Mesoporous Zn/MgO Hexagonal Nano-Plates as a Catalyst for Camelina Oil Biodiesel Synthesis
Abstract
:1. Introduction
2. Materials and Methods
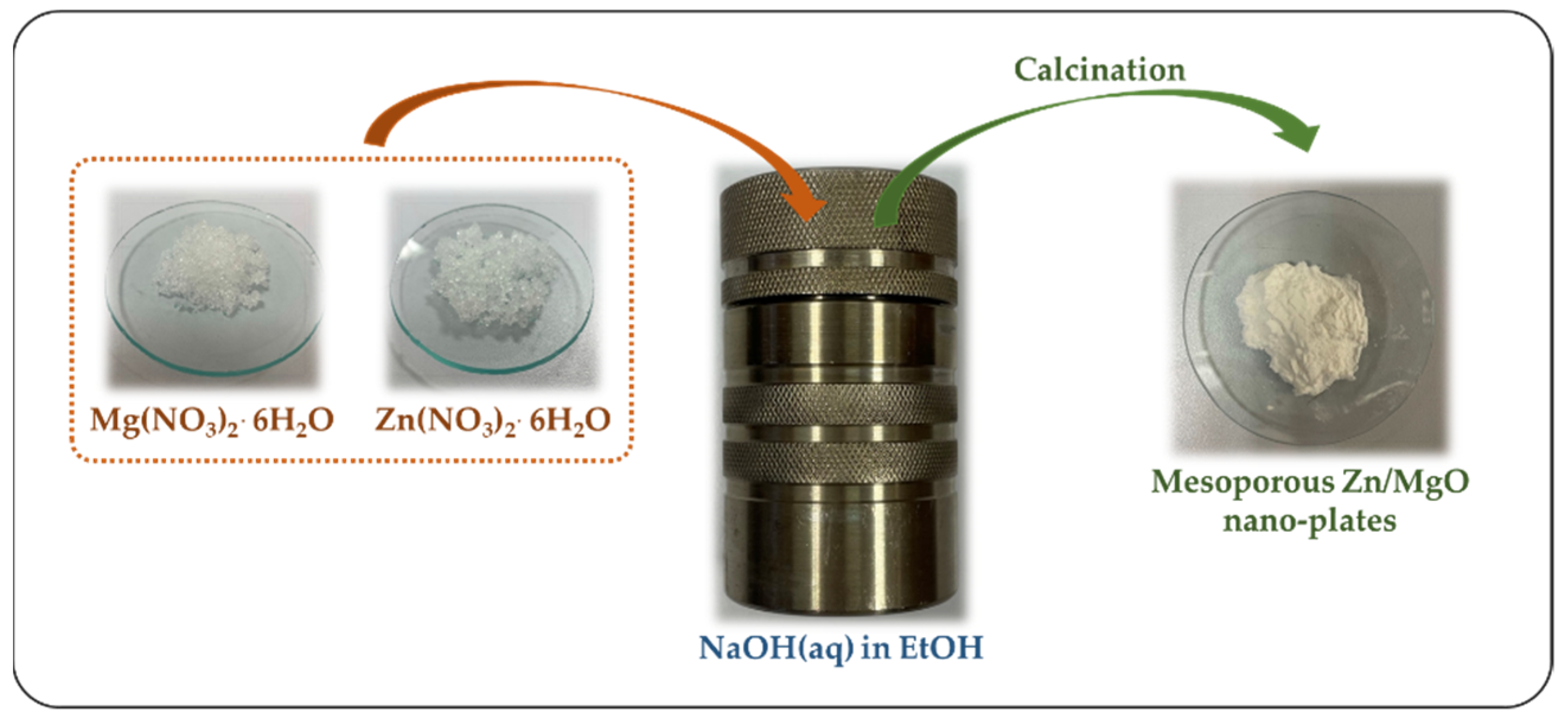
2.1. Synthesis of the Catalysts
2.2. Characterization of the Catalysts
2.3. Catalytic Reactions and Biodiesel Determination
3. Results and Discussions
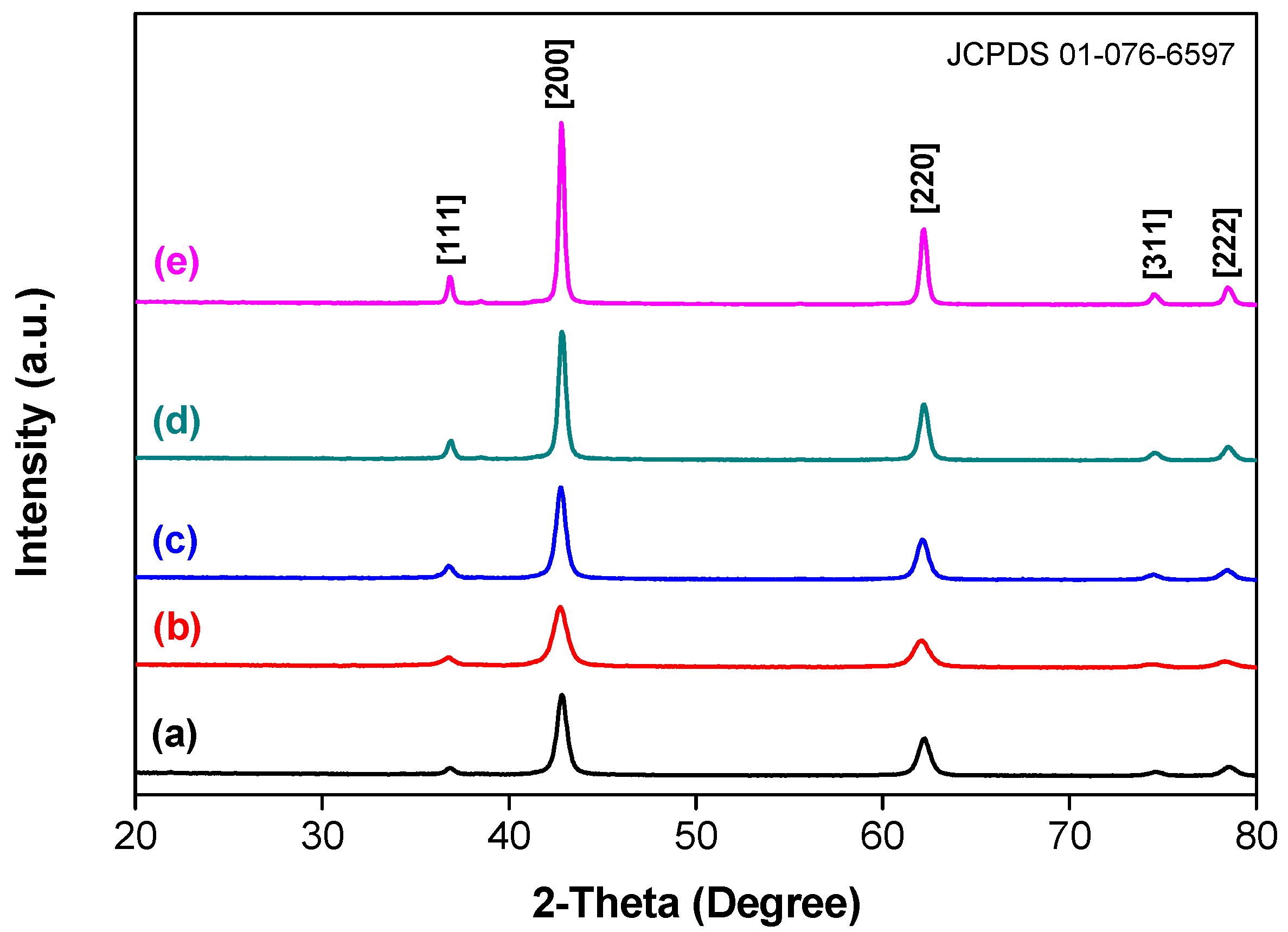
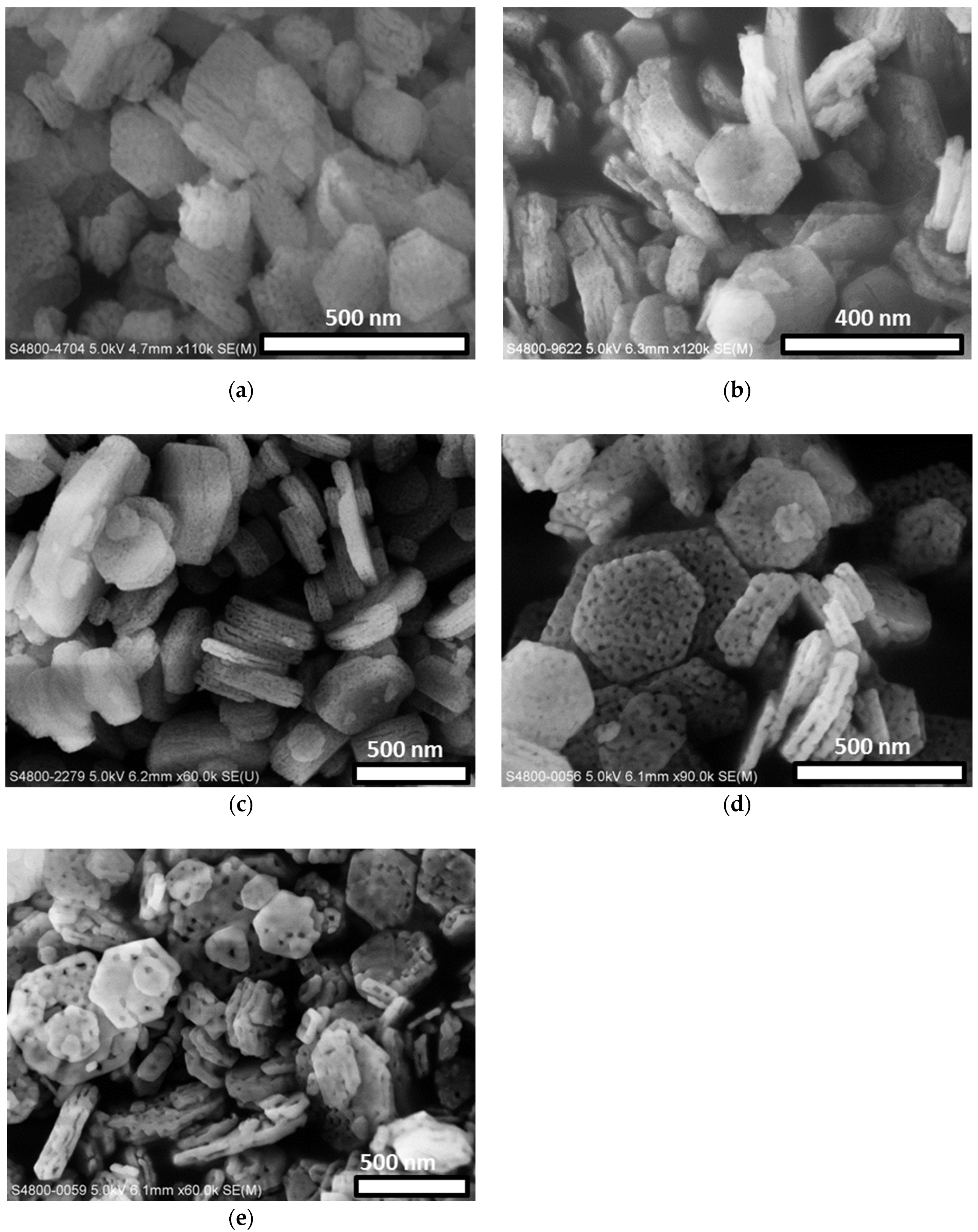
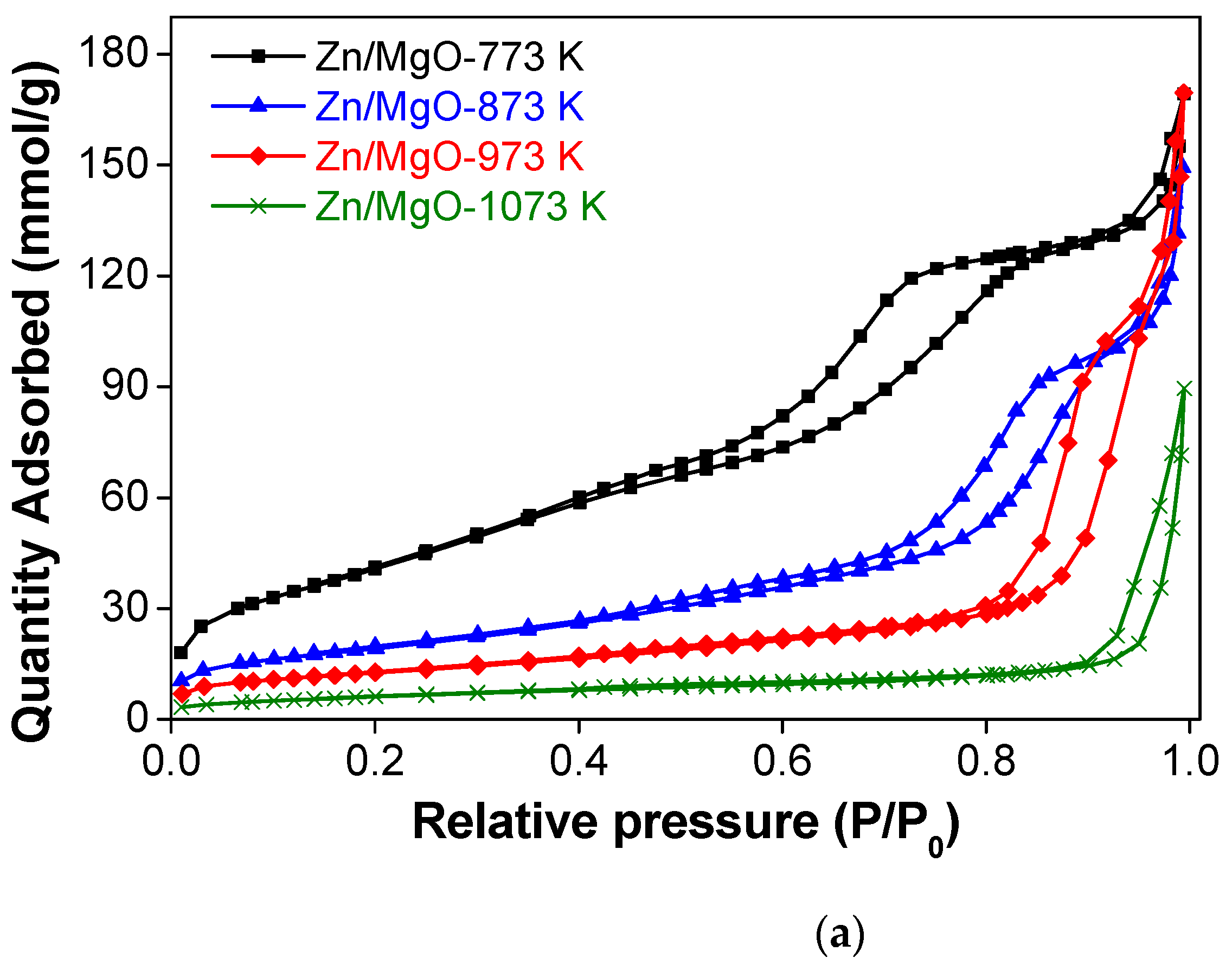
3.1. Characterization of the Catalysts
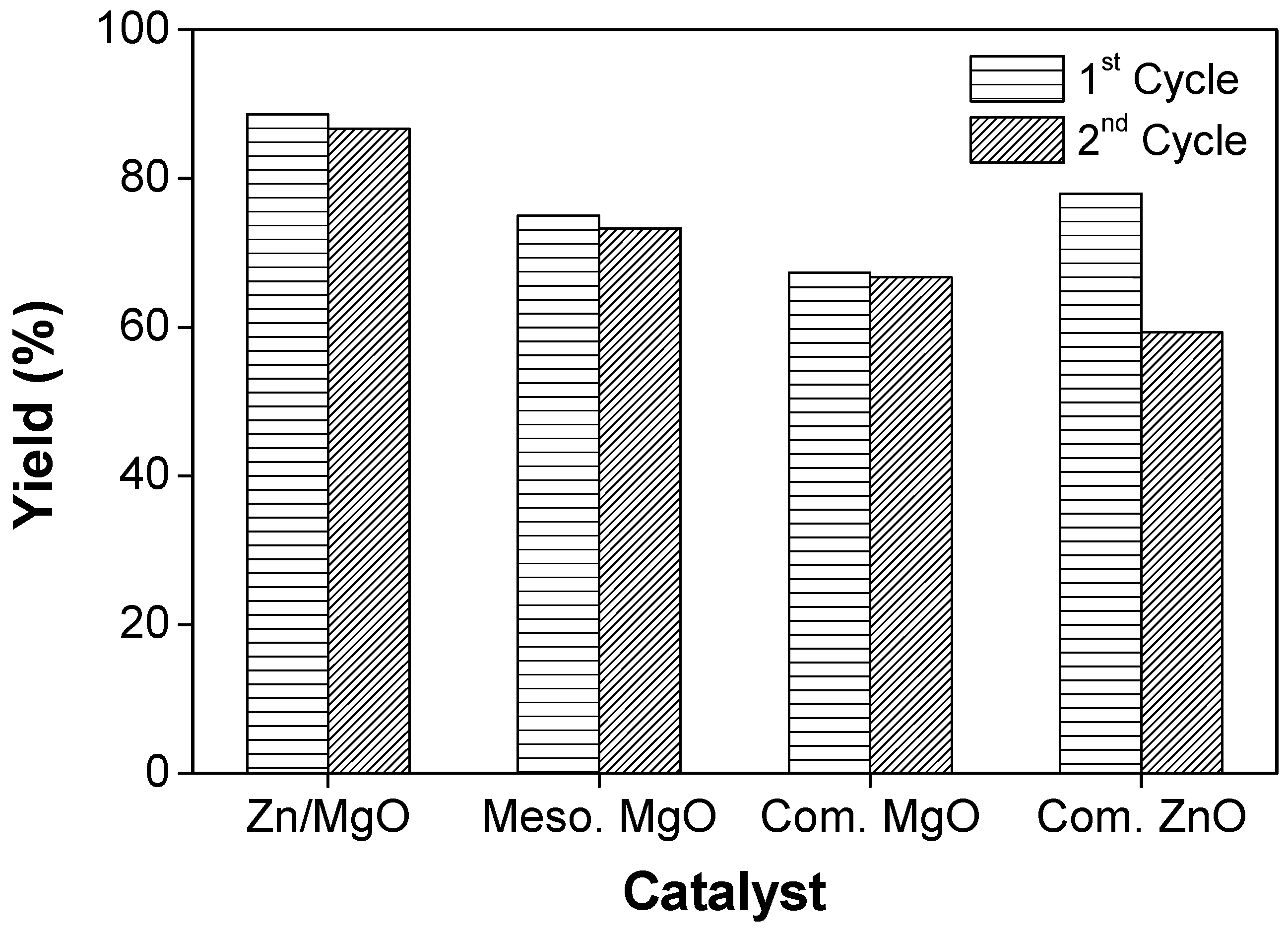
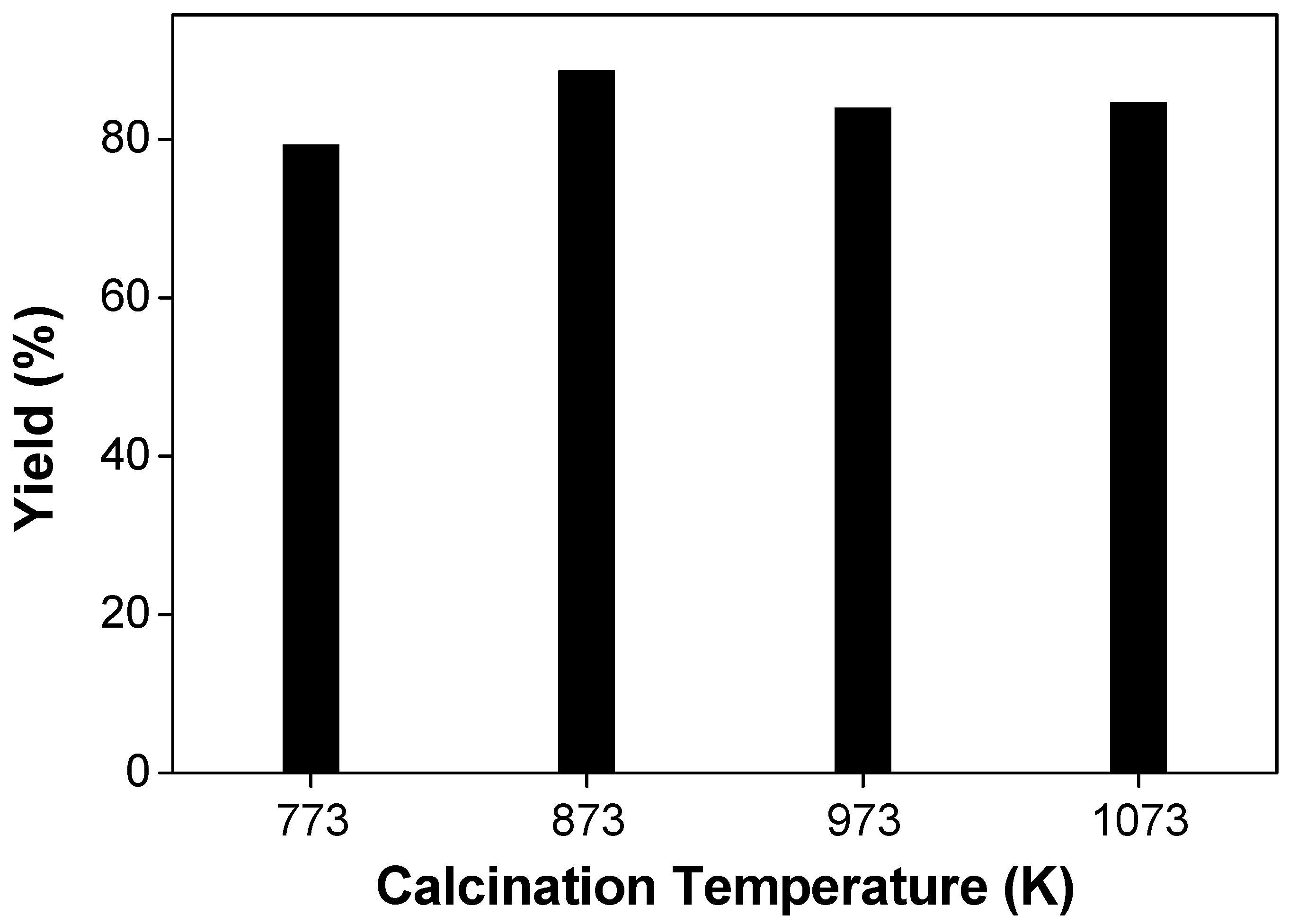
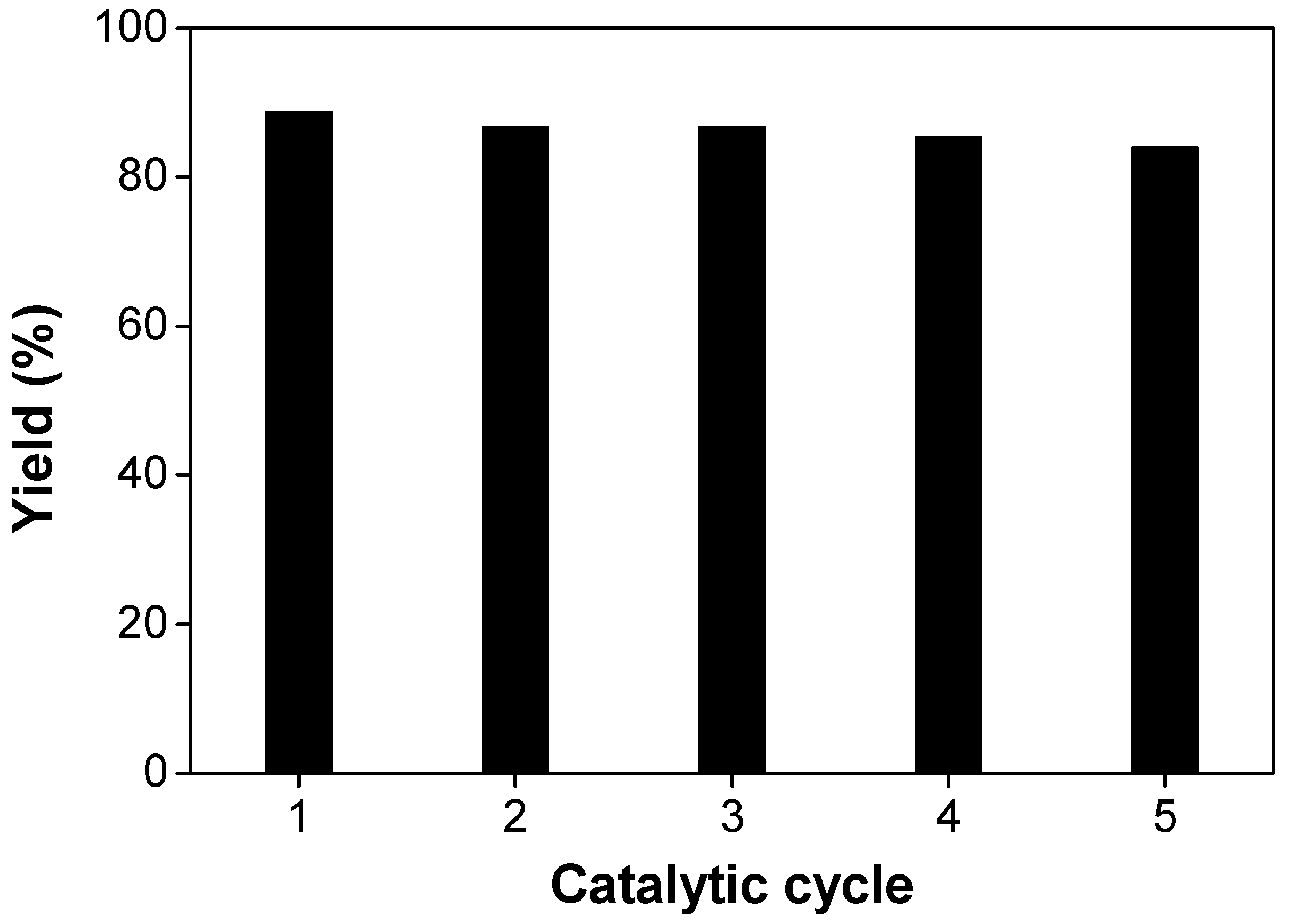
3.2. Catalytic Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahmanian, B.; Safaei, M.R.; Kazi, S.N.; Ahmadi, G.; Oztop, H.F.; Vafai, K. Investigation of pollutant reduction by simulation of turbulent non-premixed pulverized coal combustion. Appl. Therm. Eng. 2014, 73, 1222–1235. [Google Scholar] [CrossRef]
- Sateesh, K.; Yaliwal, V.; Soudagar, M.E.M.; Banapurmath, N.; Fayaz, H.; Safaei, M.R.; Elfasakhany, A.; EL-Seesy, A.I. Utilization of biodiesel/Al2O3 nanoparticles for combustion behavior enhancement of a diesel engine operated on dual fuel mode. J. Therm. Anal. Calorim. 2021, 1–15. [Google Scholar] [CrossRef]
- Akkoli, K.; Banapurmath, N.; Shivashimpi, M.; Soudagar, M.E.M.; Badruddin, I.A.; Alazwari, M.A.; Yaliwal, V.; Mujtaba, M.; Akram, N.; Goodarzi, M. Effect of injection parameters and producer gas derived from redgram stalk on the performance and emission characteristics of a diesel engine. Alex. Eng. J. 2021, 60, 3133–3142. [Google Scholar] [CrossRef]
- Khan, H.; Soudagar, M.E.M.; Kumar, R.H.; Safaei, M.R.; Farooq, M.; Khidmatgar, A.; Banapurmath, N.R.; Farade, R.A.; Abbas, M.M.; Afzal, A. Effect of nano-graphene oxide and n-butanol fuel additives blended with diesel—Nigella sativa biodiesel fuel emulsion on diesel engine characteristics. Symmetry 2020, 12, 961. [Google Scholar] [CrossRef]
- Gavhane, R.S.; Kate, A.M.; Pawar, A.; Safaei, M.R.; Soudagar, M.E.; Mujtaba Abbas, M.; Muhammad Ali, H.; Banapurmath, N.R.; Goodarzi, M.; Badruddin, I.A. Effect of zinc oxide nano-additives and soybean biodiesel at varying loads and compression ratios on VCR diesel engine characteristics. Symmetry 2020, 12, 1042. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Kazi, S.; Sadeghinejad, F.; Badarudin, A.; Mehrali, M.; Sadri, R.; Safaei, M.R. A comprehensive literature review of bio-fuel performance in internal combustion engine and relevant costs involvement. Renew. Sustain. Energy Rev. 2014, 30, 29–44. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Wategave, S.; Banapurmath, N.; Sawant, M.; Soudagar, M.E.M.; Mujtaba, M.; Afzal, A.; Basha, J.S.; Alazwari, M.A.; Safaei, M.R.; Elfasakhany, A. Clean combustion and emissions strategy using reactivity controlled compression ignition (RCCI) mode engine powered with CNG-Karanja biodiesel. J. Taiwan Inst. Chem. Eng. 2021, 124, 116–131. [Google Scholar] [CrossRef]
- Banapurmath, N.; Chandrashekar, T.; Soudagar, M.E.M.; Anqi, A.E.; Mujtaba, M.; Goodarzi, M.; Elfasakhany, A.; Siddiqui, M.I.H.; Ali, M.A. Effect of Parameters Behavior of Simarouba Methyl Ester Operated Diesel Engine. Energies 2021, 14, 4973. [Google Scholar]
- Bansal, S.; Durrett, T.P. Camelina sativa: An ideal platform for the metabolic engineering and field production of industrial lipids. Biochimie 2016, 120, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, A.; Howard-Hildige, R.; O’Connell, A.; Nichol, R.; Ryan, J.; Rice, B.; Roche, E.; Leahy, J.J. Camelina oil as a fuel for diesel transport engines. Ind. Crops Prod. 2003, 17, 191–197. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Deng, S. Biodiesel Production from Jatropha Curcas, Waste Cooking, and Camelina Sativa Oils. Ind. Eng. Chem. Res. 2009, 48, 10850–10856. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Almerindo, G.I.; Buratto, S.C.; Wanderlind, E.H.; Nicolazi, L.M.; Sangaletti, P.; Medeiros, M.; Schneider, F.S.; Caramori, G.F.; Parreira, R.L.; Micke, G.A. Kinetics and adsorption calculations: Insights into the MgO-catalyzed detoxification of simulants of organophosphorus biocides. J. Mater. Chem. A 2020, 8, 19011–19021. [Google Scholar] [CrossRef]
- Montero, J.; Isaacs, M.; Lee, A.; Lynam, J.; Wilson, K. The surface chemistry of nanocrystalline MgO catalysts for FAME production: An in situ XPS study of H2O, CH3OH and CH3OAc adsorption. Surf. Sci. 2016, 646, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Poosumas, J.; Ngaosuwan, K.; Quitain, A.T.; Assabumrungrat, S. Role of ultrasonic irradiation on transesterification of palm oil using calcium oxide as a solid base catalyst. Energy Convers. Manag. 2016, 120, 62–70. [Google Scholar] [CrossRef]
- Qu, T.; Niu, S.; Gong, Z.; Han, K.; Wang, Y.; Lu, C. Wollastonite decorated with calcium oxide as heterogeneous transesterification catalyst for biodiesel production: Optimized by response surface methodology. Renew. Energy 2020, 159, 873–884. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; Yuan, C.; Wang, W.; Hu, J.; Li, Q.; He, J. Surface-modification effect of MgO nanoparticles on the electrical properties of polypropylene nanocomposite. High Volt. 2020, 5, 249–255. [Google Scholar] [CrossRef]
- Qasim, M.K. Modified nanostructure MgO superbasicity with CaO in heterogeneous transesterification of sunflower oil. Egypt. J. Chem. 2019, 62, 475–485. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Ramavandi, B.; Boffito, D.C. One-pot transesterification of non-edible Moringa oleifera oil over a MgO/K2CO3/HAp catalyst derived from poultry skeletal waste. Environ. Technol. Innov. 2021, 21, 101250. [Google Scholar] [CrossRef]
- Barzegari, F.; Farhadi, F.; Rezaei, M.; Kazemeini, M.; Keshavarz, A. Influence of metal loading and reduction temperature on the performance of mesoporous NiO–MgO–SiO2 catalyst in propane steam reforming. J. Energy Inst. 2021, 96, 38–51. [Google Scholar] [CrossRef]
- Manríquez-Ramírez, M.E.; Elizalde, I.; Ortiz-Islas, E. Synthesis of MgO and MgO–CeO2 by co-precipitation for the catalytic conversion of acetone by aldol condensation. React. Kinet. Mech. Catal. 2020, 131, 769–780. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Synthesis of biodiesel from vegetable oil with methanol catalyzed by Li-doped magnesium oxide catalysts. Appl. Energy 2010, 87, 743–748. [Google Scholar] [CrossRef]
- Ab Rahman, N.A.; Olutoye, M.A.; Hameed, B.H. Synthesis of methyl esters from palm (Elaeis guineensis) oil using cobalt doped MgO as solid oxide catalyst. Bioresour. Technol. 2011, 102, 9749–9754. [Google Scholar] [CrossRef] [PubMed]
- Mahraz, Z.A.S.; Sahar, M.; Ghoshal, S.; Saad, A.P.M.; Syahrom, A. Sol–gel grown MgO-ZnO-tricalcium-phosphate nanobioceramics: Evaluation of mechanical and degradation attributes. Corros. Sci. 2018, 138, 179–188. [Google Scholar] [CrossRef]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, D.; Enache, C.; Iordache, F.; Jinga, S.-I. Akermanite-based coatings grown by pulsed laser deposition for metallic implants employed in orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Evstropiev, S.; Soshnikov, I.; Kolobkova, E.; Evstropyev, K.; Nikonorov, N.; Khrebtov, A.; Dukelskii, K.; Kotlyar, K.; Oreshkina, K.; Nashekin, A. Polymer-salt synthesis and characterization of MgO-ZnO ceramic coatings with the high transparency in UV spectral range. Opt. Mater. 2018, 82, 81–87. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Damiani, D.E.; Tonetto, G.M. Efficient production of biodiesel from low-cost feedstock using zinc oleate as catalyst. Fuel Process. Technol. 2015, 134, 26–31. [Google Scholar] [CrossRef]
- Kwong, T.-L.; Yung, K.-F. One-step production of biodiesel through simultaneous esterification and transesterification from highly acidic unrefined feedstock over efficient and recyclable ZnO nanostar catalyst. Renew. Energy 2016, 90, 450–457. [Google Scholar] [CrossRef]
- Lau, P.-C.; Kwong, T.-L.; Yung, K.-F. Effective heterogeneous transition metal glycerolates catalysts for one-step biodiesel production from low grade non-refined Jatropha oil and crude aqueous bioethanol. Sci. Rep. 2016, 6, 23822. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, V.; Sharma, Y.C. Methyl transesterification of waste cooking oil using a laboratory synthesized reusable heterogeneous base catalyst: Process optimization and homogeneity study of catalyst. Energy Convers. Manag. 2017, 148, 1438–1452. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ohtomo, A.; Kawasaki, M.; Koida, T.; Masubuchi, K.; Koinuma, H.; Sakurai, Y.; Yoshida, Y.; Yasuda, T.; Segawa, Y. MgxZn1−xO as a II–VI widegap semiconductor alloy. Appl. Phys. Lett. 1998, 72, 2466–2468. [Google Scholar] [CrossRef] [Green Version]
- Park, W.I.; Yi, G.-C.; Jang, H.M. Metalorganic vapor-phase epitaxial growth and photoluminescent properties of Zn1−xMgxO (0 ≤ x ≤ 0.49) thin films. Appl. Phys. Lett. 2001, 79, 2022–2024. [Google Scholar] [CrossRef] [Green Version]
- Berger, T.; Schuh, J.; Sterrer, M.; Diwald, O.; Knözinger, E. Lithium ion induced surface reactivity changes on MgO nanoparticles. J. Catal. 2007, 247, 61–67. [Google Scholar] [CrossRef]
- Ling, Z.; Zheng, M.; Du, Q.; Wang, Y.; Song, J.; Dai, W.; Zhang, L.; Ji, G.; Cao, J. Synthesis of mesoporous MgO nanoplate by an easy solvothermal–annealing method. Solid State Sci. 2011, 13, 2073–2079. [Google Scholar] [CrossRef]
- Montero, J.M.; Brown, D.R.; Gai, P.L.; Lee, A.F.; Wilson, K. In situ studies of structure–reactivity relations in biodiesel synthesis over nanocrystalline MgO. Chem. Eng. J. 2010, 161, 332–339. [Google Scholar] [CrossRef]
- Montero, J.M.; Gai, P.; Wilson, K.; Lee, A.F. Structure-sensitive biodiesel synthesis over MgO nanocrystals. Green Chem. 2009, 11, 265–268. [Google Scholar] [CrossRef]
- Yu, X.; Wen, Z.; Li, H.; Tu, S.-T.; Yan, J. Transesterification of Pistacia chinensis oil for biodiesel catalyzed by CaO–CeO2 mixed oxides. Fuel 2011, 90, 1868–1874. [Google Scholar] [CrossRef]
- Li, W.-C.; Lu, A.-H.; Weidenthaler, C.; Schüth, F. Hard-Templating Pathway To Create Mesoporous Magnesium Oxide. Chem. Mater. 2004, 16, 5676–5681. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Dai, H.; Deng, J.; Liu, C.; He, H.; Au, C.T. P123-Assisted Hydrothermal Synthesis and Characterization of Rectangular Parallelepiped and Hexagonal Prism Single-Crystalline MgO with Three-Dimensional Wormholelike Mesopores. Inorg. Chem. 2008, 47, 4015–4022. [Google Scholar] [CrossRef]
- Etacheri, V.; Roshan, R.; Kumar, V. Mg-Doped ZnO Nanoparticles for Efficient Sunlight-Driven Photocatalysis. ACS Appl. Mater. Interfaces 2012, 4, 2717–2725. [Google Scholar] [CrossRef]
- Hattori, H. Heterogeneous Basic Catalysis. Chem. Rev. 1995, 95, 537–558. [Google Scholar] [CrossRef]
- Rheinheimer, V.; Unluer, C.; Liu, J.; Ruan, S.; Pan, J.; Monteiro, P.J. XPS study on the stability and transformation of hydrate and carbonate phases within MgO systems. Materials 2017, 10, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerpen, J.V. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Chiesa, M.; Paganini, M.C.; Giamello, E.; Di Valentin, C.; Pacchioni, G. First Evidence of a Single-Ion Electron Trap at the Surface of an Ionic Oxide. Angew. Chem. Int. Ed. 2003, 42, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Olutoye, M.; Hameed, B. Synthesis of fatty acid methyl ester from used vegetable cooking oil by solid reusable Mg1− xZn1+xO2 catalyst. Bioresour. Technol. 2011, 102, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Taufiq-Yap, Y.; Hussein, M.; Yunus, R. Transesterification of jatropha oil with methanol over Mg–Zn mixed metal oxide catalysts. Energy 2013, 49, 12–18. [Google Scholar] [CrossRef]
- Kwong, T.-L.; Yung, K.-F. Heterogeneous alkaline earth metal–transition metal bimetallic catalysts for synthesis of biodiesel from low grade unrefined feedstock. RSC Adv. 2015, 5, 83748–83756. [Google Scholar] [CrossRef]

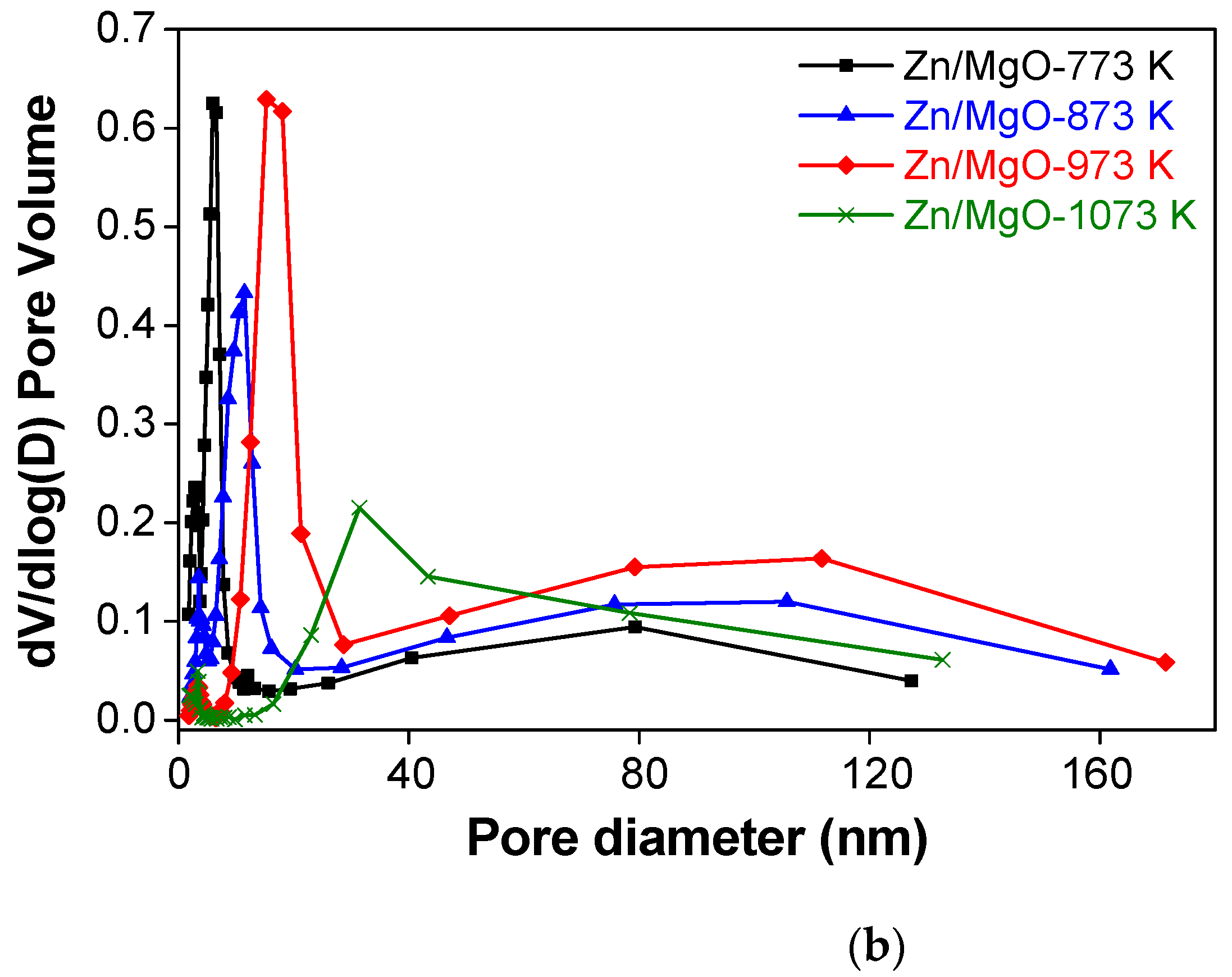
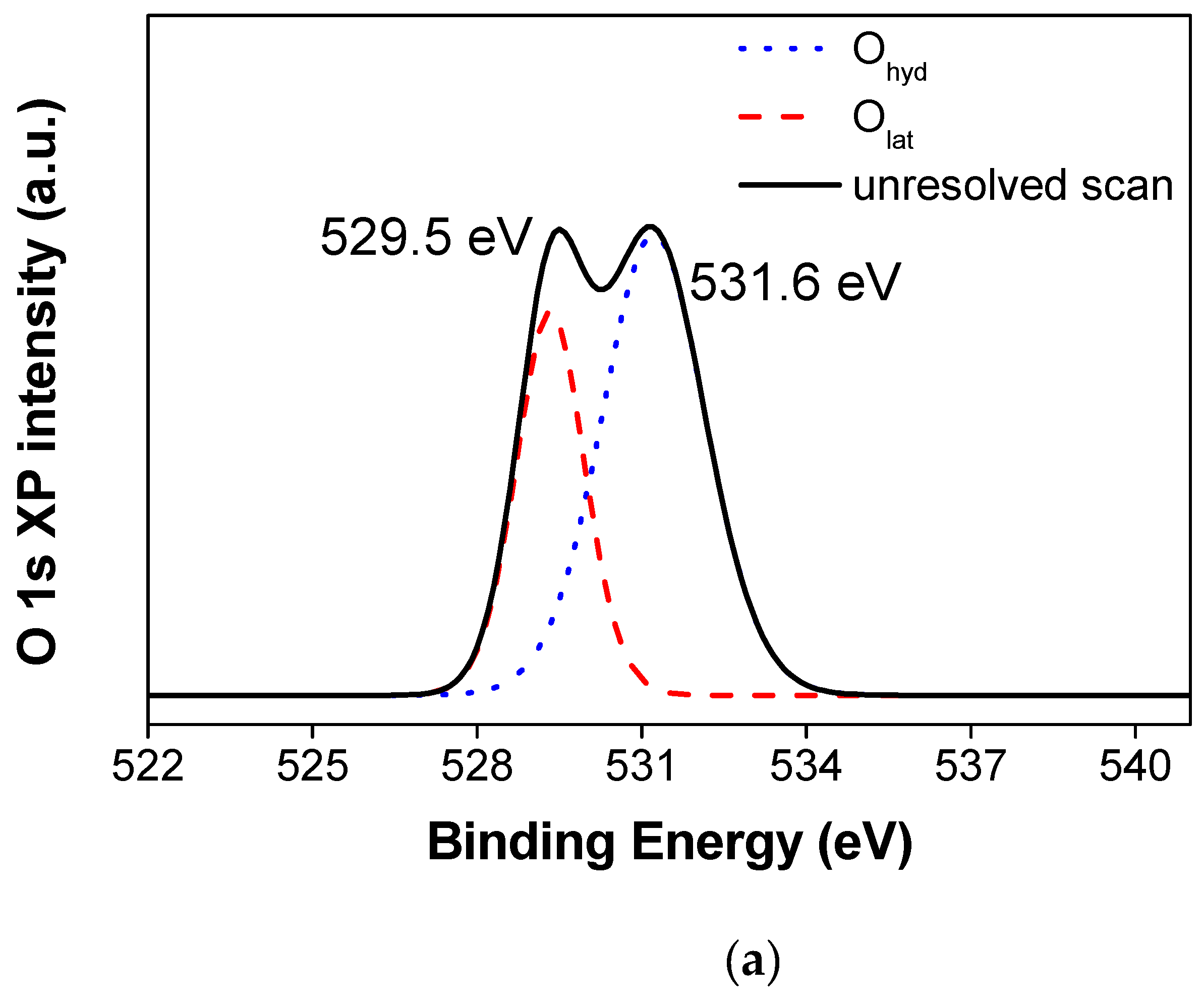

| Catalyst | Crystallite Size 1 (nm) | SBET 2 (m2 g−1) | V 3 (cm3 g−1) | Daver 4 (nm) |
|---|---|---|---|---|
| Meso. MgO-873 K | 12.6 | 73.1 | 0.267 | 12.0 |
| Zn/MgO-773 K | 9.7 | 151.1 | 0.269 | 5.4 |
| Zn/MgO-873 K | 12.7 | 69.1 | 0.233 | 10.4 |
| Zn/MgO-973 K | 17.8 | 45.3 | 0.263 | 18.8 |
| Zn/MgO-1073 K | 24.1 | 22.6 | 0.140 | 22.7 |
| Catalyst | Basic Strength by Hammett Indicator | Basicity by Benzoic Titration (mmol g−1) | |||
|---|---|---|---|---|---|
| 6.8 < H_ | 6.8 < H_ < 7.2 | 7.2 < H_ < 9.3 | Total Basicity | ||
| MgO-873 K | 9.3 < H_ < 10.1 | 0.79 | 0.25 | 0.01 | 1.05 |
| Zn/MgO-773 K | 9.3 < H_ < 10.1 | 0.60 | 0.15 | 0.32 | 1.07 |
| Zn/MgO-873 K | 9.3 < H_ < 10.1 | 0.90 | 0.15 | 0.47 | 1.52 |
| Zn/MgO-973 K | 9.3 < H_ < 10.1 | 0.67 | 0.30 | 0.45 | 1.42 |
| Zn/MgO-1073 K | 9.3 < H_ < 10.1 | 0.59 | 0.34 | 0.54 | 1.47 |
| Catalyst | Binding Energy (eV) | ||||||
|---|---|---|---|---|---|---|---|
| Mg 2p | Zn 2p1/2 | Zn 2p3/2 | Zn LMM | Auger Parameter 1 | Olat | Ohyd | |
| MgO | 49.3 | - | - | - | - | 529.5 | 531.2 |
| ZnO | - | 1045.2 | 1022.1 | 498.9 | 2009.8 | 531.0 | 532.5 |
| Zn/MgO | 49.3 | 1044.2 | 1021.1 | 497.7 | 2010.0 | 529.6 | 531.6 |
| Catalyst | Surface Percentage of Olat (at.%) |
|---|---|
| Mesoporous MgO | 23.5 |
| Zn/MgO | 32.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, L.-F.; Kwong, T.-L.; Wong, W.-T.; Yung, K.-F. Mesoporous Zn/MgO Hexagonal Nano-Plates as a Catalyst for Camelina Oil Biodiesel Synthesis. Nanomaterials 2021, 11, 2690. https://doi.org/10.3390/nano11102690
Man L-F, Kwong T-L, Wong W-T, Yung K-F. Mesoporous Zn/MgO Hexagonal Nano-Plates as a Catalyst for Camelina Oil Biodiesel Synthesis. Nanomaterials. 2021; 11(10):2690. https://doi.org/10.3390/nano11102690
Chicago/Turabian StyleMan, Lai-Fan, Tsz-Lung Kwong, Wing-Tak Wong, and Ka-Fu Yung. 2021. "Mesoporous Zn/MgO Hexagonal Nano-Plates as a Catalyst for Camelina Oil Biodiesel Synthesis" Nanomaterials 11, no. 10: 2690. https://doi.org/10.3390/nano11102690
APA StyleMan, L.-F., Kwong, T.-L., Wong, W.-T., & Yung, K.-F. (2021). Mesoporous Zn/MgO Hexagonal Nano-Plates as a Catalyst for Camelina Oil Biodiesel Synthesis. Nanomaterials, 11(10), 2690. https://doi.org/10.3390/nano11102690






