Photocatalytic Activity of Titanium Dioxide Nanotubes Following Long-Term Aging
Abstract
:1. Introduction
2. Methodology
2.1. Material Synthesis
2.2. Aging Test Setup
2.3. Preparation of Synthetic Greywater
2.4. Sample Handling and Testing
2.4.1. Morphological Investigation
2.4.2. Methylene Blue Dye Degradation Tests
3. Results and Discussion
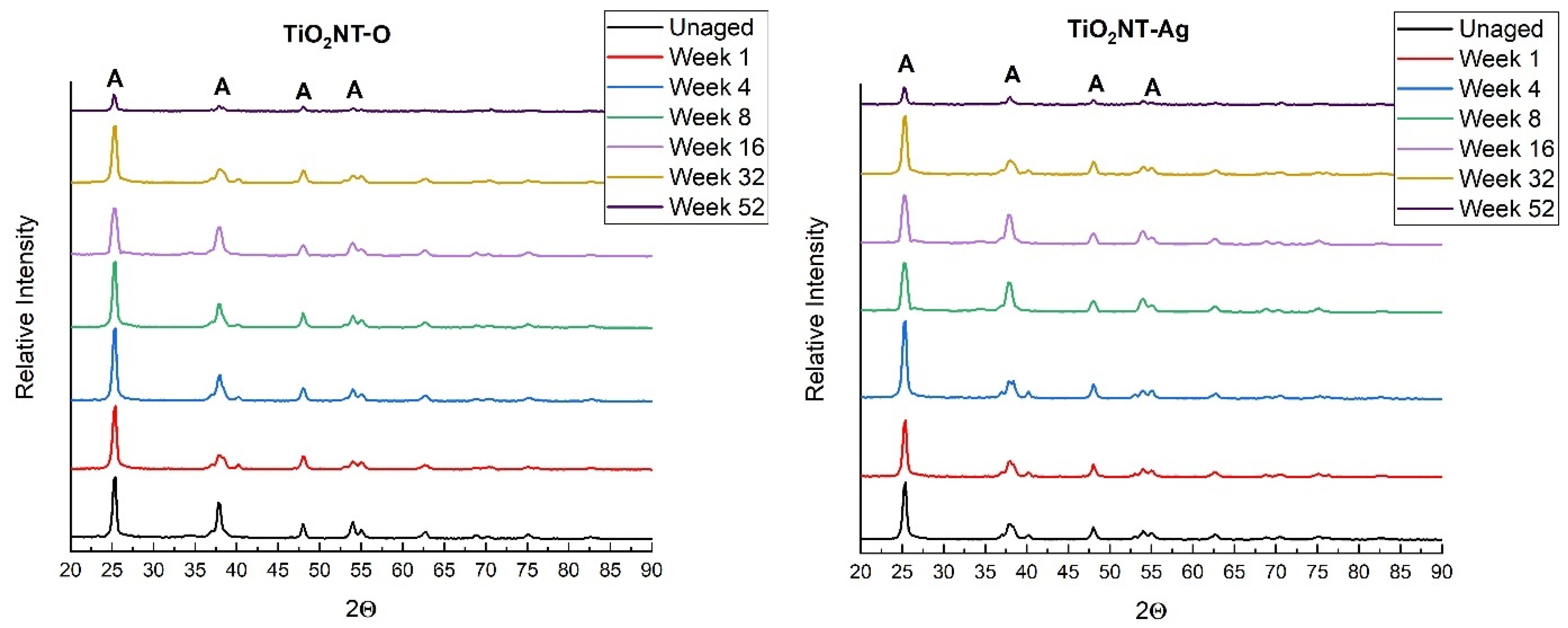
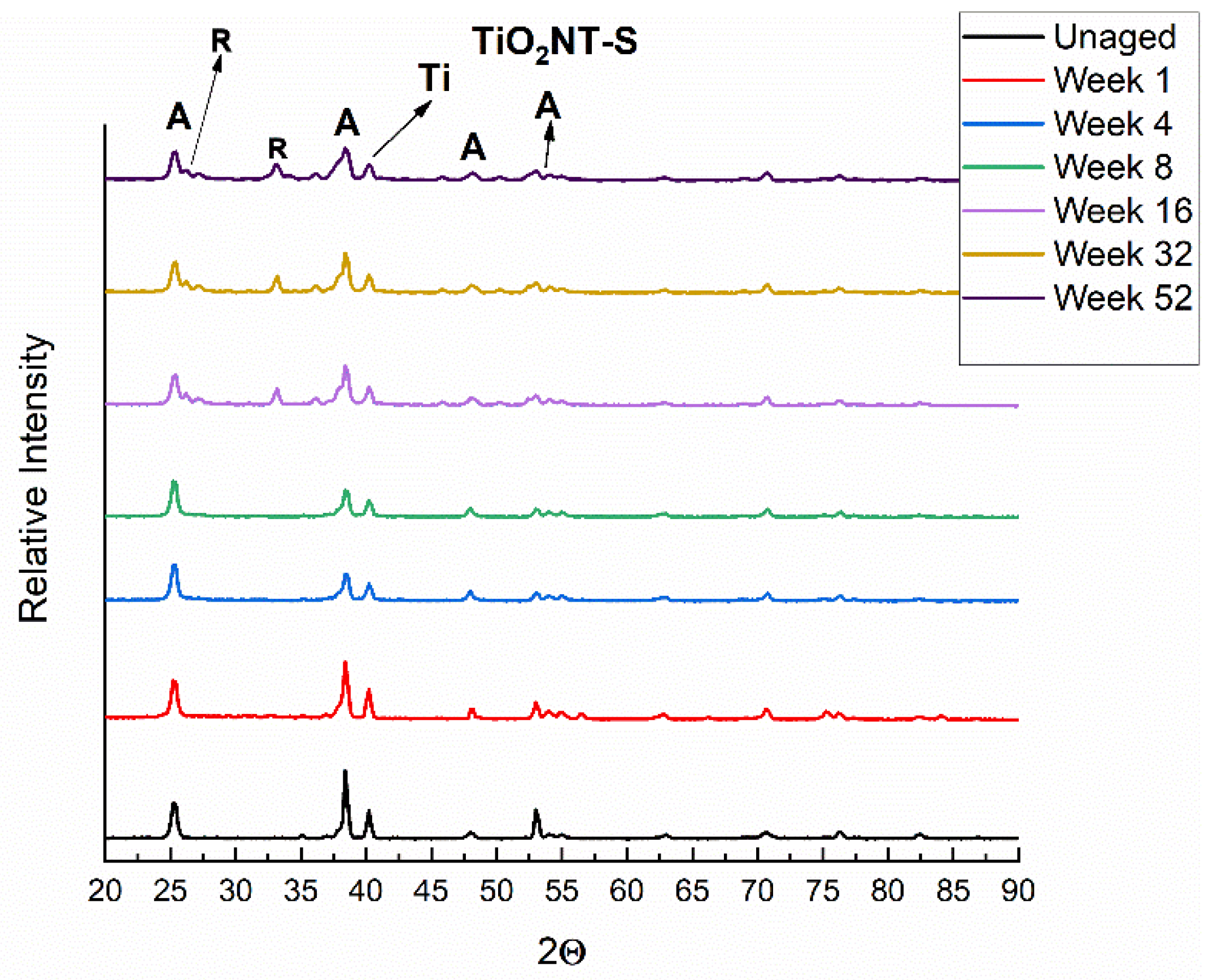
3.1. Morphology and Chemical Composition
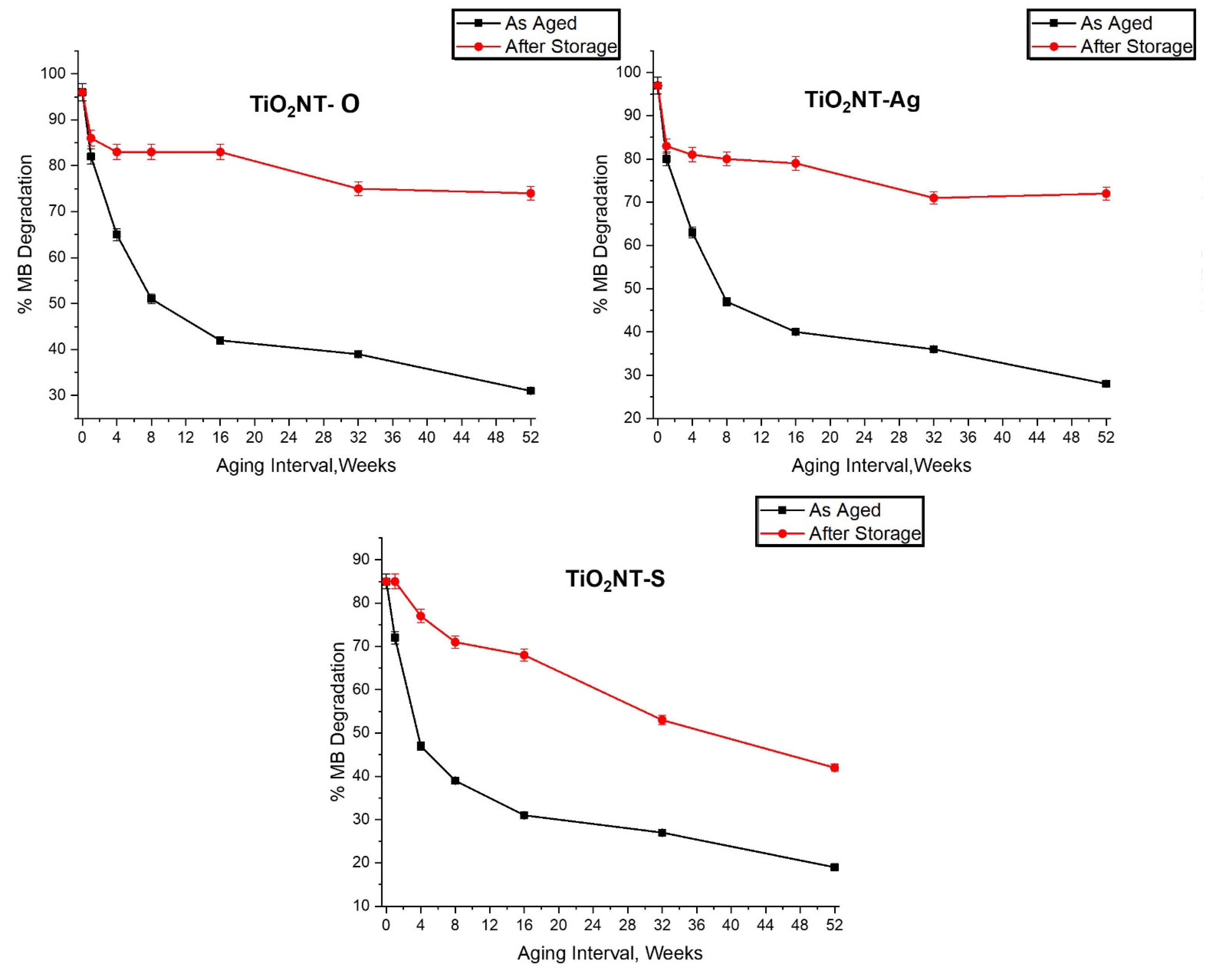
3.2. Effect of Aging on the Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Water Scarcity and Drought in the European Union. Available online: https://ec.europa.eu/environment/pubs/pdf/factsheets/water_scarcity.pdf (accessed on 24 August 2021).
- Adán, C.; Marugán, J.; Sánchez, E.; Pablos, C.; van Grieken, R. Understanding the effect of morphology on the photocatalytic activity of TiO2 nanotube array electrodes. Electrochim. Acta 2016, 191, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.-D.; Wang, Y.; Sun, R.-J.; Zhou, D.-M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Wang, X.; Yao, M.; Yao, X. Band structure design of semiconductors for enhanced photocatalytic activity: The case of TiO2. Prog. Nat. Sci. 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Du, P.; Carneiro, J.T.; Moulijn, J.A.; Mul, G. A novel photocatalytic monolith reactor for multiphase heterogeneous photocatalysis. Appl. Catal. A Gen. 2008, 334, 119–128. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, A.; Lassaletta, G.; Jiménez, V.; Justo, A.; Gonzalez-Elipe, A.; Herrmann, J.-M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B Environ. 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Sanz, R.; Buccheri, M.A.; Zimbone, M.; Scuderi, V.; Amiard, G.; Impellizzeri, G.; Romano, L.; Privitera, V. Photoactive layered nanocomposites obtained by direct transferring of anodic TiO 2 nanotubes to commodity thermoplastics. Appl. Surf. Sci. 2017, 399, 451–462. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.; Ghicov, A.; Taveira, L.; Schmuki, P. Self-organized porous TiO2 and ZrO2 produced by anodization. Corros. Sci. 2005, 47, 3324–3335. [Google Scholar] [CrossRef]
- Padiyan, D.P.; Raja, D.H. Synthesis of Various Generations Titania Nanotube Arrays by Electrochemical Anodization for H2 Production. Energy Procedia 2012, 22, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [PubMed]
- Panaitescu, E.; Richter, C.; Menon, L. A Study of Titania Nanotube Synthesis in Chloride-Ion-Containing Media. J. Electrochem. Soc. 2008, 155, E7–E13. [Google Scholar] [CrossRef] [Green Version]
- Cheong, Y.L.; Yam, F.K.; Ng, S.; Hassan, Z.; Ng, S.S.; Low, I. Fabrication of titanium dioxide nanotubes in fluoride-free electrolyte via rapid breakdown anodization. J. Porous Mater. 2015, 22, 1437–1444. [Google Scholar] [CrossRef]
- Ng, S.W.; Yam, F.K.; Beh, K.P.; Hassan, Z. Titanium Dioxide Nanotubes in Chloride Based Electrolyte: An Alternative to Fluoride Based Electrolyte. Sains Malays. 2014, 43, 947–951. [Google Scholar]
- Hahn, R.; Macak, J.; Schmuki, P. Rapid anodic growth of TiO2 and WO3 nanotubes in fluoride free electrolytes. Electrochem. Commun. 2007, 9, 947–952. [Google Scholar] [CrossRef]
- Regoninia, D.; Chen, G.; Leach, C.; Clemens, F.J. Comparison of photoelectrochemical properties of TiO2 Nanotubes and sol-gel. Electrochim. Acta 2016, 213, 31–36. [Google Scholar] [CrossRef]
- Robertson, J.M.; Robertson, P.K.; Lawton, L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J. Photochem. Photobiol. A Chem. 2005, 175, 51–56. [Google Scholar] [CrossRef]
- Nagamine, S.; Inohara, K. Photocatalytic microreactor using anodized TiO2 nanotube array. Adv. Powder Technol. 2018, 29, 3100–3106. [Google Scholar] [CrossRef]
- Teodorescu-Soare, C.T.; Catrinescu, C.; Dobromir, M.; Stoian, G.; Arvinte, A.; Luca, D. Growth and characterization of TiO2 nanotube arrays under dynamic anodization. Photocatalytic activity. J. Electroanal. Chem. 2018, 823, 388–396. [Google Scholar]
- Riboni, F.; Nguyen, N.T.; So, S.; Schmuki, P. Aligned metal oxide nanotube arrays: Key-aspects of anodic TiO2 nanotube formation and properties. Nanoscale Horiz. 2016, 1, 445–466. [Google Scholar] [PubMed] [Green Version]
- Gomes, J.; Lincho, J.; Domingues, E.; Gmurek, M.; Mazierski, P.; Zaleska-Medynska, A.; Klimczuk, T.; Quinta-Ferreira, R.M.; Martins, R.C. TiO2 nanotube arrays-based reactor for photocatalytic oxidation of parabens mixtures in ultrapure water: Effects of photocatalyst properties, operational parameters and light source. Sci. Total Environ. 2019, 689, 79–89. [Google Scholar] [CrossRef] [PubMed]
- McMichael, S.; Waso, M.; Reyneke, B.; Khan, W.; Byrne, J.A.; Fernandez-Ibanez, P. Electrochemically assisted photocatalysis for the disinfection of rainwater under solar irradiation. Appl. Catal. B Environ. 2021, 281, 119485. [Google Scholar]
- Kim, H.-I.; Kim, D.; Kim, W.; Ha, Y.-C.; Sim, S.-J.; Kim, S.; Choi, W. Anodic TiO2 nanotube layer directly formed on the inner surface of Ti pipe for a tubular photocatalytic reactor. Appl. Catal. A Gen. 2016, 521, 174–181. [Google Scholar] [CrossRef]
- Prototype Greywater Treatment Units. Available online: https://microwatts-water.com/research/prototype-greywater-treatment-units/ (accessed on 23 October 2021).
- Kaneco, S.; Chen, Y.; Westerhoff, P.; Crittenden, J.C. Fabrication of uniform size titanium oxide nanotubes: Impact of current density and solution conditions. Scr. Mater. 2007, 56, 373–376. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. A New Benchmark for TiO2 Nanotube Array Growth by Anodization. J. Phys. Chem. C 2007, 111, 7235–7241. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Kerr, J.; Fioletov, V. Surface ultraviolet radiation. Atmosphere-Ocean 2008, 46, 159–184. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, C.; Di Mauro, A.; Lia, F.; Zammit, E.; Rizzo, A.; Privitera, V.; Impellizzeri, G.; Buccheri, M.; Rappazzo, G.; Grech, M.; et al. Suitability of Different Titanium Dioxide Nanotube Morphologies for Photocatalytic Water Treatment. Nanomaterials 2021, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Lia, F.; Zammit, E.; Rizzo, A.; Privitera, V.; Impellizzeri, G.; Di Mauro, A.; Buccheri, M.; Rapazzo, G.; Grech, M.; et al. Aging of anodic titanium dioxide nanotubes in synthetic greywater: Assessment of stability and retention of photocatalytic activity. Mater. Chem. Phys. 2021, 272, 124986. [Google Scholar] [CrossRef]
- Mohamed, A.E.R.; Rohani, S. Modified TiO2 nanotube arrays (TNTAs): Progressive strategies towards visible light responsive photoanode, a review. Energy Environ. Sci. 2011, 4, 1065–1086. [Google Scholar]
- Bauer, S.; Kleber, S.; Schmuki, P. TiO2 nanotubes: Tailoring the geometry in H3PO4/ HF electrolytes. Electrochem. Commun. 2006, 8, 1321–1325. [Google Scholar] [CrossRef]
- Ruan, C.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced photoelectrochemical-response in highly ordered TiO2 nanotube-arrays anodized in boric acid containing electrolyte. Sol. Energy Mater. Sol. Cells 2006, 90, 1283–1295. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Yoriya, S.; Prakasam, H.E.; Varghese, O.K.; Mor, G.K.; Latempa, T.A.; Fitzgerald, A.; Grimes, C.A. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 μm in Length. J. Phys. Chem. B 2006, 110, 16179–16184. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Summers, R.S.; Cook, S.M. Development and experimental validation of the composition and treatability of a new synthetic bathroom greywater (SynGrey). Environ. Sci. Water Res. Technol. 2017, 3, 1120–1131. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Standardisation, I.O.f. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue, 1st ed.; ISO 10678; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Chen, B.; Hou, J.; Lu, K. Formation Mechanism of TiO2 Nanotubes and Their Applications in Photoelectrochemical Water Splitting and Supercapacitors. Langmuir 2013, 29, 5911–5919. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, J.; Butler, K.T.; Catlow, C.R.A.; Logsdail, A.; Scanlon, D.; Shevlin, S.A.; Woodley, S.M.; Sokol, A.; Walsh, A. Polymorph Engineering of TiO2: Demonstrating How Absolute Reference Potentials Are Determined by Local Coordination. Chem. Mater. 2015, 27, 3844–3851. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chen, Z.; Li, Y.; Wang, Q.; Wan, L. Photocatalytic degradation of methylene blue in a sparged tube reactor with TiO2 fibres prepared by a properly two-step method. Catal. Commun. 2008, 9, 1178–1183. [Google Scholar] [CrossRef]
- Talapko, J.; Matijevi’c, T.; Juzbaši´c, M.; Antolovi´c-Požgain, A.; Škrlec, I. Antibacterial Activity of Silver and Its Application in Dentistry, Cardiology and Dermatology. Microorganisms 2020, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Ricci, P.C.; Carbonaro, C.M.; Stagi, L.; Salis, M.; Casu, A.; Enzo, S.; Delogu, F. Anatase-to-Rutile Phase Transition in TiO2 Nanoparticles Irradiated by Visible Light. J. Phys. Chem. C 2013, 117, 7850–7857. [Google Scholar] [CrossRef]
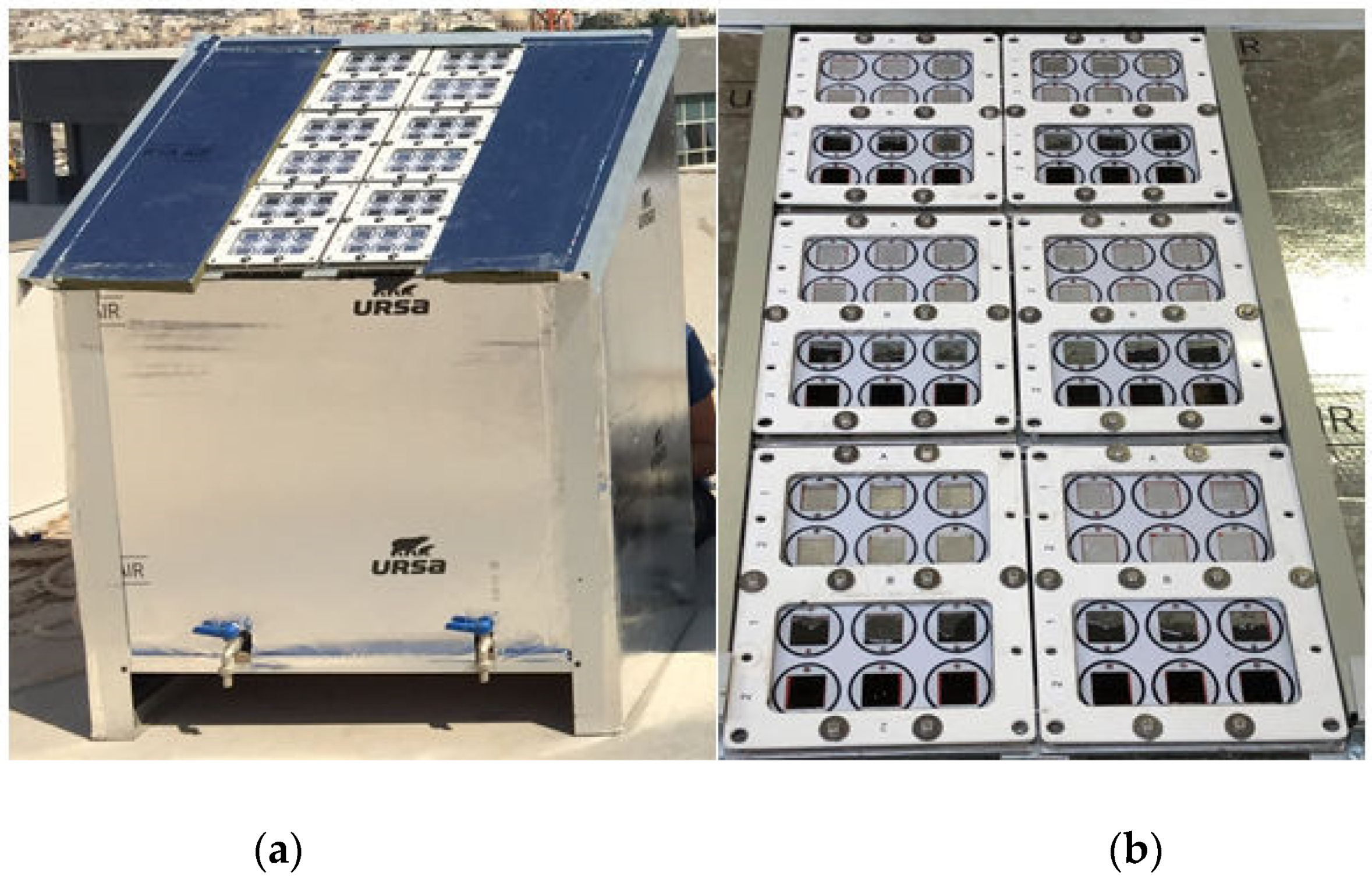
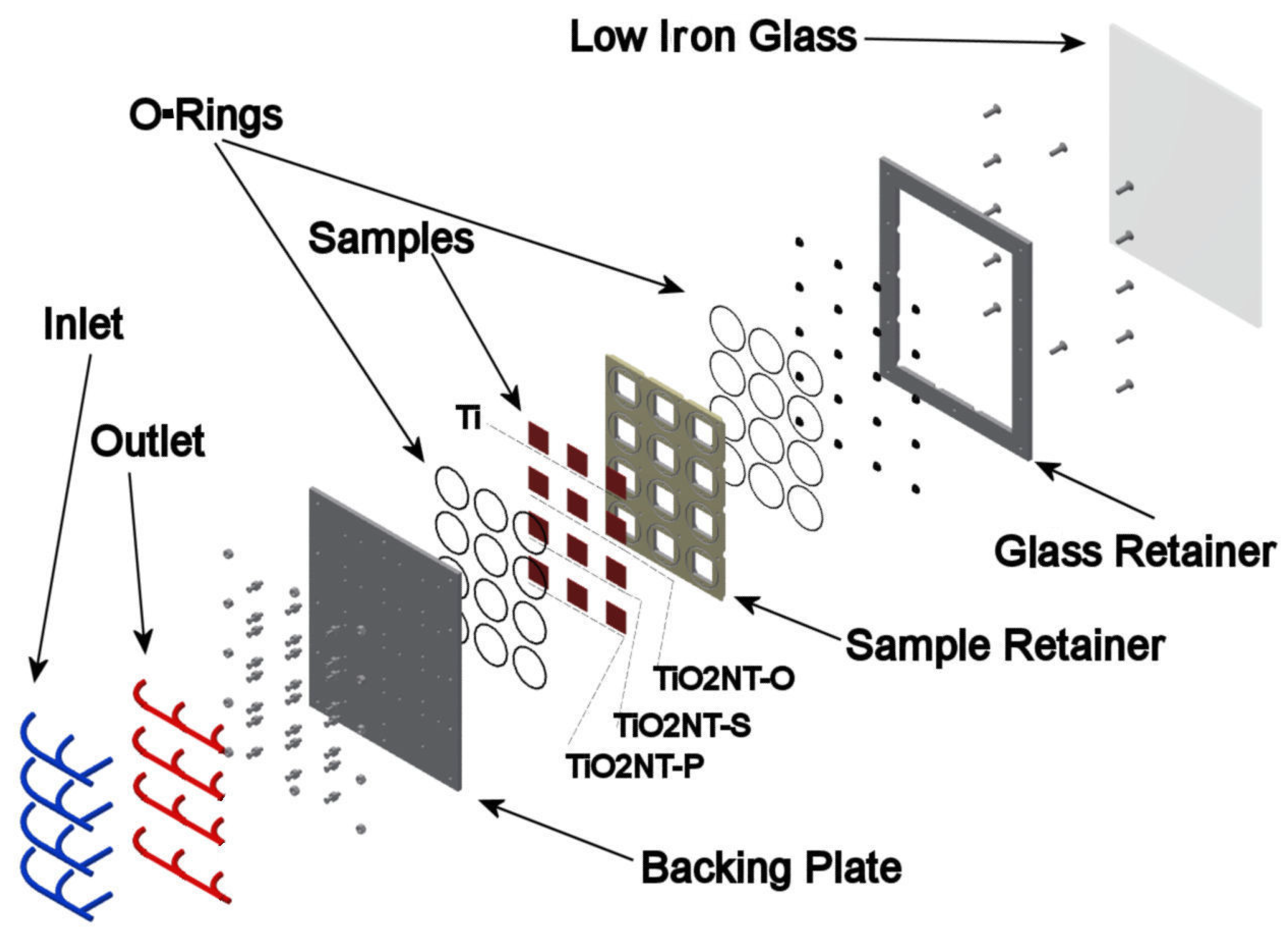

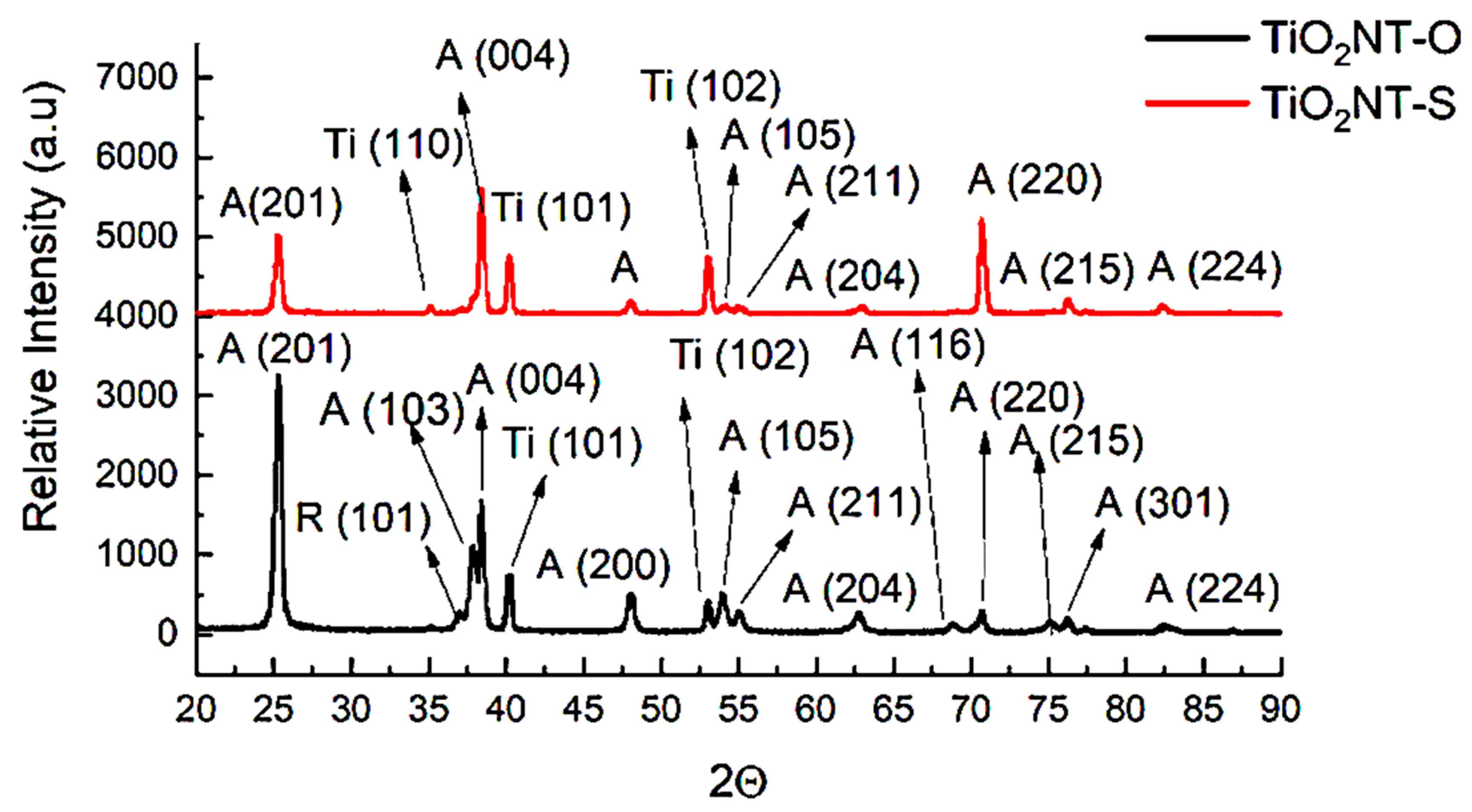


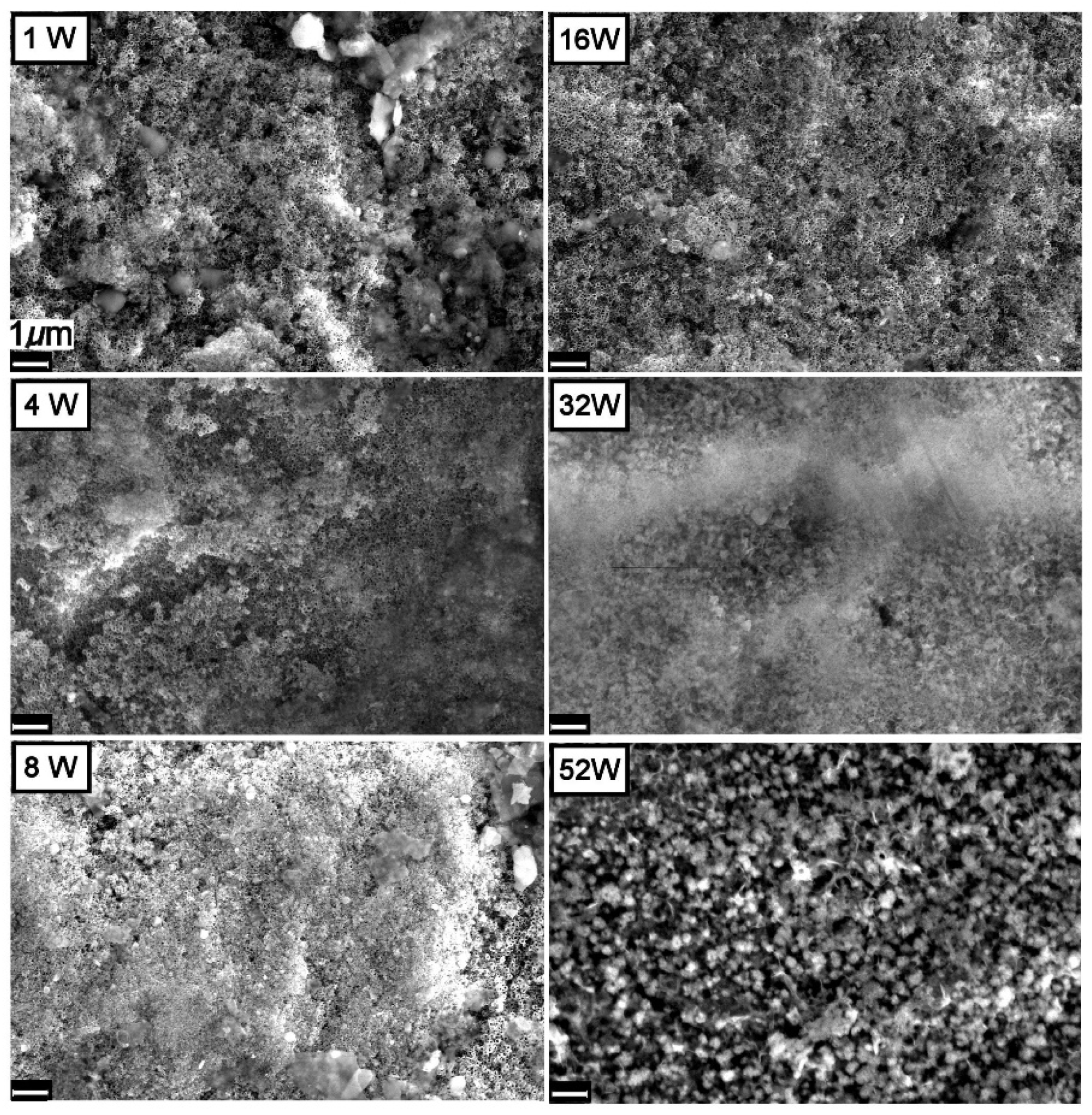

| Chemical | Concentration in the Final Solution mg/L | Working Concentration 1:100 Preparation of 10 L Stock (g) |
|---|---|---|
| Ammonium Chloride | 12.2 | 12.2 |
| Calcium Carbonate | 2.1 | 2.1 |
| Calcium Chloride | 63.5 | 63.5 |
| CAPB | 4.0 | 4.0 |
| Polyquaterium-10 | 4.0 | 4.0 |
| Magnesium Sulfate anhydrous | 110 | 110 |
| Potassium Chloride | 7.3 | 7.3 |
| Sodium hydrogen carbonate | 29.5 | 29.5 |
| Sodium Chloride | 120 | 120 |
| SDS | 15 | 15 |
| Sodium nitrate | 4.0 | 4.0 |
| Sodium Sulfate | 100 | 100 |
| Yeast extract | 49.0 | 49.0 |
| Testing Period | Test Duration |
|---|---|
| 1 | After: 4 Weeks |
| 2 | 8 Weeks |
| 3 | 16 Weeks |
| 4 | 32 Weeks |
| 5 | 52 Weeks |
| Material | Parameters | Layer Thickness (µm) | Tube Diameter (nm) | Wall Thickness (nm) | Aspect Ratio |
|---|---|---|---|---|---|
| TiO2NT-O | 70 V, 1 h | 9.99 ± 0.48 | 85–125 | 10.0 ± 2.00 | 80–117 |
| TiO2NT-S | 20 V, 6 h | 1.45 ± 0.07 | 60–100 | 14 ± 2.00 | 14–24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abela, S.; Farrugia, C.; Xuereb, R.; Lia, F.; Zammit, E.; Rizzo, A.; Refalo, P.; Grech, M. Photocatalytic Activity of Titanium Dioxide Nanotubes Following Long-Term Aging. Nanomaterials 2021, 11, 2823. https://doi.org/10.3390/nano11112823
Abela S, Farrugia C, Xuereb R, Lia F, Zammit E, Rizzo A, Refalo P, Grech M. Photocatalytic Activity of Titanium Dioxide Nanotubes Following Long-Term Aging. Nanomaterials. 2021; 11(11):2823. https://doi.org/10.3390/nano11112823
Chicago/Turabian StyleAbela, Stephen, Clayton Farrugia, Ryan Xuereb, Frederick Lia, Edwin Zammit, Alex Rizzo, Paul Refalo, and Maurice Grech. 2021. "Photocatalytic Activity of Titanium Dioxide Nanotubes Following Long-Term Aging" Nanomaterials 11, no. 11: 2823. https://doi.org/10.3390/nano11112823
APA StyleAbela, S., Farrugia, C., Xuereb, R., Lia, F., Zammit, E., Rizzo, A., Refalo, P., & Grech, M. (2021). Photocatalytic Activity of Titanium Dioxide Nanotubes Following Long-Term Aging. Nanomaterials, 11(11), 2823. https://doi.org/10.3390/nano11112823







