Renewable Carbon Nanomaterials: Novel Resources for Dental Tissue Engineering
Abstract
:1. Introduction
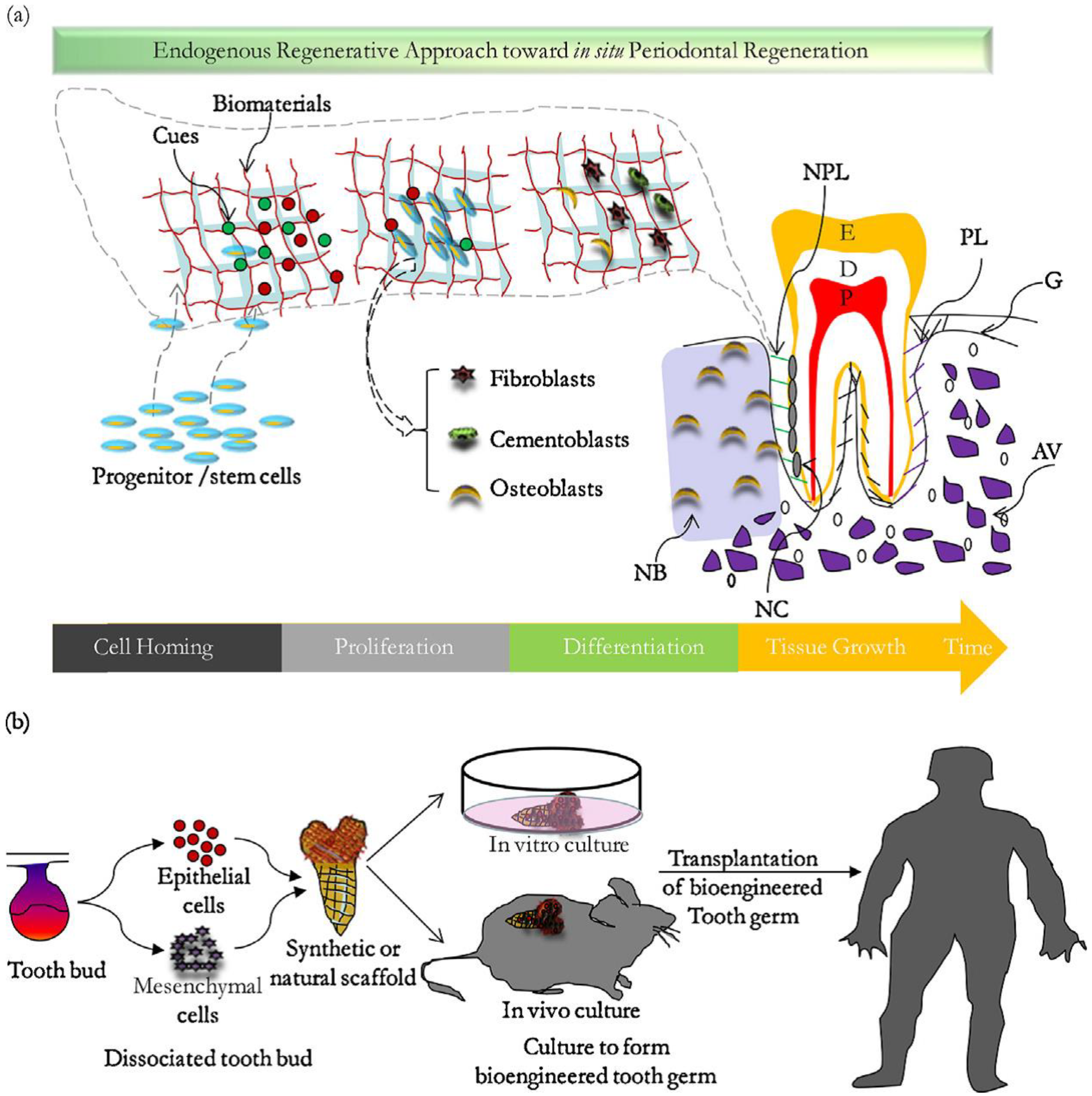
2. Tissue Engineering in Dentistry
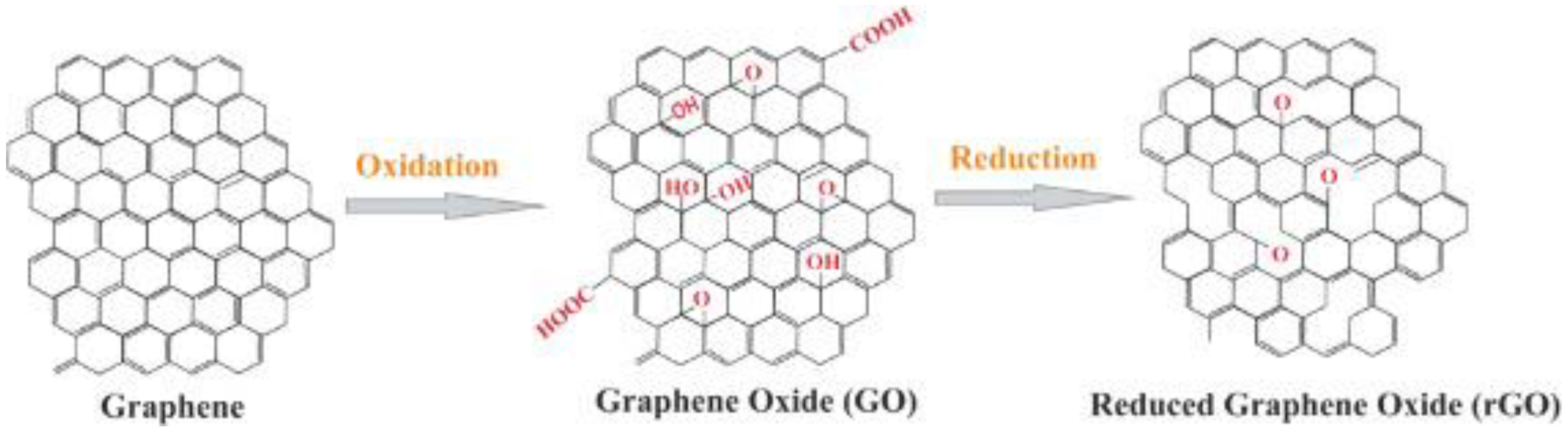
3. Graphene-Based Nanostructures
3.1. Biocompatibility and Cytotoxicity of Graphene-Based Materials
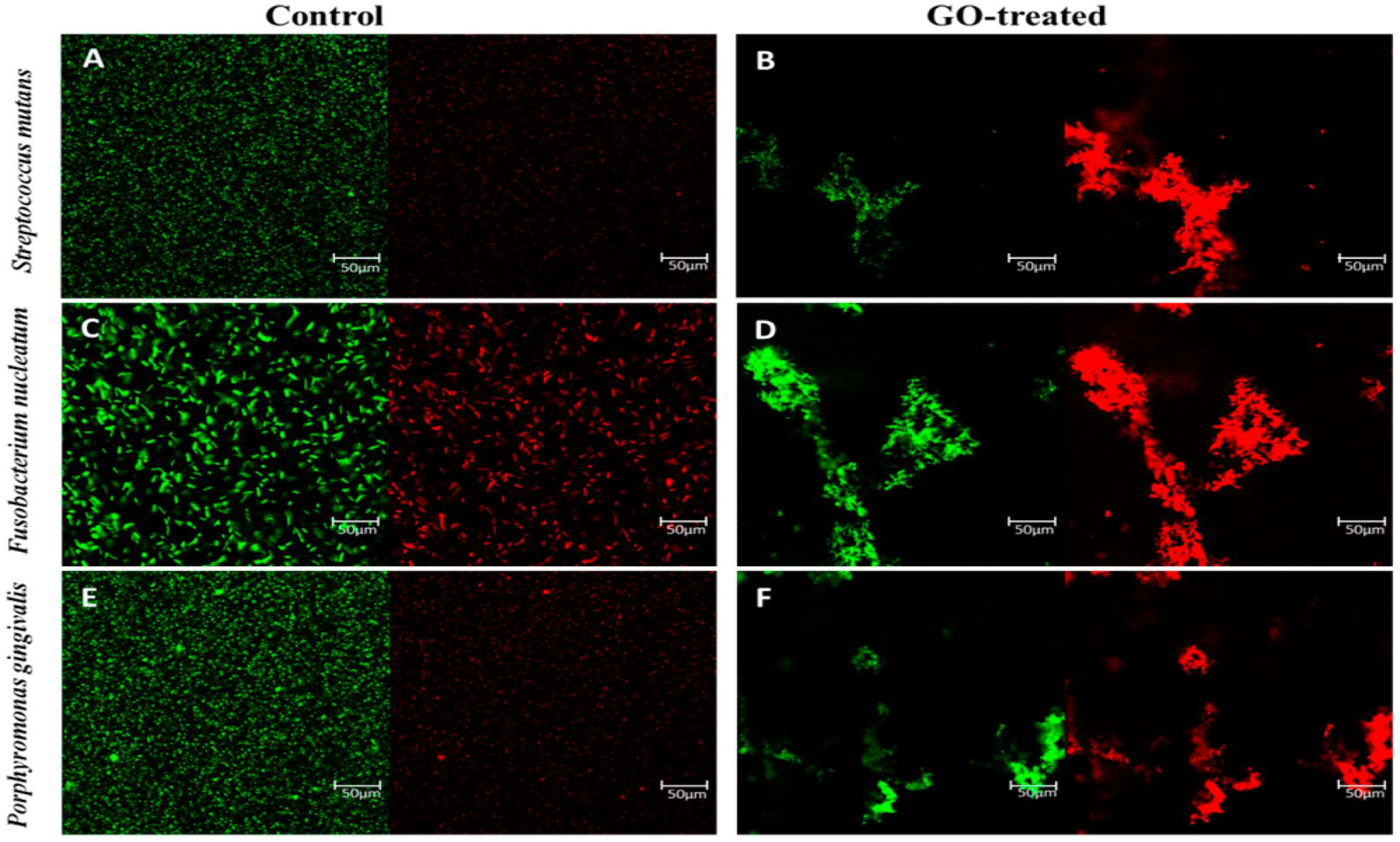
3.2. Anti-Bacterial Activity of Graphene-Based Nanomaterials

4. Applications of Graphene and Its Derivatives in the Tissue of Dentistry
5. Carbon Nanotube
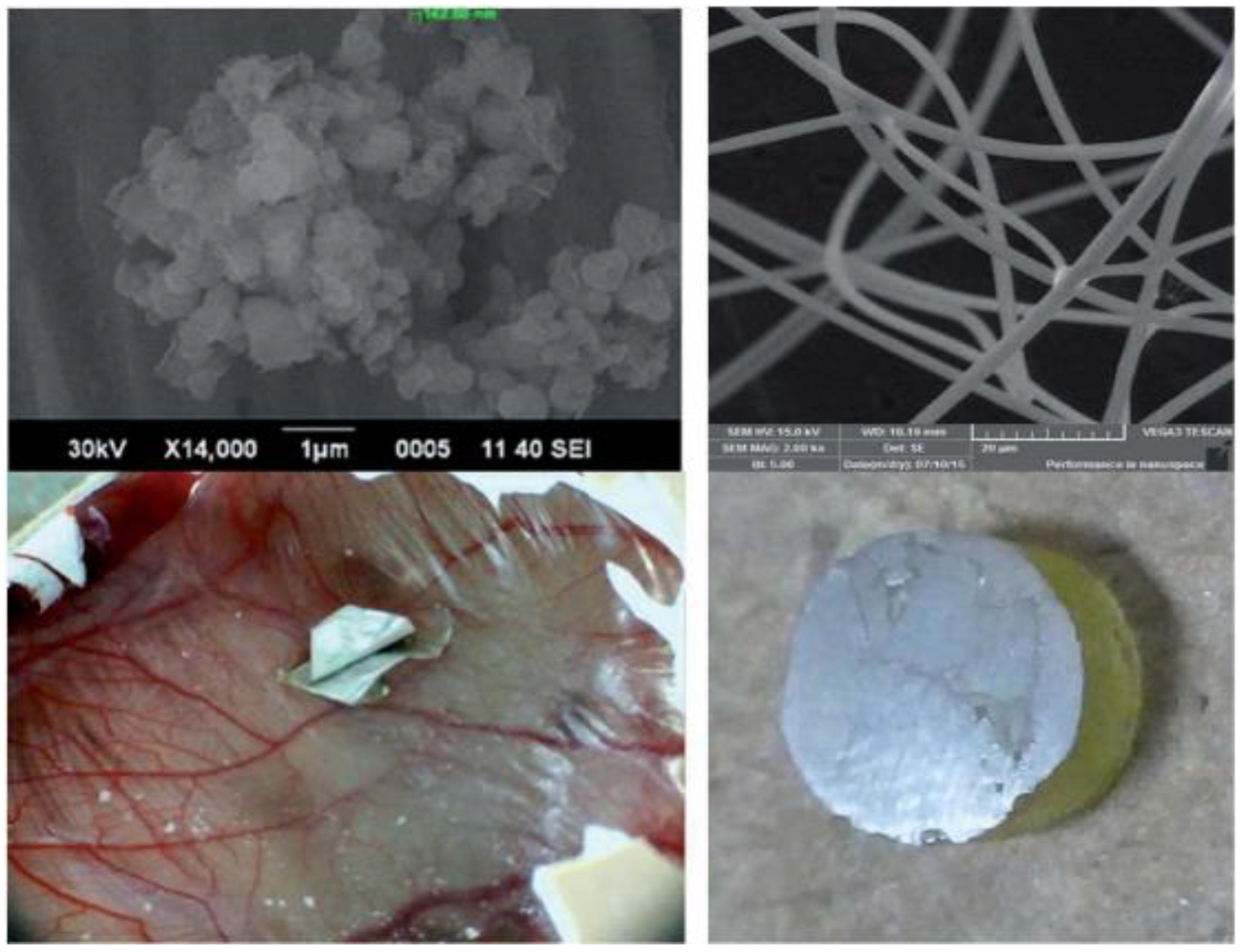
5.1. Mechanical Properties of Carbon Nanotubes on Dental Materials
5.2. Biocompatibility of Carbon Nanotubes
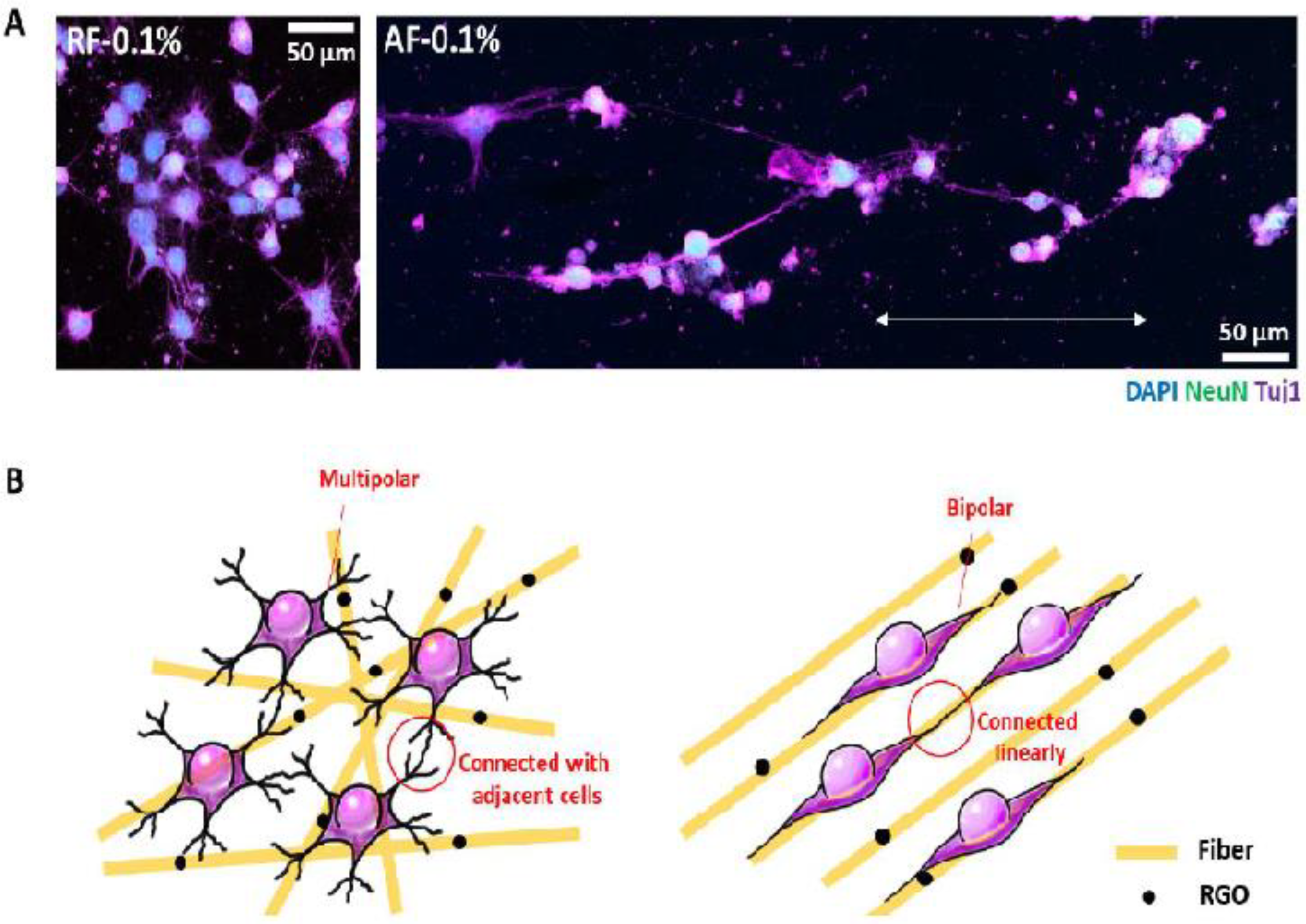
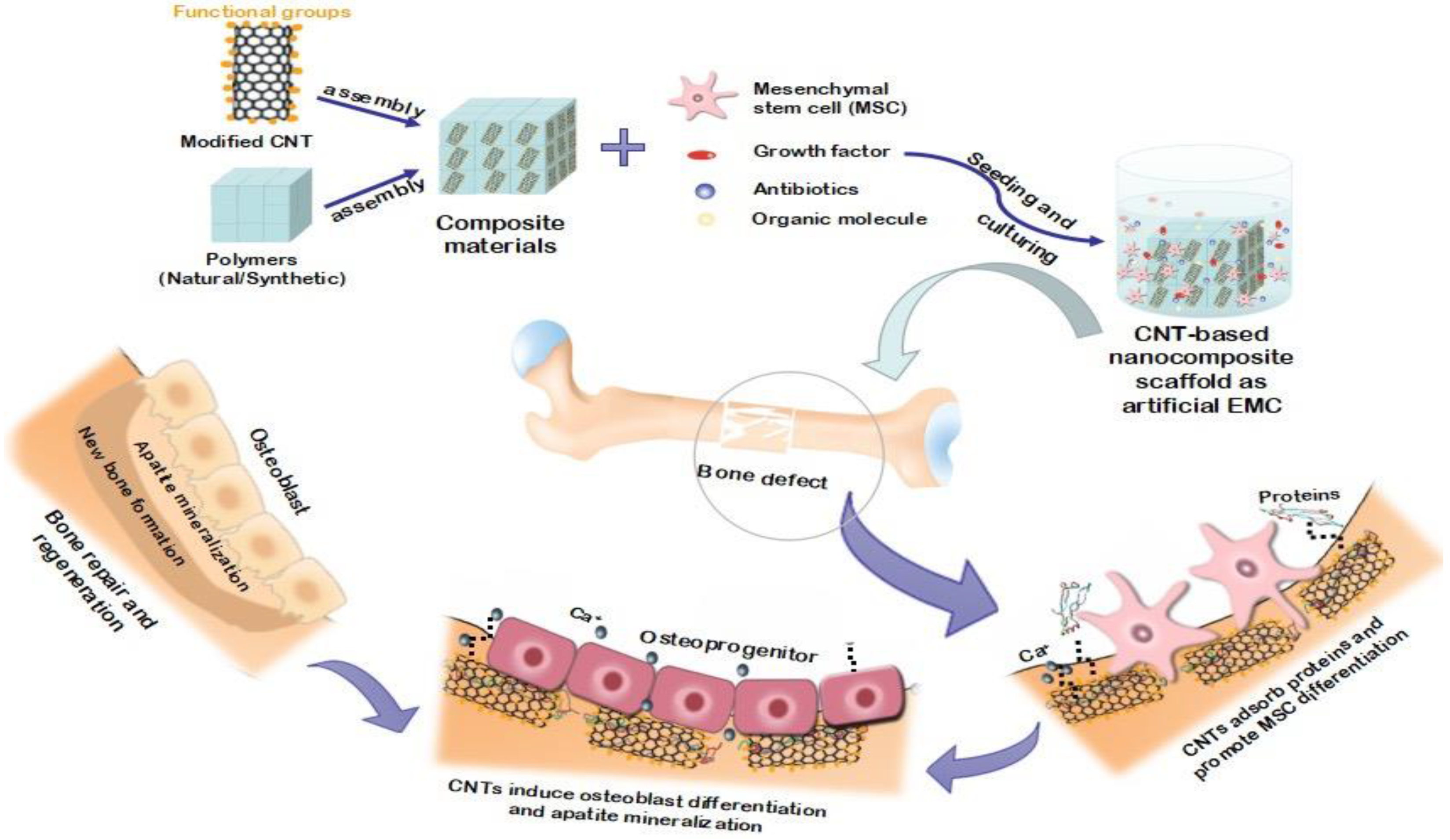
5.3. Bone Growth and Proliferation in the Presence of Carbon Nanotubes
5.4. The Antibacterial Activity of Nano Carbon
- Levels of anti-microorganisms.
- The manufacture of substances with antibacterial properties.
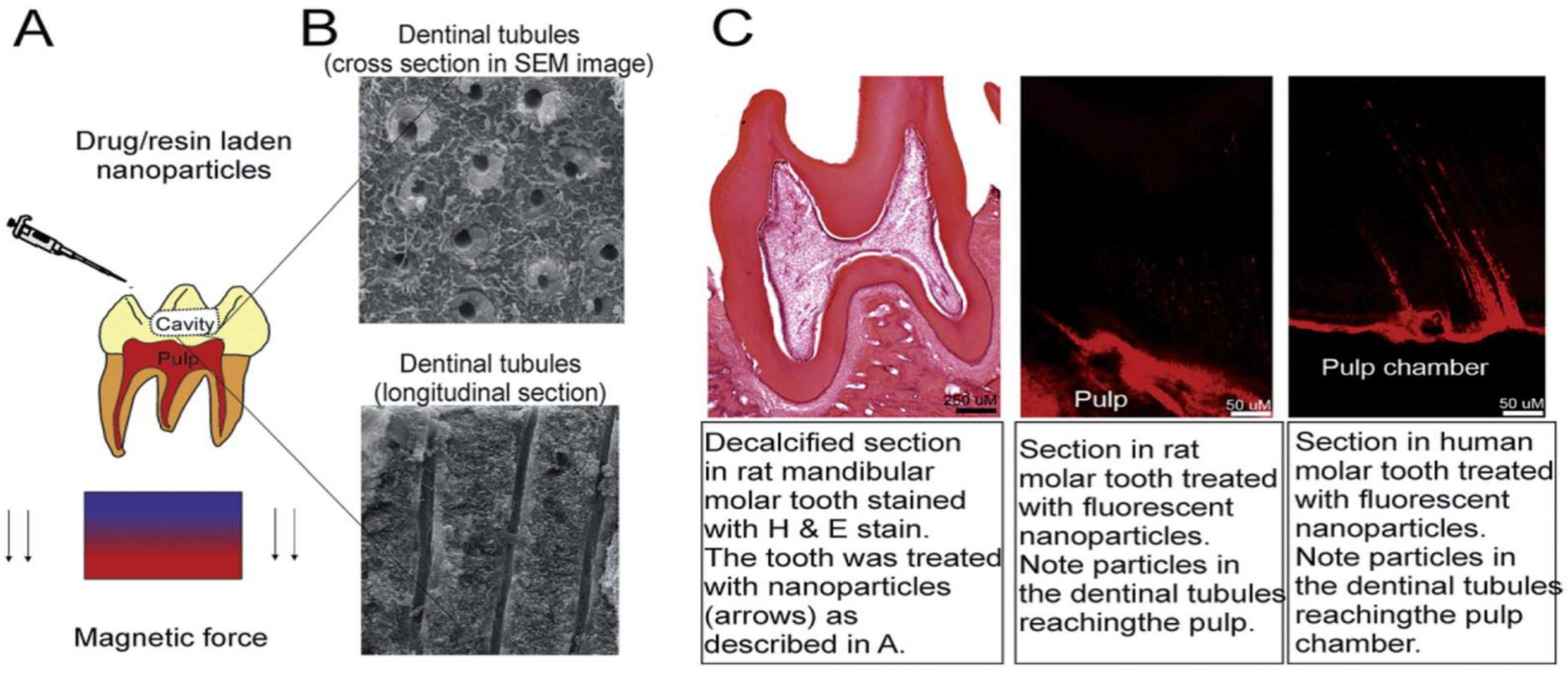
5.5. Application of Carbon Nanotubes in Dental Tissue
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitsiadis, T.A.; Woloszyk, A.; Jiménez-Rojo, L. Nanodentistry: Combining nanostructured materials and stem cells for dental tissue regeneration. Nanomedicine 2012, 7, 1743–1753. [Google Scholar] [CrossRef] [Green Version]
- Rosa, V.; Della Bona, A.; Cavalcanti, B.; Nör, J.E. Tissue engineering: From research to dental clinics. Dent. Mater. 2012, 28, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A. Gold nanostars-diagnosis, bioimaging and biomedical applications. Drug Metab. Rev. 2020, 52, 299–318. [Google Scholar] [CrossRef]
- Mousavi, S.; Hashemi, S.; Mazraedoost, S.; Yousefi, K.; Gholami, A.; Behbudi, G.; Ramakrishna, S.; Omidifar, N.; Alizadeh, A.; Chiang, W.-H. Multifunctional gold nanorod for therapeutic applications and pharmaceutical delivery considering cellular metabolic responses, oxidative stress and cellular longevity. Nanomaterials 2021, 11, 1868. [Google Scholar] [CrossRef]
- Ghahramani, Y.; Javanmardi, N. Graphene oxide quantum dots and their applications via stem cells: A mini-review. Adv. Appl. NanoBio-Technol. 2021, 2, 54–56. [Google Scholar]
- Moradlou, O.; Rabiei, Z.; Delavari, N. Antibacterial effects of carbon quantum dots@ hematite nanostructures deposited on titanium against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A Chem. 2019, 379, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Son, S.-A.; Kim, D.-H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, Y.-I. Mesoporous bioactive glass combined with graphene oxide quantum dot as a new material for a new treatment option for dentin hypersensitivity. Nanomaterials 2020, 10, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Li, D.; Mou, X.; Li, J.; Guo, W.; Wang, S.; Yu, X.; Ma, B.; Zhang, S.; Tang, W.; et al. Effects of graphene quantum dots on the self-renewal and differentiation of mesenchymal stem cells. Adv. Health Mater. 2016, 5, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Hashemi, S.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.; Gholami, A.; Chiang, W.-H. Recent advancements in polythiophene-based materials and their biomedical, geno sensor and DNA detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef] [PubMed]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [Green Version]
- Neel, E.A.A.; Chrzanowski, W.; Salih, V.M.; Kim, H.-W.; Knowles, J.C. Tissue engineering in dentistry. J. Dent. 2014, 42, 915–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, R.E.; Windisch, S.I.; Eggenschwiler, A.M.; Thoma, D.S.; Weber, F.E.; Hämmerle, C.H.F. A randomized-controlled clinical trial evaluating clinical and radiological outcomes after 3 and 5 years of dental implants placed in bone regenerated by means of GBR techniques with or without the addition of BMP-2. Clin. Oral Implant. Res. 2009, 20, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Martins-Júnior, P.A.; Alcântara, C.E.; Resende, R.R.; Ferreira, A.J. Carbon nanotubes: Directions and perspectives in oral regenerative medicine. J. Dent. Res. 2013, 92, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Sun, H.-H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef] [PubMed]
- de Oliva, M.A.; Maximiano, W.M.A.; de Castro, L.M.S.; da Silva, P.E., Jr.; Fernandes, R.R.; Ciancaglini, P.; Beloti, M.M.; Nanci, A.; Rosa, A.L.; de Oliveira, P.T. Treatment with a growth factor–protein mixture inhibits formation of mineralized nodules in osteogenic cell cultures grown on titanium. J. Histochem. Cytochem. 2009, 57, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Low, F.W.; Hashemi, S.A.; Samsudin, N.A.; Shakeri, M.; Yusoff, Y.; Rahsepar, M.; Lai, C.W.; Babapoor, A.; Soroshnia, S.; et al. Development of hydrophobic reduced graphene oxide as a new efficient approach for photochemotherapy. RSC Adv. 2020, 10, 12851–12863. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2009, 110, 132–145. [Google Scholar] [CrossRef]
- Gholami, A.; Emadi, F.; Nazem, M.; Aghayi, R.; Khalvati, B.; Amini, A.; Ghasemi, Y. Expression of key apoptotic genes in hepatocellular carcinoma cell line treated with etopo-side-loaded graphene oxide. J. Drug Deliv. Sci. Technol. 2020, 57, 101725. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Emadi, F.; Gholami, A. A Comprehensive insight towards pharmaceutical aspects of graphene nanosheets. Curr. Pharm. Biotechnol. 2020, 21, 1016–1027. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Emadi, F.; Amini, A.; Shokripour, M.; Chashmpoosh, M.; Omidifar, N. Functionalization of graphene oxide nanosheets can reduce their cytotoxicity to dental pulp stem cells. J. Nanomater. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Omidifar, N.; Zarei, M.; Bahrani, S.; Yousefi, K.; Chiang, W.-H.; Babapoor, A. Bioinorganic synthesis of polyrhodanine stabilized Fe3O4/Graphene oxide in microbial supernatant media for anticancer and antibacterial applications. Bioinorg. Chem. Appl. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Aligholi, H.; Zeraatpisheh, Z.; Gholami, A.; Mirzaei, E. Collagen/chitosan-functionalized graphene oxide hydrogel provide a 3D matrix for neural stem/precursor cells survival, adhesion, infiltration and migration. J. Bioact. Compat. Polym. 2021, 36, 296–313. [Google Scholar] [CrossRef]
- Han, X.; Li, S.; Peng, Z.; Al-Yuobi, A.R.O.; Bashammakh, A.S.O.; Leblanc, R.M. Interactions between carbon nanomaterials and biomolecules. J. Oleo Sci. 2016, 65, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Mazraedoost, S.; Chiang, W.H.; Arjmand, O.; Omidifar, N.; Babapoor, A. Precise blood glucose sensing by nitrogen-doped graphene quantum dots for tight control of diabetes. J. Sens. 2021, 894, 115341. [Google Scholar]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Agharkar, M.; Kochrekar, S.; Hidouri, S.; Azeez, M.A. Trends in green reduction of graphene oxides, issues and challenges: A review. Mater. Res. Bull. 2014, 59, 323–328. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Alternative methods and nature-based reagents for the reduction of graphene oxide: A review. Carbon 2015, 94, 224–242. [Google Scholar] [CrossRef]
- De Silva, K.; Huang, H.-H.; Joshi, R.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Lu, J.; Do, I.; Drzal, L.T.; Worden, R.M.; Lee, I. Nanometal-decorated exfoliated graphite nanoplatelet based glucose biosensors with high sensitivity and fast response. ACS Nano 2008, 2, 1825–1832. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Qiu, J.; Yan, M.; Liu, H.; Ji, D.; Huang, J.; Yu, J.; Liu, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017, 33, 525–535. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Pompa, L.; Haider, W. Influence of Electropolishing and Magnetoelectropolishing on Corrosion and Biocompatibility of Titanium Implants. J. Mater. Eng. Perform. 2014, 23, 3907–3915. [Google Scholar] [CrossRef]
- Ren, L.; Pan, S.; Li, H.; Li, Y.; He, L.; Zhang, S.; Che, J.; Niu, Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- La, W.G.; Jin, M.; Park, S.; Yoon, H.H.; Jeong, G.J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9 (Suppl. 1), 107. [Google Scholar]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.-P.; Jiang, W.; Sun, B.; Hersam, M.C.; et al. Surface oxidation of graphene oxide determines membrane damage, lipid peroxidation, and cytotoxicity in macrophages in a pulmonary toxicity model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hao, L.; Huang, Q.; Zhao, D.; Li, Q.; Cai, X. Tea polyphenol-reduced graphene oxide deposition on titanium surface enhances osteoblast bioactivity. J. Nanosci. Nanotechnol. 2018, 18, 3134–3140. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Khan, S.; Meena, R.; Singh, B.R.; Khan, A.U. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling 2014, 30, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wang, C.; Jiang, C.; Sun, J.; Yu, C.; Jiao, T. Graphene Oxide Enables the reosteogenesis of previously contaminated titanium in vitro. J. Dent. Res. 2020, 99, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Ruddock, F.M.; Dowling, A.H.; Byrne, K.; Schmitt, W.; Khalakhan, I.; Nemoto, Y.; Guo, H.; Shrestha, L.K.; Ariga, K.; et al. Graphene composites with dental and biomedical applicability. Beilstein J. Nanotechnol. 2018, 9, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadrezaei, D.; Golzar, H.; Rezai Rad, M.; Omidi, M.; Rashedi, H.; Yazdian, F.; Khojasteh, A.; Tayebi, L. In vitro effect of graphene structures as an osteoinductive factor in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. Part A 2018, 106, 2284–2343. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.-L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Duan, G.; Zhang, Y.; Luan, B.; Weber, J.K.; Zhou, R.; Yang, Z.; Zhao, L.; Xu, J.; Luo, J.; Zhou, R. Graphene-induced pore formation on cell membranes. Sci. Rep. 2017, 7, 42767. [Google Scholar] [CrossRef] [Green Version]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Singh, V.; Joung, D.; Dowding, J.M.; Reid, D.; Anderson, J.; Zhai, L.; Khondaker, S.I.; Self, W.T.; et al. Oxygenated functional group density on graphene oxide: Its effect on cell toxicity. Part. Part. Syst. Charact. 2013, 30, 148–157. [Google Scholar] [CrossRef]
- Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Polymer surface adsorption as a strategy to improve the bio-compatibility of graphene nanoplatelets. Coll. Surf. B Biointerfaces 2016, 146, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Wang, S.; Ma, J.; Xu, M.; Gao, M.; Liu, R.; Chen, W.; Liu, S. Reduction of graphene oxide alters its cyto-compatibility towards primary and immortalized macrophages. Nanoscale 2018, 10, 14637–14650. [Google Scholar] [CrossRef] [PubMed]
- Chwalibog, A.; Jaworski, S.; Sawosz, E.; Kutwin, M.; Wierzbicki, M.; Hinzmann, M.; Grodzik, M.; Winnicka, A.; Lipinska, L.; Wlodyga, K. In vitro and in vivo effects of graphene oxide and reduced graphene oxide on glioblastoma. Int. J. Nanomed. 2015, 10, 1585–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of exfoliated graphite oxide under alkaline conditions: A green route to graphene preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Nagaraju, G.; Nagabhushana, H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in-vitro cytotoxicity against human cancer cell lines. Biotechnol. Rep. 2019, 24, e00376. [Google Scholar] [CrossRef]
- Shubha, P.; Namratha, K.; Aparna, H.; Ashok, N.; Mustak, M.; Chatterjee, J.; Byrappa, K. Facile green reduction of graphene oxide using Ocimum sanctum hydroalcoholic extract and evaluation of its cellular toxicity. Mater. Chem. Phys. 2017, 198, 66–72. [Google Scholar] [CrossRef]
- Thiyagarajulu, N.; Arumugam, S. Green synthesis of reduced graphene oxide nanosheets using leaf extract of lantana camara and its in-vitro biological activities. J. Clust. Sci. 2020, 32, 559–568. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, I.; Magalhães, F. Graphene-based materials biocompatibility: A review. Coll. Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Coll. Surf. B Biointerfaces 2016, 146, 70–83. [Google Scholar] [CrossRef]
- Zhao, M.; Shan, T.; Wu, Q.; Gu, L. The antibacterial effect of graphene oxide on streptococcus mutans. J. Nanosci. Nanotechnol. 2020, 20, 2095–2103. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Carpio, I.E.M.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef] [PubMed]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 1–13. [Google Scholar] [CrossRef]
- Strużycka, I. The Oral Microbiome in Dental Caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef]
- Allaker, R.P.; Memarzadeh, K. Nanoparticles and the control of oral infections. Int. J. Antimicrob. Agents 2014, 43, 95–104. [Google Scholar] [CrossRef]
- Song, C.; Yang, C.-M.; Sun, X.-F.; Xia, P.-F.; Qin, J.; Guo, B.-B.; Wang, S.-G. Influences of graphene oxide on biofilm formation of gram-negative and gram-positive bacteria. Environ. Sci. Pollut. Res. 2017, 25, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Low, F.W.; Hashemi, S.A.; Lai, C.W.; Ghasemi, Y.; Soroshnia, S.; Savardashtaki, A.; Babapoor, A.; Rumjit, N.P.; Goh, S.M.; et al. Development of graphene based nanocomposites towards medical and biological applications. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.O.; Bhandari, P.V.; Deshmukh, P.K.; Mahale, S.S.; Patil, A.G.; Bafna, H.R.; Patel, K.V.; Bari, S.B. Green synthesis of graphene based silver nanocomposite for enhanced antibacterial activity against dental pathogens. JSM Nanotechnol. SciMed. Central USA 2017, 5, 1–7. [Google Scholar]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Moazzami, F.; Ghahramani, Y.; Tamaddon, A.M.; Nazhavani, A.D.; Adl, A. A Histological comparison of a new pulp capping material and mineral trioxide aggregate in rat molars. Iran. Endod. J. 2013, 9, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, N.; Hasanzadeh, M.; Khalilzadeh, B. Graphene based scaffolds on bone tissue engineering. Bioengineered 2017, 9, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.; Zarei, M.; Hashemi, S.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.; Gholami, A.; Omidifar, N.; Shokripour, M. Asymmetric membranes: A potential scaffold for wound healing applications. Symmetry 2020, 12, 1100. [Google Scholar] [CrossRef]
- Gholami, A.; Hashemi, S.A.; Yousefi, K.; Mousavi, S.M.; Chiang, W.-H.; Ramakrishna, S.; Mazraedoost, S.; Alizadeh, A.; Omidifar, N.; Behbudi, G.; et al. 3D Nanostructures for tissue engineering, cancer therapy, and gene delivery. J. Nanomater. 2020, 2020, 1–24. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, I.R.; Kim, Y.; Kim, D.H.; Park, S.B.; Park, B.S.; Bae, M.K.; Kim, Y.I. The effect of mesoporous bioactive glass nanoparticles/graphene oxide composites on the differentiation and mineralization of human dental pulp stem cells. Nanomaterials 2020, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Dan, S.; Upadhyay, S.K.; Pant, M. Synergistic approach of graphene oxide-silver-titanium nanocomposite film in oral and dental studies: A new paradigm of infection control in dentistry. Biointerface Res. Appl. Chem. 2021, 11, 3182. [Google Scholar]
- Radunovic, M.; De Colli, M.; De Marco, P.; Di Nisio, C.; Fontana, A.; Piattelli, A.; Cataldi, A.; Zara, S. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J. Biomed. Mater. Res. Part A 2017, 105, 2312–2320. [Google Scholar] [CrossRef]
- Xie, H.; Chua, M.; Islam, I.; Bentini, R.; Cao, T.; Viana-Gomes, J.C.; Neto, A.H.C.; Rosa, V. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. 2016, 33, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, P.; Li, X.; Liu, H.; Ge, S. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci. Rep. 2016, 6, srep19343. [Google Scholar] [CrossRef]
- Seonwoo, H.; Jang, K.J.; Lee, D.; Park, S.; Lee, M.; Park, S.; Lim, K.T.; Kim, J.; Chung, J.H. Neurogenic differentiation of human dental pulp stem cells on graphene-polycaprolactone hybrid nanofibers. Nanomaterials 2018, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M.; Nishida, E. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C.; Rao, A.M. Carbon nanotubes. In The Physics of Fullerene-Based and Fullerene-Related Materials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 331–379. [Google Scholar]
- Jia, Z.; Wang, Z.; Liang, J.; Wei, B.; Wu, D. Production of short multi-walled carbon nanotubes. Carbon 1999, 37, 903–906. [Google Scholar] [CrossRef]
- Castro-Rojas, M.A.; Vega-Cantu, Y.I.; Cordell, G.A.; Rodriguez-Garcia, A. Dental applications of carbon nanotubes. Molecules 2021, 26, 4423. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Ryu, B.; Sudha, P.N.; Kim, S.K. Preparation and characterization of chitosan–carbon nanotube scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 393–402. [Google Scholar] [CrossRef]
- Gupta, A.; Liberati, T.A.; Verhulst, S.J.; Main, B.J.; Roberts, M.H.; Potty, A.G.R.; Pylawka, T.K.; Iii, S.F.E.-A. Biocompatibility of single-walled carbon nanotube composites for bone regeneration. Bone Jt. Res. 2015, 4, 70–77. [Google Scholar] [CrossRef]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone Cell Proliferation on Carbon Nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of carbon nanotubes in bone tissue regeneration and engineering: Superiority, concerns, current advancements, and prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarrategi, A.; Gutierrez, M.C.; Moreno-Vicente, C.; Hortigüela, M.J.; Ramos, V.; López-Lacomba, J.L.; Ferrer, M.L.; del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Orlovskii, V.P.; Komlev, V.; Barinov, S.M. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg. Mater. 2002, 38, 973–984. [Google Scholar] [CrossRef]
- Pawelec, K.M.; White, A.A.; Best, S.M. Properties and characterization of bone repair materials. In Bone Repair Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 65–102. [Google Scholar] [CrossRef]
- Khan, A.S.; Hussain, A.N.; Sidra, L.; Sarfraz, Z.; Khalid, H.; Khan, M.; Manzoor, F.; Shahzadi, L.; Yar, M.; Rehman, I.U. Fabrication and in vivo evaluation of hydroxyapatite/carbon nanotube electrospun fibers for bio-medical/dental application. Mater. Sci. Eng. C 2017, 80, 387–396. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.-H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 25, 1–20. [Google Scholar]
- Li, Y.; Hu, X.; Xia, Y.; Ji, Y.; Ruan, J.; Weir, M.D.; Lin, X.; Nie, Z.; Gu, N.; Masri, R.; et al. Novel magnetic nanoparticle-containing adhesive with greater dentin bond strength and antibacterial and remineralizing capabilities. Dent. Mater. 2018, 34, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Avval, Z.M.; Malekpour, L.; Raeisi, F.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Salari, M. Introduction of magnetic and supermagnetic nanoparticles in new approach of targeting drug delivery and cancer therapy application. Drug Metab. Rev. 2019, 52, 157–184. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, S.M.; Yousefi, K.; Hashemi, S.A.; Afsa, M.; BahranI, S.; Gholami, A.; Ghahramani, Y.; Alizadeh, A.; Chiang, W.-H. Renewable Carbon Nanomaterials: Novel Resources for Dental Tissue Engineering. Nanomaterials 2021, 11, 2800. https://doi.org/10.3390/nano11112800
Mousavi SM, Yousefi K, Hashemi SA, Afsa M, BahranI S, Gholami A, Ghahramani Y, Alizadeh A, Chiang W-H. Renewable Carbon Nanomaterials: Novel Resources for Dental Tissue Engineering. Nanomaterials. 2021; 11(11):2800. https://doi.org/10.3390/nano11112800
Chicago/Turabian StyleMousavi, Seyyed Mojtaba, Khadije Yousefi, Seyyed Alireza Hashemi, Marzie Afsa, Sonia BahranI, Ahmad Gholami, Yasmin Ghahramani, Ali Alizadeh, and Wei-Hung Chiang. 2021. "Renewable Carbon Nanomaterials: Novel Resources for Dental Tissue Engineering" Nanomaterials 11, no. 11: 2800. https://doi.org/10.3390/nano11112800
APA StyleMousavi, S. M., Yousefi, K., Hashemi, S. A., Afsa, M., BahranI, S., Gholami, A., Ghahramani, Y., Alizadeh, A., & Chiang, W.-H. (2021). Renewable Carbon Nanomaterials: Novel Resources for Dental Tissue Engineering. Nanomaterials, 11(11), 2800. https://doi.org/10.3390/nano11112800








