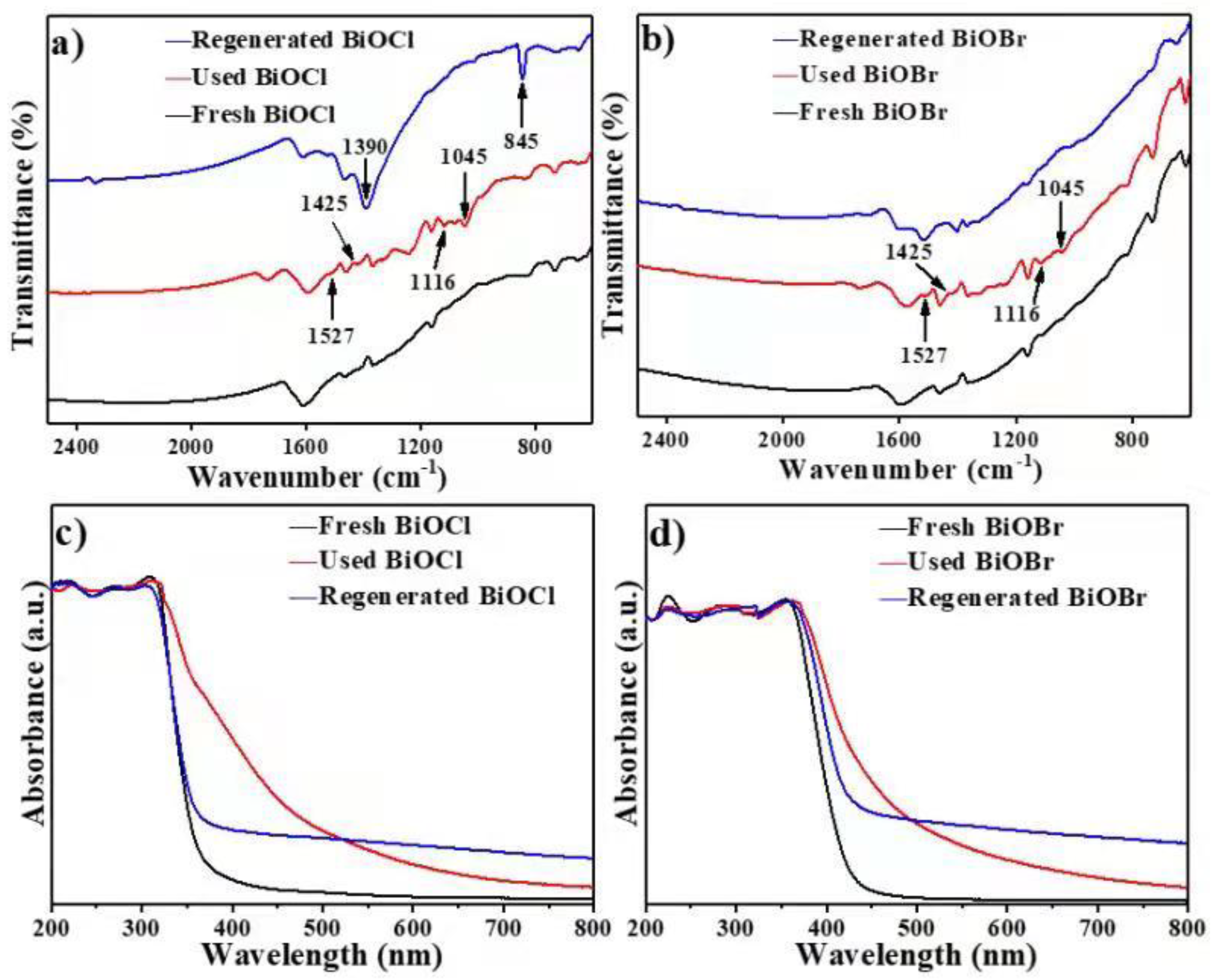
3.4. Characterization of BiOCl/BiOBr
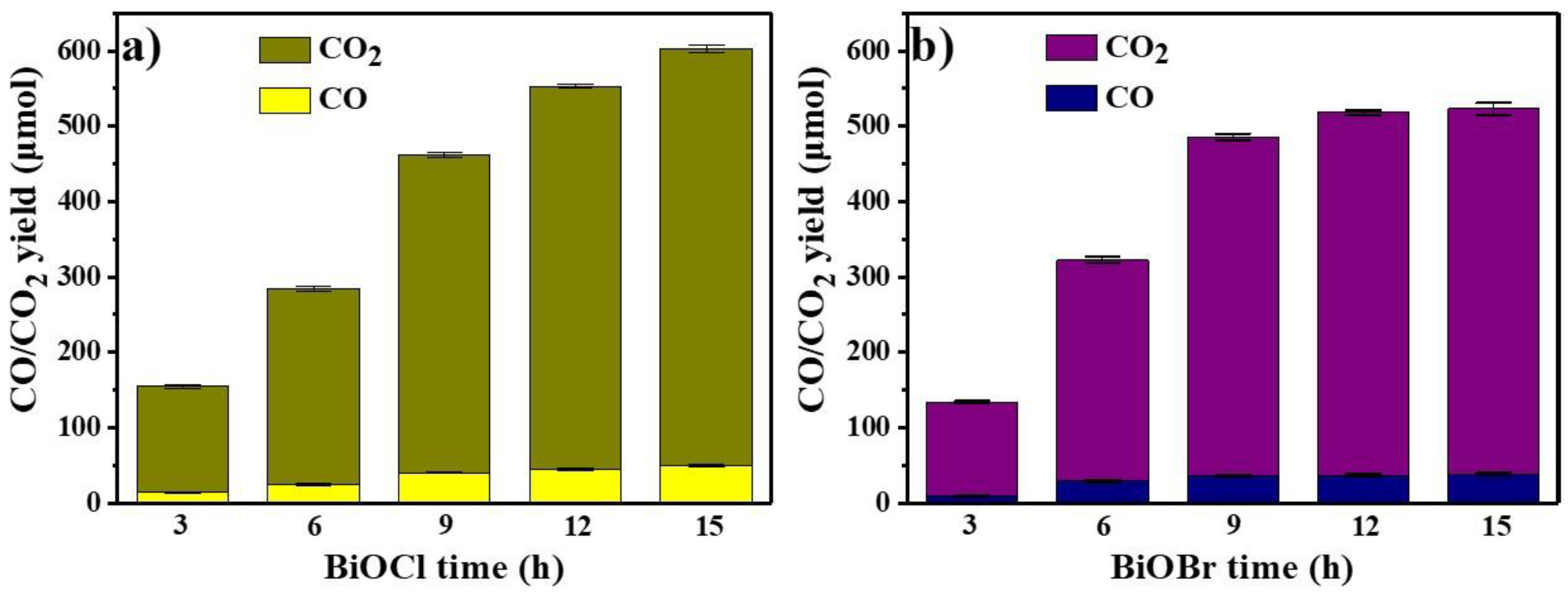
3.5. Photocatalytic Degradation of Lignin
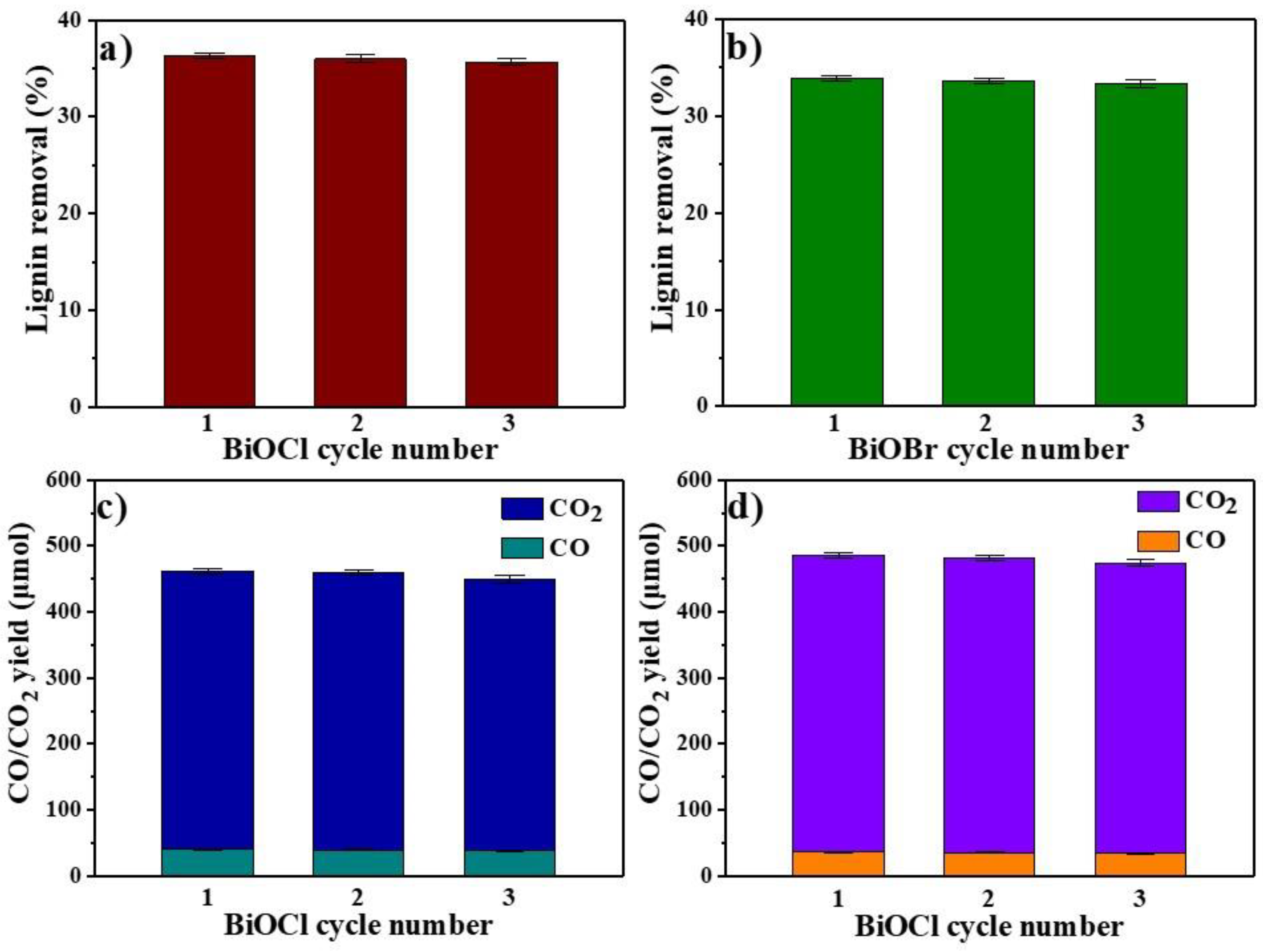
3.6. Cycle Performance of BiOCl/BiOBr
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saritha, M.; Arora, A. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Biochem. 2012, 52, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Ana, B.; Brigita, H.; Miha, G.; Uroš, N.; Blaž, L. A review of sustainable lignocellulose biorefining applying (natural) deep eutectic solvents (DESs) for separations, catalysis and enzymatic biotransformation processes. Chem. Eng. 2020, 2019, 77. [Google Scholar]
- Budnyak, T.M.; Onwumere, J.; Pylypchuk, I.V.; Jaworski, A.; Chen, J.H.; Rokicińska, A.; Lindström, M.E.; Kuśtrowski, P.; Sevastyanova, O.; Slabon, A. LignoPhot: Photoactive Hybrid Lignin/Bi4O5Br2/BiOBr Composite for Complex Pollutants Removal. 2021. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/60c753d3bdbb896669a3a515 (accessed on 18 October 2021).
- Miao, Q.X.; Chen, L.H.; Huang, L.L.; Tian, C.; Zheng, L.Q.; Ni, Y.H. A process for enhancing the accessibility and reactivity of hardwood kraft-based dissolving pulp for viscose rayon production by cellulase treatment. Bioresour. Technol. 2014, 154, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, H.; Nairy, A.; Jahan, M.S.; Yang, G.; Ni, Y.H. Viscosity of prehydrolysis liquor of a hardwood kraft-based dissolving pulp production process. Ind. Eng. Chem. Res. 2013, 52, 3974–3979. [Google Scholar] [CrossRef]
- Shen, J.; Fatehi, P.; Soleimani, P.; Ni, Y.H. Recovery of lignocelluloses from pre-hydrolysis liquor in the lime kiln of kraft-based dissolving pulp production process by adsorption to lime mud. Bioresour. Technol. 2011, 102, 10035–10039. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Fatehi, P.; Chen, J. Extraction of Technical Lignins from Pulping Spent Liquors, Challenges and Opportunities, Production of Biofuels and Chemicals from Lignin; Springer: Singapore, 2016; pp. 35–54. [Google Scholar]
- Yedro, F.M.; Grénman, H.; Rissanen, J.V.; Salmi, T.; García-Serna, J.; Cocero, M.J. Chemical composition and extraction kinetics of Holm oak (Quercus ilex) hemicelluloses using subcritical water. J. Supercrit. Fluids 2017, 129, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Hahn-Hägerdal, B.; Karhumaa, K.; Fonseca, C.; Spencer-Martins, I.; Gorwa-Grauslund, M.F. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 2007, 74, 937–953. [Google Scholar] [CrossRef]
- Liu, Z.; Fatehi, P.; Jahan, M.S.; Ni, Y.H. Separation of lignocellulosic materials by combined processes of pre-hydrolysis and ethanol extraction. Bioresour. Technol. 2011, 102, 1264–1269. [Google Scholar] [CrossRef]
- Shi, H.; Fatehi, P.; Xiao, H.; Ni, Y.H. A combined acidification/PEO flocculation process to improve the lignin removal from the pre-hydrolysis liquor of kraft-based dissolving pulp production process. Bioresour. Technol. 2011, 102, 5177–5182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, H.; Hou, J.P.; Jia, C.M.; Yao, S.J. Systematic isolation and utilization of lignocellulosic components from sugarcane bagasse. Bioresour. Technol. 2013, 48, 2217–2224. [Google Scholar] [CrossRef]
- Shen, J.; Fatehi, P.; Soleimani, P.; Ni, Y.H. Lime treatment of prehydrolysis liquor from the kraft-based dissolving pulp production process. Ind. Eng. Chem. Res. 2012, 51, 662–667. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Fu, Y.; Yuan, Z.; Qin, M.J. Saccharide separation from wood prehydrolysis liquor: Comparison of selectivity toward non-saccharide compounds with separate techniques. RSC Adv. 2015, 5, 28925–28931. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuang, J.; Wang, X.; Li, Z.; Fu, Y.; Qin, M.H. Limited adsorption selectivity of active carbon toward non-saccharide compounds in lignocellulose hydrolysate. Bioresour. Technol. 2016, 208, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jahan, M.S.; Liu, H.; Ni, Y.H. Acid hydrolysis of prehydrolysis liquor produced from the kraft-based dissolving pulp production process. Ind. Eng. Chem. Res. 2012, 51, 13902–13907. [Google Scholar] [CrossRef]
- Liu, S.; He, H.; Fu, X.; Yuan, T.; Wang, Q.; Yang, G.; Zhang, H.; Ding, M.; Liao, C.L. Xylitol Production from Prehydrolysis Liquor of Kraft-based Dissolving Pulp by Candida tropicalis. Bioresources 2019, 14, 21–30. [Google Scholar]
- Wang, X.; Zhuang, J.; Jiang, J.; Fu, Y.; Qin, M.; Wang, Z.J. Separation and purification of hemicellulose-derived saccharides from wood hydrolysate by combined process. Bioresour. Technol. 2015, 196, 426–430. [Google Scholar] [CrossRef]
- Shi, H.; Fatehi, P.; Xiao, H.; Ni, Y.H. Optimizing the polyethylene oxide flocculation process for isolating lignin of prehydrolysis liquor of a kraft-based dissolving pulp production process. Ind. Eng. Chem. Res. 2012, 51, 5330–5335. [Google Scholar] [CrossRef]
- Duarte, G.; Ramarao, B.; Amidon, T.J. Polymer induced flocculation and separation of particulates from extracts of lignocellulosic materials. Bioresour. Technol. 2010, 101, 8526–8534. [Google Scholar] [CrossRef]
- Hu, R.; Lin, L.; Liu, T.; Liu, S.J. Dilute sulfuric acid hydrolysis of sugar maple wood extract at atmospheric pressure. Bioresour. Technol. 2010, 101, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Q.; Si, C.L.; Wang, Z.; Huo, D.; Hong, Y.; Li, Z.J. Recovery of oligosaccharides from prehydrolysis liquors of poplar by microfiltration/ultrafiltration membranes and anion exchange resin. ACS Sustain. Chem. Eng. 2016, 4, 937–943. [Google Scholar] [CrossRef]
- Zhuang, J.; Xu, J.; Wang, X.; Li, Z.; Han, W.; Wang, Z.J. Improved microfiltration of prehydrolysis liquor of wood from dissolving pulp mill by flocculation treatments for hemicellulose recovery. Sep. Purif. Technol. 2017, 176, 159–163. [Google Scholar] [CrossRef]
- Wang, Q.; Jahan, M.S.; Liu, S.; Miao, Q.; Ni, Y.H. Lignin removal enhancement from prehydrolysis liquor of kraft-based dissolving pulp production by laccase-induced polymerization. Bioresour. Technol. 2014, 164, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.S.; Yang, G.H.; Chen, J.C. Modeling laccase-induced lignin removal in prehydrolysis liquor from kraft-based dissolving pulp production. Bioresour. Technol. 2015, 175, 638–641. [Google Scholar] [CrossRef]
- Yang, D.; Chang, Y.; Wu, X.; Qiu, X.; Lou, H.J. Modification of sulfomethylated alkali lignin catalyzed by horseradish peroxidase. RSC Adv. 2014, 4, 53855–53863. [Google Scholar] [CrossRef]
- Li, Z.Q.; Qiu, C.L.; Gao, J.J.; Wang, H.W.; Fu, Y.J.; Qin, M.H. Improving lignin removal from pre-hydrolysis liquor by horseradish peroxidase-catalyzed polymerization. Sep. Purif. Technol. 2019, 212, 273–279. [Google Scholar] [CrossRef]
- Xu, F.; Chen, J.C.; Yang, G.H.; Ji, X.X.; Wang, Q.; Liu, S.S.; Ni, Y.H. Combined treatments consisting of calcium hydroxide and activate cccarbon for purification of xylo-oligosaccharides of pre-hydrolysis liquor. Polymers 2019, 11, 1558. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Dong, J.R.; Yang, G.H.; He, M.; Xu, F.; Fatehi, P. A process for purifying xylosugars of pre-hydrolysis liquor from kraft-based dissolving pulp production process. Biotechnol. Biofuels 2018, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- Gütsch, J.S.; Sixta, H. Research, Regeneration of spent activated charcoals used for lignin removal from prehydrolysis-kraft prehydrolyzates. Ind. Eng. Chem. Res. 2012, 51, 8624–8630. [Google Scholar] [CrossRef]
- Carratalá-Abril, J.; Lillo-Ródenas, M.; Linares-Solano, A.; Cazorla-Amorós, D. Regeneration of activated carbons saturated with benzene or toluene using an oxygen-containing atmosphere. Chem. Eng. Sci. 2010, 65, 2190–2198. [Google Scholar] [CrossRef]
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The role of polarization in photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 10061–10073. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef]
- Jiang, Z.; Liang, X.; Liu, Y.; Jing, T.; Wang, Z.; Zhang, X.; Qin, X.; Dai, Y.; Huang, B. Enhancing visible light photocatalytic degradation performance and bactericidal activity of BiOI via ultrathin-layer structure. Appl. Catal. B 2017, 211, 252–257. [Google Scholar] [CrossRef]
- Mills, A.; LeHunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Sun, W.; Miao, W.K.; Yuan, Z.M.; Yang, G.H.; Kong, F.G.; Yan, T.J.; Chen, J.C.; Huang, B.B.; An, C.H. Living atomically dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.H.; Miao, W.K.; Yuan, Z.M.; Jiang, Z.Y.; Huang, B.B.; Wang, P.; Chen, J.C. Bi quantum dots obtained via in situ photodeposition method as a new photocatalytic CO2 reduction cocatalyst instead of noble metals: Borrowing redox conversion between Bi2O3 and Bi. Appl. Catal. B 2018, 237, 302–308. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zhang, X.H.; Sun, W.; Yang, D.R.; Duchesne, P.N.; Gao, Y.G.; Wang, Z.Y.; Yan, T.J.; Yuan, Z.M.; Yang, G.H.; et al. Building a bridge from papermaking to solar fuels. Angew. Chem. Int. Ed. 2019, 58, 14850–14854. [Google Scholar] [CrossRef]
- Shen, J.; Kaur, I.; Baktash, M.; He, Z.; Ni, Y. A combined process of activated carbon adsorption, ion exchange resin treatment and membrane concentration for recovery of dissolved organics in pre-hydrolysis liquor of the kraft-based dissolving pulp production proce. Bioresour. Technol. 2013, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Kadla, J.F. Hydrogen bonding in lignin: A Fourier transform infrared model compound study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.D.; Wang, L.; Xu, K.; Ren, L.; Wang, L.; Yu, Y.X.; Du, Y.; Hao, W.C. Band-gap engineering of BiOCl with oxygen vacancies for efficient photooxidation properties under visible-light irradiation. J. Mater. Chem. A 2018, 6, 2193–2199. [Google Scholar] [CrossRef]
| Initial pH | Zeta Potential (mV) | |
|---|---|---|
| PHL | 3.48 | −2.84 |
| DI water | 5.50 | −1.02 |
| 1 g/L lignin suspension | 7.31 | −21.2 |
| 1 g/L sugar solution | 5.42 | −2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, J.; Jia, Q.; Jiang, Q.; Yan, J.; Yang, G. A Novel and Effective Recyclable BiOCl/BiOBr Photocatalysis for Lignin Removal from Pre-Hydrolysis Liquor. Nanomaterials 2021, 11, 2836. https://doi.org/10.3390/nano11112836
Zhang S, Chen J, Jia Q, Jiang Q, Yan J, Yang G. A Novel and Effective Recyclable BiOCl/BiOBr Photocatalysis for Lignin Removal from Pre-Hydrolysis Liquor. Nanomaterials. 2021; 11(11):2836. https://doi.org/10.3390/nano11112836
Chicago/Turabian StyleZhang, Shengyu, Jiachuan Chen, Qianqian Jia, Qimeng Jiang, Jiaqiang Yan, and Guihua Yang. 2021. "A Novel and Effective Recyclable BiOCl/BiOBr Photocatalysis for Lignin Removal from Pre-Hydrolysis Liquor" Nanomaterials 11, no. 11: 2836. https://doi.org/10.3390/nano11112836
APA StyleZhang, S., Chen, J., Jia, Q., Jiang, Q., Yan, J., & Yang, G. (2021). A Novel and Effective Recyclable BiOCl/BiOBr Photocatalysis for Lignin Removal from Pre-Hydrolysis Liquor. Nanomaterials, 11(11), 2836. https://doi.org/10.3390/nano11112836






