Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application
Abstract
1. Introduction
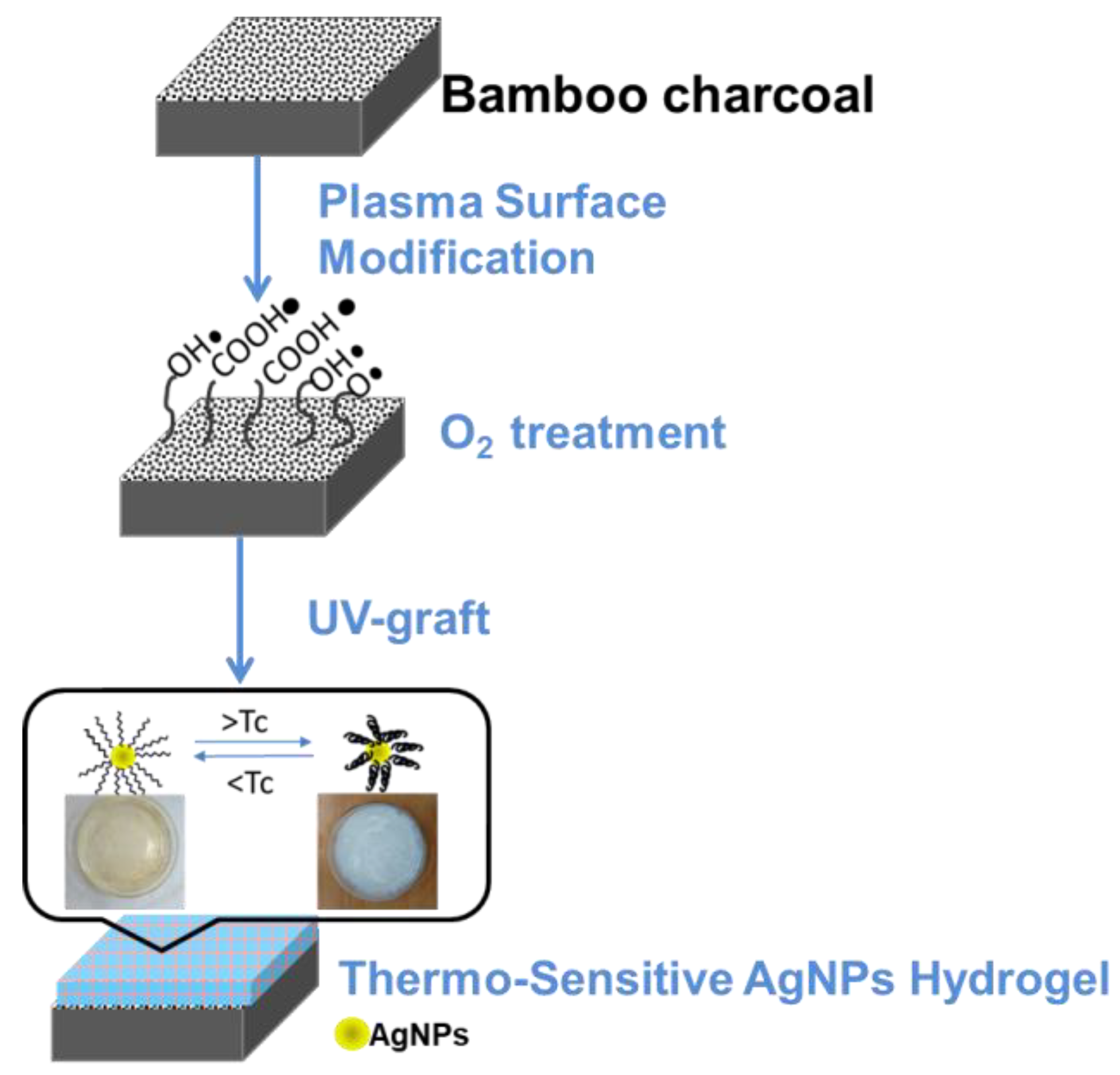
2. Materials and Methods
2.1. Pretreatment of Materials
2.2. Synthesis of Silver Nanoparticles (AgNPs)
2.3. O2 Plasma Activation Pre-Treatment
2.4. Thermo-Sensitive AgNPs Hydrogel by UV Light Surface Graft Polymerization
2.5. Characterization Analysis
2.5.1. UV-VIS Spectra
2.5.2. Wettability (Surface Hydrophobicity/Hydrophilicity) Test
2.5.3. Surface Characterization
2.5.4. Swelling Studies of the Treatment BC
2.6. Cytotoxicity Test of Treatment BC
2.7. Antibacterial Efficacy Test
3. Results and Discussion
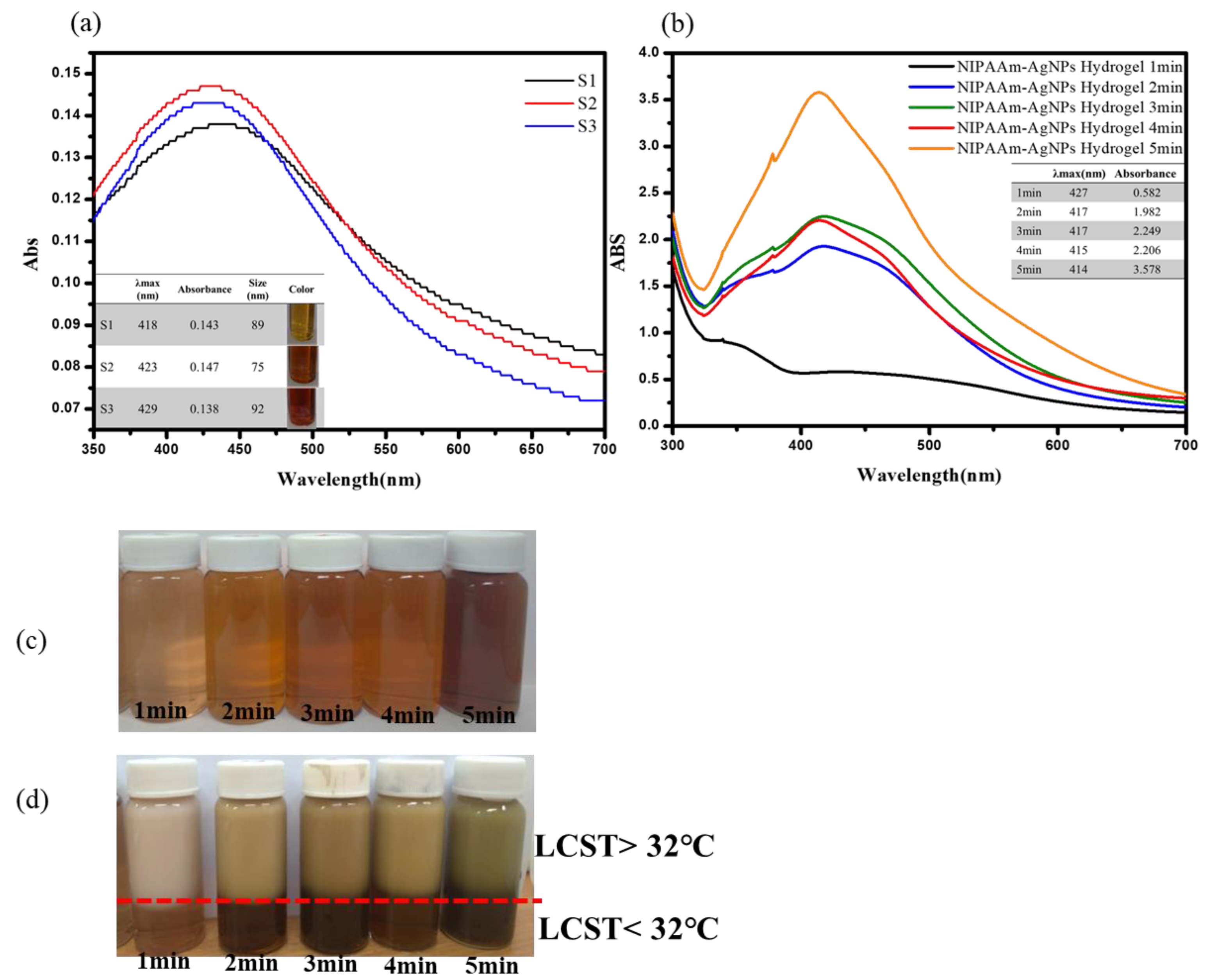
3.1. UV-Visible Spectroscopy Characterization of the AgNPs and Thermo-Sensitive AgNPs Hydrogels
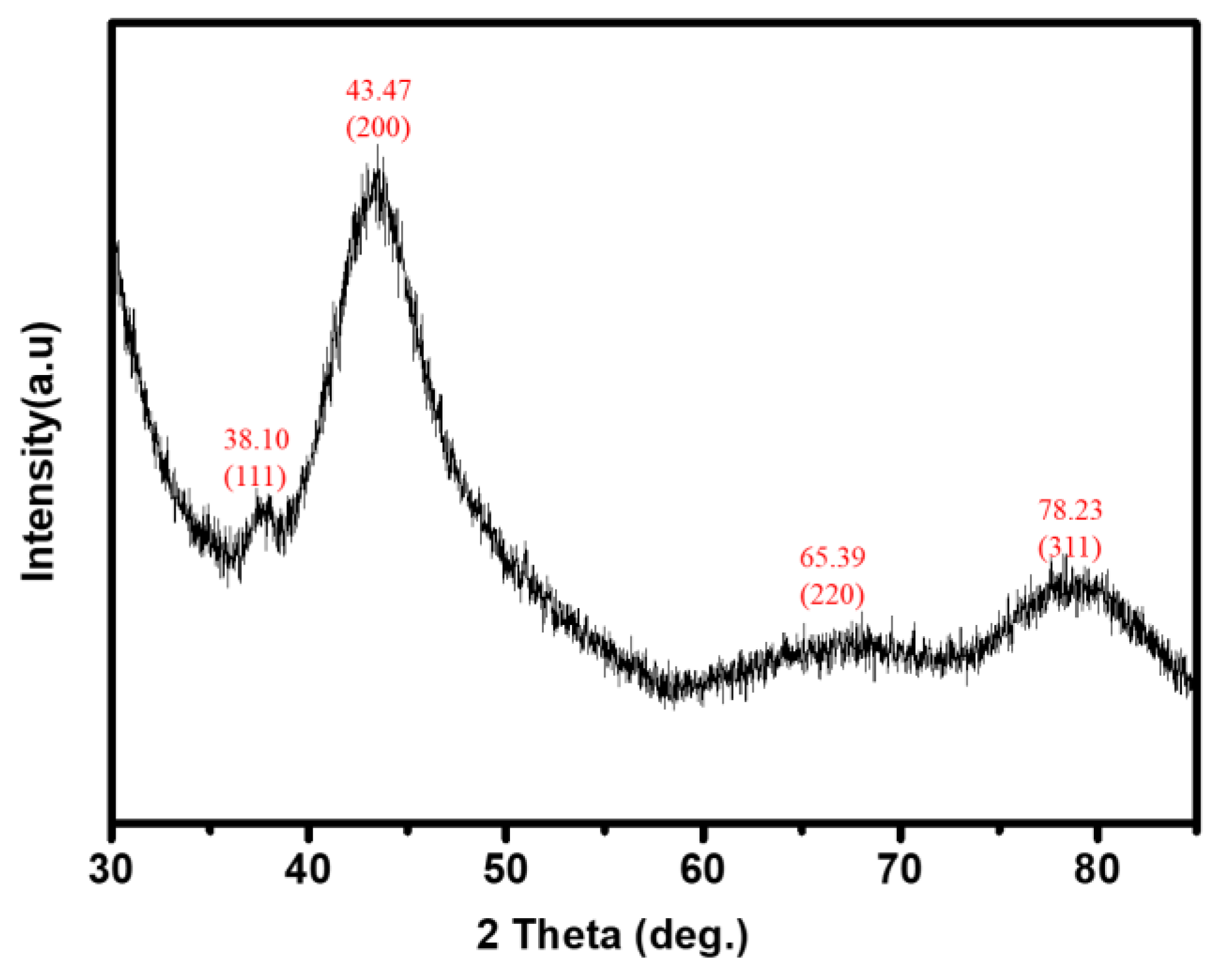
3.2. XRD Characterization of Thermo-Sensitive AgNPs Hydrogels
3.3. Wettability of Surface-Modified Bamboo Charcoal
3.4. Swelling Ratio of Surface-Modified Bamboo Charcoal
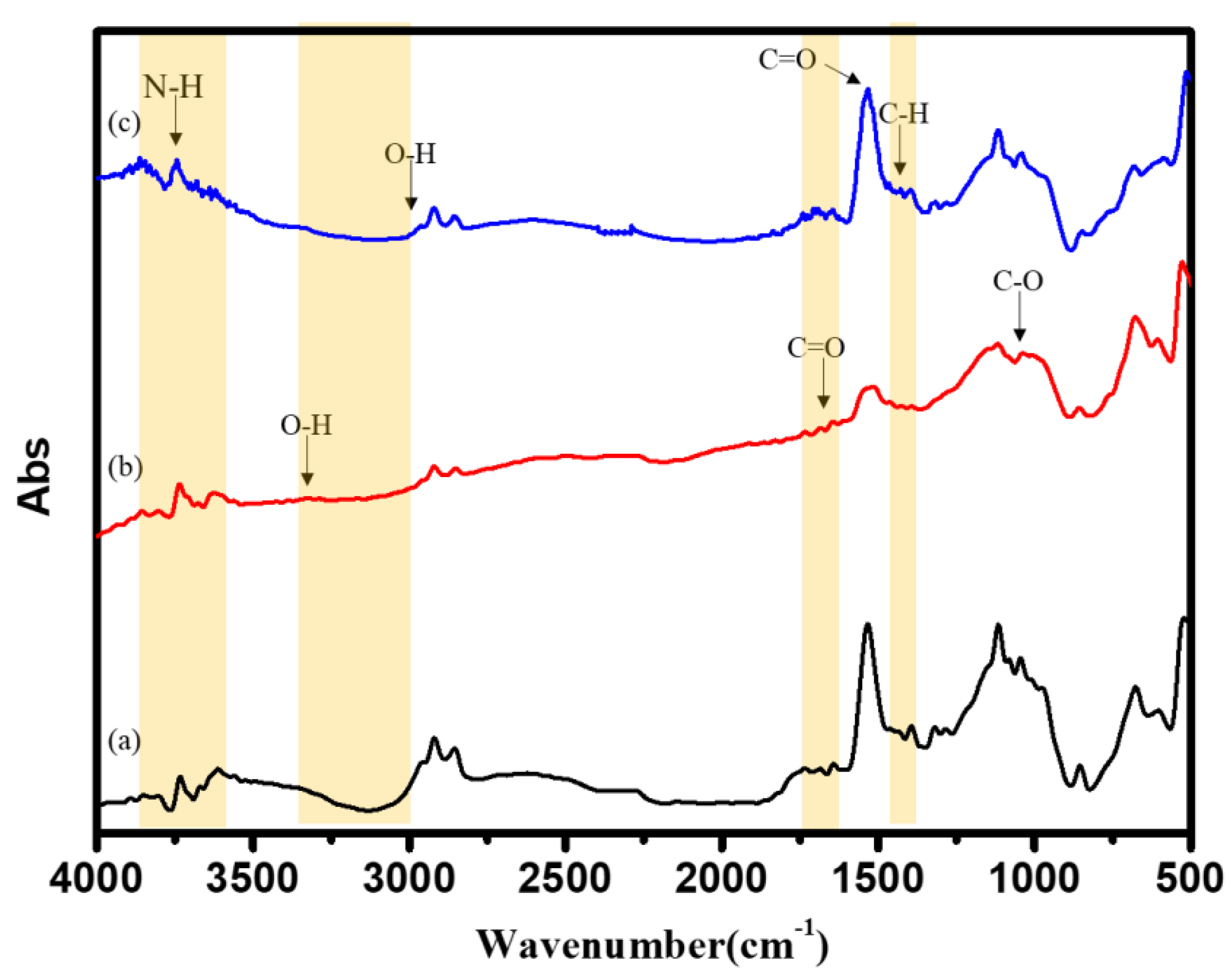
3.5. FTIR Characterization of Surface-Modified Bamboo Charcoal
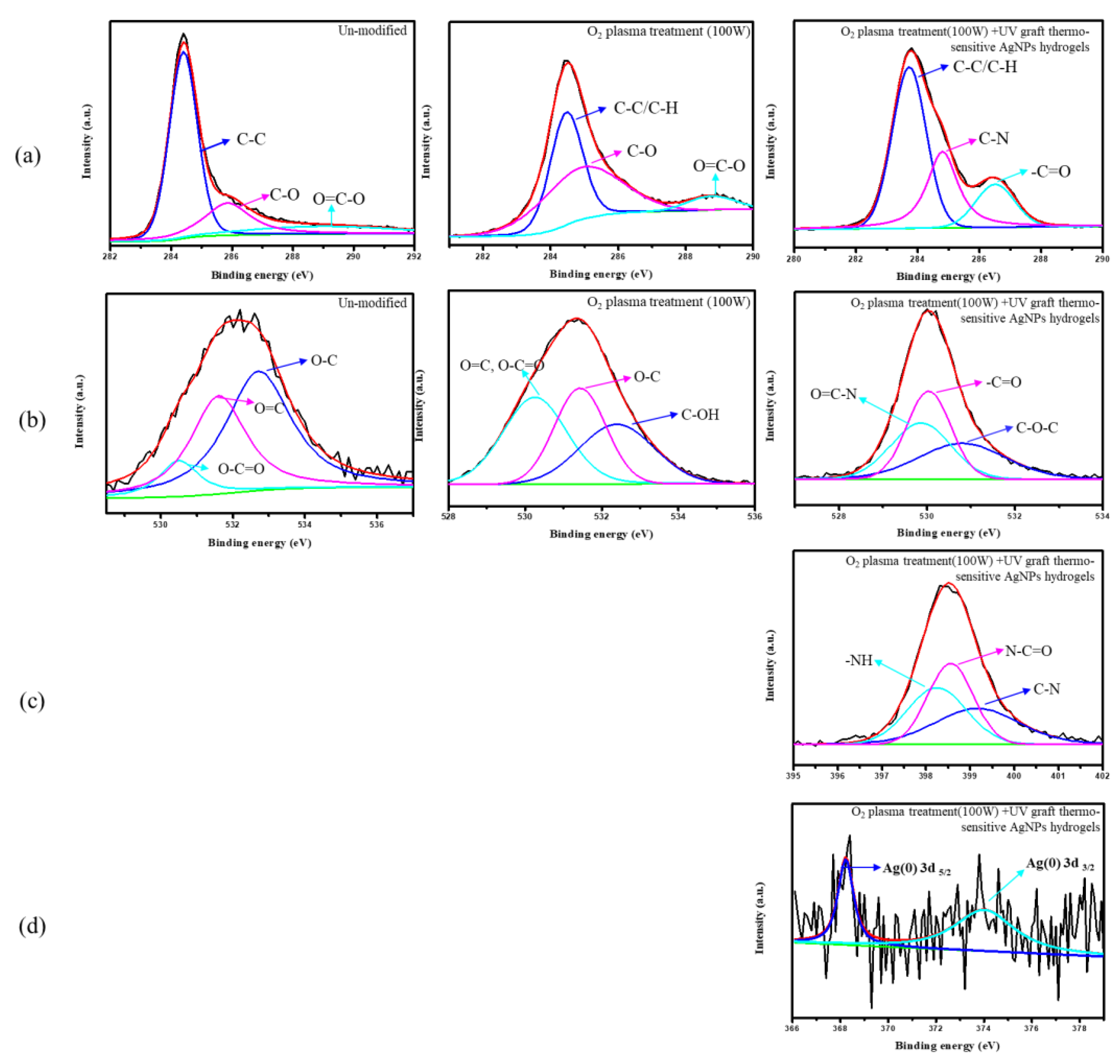
3.6. Chemical Composition Analysis of Surface-Modified Bamboo Charcoal
3.7. Surface Morphology of Surface-Modified Bamboo Charcoal
3.8. In Vitro Cytocompatibility Assay of Surface-Modified Bamboo Charcoal
3.9. Antibacterial Characteristics of Surface-Modified Bamboo Charcoal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isa, S.; Ramli, M.; Hambali, N.; Kasjoo, S.; Isa, M.; Nor, N.; Khalid, N.; Ahmad, N. Adsorption Properties and Potential Applications of Bamboo Charcoal: A Review. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 78, p. 01097. [Google Scholar]
- Ye, Y.; Zhang, Z. Research Progress of the Properties and Application of Bamboo Charcoal. Appl. Mech. Mater. 2013, 395, 646–649. [Google Scholar] [CrossRef]
- Yang, F.; Wu, K.; Lin, W.; Hu, M.; Sheu, T.; Horng, D. Preparation and Antibacterial Effect of Bamboo Charcoal/Ag on Staphylococcus Aureusand Pseudomonas Aeruginosa. J. Chin. Chem. Soc. 2009, 56, 327–334. [Google Scholar] [CrossRef]
- Wang, R.; Amano, Y.; Machida, M. Surface properties and water vapor adsorption–desorption characteristics of bamboo-based activated carbon. J. Anal. Appl. Pyrolysis 2013, 104, 667–674. [Google Scholar] [CrossRef]
- Yang, F.; Wu, K.; Liu, M.; Lin, W.; Hu, M. Evaluation of the antibacterial efficacy of bamboo charcoal/silver biological protective material. Mater. Chem. Phys. 2009, 113, 474–479. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Wu, S. Preparation of Bamboo Charcoal/Polyvinyl Formal Foam as Biological Support Material. Adv. Mater. Res. 2012, 466, 106–110. [Google Scholar] [CrossRef]
- Lin, C.; Lin, J. Evaluation of the Function and Manufacturing Technique of Bamboo Charcoal Complex Yarns and Knitted Fabrics. J. Eng. Fibers Fabr. 2012, 7, 155892501200700. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.; Dai, C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses. Polymers 2020, 12, 2217. [Google Scholar] [CrossRef]
- Hsieh, M.; Wen, H.; Shyu, C.; Chen, S.; Li, W.; Wang, W.; Chen, W. Synthesis, in vitro macrophage response and detoxification of bamboo charcoal beads for purifying blood. J. Biomed. Mater. Res. Part A 2010, 94, 1133–1140. [Google Scholar]
- Nitayaphat, W.; Jiratumnukul, N.; Charuchinda, S.; Kittinaovarat, S. Mechanical properties of chitosan/bamboo charcoal composite films made with normal and surface oxidized charcoal. Carbohydr. Polym. 2009, 78, 444–448. [Google Scholar] [CrossRef]
- Lin, C.; Ni, M.; Chang, Y.; Yeh, H.; Lin, F. A cell sorter with modified bamboo charcoal for the efficient selection of specific antibody-producing hybridomas. Biomaterials 2010, 31, 8445–8453. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.; Dufresne, A.; Danquah, M. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Kim, J.; Kuk, E.; Yu, K.; Kim, J.; Park, S.; Lee, H.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Thakkar, K.; Mhatre, S.; Parikh, R. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Singh, A.; Gaud, B.; Jaybhaye, S. Optimization of Synthesis Parameters of Silver Nanoparticles and Its Antimicrobial Activity. Mater. Sci. Energy Technol. 2019, 3, 232–236. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2017, 13, 65–71. [Google Scholar] [CrossRef]
- Sood, R.; Chopra, D. Optimization of reaction conditions to fabricate Ocimum sanctum synthesized silver nanoparticles and its application to nano-gel systems for burn wounds. Mater. Sci. Eng. C 2018, 92, 575–589. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Siddiqui, A.; Shakoor, A.; Zohra, R.; Ahmad, M.; Zohra, R. Green Synthesis of Antibacterial Silver Nanoparticles against Drug-Resistant Bacteria. Anti-Infect. Agents 2021, 19. [Google Scholar] [CrossRef]
- Chang, B.; Pan, L.; Lin, H.; Chang, H. Nanodiamond-supported silver nanoparticles as potent and safe antibacterial agents. Sci. Rep. 2019, 9, 13164. [Google Scholar] [CrossRef]
- Miyan, M.N.; Durg, V. Study of Silver Complexes as Potential Antibacterial and Antifungal Agents. Strad Res. 2020, 7, 710–716. [Google Scholar]
- Hyeon, T. Designed chemical synthesis and assembly of uniform-sized nanoparticles for medical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1800–1801. [Google Scholar] [CrossRef]
- Sun, S. Monodisperse magnetic nanoparticles for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 288. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.; Omray, P.; Khandelwal, S.; Paknikar, K. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, E. Green synthesis and characterization of silver nanoparticles using fructose. Asian J. Green Chem. 2017, 3, 41–50. [Google Scholar] [CrossRef]
- Zhong, Z.; Luo, S.; Yang, K.; Wu, X.; Ren, T. High-performance anionic waterborne polyurethane/Ag nanocomposites with excellent antibacterial property via in situ synthesis of Ag nanoparticles. RSC Adv. 2017, 7, 42296–42304. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Huang, J.; Chen, C.; Wang, Z.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.; Silva, R.; Morris, B.; Scheckel, K.; Suidan, M.; Tolaymat, T. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Raza, M.; Kanwal, Z.; Rauf, A.; Sabri, A.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Das, T.; Ghosh, S.; Das, S.; Bose, M.; Mondal, M.; Das, A.; Das, N. Acoustic cavitation assisted destratified clay tactoid reinforced in situ elastomer mimetic semi-IPN hydrogel for catalytic and bactericidal application. Ultrason. Sonochem. 2020, 60, 104797. [Google Scholar] [CrossRef] [PubMed]
- Griessner, H.; Hodgkin, J. Biopolymer surface modification using plasma grafting. Biomaterials 1988, 9, 292. [Google Scholar] [CrossRef]
- Steckelmacher, W. Film deposition by plasma techniques. Vacuum 1993, 44, 1069. [Google Scholar] [CrossRef]
- Inagaki, N. Plasma Surface Modification and Plasma Polymerization; Technomic Pub. Co.: Lancaster, UK, 1996. [Google Scholar]
- Liao, S.; Chen, K.; Chen, W.; Chou, C.; Wai, K. Surface Graft Polymerization of Acrylamide onto Plasma Activated Nylon Microfiber Artificial Leather for Improving Dyeing Properties. Int. J. Chem. Eng. Appl. 2013, 4, 78–81. [Google Scholar] [CrossRef][Green Version]
- Wolf, R.; Sparavigna, A. Role of Plasma Surface Treatments on Wetting and Adhesion. Engineering 2010, 2, 397–402. [Google Scholar] [CrossRef]
- Chaiwong, C.; Rachtanapun, P.; Wongchaiya, P.; Auras, R.; Boonyawan, D. Effect of plasma treatment on hydrophobicity and barrier property of polylactic acid. Surf. Coat. Technol. 2010, 204, 2933–2939. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.; González-Martín, M. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Liao, S.C.; Chen, K.S.; Chien, J.L.; Chen, S.C.; Lin, W.L. Acetic-Acid Plasma-Polymerization on Polymeric Substrates for Biomedical Application. Nanomaterials 2019, 9, 941. [Google Scholar] [CrossRef]
- Razavi, A. Plasma Surface Modification of Polymeric Materials. In MRS Proceedings; 2005; Volume 890. [Google Scholar]
- Tabares, F.; Junkar, I. Cold Plasma Systems and Their Application in Surface Treatments for Medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef]
- Vesel, A.; Primc, G.; Zaplotnik, R.; Mozetič, M. Applications of highly non-equilibrium low-pressure oxygen plasma for treatment of polymers and polymer composites on an industrial scale. Plasma Phys. Control. Fusion 2020, 62, 024008. [Google Scholar] [CrossRef]
- Kariž, M.; Tomec, D.; Dahle, S.; Kuzman, M.; Šernek, M.; Žigon, J. Effect of Sanding and Plasma Treatment of 3D-Printed Parts on Bonding to Wood with PVAc Adhesive. Polymers 2021, 13, 1211. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.; Bueno-Ferrer, C.; Misra, N.; Milosavljević, V.; O’Donnell, C.; Bourke, P.; Keener, K.; Cullen, P. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Shishoo, R. Plasma Treatment—Industrial Applications and Its Impact on the C&L Industry. J. Coat. Fabr. 1996, 26, 26–35. [Google Scholar]
- Tiede, R.; Hirschberg, J.; Daeschlein, G.; von Woedtke, T.; Vioel, W.; Emmert, S. Plasma Applications: A Dermatological View. Contrib. Plasma Phys. 2014, 54, 118–130. [Google Scholar] [CrossRef]
- Virtanen, J.; Tenhu, H. Thermal Properties of poly(N-isopropylacrylamide)-g-poly (ethylene oxide) in Aqueous Solutions: Influence of the Number and Distribution of the Grafts. Macromolecules 2000, 33, 5970–5975. [Google Scholar] [CrossRef]
- Naddaf, A.; Bart, H. Diffusion Coefficients in Thermosensitive Poly (NIPAAm) Hydrogels. Macromol. Symp. 2011, 306, 150–165. [Google Scholar] [CrossRef]
- Chou, C.; Chen, K.; Lin, W.; Ye, Y.; Liao, S. Plasma Polymerization of SnOxCy Organic-Like Films and Grafted PNIPAAm Composite Hydrogel with Nanogold Particles for Promotion of Thermal Resistive Properties. Micromachines 2016, 8, 5. [Google Scholar] [CrossRef]
- Frazar, E.; Shah, R.; Dziubla, T.; Hilt, J. Multifunctional temperature-responsive polymers as advanced biomaterials and beyond. J. Appl. Polym. Sci. 2020, 137, 48770. [Google Scholar] [CrossRef]
- Haq, M.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Kim, S. Synthesis and characteristics of interpenetrating polymer network hydrogels composed of poly (vinyl alcohol) and poly(N-isopropylacrylamide). React. Funct. Polym. 2003, 55, 61–67. [Google Scholar] [CrossRef]
- Chen, K.; Chang, S.; Feng, C.; Lin, W.; Liao, S. Plasma Deposition and UV Light Induced Surface Grafting Polymerization of NIPAAm on Stainless Steel for Enhancing Corrosion Resistance and Its Drug Delivery Property. Polymers 2018, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Ankareddi, I.; Brazel, C. Development of a thermosensitive grafted drug delivery system-synthesis and characterization of NIPAAm-based grafts and hydrogel structure. J. Appl. Polym. Sci. 2010, 120, 1597–1606. [Google Scholar] [CrossRef]
- Suradi, S.; Naemuddin, N.; Hashim, S.; Adrus, N. Impact of carboxylation and hydrolysis functionalisations on the anti-oil staining behaviour of textiles grafted with poly(N-isopropylacrylamide) hydrogel. RSC Adv. 2018, 8, 13423–13432. [Google Scholar] [CrossRef]
- Conzatti, G.; Cavalie, S.; Combes, C.; Torrisani, J.; Carrere, N.; Tourrette, A. PNIPAM grafted surfaces through ATRP and RAFT polymerization: Chemistry and bioadhesion. Colloids Surf. B Biointerfaces 2017, 151, 143–155. [Google Scholar] [CrossRef]
- Li, Z.; Guan, J. Thermosensitive hydrogels for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 991–1007. [Google Scholar] [CrossRef]
- Chen, K.; Chou, C.; Hsu, S.; Lin, H. Surface Grafting Polymerization and Crosslinking of Thermosensitive NIPAAm Hydrogels onto Plasma Pretreated Substrates and Drug Delivery Properties. Mater. Sci. Forum 2003, 426, 3267–3272. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Ohya, S.; Nakayama, Y.; Matsuda, T. Thermoresponsive Artificial Extracellular Matrix for Tissue Engineering: Hyaluronic Acid Bioconjugated with poly(N-isopropylacrylamide) Grafts. Biomacromolecules 2001, 2, 856–863. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Zhang, J.; Ju, J. Preparation and Characterization of Thermoresponsive poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, X.; Chen, B.; Qiu, G. Low temperature plasma vapor treatment of thermo-sensitive poly(N-isopropylacrylamide) and its application. Appl. Surf. Sci. 2013, 268, 332–336. [Google Scholar] [CrossRef]
- Cui, Y.; Xing, Z.; Yan, J.; Lu, Y.; Xiong, X.; Zheng, L. Thermosensitive Behavior and Super-Antibacterial Properties of Cotton Fabrics Modified with a Sercin-NIPAAm-AgNPs Interpenetrating Polymer Network Hydrogel. Polymers 2018, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Byth, H.; Mchunu, B.; Dubery, I.; Bornman, L. Assessment of a simple, non-toxic alamar blue cell survival assay to monitor tomato cell viability. Phytochem. Anal. 2001, 12, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Chang, C.; Chen, C.; Lee, C.; Lin, W. Functionalization of pure titanium MAO coatings by surface modifications for biomedical applications. Surf. Coat. Technol. 2020, 394, 125812. [Google Scholar] [CrossRef]
- Paladini, F.; Di Franco, C.; Panico, A.; Scamarcio, G.; Sannino, A.; Pollini, M. In Vitro Assessment of the Antibacterial Potential of Silver Nano-Coatings on Cotton Gauzes for Prevention of Wound Infections. Materials 2016, 9, 411. [Google Scholar] [CrossRef]
- Pollini, M.; Russo, M.; Licciulli, A.; Sannino, A.; Maffezzoli, A. Characterization of antibacterial silver coated yarns. J. Mater. Sci. Mater. Med. 2009, 20, 2361–2366. [Google Scholar] [CrossRef]



| Type | AgNO3 (mM) | Na3C6H5O7 (mM) | PVP (μM) |
|---|---|---|---|
| S1 | 10 | 10 | 75 |
| S2 | 10 | 50 | 75 |
| S3 | 10 | 100 | 75 |
| Untreated | Treatment A | Treatment B | Treatment C | Treatment D | |
|---|---|---|---|---|---|
| θH2O | 63.5 ± 7.8° | <0° | <0° | 32.1 ± 1.1° | 34 ± 1.5° |
| Temperature (°C) | ||||
| 25 | 37 | 42 | ||
| RO water | Untreated | 103.2 | 103.9 | 106.9 |
| A | 91.5 | 69.8 | 72.6 | |
| B | 134.8 | 51.2 | 54.8 | |
| Temperature (°C) | ||||
| 25 | 37 | 42 | ||
| SBF solution | Untreated | 39.6 | 33.7 | 33.0 |
| A | 64.7 | 59.8 | 54.3 | |
| B | 87.8 | 71.1 | 67.8 | |
| Test Organism | Diameter Zone (mm), Mean (n = 3) | |
|---|---|---|
| Un-Modified | O2 Plasma Treatment (100 W) +UV Graft Thermo-Sensitive AgNPs Hydrogels | |
| E. coli | 0 | 15.7 ± 0.2 (mm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-J.; Liao, S.-C. Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application. Nanomaterials 2021, 11, 2697. https://doi.org/10.3390/nano11102697
Liu S-J, Liao S-C. Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application. Nanomaterials. 2021; 11(10):2697. https://doi.org/10.3390/nano11102697
Chicago/Turabian StyleLiu, Shih-Ju, and Shu-Chuan Liao. 2021. "Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application" Nanomaterials 11, no. 10: 2697. https://doi.org/10.3390/nano11102697
APA StyleLiu, S.-J., & Liao, S.-C. (2021). Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application. Nanomaterials, 11(10), 2697. https://doi.org/10.3390/nano11102697





