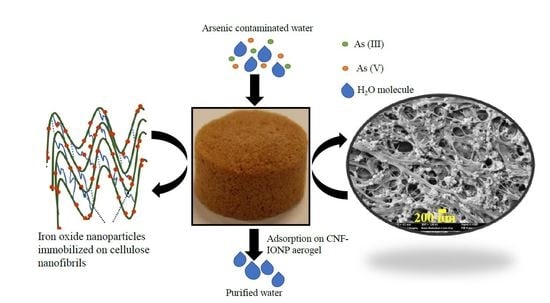
Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mg-Doped Amorphous IONPs
2.3. Preparation of CNF-IONP Adsorbent
2.4. Characterization
2.5. Arsenic Adsorption Experiments
3. Results and Discussion
3.1. Arsenic Adsorption Experiments
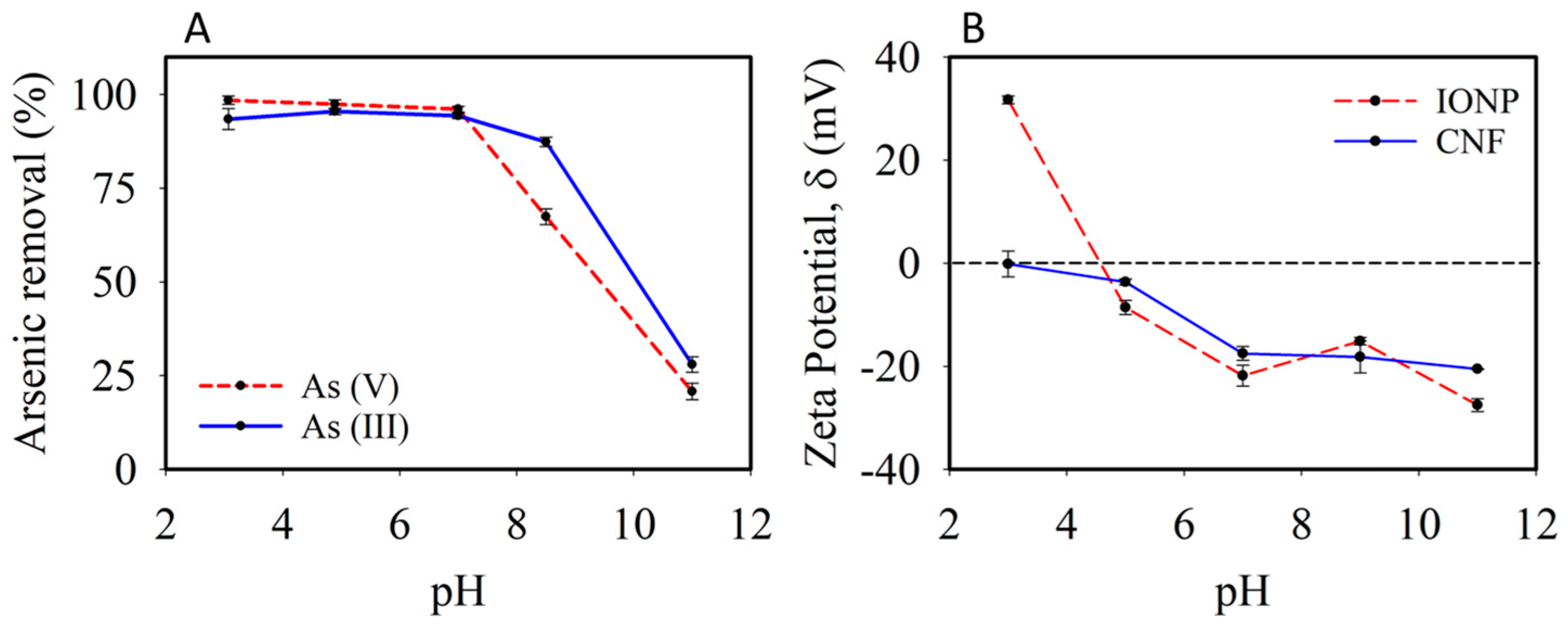
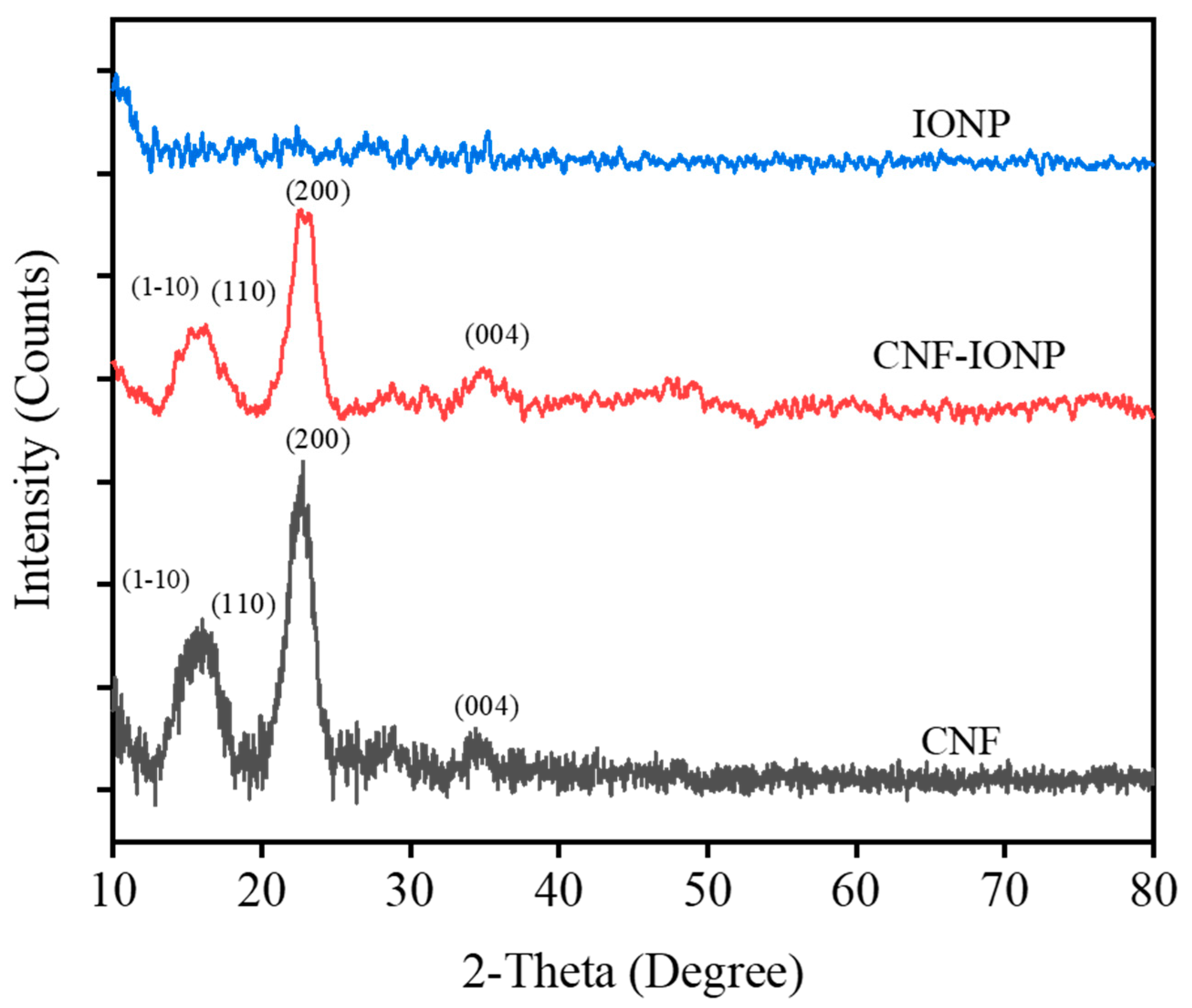
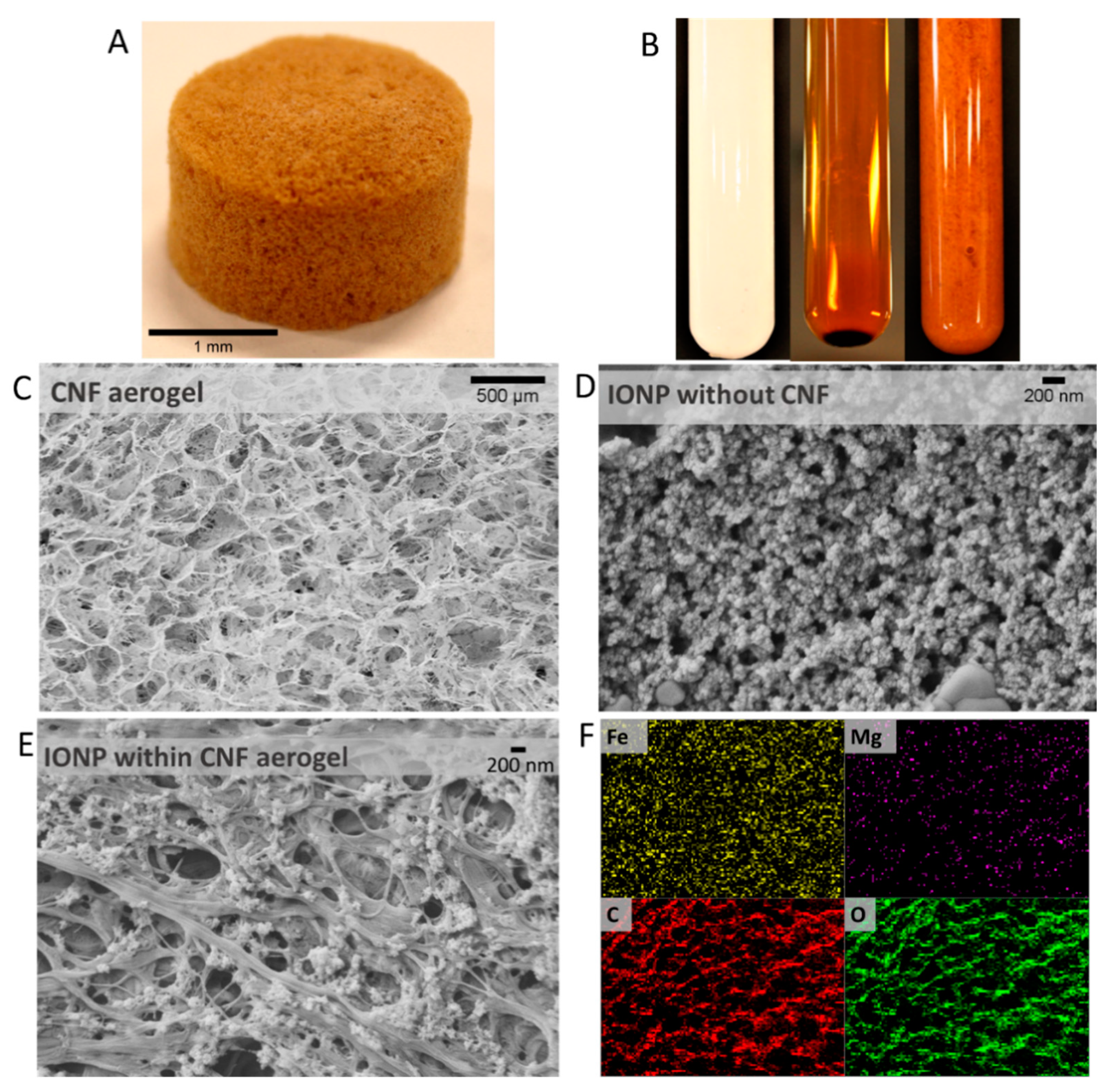
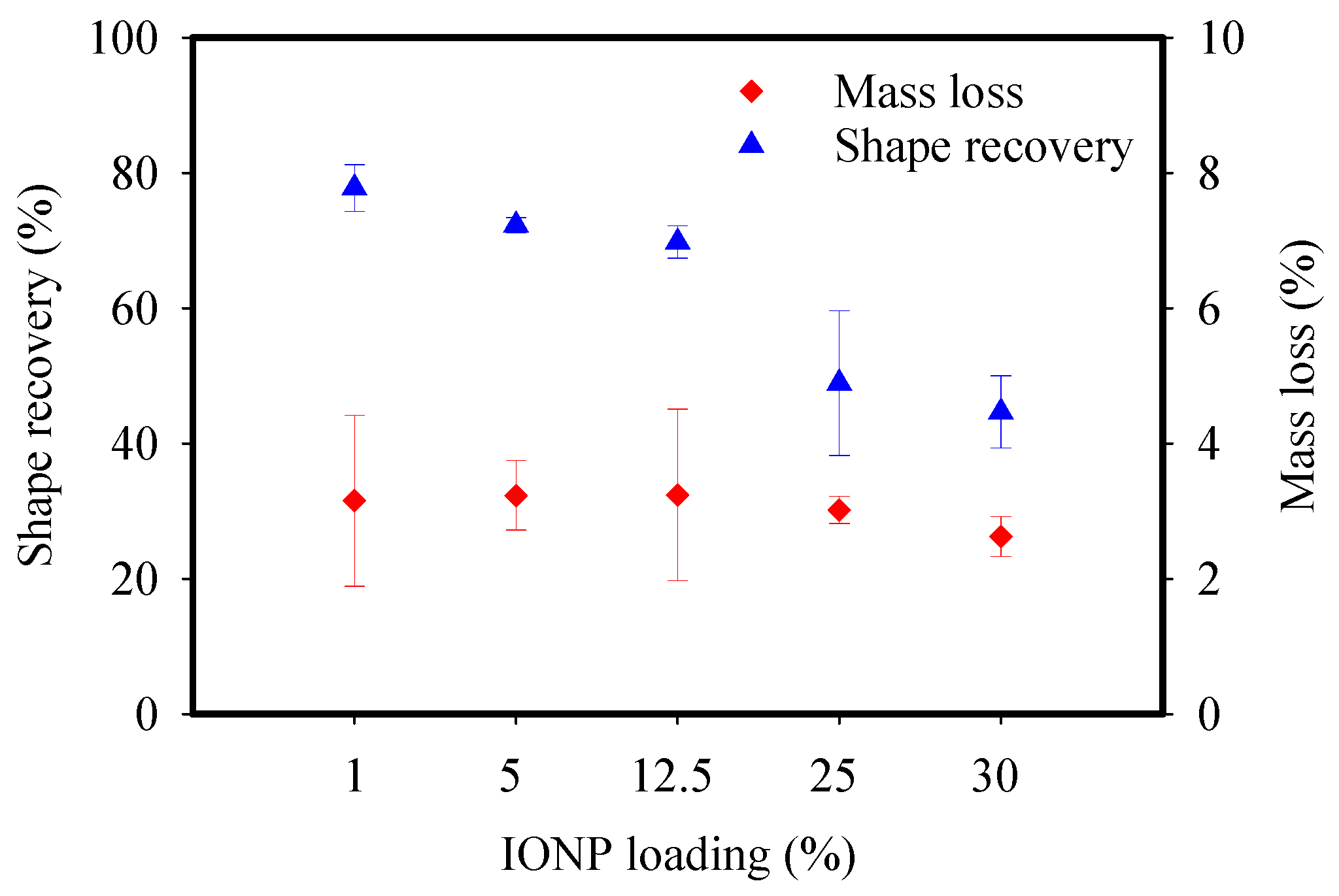
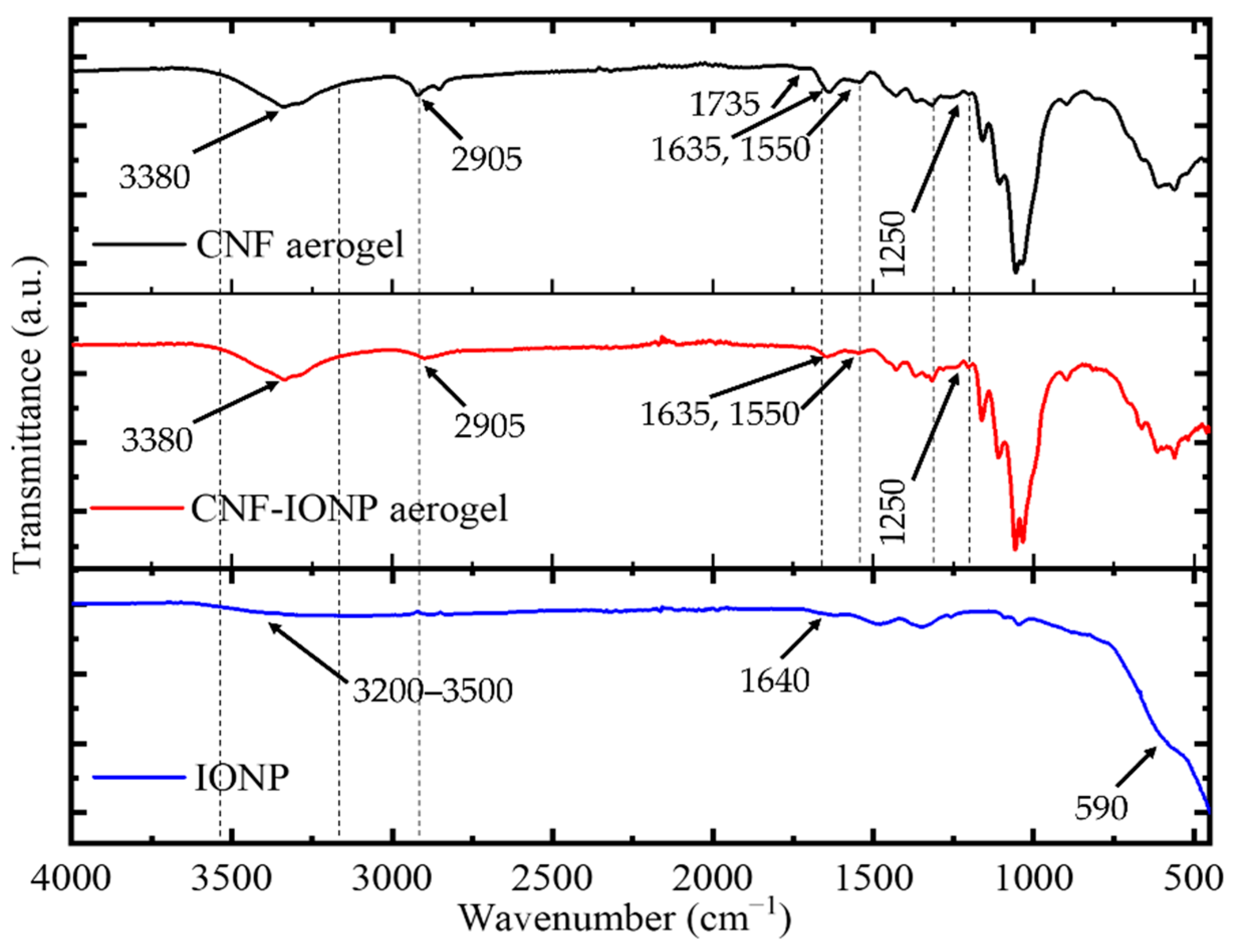
3.2. Characterization of the Adsorbent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shankar, S.; Shanker, U. Shikha Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Nicomel, N.R.; Leus, K.; Folens, K.; van der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Macera, L.; Taglieri, G.; Daniele, V.; Passacantando, M.; D’Orazio, F. Nano-Sized Fe(III) Oxide Particles Starting from an Innovative and Eco-Friendly Synthesis Method. Nanomaterials 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of Two-Line Ferrihydrite to Goethite and Hematite as a Function of pH and Temperature. Environ. Sci. Technol. 2011, 45, 268–275. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, J.; Zhong, W.; Chen, H.-Y. Synthesis of amorphous Fe2O3 nanoparticles by microwave irradiation. Mater. Lett. 2001, 50, 341–346. [Google Scholar] [CrossRef]
- Phu, N.D.; Ngo, D.-T.; Hoang, L.H.; Luong, N.H.; Chau, N.; Hai, N.H. Crystallization process and magnetic properties of amorphous iron oxide nanoparticles. J. Phys. D Appl. Phys. 2011, 44, 345002. [Google Scholar] [CrossRef]
- Baltpurvins, K.A.; Burns, R.C.; Lawrance, G.A.; Stuart, A.D. Effect of Ca2+, Mg2+, and Anion Type on the Aging of Iron(III) Hydroxide Precipitates. Environ. Sci. Technol. 1997, 31, 1024–1032. [Google Scholar] [CrossRef]
- Iacob, M.; Cazacu, M.; Turta, C.; Doroftei, F.; Botko, M.; Čižmár, E.; Zeleňáková, A.; Feher, A. Amorphous iron–chromium oxide nanoparticles with long-term stability. Mater. Res. Bull. 2015, 65, 163–168. [Google Scholar] [CrossRef]
- Bushell, M.; Beauchemin, S.; Kunc, F.; Gardner, D.; Ovens, J.; Toll, F.; Kennedy, D.; Nguyen, K.; Vladisavljevic, D.; Rasmussen, P.E.; et al. Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area. Nanomaterials 2020, 10, 1812. [Google Scholar] [CrossRef] [PubMed]
- Casentini, B.; Gallo, M.; Baldi, F. Arsenate and arsenite removal from contaminated water by iron oxides nanoparticles formed inside a bacterial exopolysaccharide. J. Environ. Chem. Eng. 2019, 7, 102908. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Chakrabarti, S.; Ghosh, U.C. Fixed-bed column performance of Mn-incorporated iron(III) oxide nanoparticle agglomerates on As(III) removal from the spiked groundwater in lab bench scale. Chem. Eng. J. 2014, 248, 18–26. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2014, 68, 206–216. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Grishkewich, N.; Tam, K.C. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J. Mater. Chem. A 2012, 1, 959–965. [Google Scholar] [CrossRef]
- Dong, F.; Xu, X.; Shaghaleh, H.; Guo, J.; Guo, L.; Qian, Y.; Liu, H.; Wang, S. Factors influencing the morphology and adsorption performance of cellulose nanocrystal/iron oxide nanorod composites for the removal of arsenic during water treatment. Int. J. Biol. Macromol. 2019, 156, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Su, Y.; Li, Q.; Gao, S.; Shang, J.K. Superparamagnetic magnesium ferrite nanoadsorbent for effective arsenic (III, V) removal and easy magnetic separation. Water Res. 2013, 47, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Smedley, P.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Wu, M.; Liu, R.; Wang, D.; Lin, X.; Liu, W.; Ma, L.; Li, Y.; Huang, Y. Modified native cellulose fibers—A novel efficient adsorbent for both fluoride and arsenic. J. Hazard. Mater. 2011, 185, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, P.; Qiu, F.; Zhang, T.; Xu, J. Controllable preparation of FeOOH/CuO@WBC composite based on water bamboo cellulose applied for enhanced arsenic removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Singh, K.; Sinha, T.; Srivastava, S. Functionalized nanocrystalline cellulose: Smart biosorbent for decontamination of arsenic. Int. J. Miner. Process. 2015, 139, 51–63. [Google Scholar] [CrossRef]
- Najib, N.; Christodoulatos, C. Removal of arsenic using functionalized cellulose nanofibrils from aqueous solutions. J. Hazard. Mater. 2018, 367, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Synthesis and characterization of multi-amino-functionalized cellulose for arsenic adsorption. Carbohydr. Polym. 2013, 92, 380–387. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Senan, P.; Suchithra, P.S. Evaluation of Iron(III)-Coordinated Amino-Functionalized Poly(Glycidyl Methacrylate)-Grafted Cellulose for Arsenic(V) Adsorption from Aqueous Solutions. Water Air Soil Pollut. 2011, 220, 101–116. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Divya, L.; Parvathy, J. Arsenic adsorption from contaminated water on Fe(III)-coordinated amino-functionalized poly(glycidylmethacrylate)-grafted TiO2-densified cellulose. J. Chem. Technol. Biotechnol. 2012, 88, 878–886. [Google Scholar] [CrossRef]
- Yousif, A.M.; Zaid, O.F.; Ibrahim, I. Fast and selective adsorption of As(V) on prepared modified cellulose containing Cu(II) moieties. Arab. J. Chem. 2016, 9, 607–615. [Google Scholar] [CrossRef]
- Zeng, H.; Zhai, L.; Qiao, T.; Yu, Y.; Zhang, J.; Li, D. Efficient removal of As(V) from aqueous media by magnetic nanoparticles prepared with Iron-containing water treatment residuals. Sci. Rep. 2020, 10, 9335. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, A.; Banjara, A.; Sharma, M. Use of iron oxide/activated carbon magnetic composite for adsorptive removal of arsenic from water. Int. J. Adv. Eng. 2019, 1, 9–16. [Google Scholar]
- Wen, Z.; Zhang, Y.; Dai, C.; Chen, B.; Guo, S.; Yu, H.; Wu, D. Synthesis of ordered mesoporous iron manganese bimetal oxides for arsenic removal from aqueous solutions. Microporous Mesoporous Mater. 2014, 200, 235–244. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2013, 21, 449–461. [Google Scholar] [CrossRef]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Mohapatra, M.; Hariprasad, D.; Anand, S.; Mishra, B. Mg-doped nano ferrihydrite—A new adsorbent for fluoride removal from aqueous solutions. Appl. Surf. Sci. 2012, 258, 4228–4236. [Google Scholar] [CrossRef]
- Farrell, J.; Chaudhary, B.K. Understanding Arsenate Reaction Kinetics with Ferric Hydroxides. Environ. Sci. Technol. 2013, 47, 8342–8347. [Google Scholar] [CrossRef]
- Mondal, P.; Balomajumder, C.; Mohanty, B. A laboratory study for the treatment of arsenic, iron, and manganese bearing ground water using Fe3+ impregnated activated carbon: Effects of shaking time, pH and temperature. J. Hazard. Mater. 2007, 144, 420–426. [Google Scholar] [CrossRef]
- Padmavathy, K.; Madhu, G.; Haseena, P. A study on Effects of pH, Adsorbent Dosage, Time, Initial Concentration and Adsorption Isotherm Study for the Removal of Hexavalent Chromium (Cr (VI)) from Wastewater by Magnetite Nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Hafez, I.; Amini, E.; Tajvidi, M. The synergy between cellulose nanofibrils and calcium carbonate in a hybrid composite system. Cellulose 2020, 27, 3773–3787. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Lu, C.; Deng, Y. Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J. Mater. Chem. 2012, 22, 11642–11650. [Google Scholar] [CrossRef]
- González-Ugarte, A.S.; Hafez, I.; Tajvidi, M. Characterization and properties of hybrid foams from nanocellulose and kaolin-microfibrillated cellulose composite. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Qing, Y.; Wu, Y.; Cai, Z.; Li, X. Water-Triggered Dimensional Swelling of Cellulose Nanofibril Films: Instant Observation Using Optical Microscope. J. Nanomater. 2013, 2013, 594734. [Google Scholar] [CrossRef] [Green Version]
- Battisha, I.; Afify, H.; Ibrahim, M. Synthesis of Fe2O3 concentrations and sintering temperature on FTIR and magnetic susceptibility measured from 4 to 300K of monolith silica gel prepared by sol–gel technique. J. Magn. Magn. Mater. 2006, 306, 211–217. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Gadri, A.; Ammar, S. Structural, optical and morphological characterization of Cu-doped α-Fe2O3 nanoparticles synthesized through co-precipitation technique. J. Mol. Struct. 2017, 1148, 276–281. [Google Scholar] [CrossRef]
- Darezereshki, E. One-step synthesis of hematite (α-Fe2O3) nano-particles by direct thermal-decomposition of maghemite. Mater. Lett. 2011, 65, 642–645. [Google Scholar] [CrossRef]
- Kondo, T. The assignment of IR absorption bands due to free hydroxyl groups in cellulose. Cellulose 1997, 4, 281–292. [Google Scholar] [CrossRef]
- Rodriguez, D.M.P. Aminosilane-Functionalized Cellulosic Polymers for Increased Carbon Dioxide Sorption. Master’s Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2010. [Google Scholar]
- Zhang, C.; Su, J.; Zhu, H.; Xiong, J.; Liu, X.; Li, D.; Chen, Y.; Li, Y. The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H2O2. RSC Adv. 2017, 7, 34182–34191. [Google Scholar] [CrossRef] [Green Version]
- Obokata, T.; Isogai, A. The mechanism of wet-strength development of cellulose sheets prepared with polyamideamine-epichlorohydrin (PAE) resin. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 525–531. [Google Scholar] [CrossRef]
- Zhangab, Y.; Liab, J.; Maab, N.; Mengab, Z.; Suiab, G. Processing cellulose@Fe3O4 into mechanical, magnetic and biodegradable synapse-like material. Compos. Part. B Eng. 2019, 177, 107432. [Google Scholar] [CrossRef]
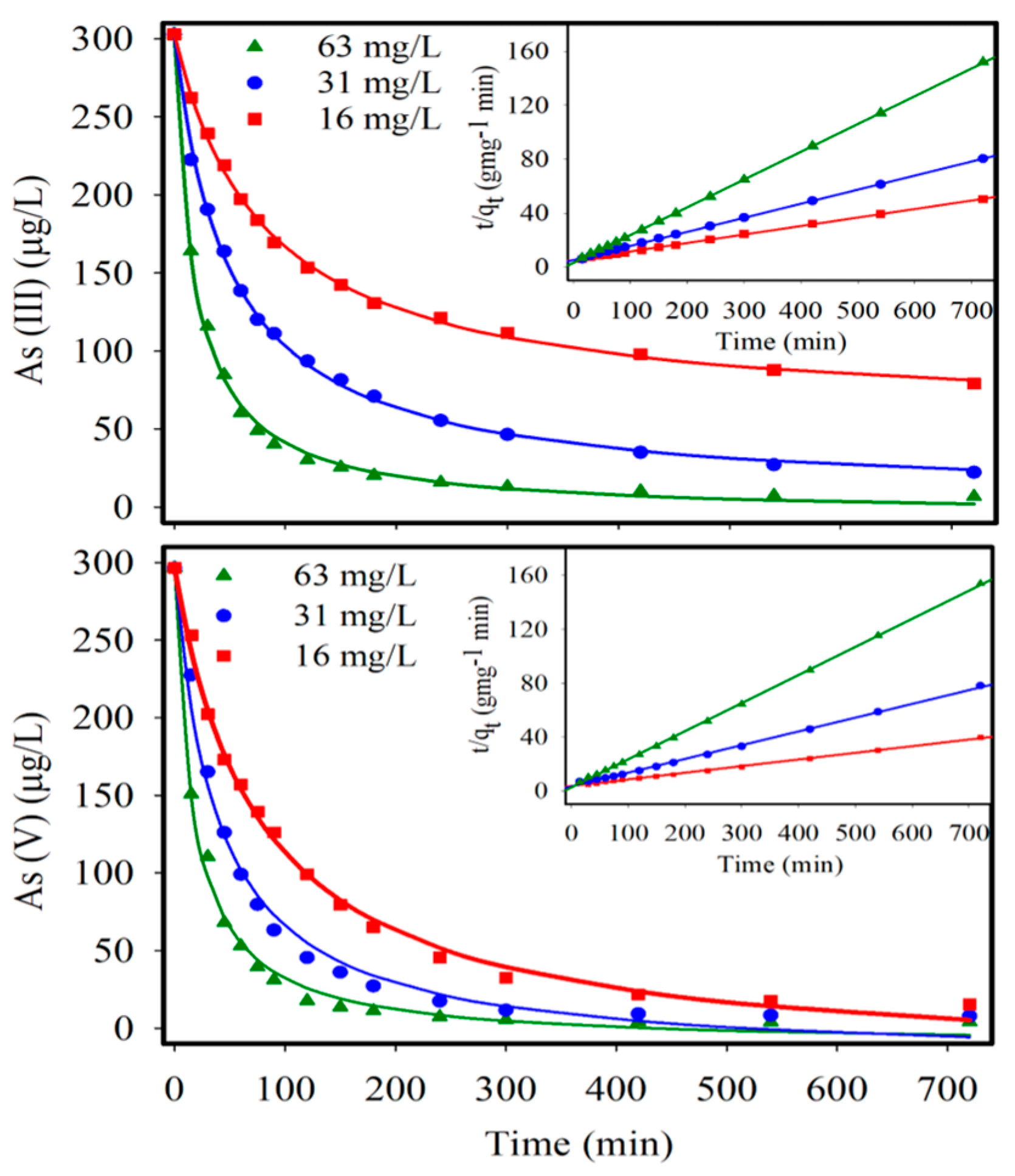

| Adsorption Parameters | As(III) | As(V) | ||||
|---|---|---|---|---|---|---|
| Initial As Concentration (mg-As L−1) | 0.0296 | 0.0302 | ||||
| Adsorbent Loading (mg-IONP L-As−1) | 16 | 31 | 63 | 16 | 31 | 63 |
| qe (mg-As g-IONP−1) | 15.87 | 9.57 | 4.85 | 20.41 | 9.78 | 4.78 |
| Kad (g-IONP mg-As·min−1) | 7.6 × 10−4 | 2.1 × 10−3 | 1.35 × 10−2 | 6.7 × 10−4 | 3.2 × 10−3 | 1.67 × 10−2 |
| r2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| As(III) | As(V) | |
|---|---|---|
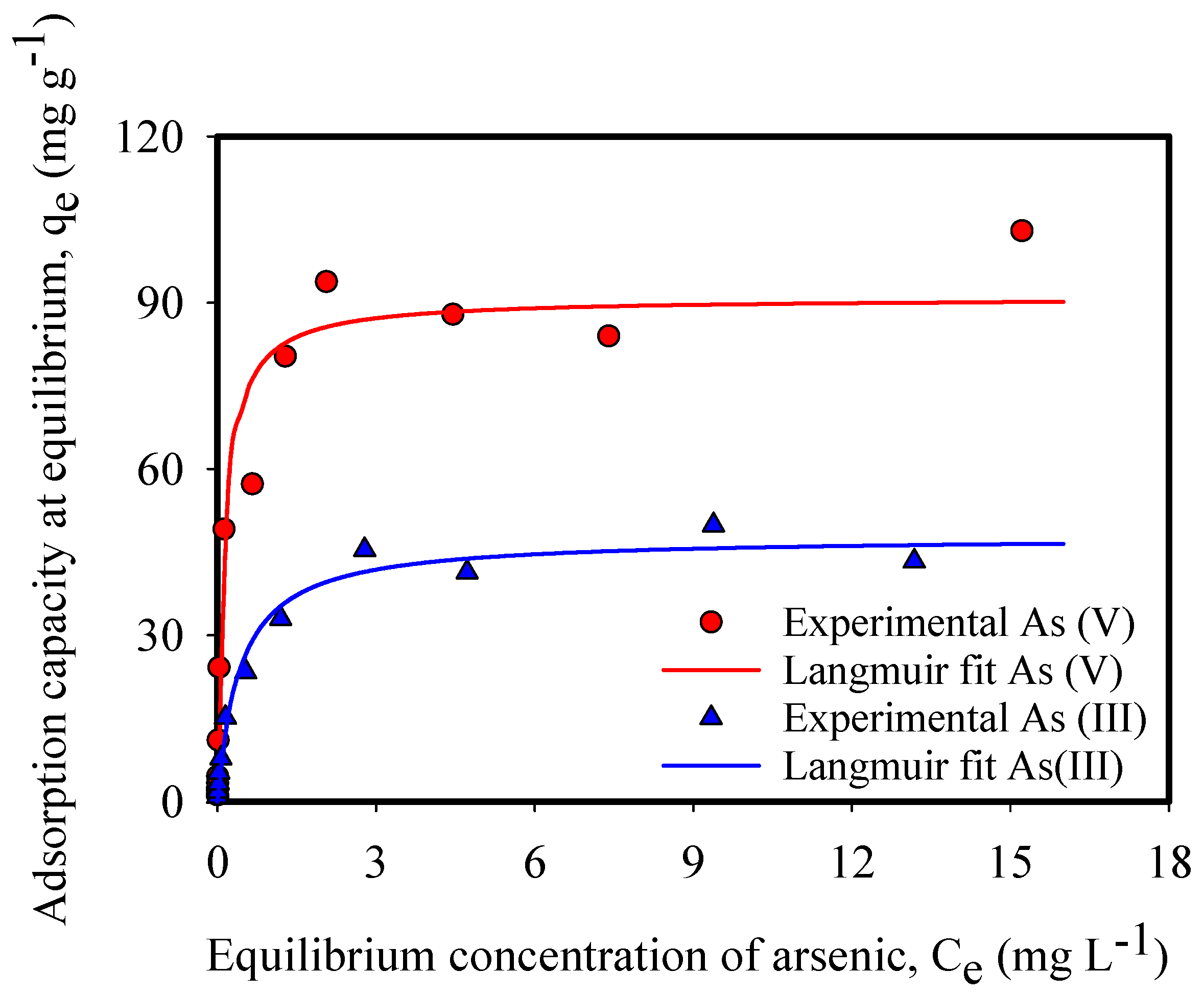
| qmax (mg-As g-IONP−1) | 47.75 | 90.90 |
| KL | 2.36 | 7.95 |
| r2 | 0.99 | 0.99 |
| Adsorbent | As Concentration Range, C0 (mg L−1) | Maximum as Adsorption Capacity, qmax (mg g−1) | BET Surface Area (m2 g−1) | pH | Reference |
|---|---|---|---|---|---|
| Cellulose-g-PDMAEMA | As(III) & As(V): 0.05–8.9 | As(III): 8.96; As(V): 27.93 | — | <10 | [25] |
| FeOOH/CuO@WBC | As(III): 20–200 | As(III): 76.1 | — | 3.5 | [26] |
| DETA-g-DA-NCC | As(III) & As(V): 0.005–50 | As(III): 10.56; As(V): 12.06 | — | 7.5 | [27] |
| Functionalized CNFs | As(V): 0.025–40 | As(V): 24.9 | 0.16 | 4–8 | [28] |
| Cellulose-g-GMA-b-TEPA | As(III): ~5–35; As(V): ~20–100 | As(III): 5.71; As(V): 75.13 | 3.68 | As(III): 7 & As(V): 5 | [29] |
| Fe(III)-AM-PGMACell | As(V): 25–400 | As(V): 78.8 | 39.9 | 6 | [30] |
| AM-Fe-PGDC | As(V): 10–400 | As(V): 105.47 | 31.6 | 6 | [31] |
| Cell-N-Cu | As(V): 100–700 | As(V): 98.9 | — | 8.4 | [32] |
| CNs/Fe2O3 nanorod | — | As(III): 13.87, As(V): 15.71 | — | 7 & 3 | [21] |
| Cellulose@Fe2O3 composites * | — | As(III): 64.33, As(V): 89.19 | 113 | 7 | [20] |
| iMNP | As(V): 1–10 | As(V): 12.74 | 145.5 | 6.6 | [33] |
| Fe3O4/AC composite | As(III): 2–120 | As(III): 7.5 | — | 8 | [34] |
| OMIM | As(III) & As(V): 1–100 | As(III): 67.89, As(V): 93.54 | 154 | 3 | [35] |
| IONP@CNF-IONP aerogel * | As(III): 0.055–15.9; As(V): 0.073–21.7 | As(III): 47.75; As(V): 90.90 | 165 | 7 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Hafez, I.; Tajvidi, M.; Amirbahman, A. Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water. Nanomaterials 2021, 11, 2818. https://doi.org/10.3390/nano11112818
Rahman MM, Hafez I, Tajvidi M, Amirbahman A. Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water. Nanomaterials. 2021; 11(11):2818. https://doi.org/10.3390/nano11112818
Chicago/Turabian StyleRahman, Md Musfiqur, Islam Hafez, Mehdi Tajvidi, and Aria Amirbahman. 2021. "Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water" Nanomaterials 11, no. 11: 2818. https://doi.org/10.3390/nano11112818
APA StyleRahman, M. M., Hafez, I., Tajvidi, M., & Amirbahman, A. (2021). Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water. Nanomaterials, 11(11), 2818. https://doi.org/10.3390/nano11112818