Development of a Strategy for Enhancing the Biomass Growth and Lipid Accumulation of Chlorella sp. UJ-3 Using Magnetic Fe3O4 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strain and Culture Conditions
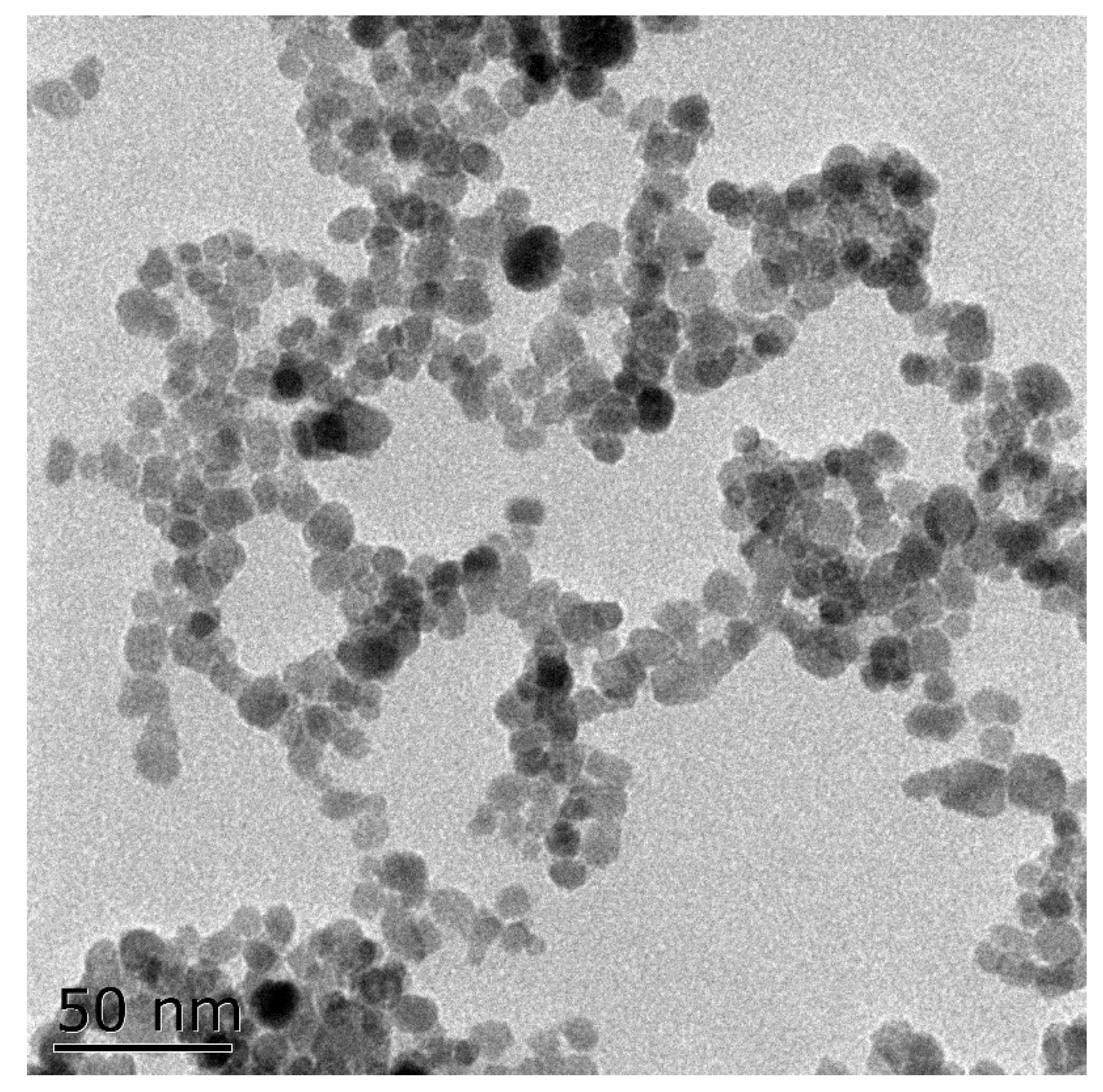
2.2. Synthesis of Nanoparticles
2.3. Experimental Design
2.4. Determination of Biomass and Total Lipids Content
2.5. Fatty Acids Analysis
2.6. Oxidative Stress Assessment and Determination of Antioxidant Enzyme Activities
2.7. Statistical Analysis
3. Results and Discussion
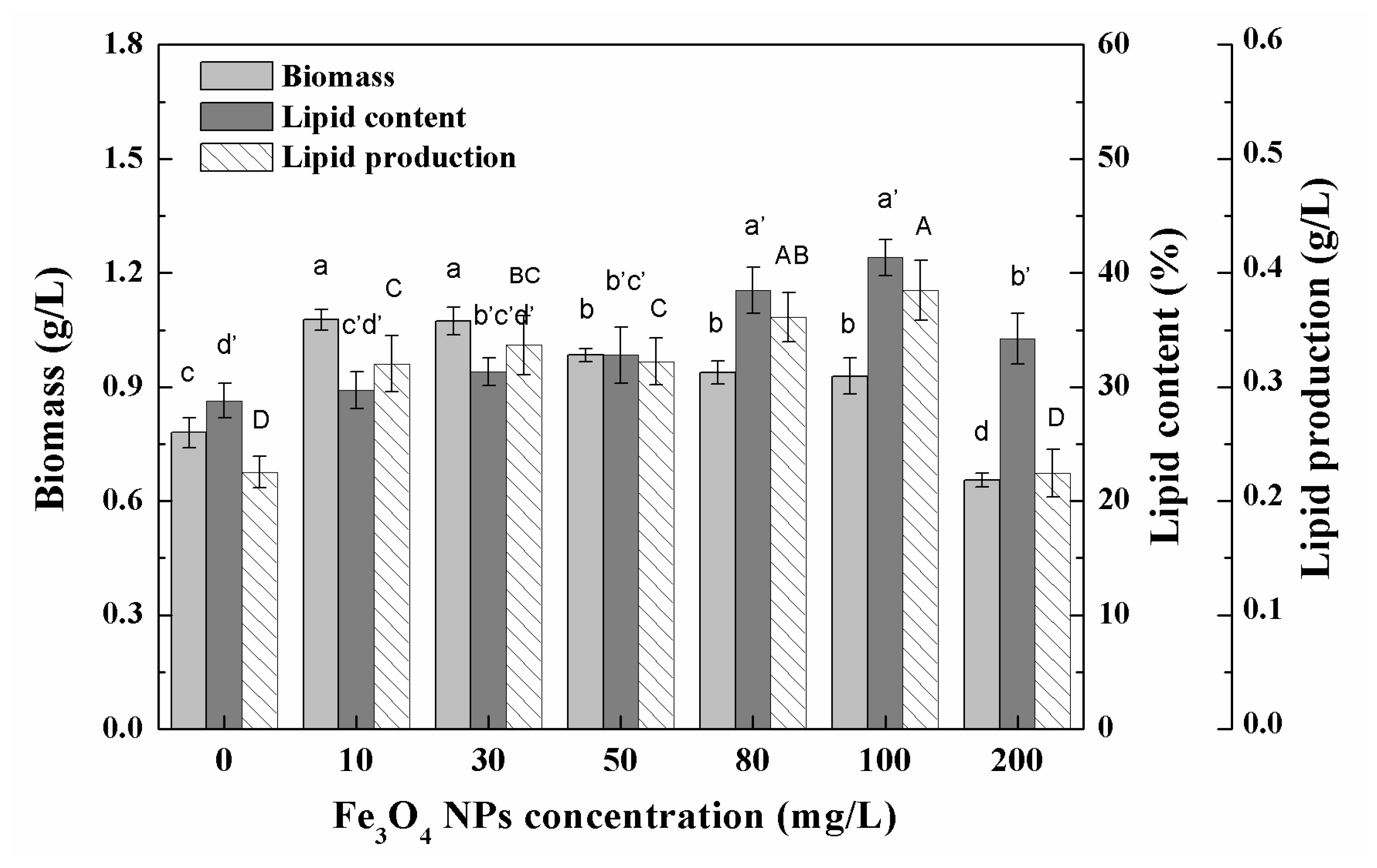
3.1. Growth Characteristics and Lipid Accumulation of Microalgae under Different Nanoparticle Concentrations
3.2. Effects of Low Concentration NPs on the Growth of Chlorella sp. UJ-3
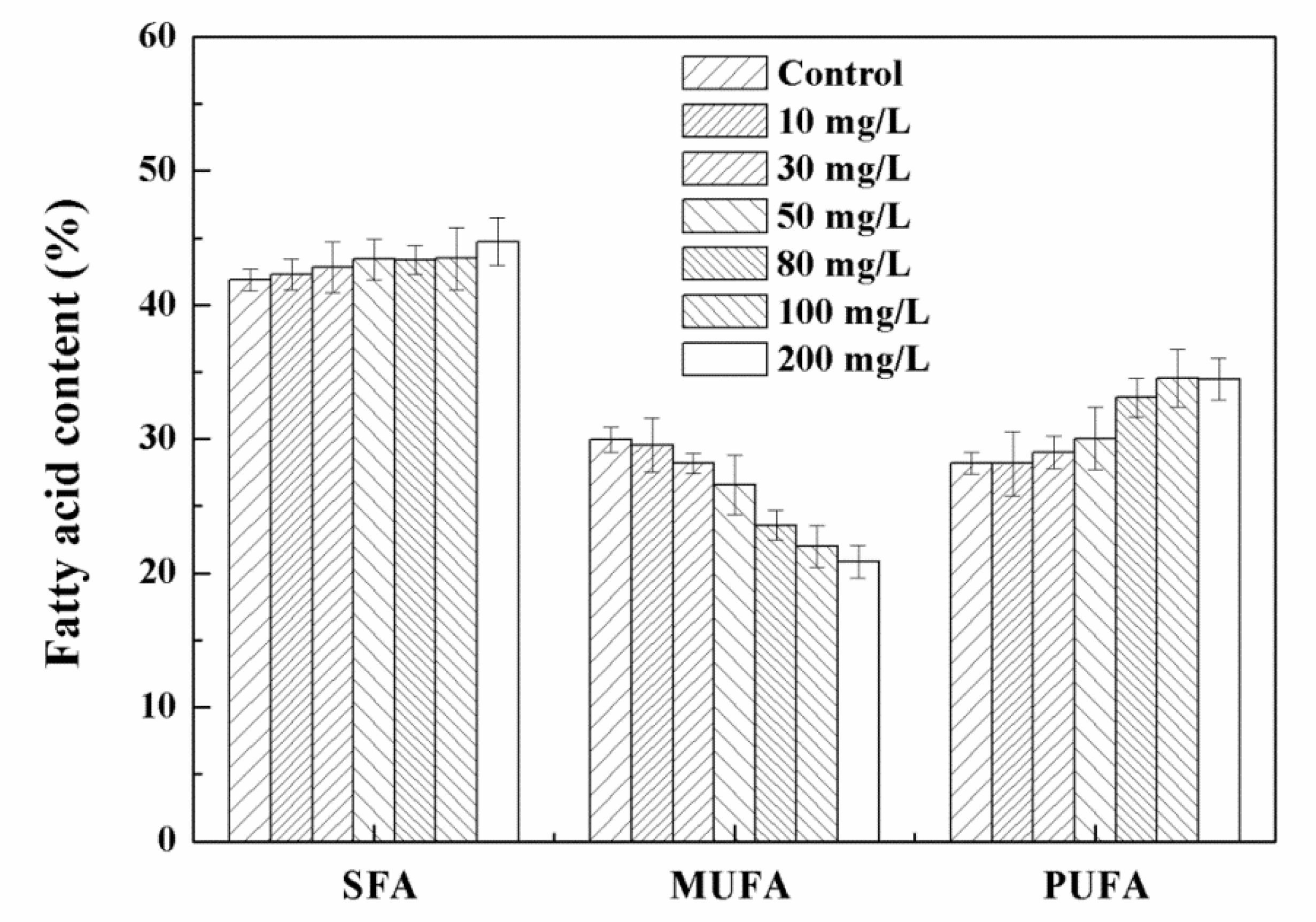
3.3. Effects of High Concentration NPs on Lipid Accumulation in Chlorella sp. UJ-3
3.4. NPs Induced Oxidative Stress and Cellular Antioxidant Defenses
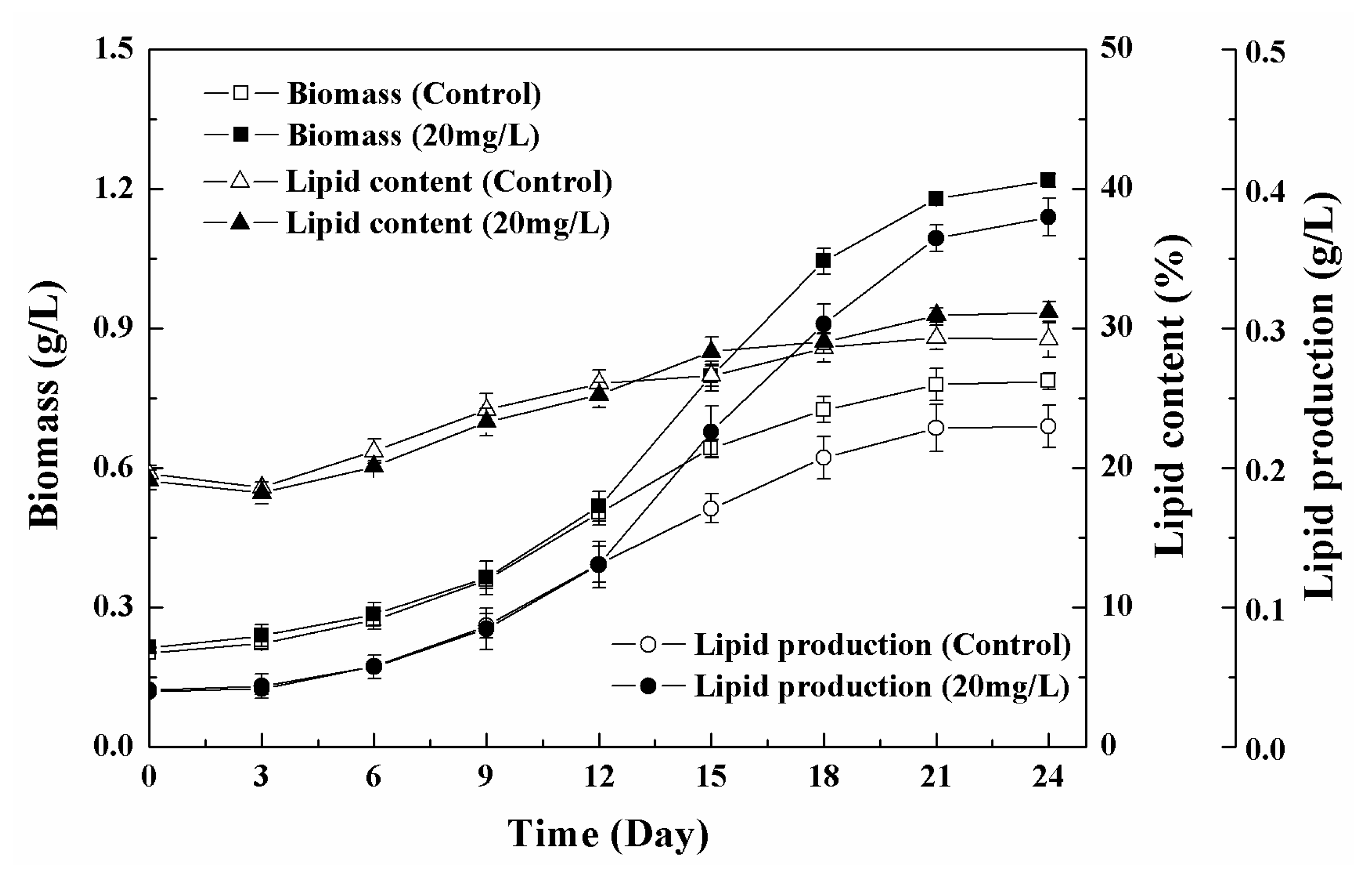
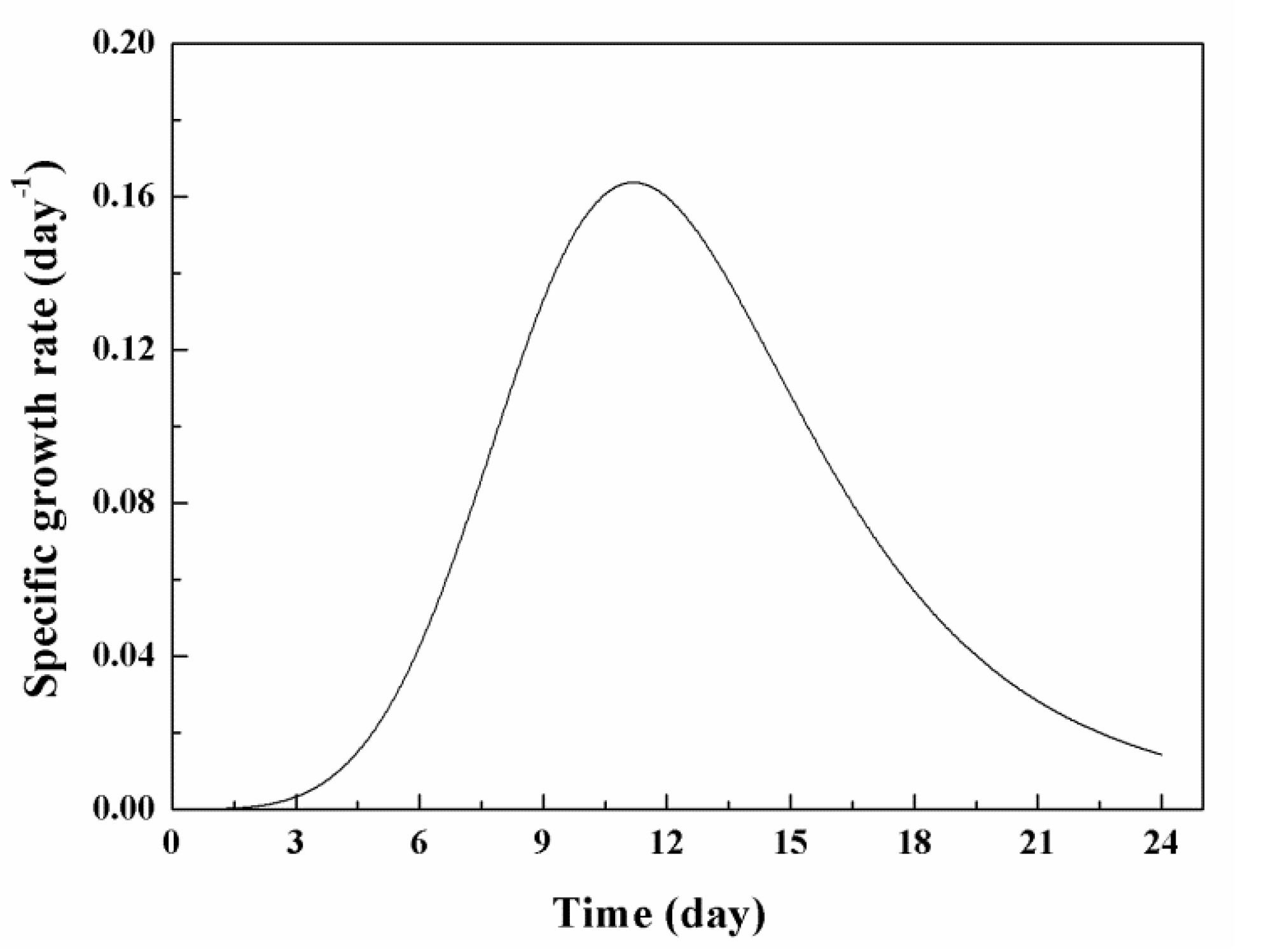
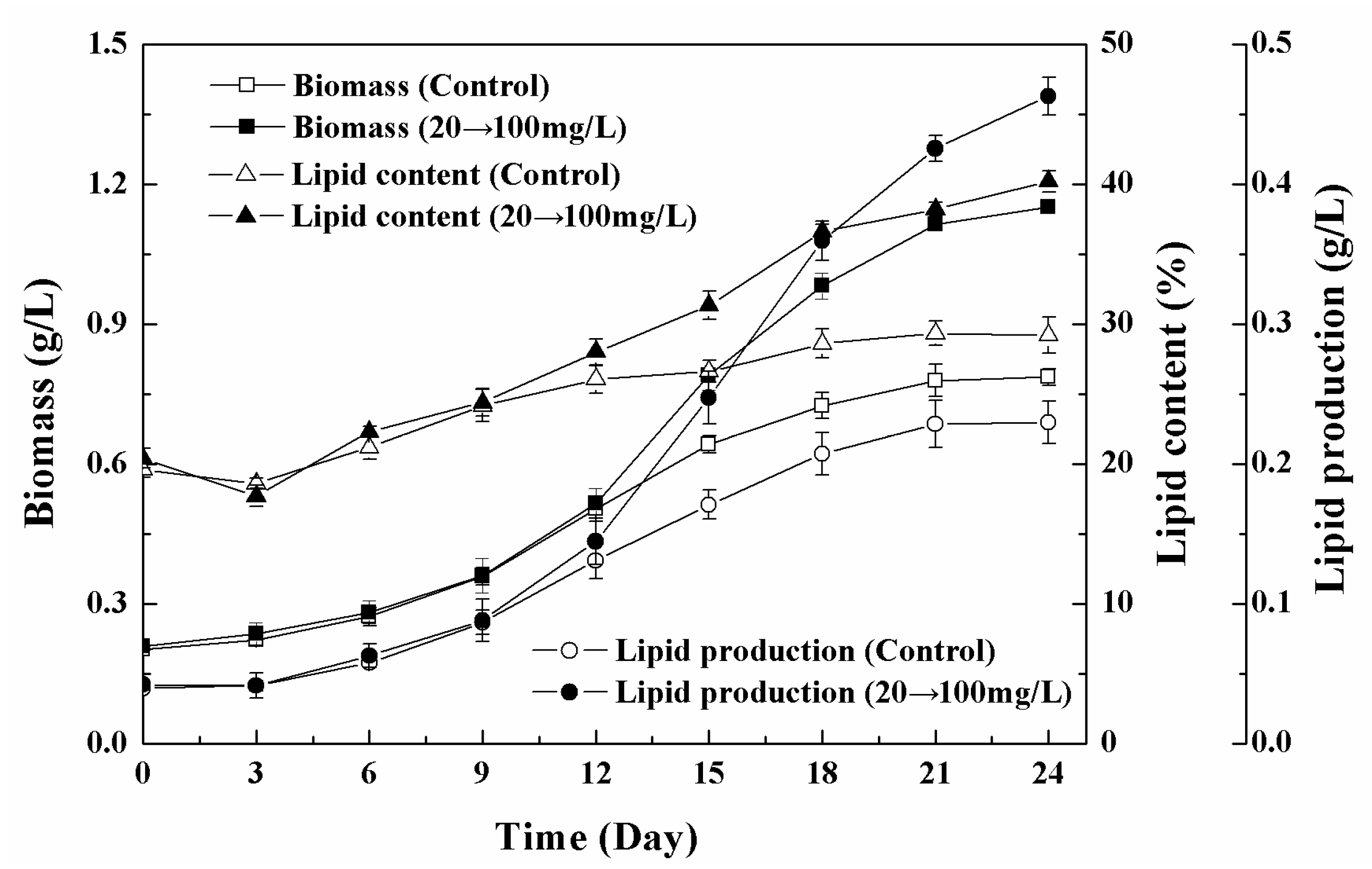
3.5. Effects of Low-High Concentration NPs Treatment on Growth and Lipid Accumulation of Chlorella sp. UJ-3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargas-Estrada, L.; Torres-Arellano, S.; Longoria, A.; Arias, D.M.; Okoye, U.; Sebastian, P. Role of nanoparticles on microalgal cultivation: A review. Fuel 2020, 280, 118598. [Google Scholar] [CrossRef]
- Georgantzopoulou, A.; Balachandran, Y.L.; Rosenkranz, P.; Dusinska, M.; Lankoff, A.; Wojewódzka, M.; Kruszewski, M.; Guignard, C.; Audinot, J.-N.; Girija, S.; et al. Ag nanoparticles: Size- and surface-dependent effects on model aquatic organisms and uptake evaluation with NanoSIMS. Nanotoxicology 2012, 7, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Besha, A.T.; Liu, Y.; Bekele, D.N.; Dong, Z.; Naidu, R.; Gebremariam, G.N. Sustainability and environmental ethics for the application of engineered nanoparticles. Environ. Sci. Policy 2020, 103, 85–98. [Google Scholar] [CrossRef]
- Pádrová, K.; Lukavský, J.; Nedbalová, L.; Čejková, A.; Cajthaml, T.; Sigler, K.; Vítová, M.; Řezanka, T. Trace concentrations of iron nanoparticles cause overproduction of biomass and lipids during cultivation of cyanobacteria and microalgae. J. Appl. Phycol. 2015, 27, 1443–1451. [Google Scholar] [CrossRef]
- He, M.; Yan, Y.; Pei, F.; Wu, M.; Gebreluel, T.; Zou, S.; Wang, C. Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rostamizadeh, E.; Iranbakhsh, A.; Majd, A.; Arbabian, S.; Mehregan, I. Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in Barley. J. Nanostructure Chem. 2020, 10, 125–130. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total. Environ. 2021, 769, 145221. [Google Scholar] [CrossRef] [PubMed]
- Elbakatoushi, R.; Tammam, A.; El-Sadekc, L.; Alframawyb, A.M. Hematite nanoparticles influence ultrastructure, antioxidant defenses, gene expression, and alleviate cadmium toxicity in zea mays. J. Plant Interact. 2020, 15, 54–74. [Google Scholar]
- Yu, Q.; Liu, Z.; Xu, H.; Zhang, B.; Zhang, M.; Li, M. TiO2 nanoparticles promote the production of unsaturated fatty acids (UFAs) fighting against oxidative stress in Pichia pastoris. RSC Adv. 2015, 5, 41033–41040. [Google Scholar] [CrossRef]
- Kazempour, Z.B.; Yazdi, M.H.; Rafii, F.; Shahverdi, A.R. Sub-inhibitory concentration of biogenic selenium nanoparticles lacks post antifungal effect for Aspergillus niger and Candida albicans and stimulates the growth of Aspergillus niger. Iran. J. Microbiol. 2013, 5, 81–85. [Google Scholar] [PubMed]
- Jeon, H.-S.; Park, S.E.; Ahn, B.; Kim, Y.-K. Enhancement of biodiesel production in Chlorella vulgaris cultivation using silica nanoparticles. Biotechnol. Bioprocess Eng. 2017, 22, 136–141. [Google Scholar] [CrossRef]
- Ren, H.Y.; Dai, Y.Q.; Kong, F.; Xing, D.; Zhao, L.; Ren, N.Q.; Ma, J.; Liu, B.F. Enhanced microalgal growth and lipid accumu-lation by addition of different nanoparticles under xenon lamp illumination. Bioresour. Technol. 2019, 297, 122409. [Google Scholar] [CrossRef]
- Hazeem, L.J.; Waheed, F.A.; Rashdan, S.; Bououdina, M.; Brunet, L.; Slomianny, C.; Boukherroub, R.; Elmeselmani, W.A. Effect of magnetic iron oxide (Fe3O4) nanoparticles on the growth and photosynthetic pigment content of Picochlorum sp. Environ. Sci. Pollut. Res. 2015, 22, 11728–11739. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Razeghifard, R. Algal biofuels. Photosynth. Res. 2013, 117, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, H.; Luo, Y.; Wan, M.; Huang, J.; Wang, W.; Li, Y. Impacts of CO2 concentration on growth, lipid accumulation, and carbon-concentrating-mechanism-related gene expression in oleaginous Chlorella. Appl. Microbiol. Biotechnol. 2015, 99, 2451–2462. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Sun, Z.; Dou, X.; Wu, J.; He, B.; Wang, Y.; Chen, Y.-F. Enhanced lipid accumulation of photoautotrophic microalgae by high-dose CO2 mimics a heterotrophic characterization. World J. Microbiol. Biotechnol. 2015, 32, 1–11. [Google Scholar] [CrossRef]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; Cha, T.S. Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 2015, 111, 65–71. [Google Scholar] [CrossRef]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; Cha, T.S. Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris (Trebouxiophyceae) during early stationary growth phase. Algal Res. 2015, 9, 14–20. [Google Scholar] [CrossRef]
- Josephine, A.; Niveditha, C.; Radhika, A.; Shali, A.B.; Kumar, T.; Dharani, G.; Kirubagaran, R. Analytical evaluation of different carbon sources and growth stimulators on the biomass and lipid production of Chlorella vulgaris–Implications for biofuels. Biomass- Bioenergy 2015, 75, 170–179. [Google Scholar] [CrossRef]
- Agirman, N.; Cetin, A.K. Effects of nitrogen starvations on biomass growth, protein and lipid amount of Chlorella vulgaris. Fresen. Environ. Bull. 2015, 24, 3643–3648. [Google Scholar]
- Zhu, S.; Wang, Y.; Shang, C.; Wang, Z.; Xu, J.; Yuan, Z. Characterization of lipid and fatty acids composition of Chlorella zofingiensis in response to nitrogen starvation. J. Biosci. Bioeng. 2015, 120, 205–209. [Google Scholar] [CrossRef]
- Sarayloo, E.; Simsek, S.; Ünlü, Y.S.; Cevahir, G.; Erkey, C.; Kavakli, I.H. Enhancement of the lipid productivity and fatty acid methyl ester profile of Chlorella vulgaris by two rounds of mutagenesis. Bioresour. Technol. 2018, 250, 764–769. [Google Scholar] [CrossRef]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano. 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Raven, J.A. Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol. 1990, 116, 1–18. [Google Scholar] [CrossRef]
- Wan, M.; Jin, X.; Xia, J.; Rosenberg, J.N.; Yu, G.; Nie, Z.; Oyler, G.A.; Betenbaugh, M.J. The effect of iron on growth, lipid accu-mulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2014, 22, 9473–9481. [Google Scholar] [CrossRef] [PubMed]
- Bibi, M.; Zhu, X.; Munir, M.; Angelidaki, I. Bioavailability and effect of α-Fe2O3 nanoparticles on growth, fatty acid composition and morphological indices of Chlorella vulgaris. Chemosphere 2021, 282, 131044. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Stanier, R.Y.; Deruelles, J.; Herdman, M.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Bligh, G.; Dyer, W. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, L.D.; Schmitz, A.A.; Pelka, J.R. Rapid Preparation of Fatty Acid Esters from Lipids for Gas Chromatographic Analysis. Anal. Chem. 1966, 38, 514–515. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E.; Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Aeby, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide disumtase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Melegari, S.; Perreault, F.; Costa, R.; Popovic, R.; Matias, W.G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2013, 142–143, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental be-havior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadar, E.; Rooks, P.; Lakey, C.; White, D. The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Sci. Total. Environ. 2012, 439, 8–17. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Schenk, P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014, 26, 14–18. [Google Scholar] [CrossRef]
- Kang, N.K.; Lee, B.; Choi, G.-G.; Moon, M.; Park, M.S.; Lim, J.; Yang, J.-W. Enhancing lipid productivity of Chlorella vulgaris using oxidative stress by TiO2 nanoparticles. Korean J. Chem. Eng. 2014, 31, 861–867. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Pérez, M.E.; Lemaire, S.; Crespo, J.L. Reactive oxygen species and autophagy in plants and algae. Plant Physiol. 2012, 160, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Redox Regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Xiang, Q.; Zhou, Y.; Xu, X.; Qiu, X.; Yu, Y.; Ahmad, F. Evaluation of the toxicity of herbicide topramezone to Chlorella vulgaris: Oxidative stress, cell morphology and photosynthetic activity. Ecotoxicol. Environ. Saf. 2017, 143, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Vranová, E.; Dat, J.F.; Inzé, D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001, 161, 405–414. [Google Scholar] [CrossRef]
- Vital, S.A.; Fowler, R.W.; Virgen, A.; Gossett, D.R.; Banks, S.W.; Rodriguez, J. Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue. Environ. Exp. Bot. 2008, 62, 60–68. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Iavicoli, I.; Calabrese, V. Hormesis: Why it is important to biogerontologists. Biogerontology 2012, 13, 215–235. [Google Scholar] [CrossRef] [PubMed]

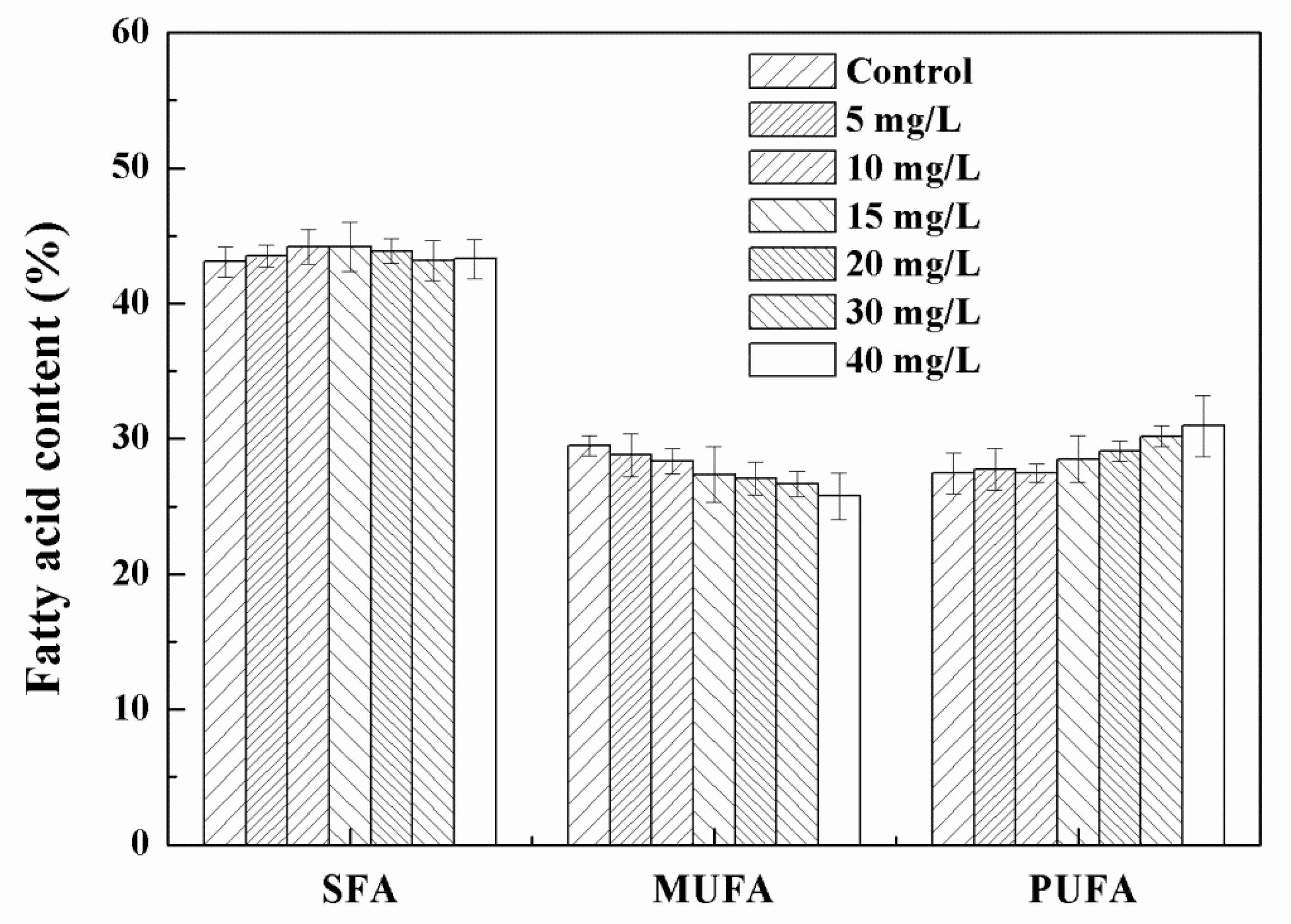

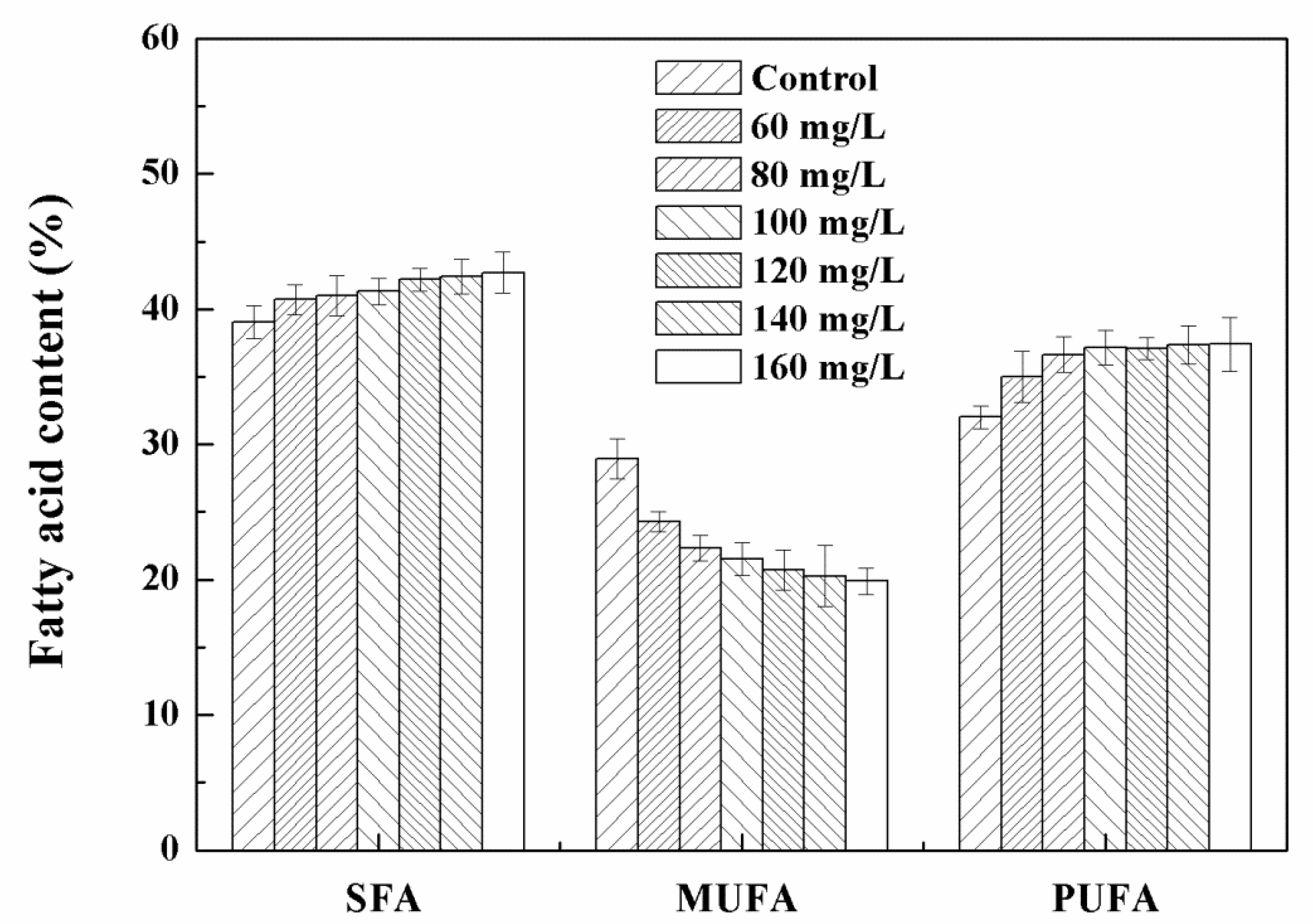
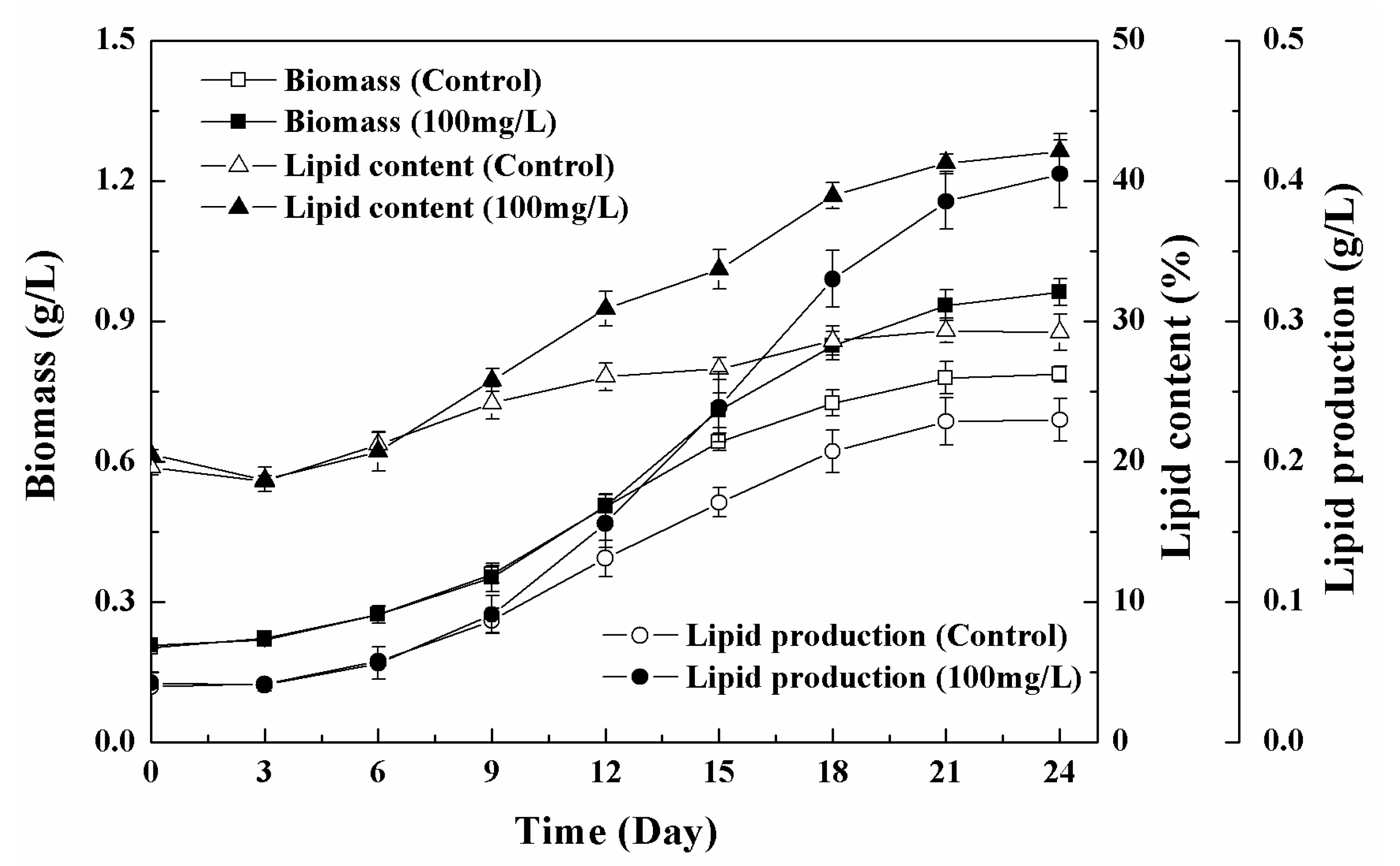
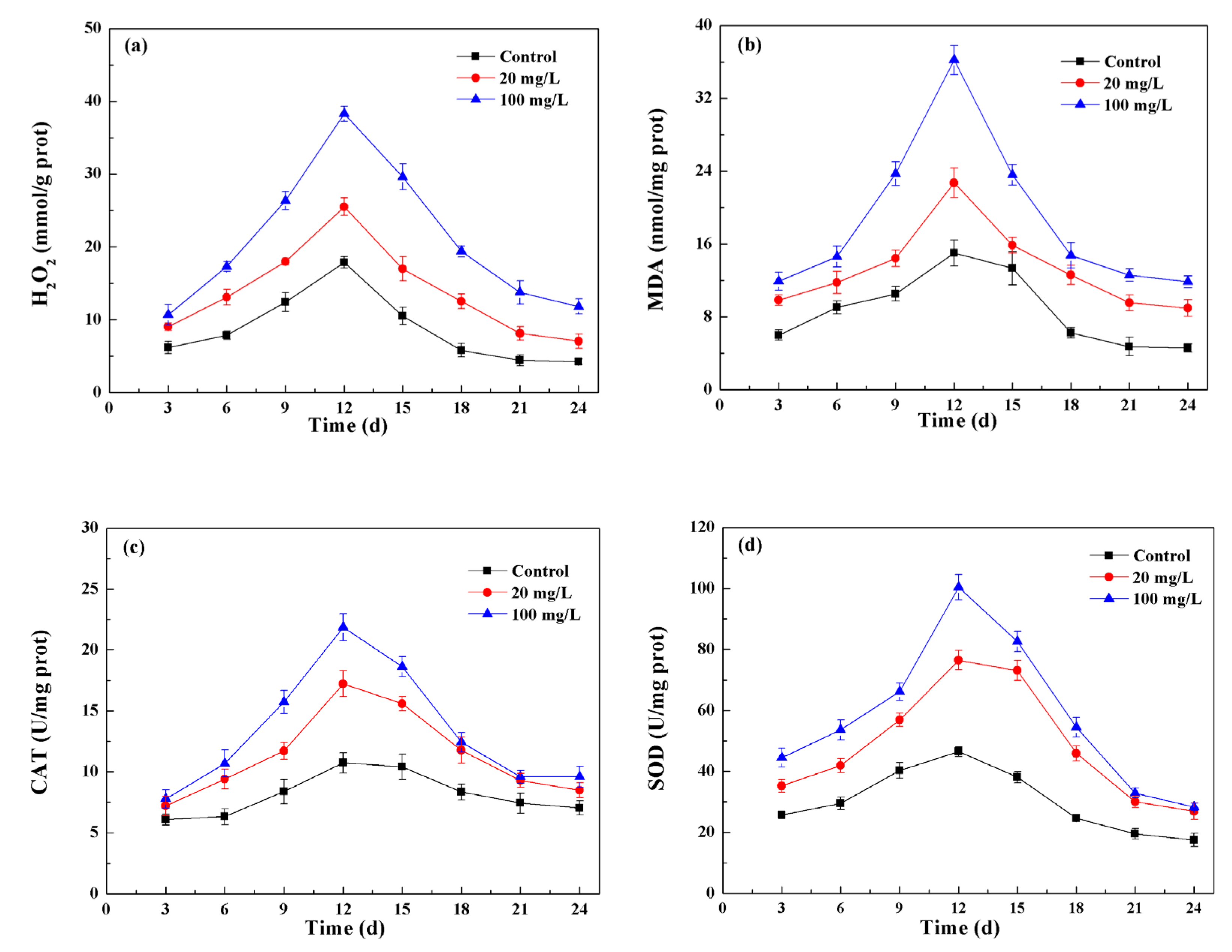
| Condition | Control | NPs Stress | ||
|---|---|---|---|---|
| Fatty Acids | Content (mg/gDW) | Composition (%Total FA) | Content (mg/gDW) | Composition (%Total FA) |
| C12:0 | 2.07 ± 0.08 | 1.35 ± 0.22 | 3.42 ± 0.10 | 1.55 ± 0.25 |
| C14:0 | 1.32 ± 0.14 | 0.86 ± 0.04 | 0.98 ± 0.03 | 0.45 ± 0.02 |
| C16:0 | 54.12 ± 1.52 | 35.20 ± 0.38 | 72.68 ± 1.89 | 32.98 ± 0.55 |
| C16:1 | 2.96 ± 0.23 | 1.92 ± 0.08 | 5.80 ± 0.43 | 2.63 ± 0.09 |
| C18:0 | 6.29 ± 0.79 | 4.09 ± 0.14 | 18.78 ± 1.65 | 8.52 ± 0.92 |
| C18:1 | 41.13 ± 0.67 | 26.75 ± 0.24 | 40.87 ± 1.74 | 18.55 ± 0.82 |
| C18:2 | 6.59 ± 0.54 | 4.29 ± 0.23 | 8.47 ± 1.20 | 3.85 ± 0.78 |
| C18:3n6 | 3.15 ± 0.42 | 2.05 ± 0.15 | 9.61 ± 0.70 | 4.36 ± 0.24 |
| C18:3n3 | 25.24 ± 1.34 | 16.41 ± 0.85 | 36.69 ± 1.55 | 16.65 ± 0.49 |
| C20:0 | 0.22 ± 0.05 | 0.14 ± 0.04 | 0.38 ± 0.03 | 0.17 ± 0.01 |
| C20:1 | 0.56 ± 0.10 | 0.36 ± 0.03 | 2.25 ± 0.15 | 1.02 ± 0.07 |
| C20:5 | 9.24 ± 0.43 | 6.01 ± 0.22 | 18.81 ± 0.86 | 8.83 ± 0.40 |
| C22:0 | 0.88 ± 0.05 | 0.57 ± 0.03 | 1.64 ± 0.07 | 0.75 ± 0.03 |
| Total | 153.77 ± 3.45 | 100.00 | 220.39 ± 4.38 | 100.00 |
| Condition | Control | NPs Stress |
|---|---|---|
| Biomass (g/L) | 0.78 ± 0.01 | 1.17 ± 0.02 |
| Total Lipid content (% DW) | 28.85 ± 2.05 | 41.22 ± 1.62 |
| Lipid production (g/L) | 0.23 ± 0.02 | 0.48 ± 0.03 |
| µmax (d−1) | 0.13 ± 0.01 | 0.16 ± 0.01 |
| Pmax (mg L−1 d−1) | 15.65 ± 0.12 | 36.55 ± 0.18 |
| Fatty acids production (g/L) | 0.12 ± 0.02 | 0.26 ± 0.02 |
| SFA composition (%) | 42.21 ± 0.44 | 44.42 ± 2.57 |
| MUFA composition (%) | 29.04 ± 2.26 | 22.20 ± 0.71 |
| PUFA composition (%) | 28.76 ± 0.79 | 33.39 ± 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, T.; Guan, W.; Xu, L.; Huo, S.; Ma, A.; Zhuang, G.; Terry, N. Development of a Strategy for Enhancing the Biomass Growth and Lipid Accumulation of Chlorella sp. UJ-3 Using Magnetic Fe3O4 Nanoparticles. Nanomaterials 2021, 11, 2802. https://doi.org/10.3390/nano11112802
Wang F, Liu T, Guan W, Xu L, Huo S, Ma A, Zhuang G, Terry N. Development of a Strategy for Enhancing the Biomass Growth and Lipid Accumulation of Chlorella sp. UJ-3 Using Magnetic Fe3O4 Nanoparticles. Nanomaterials. 2021; 11(11):2802. https://doi.org/10.3390/nano11112802
Chicago/Turabian StyleWang, Feng, Tingting Liu, Wen Guan, Ling Xu, Shuhao Huo, Anzou Ma, Guoqiang Zhuang, and Norman Terry. 2021. "Development of a Strategy for Enhancing the Biomass Growth and Lipid Accumulation of Chlorella sp. UJ-3 Using Magnetic Fe3O4 Nanoparticles" Nanomaterials 11, no. 11: 2802. https://doi.org/10.3390/nano11112802






