Physical Surface Modification of Carbon-Nanotube/Polydimethylsiloxane Composite Electrodes for High-Sensitivity DNA Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Procedures
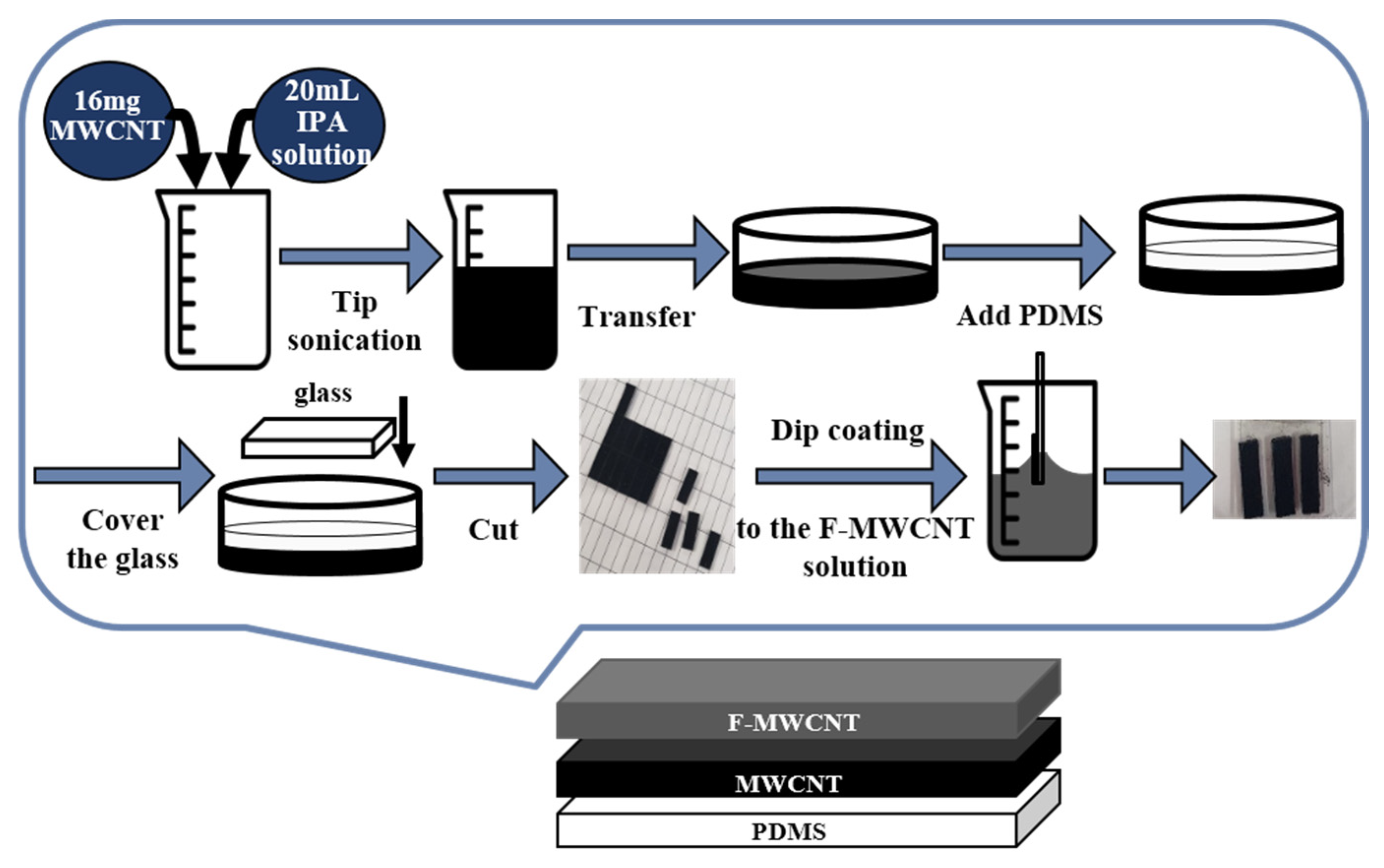
2.3.1. Preparation of Functionalized CNTs
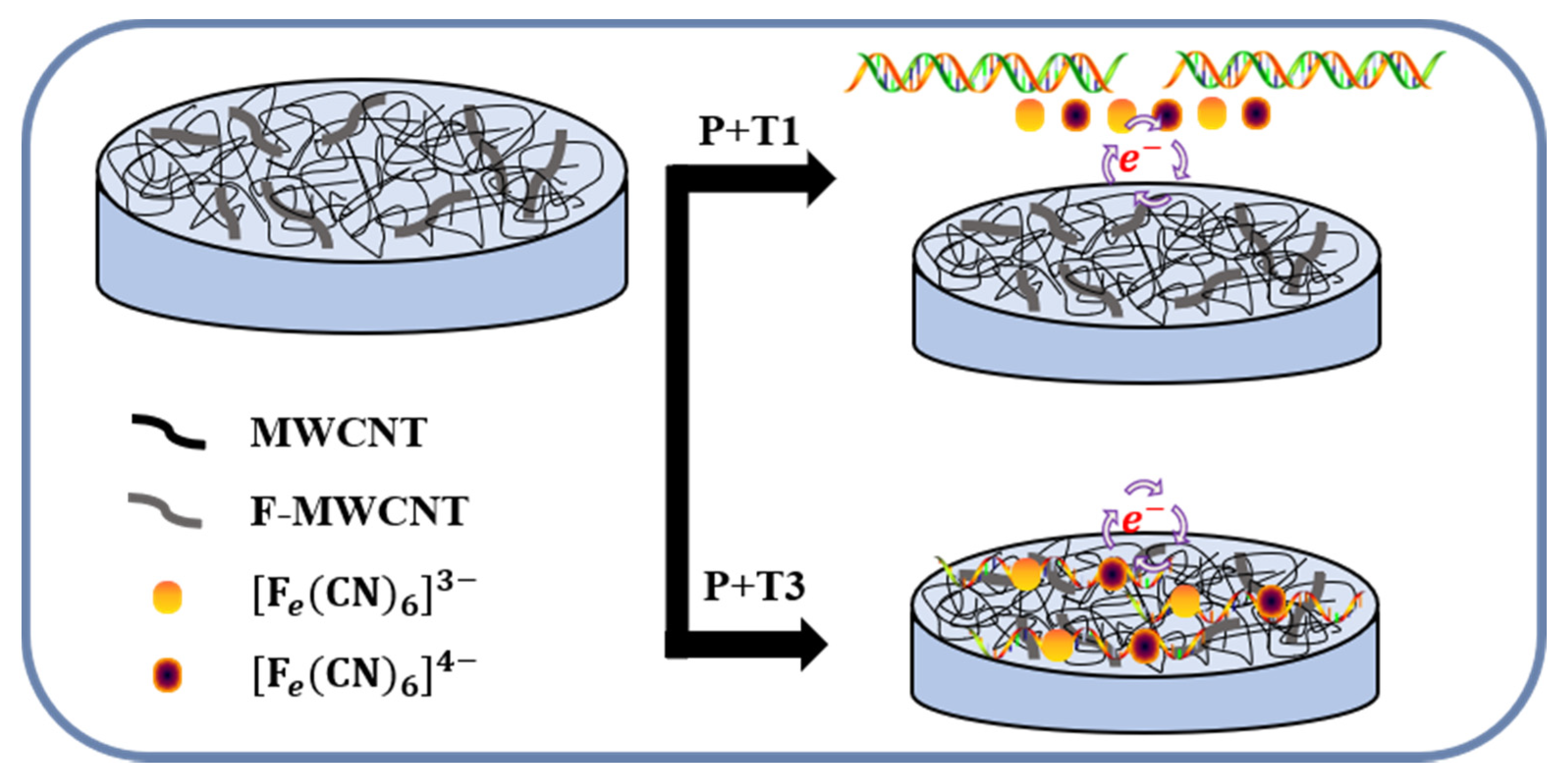
2.3.2. Process Method of the F-MWCNT/MWCNT/PDMS Electrode
2.3.3. Measurement Using EIS
3. Results and Discussion
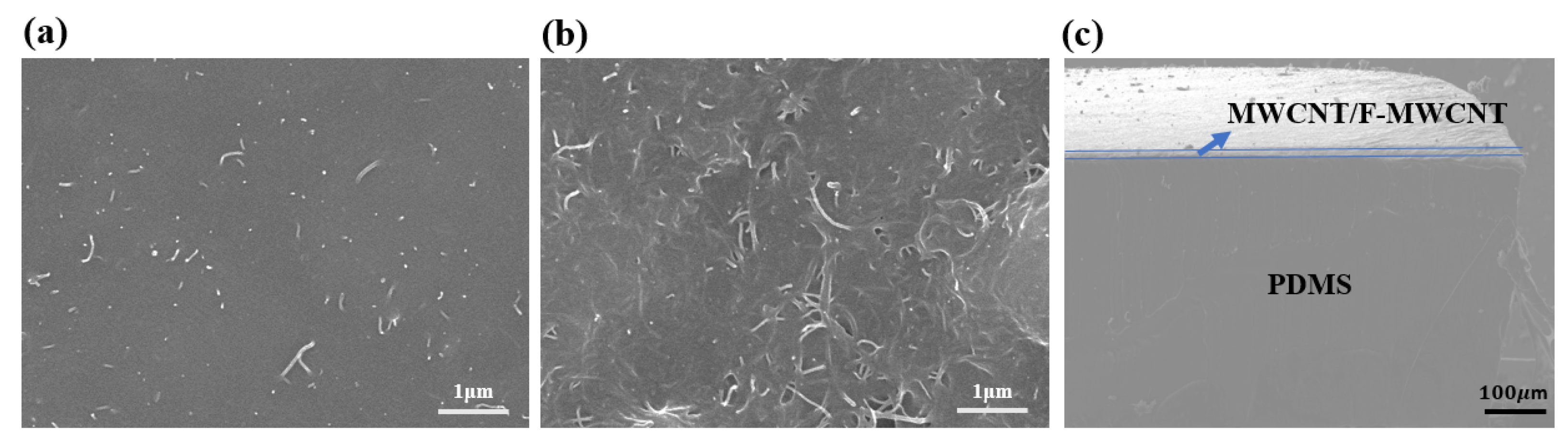
3.1. SEM Measurments of the Composite Electrode
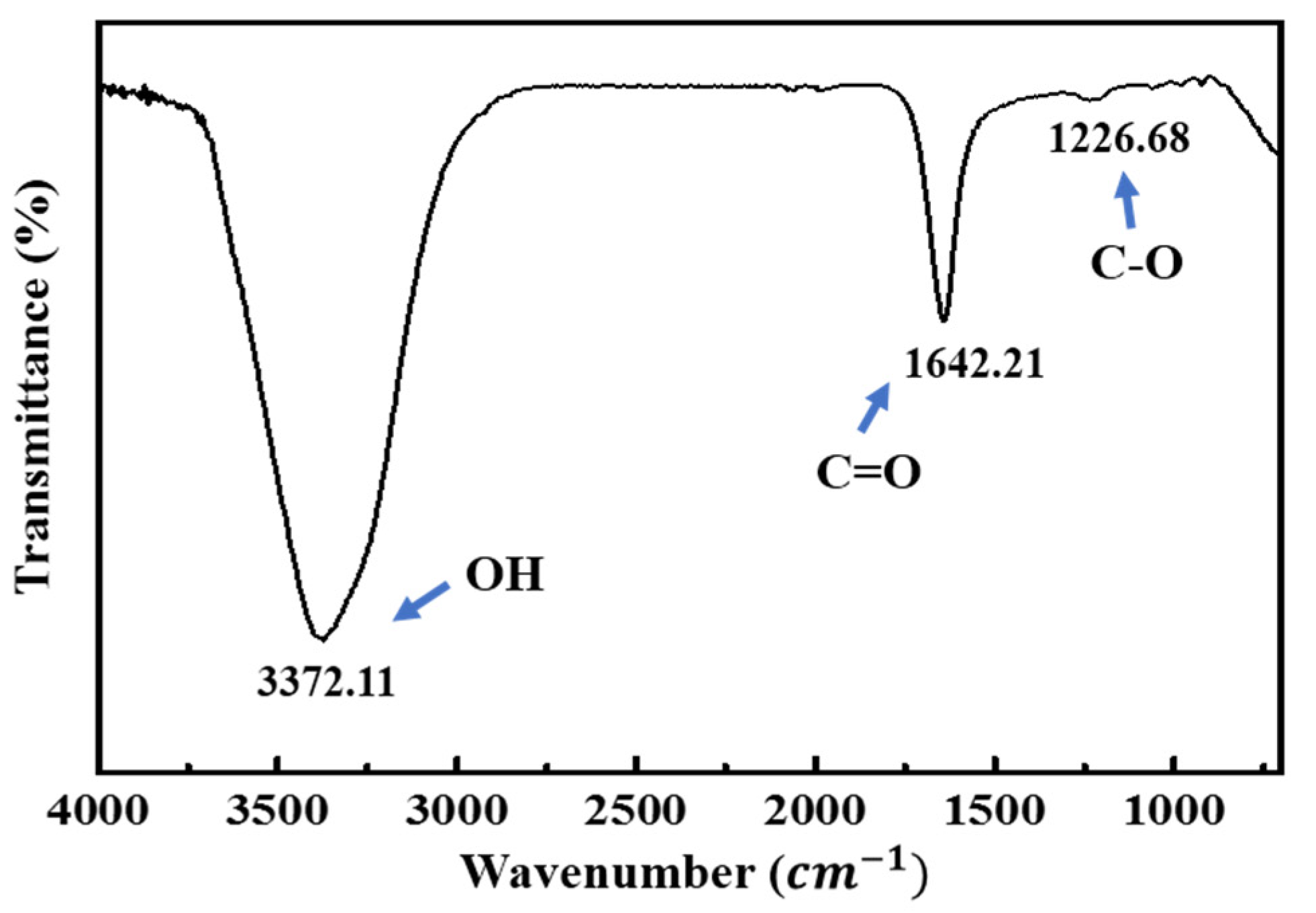
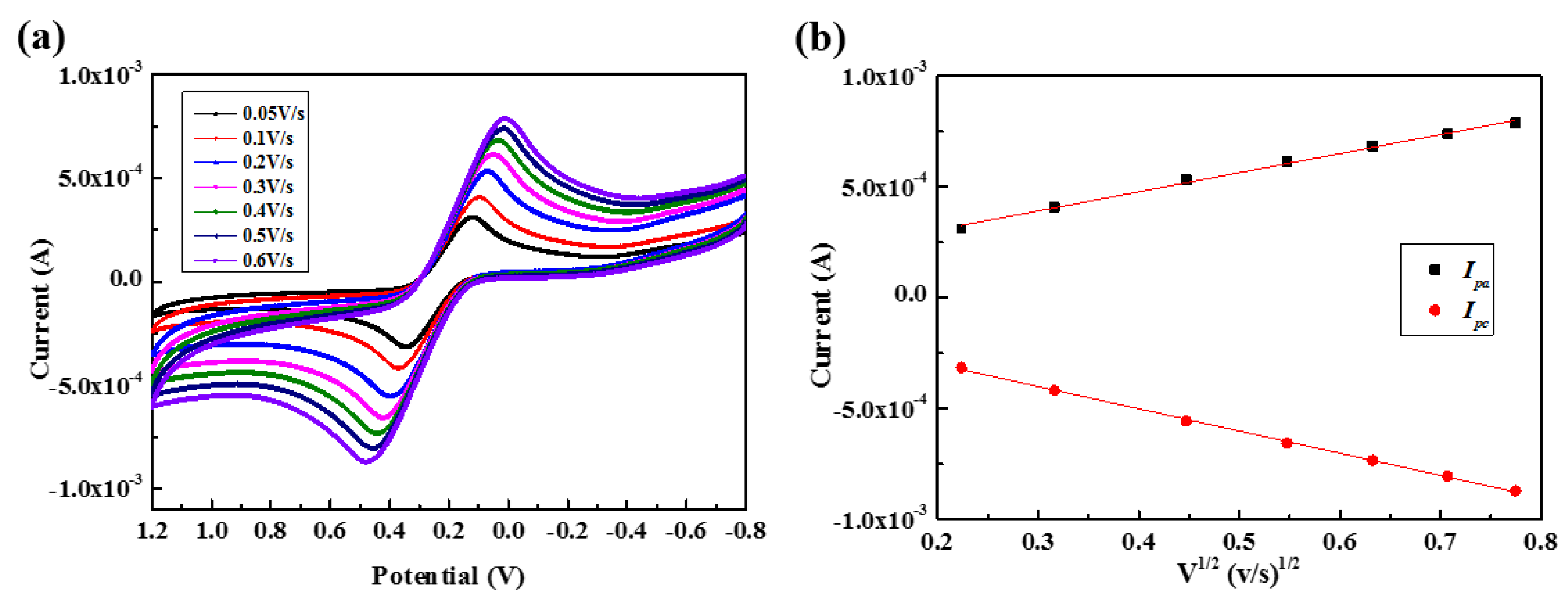
3.2. Characterization of the F-MWCNT-Modified Electrodes
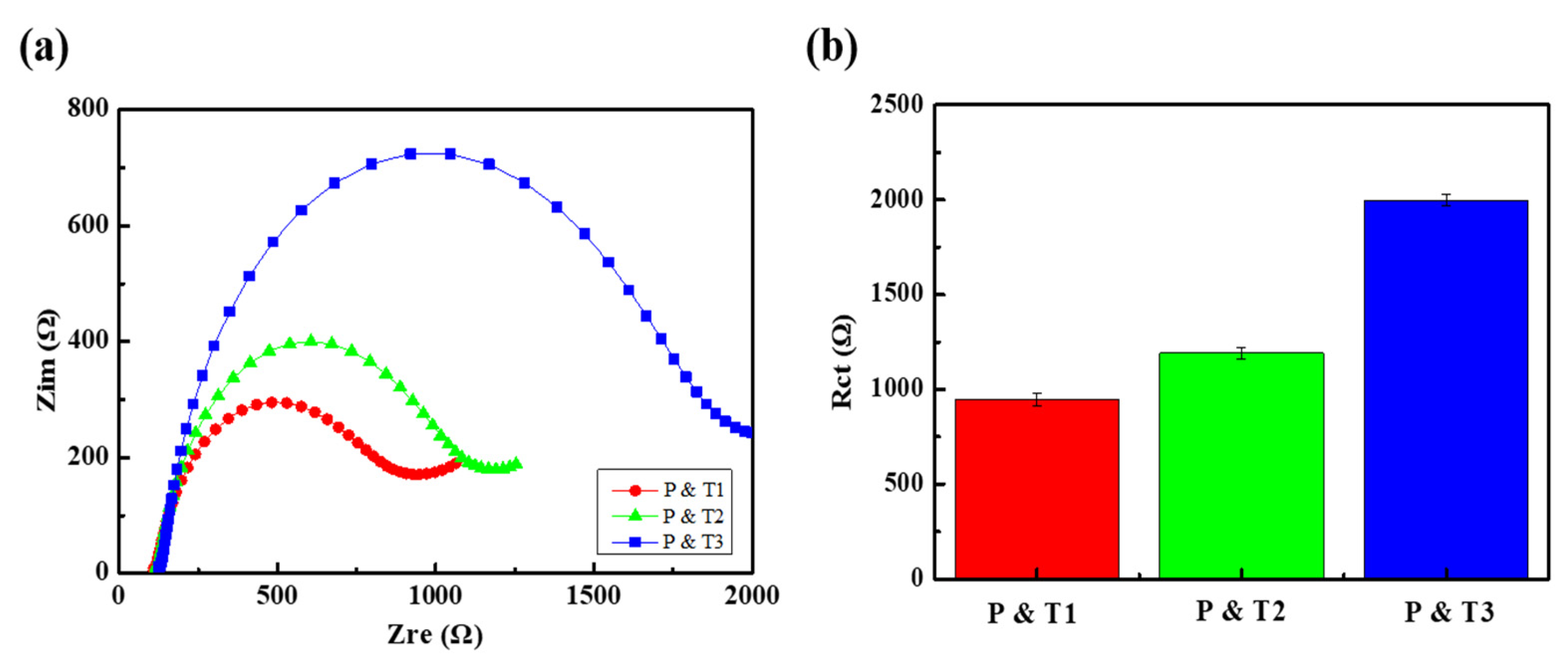
3.3. DNA Detection Using F-MWCNT-Modified Electrodes
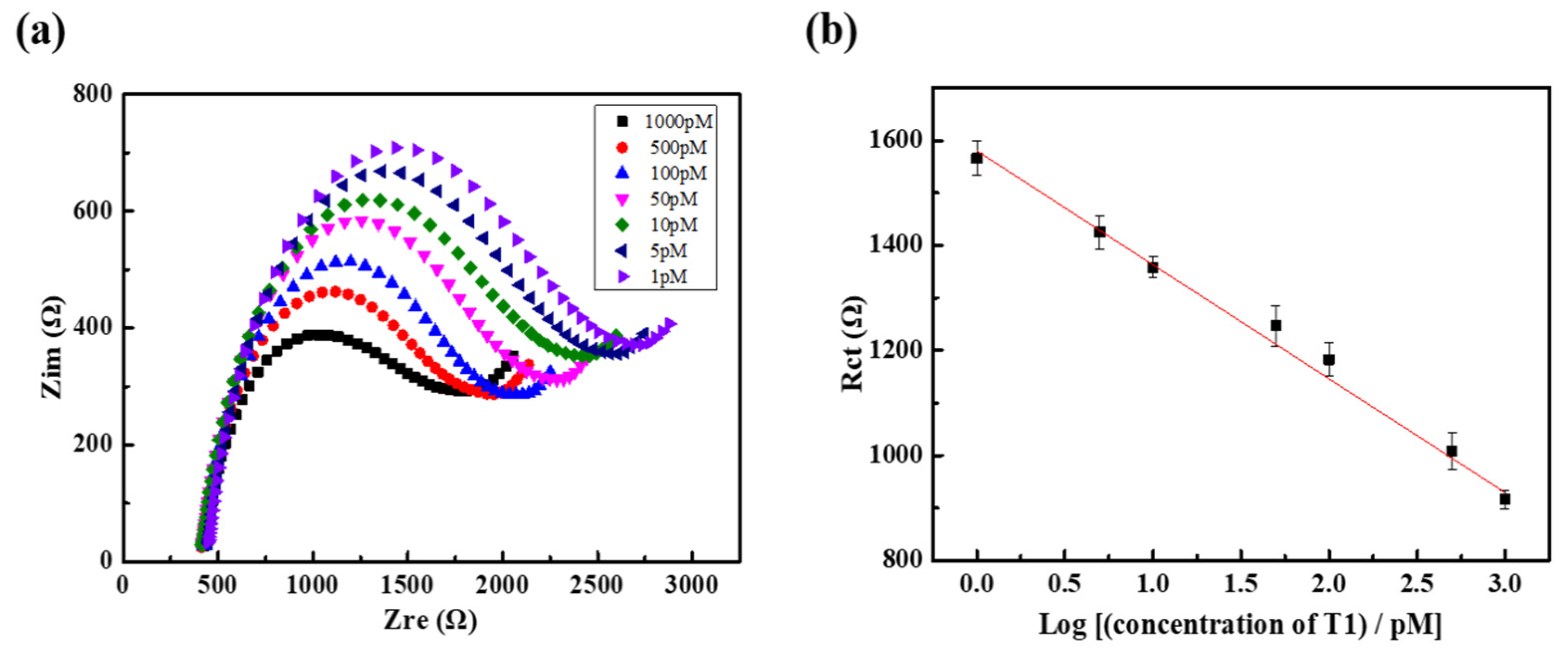
3.4. Detection Limit of the DNA Sensor
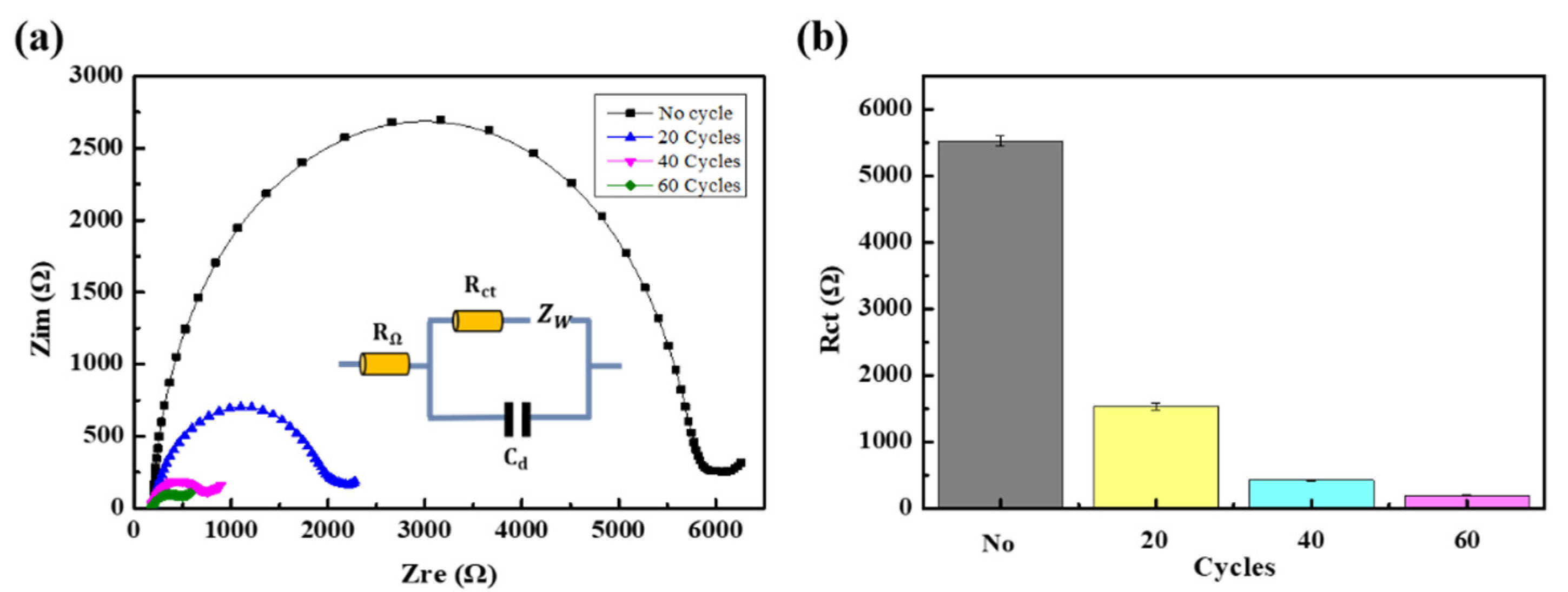
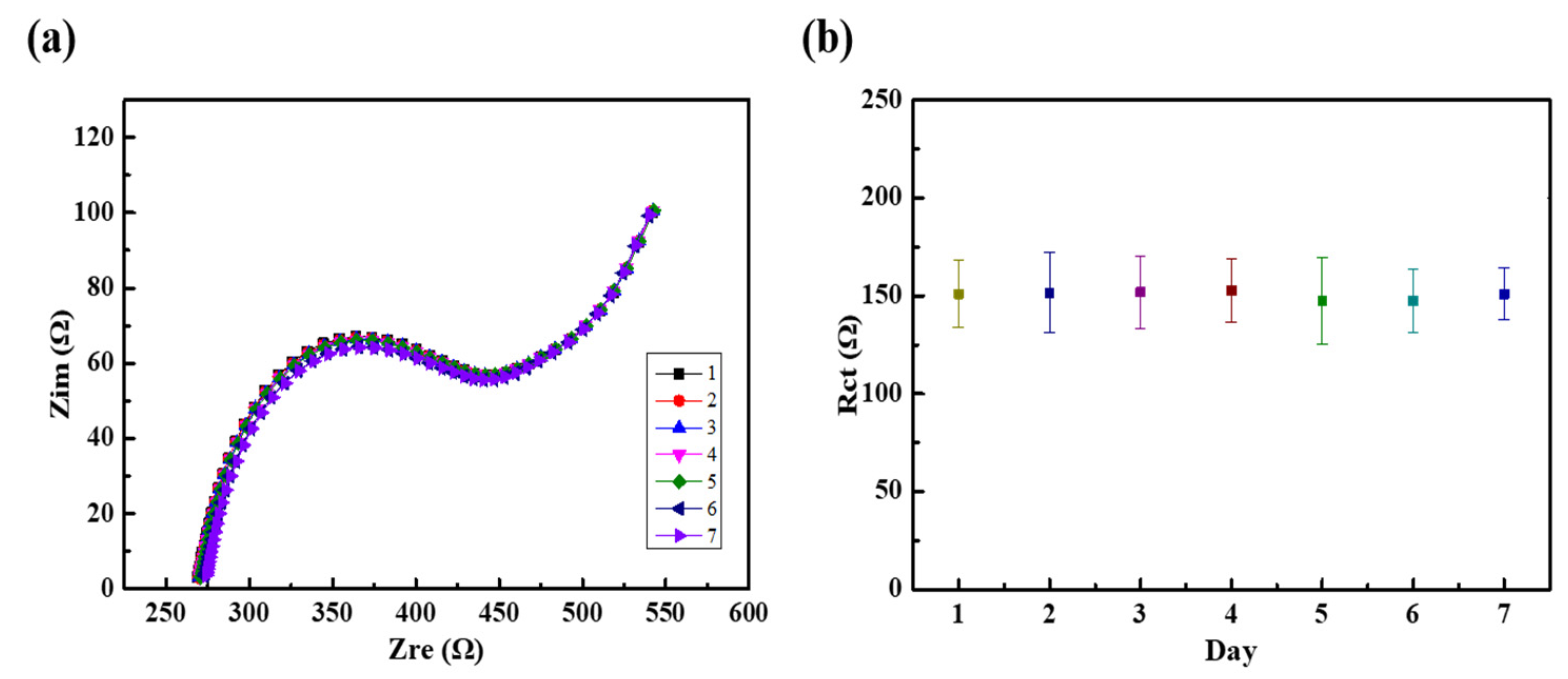
3.5. Stability of the Modified Electrode
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 68, 149–155. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Ren, J.; Qu, X. A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials 2011, 32, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Ezoji, H.; Rahimnejad, M. Electrochemical behavior of the endocrine disruptor bisphenol A and in situ investigation of its interaction with DNA. Sens. Actuators B Chem. 2018, 274, 370–380. [Google Scholar] [CrossRef]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of Staphylococcus aureus. Biosens. Bioelectron. 2015, 65, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Somerson, J.; Plaxco, K.W. Electrochemical aptamer-based sensors for rapid point-of-use monitoring of the mycotoxin ochratoxin a directly in a food stream. Molecules 2018, 23, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaylord, B.S.; Heeger, A.J.; Bazan, G.C. DNA detection using water-soluble conjugated polymers and peptide nucleic acid probes. Proc. Natl. Acad. Sci. USA 2002, 99, 10954–10957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, V.; Xiao, Y.; Shlyahovsky, B.; Willner, I. Aptamer-functionalized Au nanoparticles for the amplified optical detection of thrombin. J. Am. Chem. Soc. 2004, 126, 11768–11769. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-Free Biosensors Based on Aptamer-Modified Graphene Field-Effect. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef]
- Shin, J.-K.; Lee, J.-H.; Kim, D.-S.; Park, H.-J.; Choi, P.; Jeong, Y.-T.; Lim, G. An FET-type charge sensor for highly sensitive detection of DNA sequence. Biosens. Bioelectron. 2004, 20, 69–74. [Google Scholar] [CrossRef]
- Cai, H.; Xu, C.; He, P.; Fang, Y. Colloid Au-enhanced DNA immobilization for the electrochemical detection of sequence-specific DNA. J. Electroanal. Chem. 2001, 510, 78–85. [Google Scholar] [CrossRef]
- Wang, J.; Rivas, G.; Cai, X. Screen-Printed Electrochemical Hybridization Biosensor for the Detection of DNA Sequences from the Escherichia Coli Pathogen. Electroanalysis 1997, 9, 395–398. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Sheu, F.-S.; Al-Rubeaan, K.; Zheng, D.; Vashist, S.K. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2010, 29, 169–188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.T.; Cai, L.Y.; Zhou, Y.L.; Zhang, X.X. Immobilization-free DNA-based homogeneous electrochemical biosensors. TrAC Trends Anal. Chem. 2016, 85, 17–32. [Google Scholar] [CrossRef]
- Ahmed, R.; Reifsnider, K. Study of influence of electrode geometry on impedance spectroscopy. Int. J. Electrochem. Sci. 2011, 6, 1159–1174. [Google Scholar]
- Han, L.; Liu, P.; Petrenko, V.A.; Liu, A.H. A label-free electrochemical impedance cytosensor based on specific peptide-fused phage selected from landscape phage library. Sci. Rep. 2016, 6, 22199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, G.; Lamberti, I.; Mosiello, L.; Hianik, T. Impedimetric DNA Aptasensor for Sensitive Detection of Ochratoxin A in Food. Electroanalysis 2012, 24, 512–520. [Google Scholar] [CrossRef]
- Li, A.; Yang, F.; Ma, Y.; Yang, X. Electrochemical impedance detection of DNA hybridization based on dendrimer modified electrode. Biosens. Bioelectron. 2007, 22, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Guo, M.; Nie, Z.; Xiao, X.; Yao, S. Aptamer-based electrochemical sensor for label-free recognition and detection of cancer cells. Electroanalysis 2009, 21, 1321–1326. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, C.; Gui, R.; Gao, X.; Wang, Z. Reduced graphene oxide/nile blue/gold nanoparticles complex-modified glassy carbon electrode used as a sensitive and label-free aptasensor for ratiometric electrochemical sensing of dopamine. Anal. Chim. Acta 2018, 1025, 154–162. [Google Scholar] [CrossRef]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 2007, 363, 143–150. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, X.; Huo, Z.; He, X.; Liang, Y.; Xu, M. Electrochemical detection of dopamine in the presence of ascorbic acid using PVP/graphene modified electrodes. Talanta 2012, 97, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Cao, X.; Jiang, Y.; He, P.; Fang, Y. Carbon nanotube-enhanced electrochemical DNA biosensor for DNA hybridization detection. Anal. Bioanal. Chem. 2003, 375, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Niu, D.-J.; Sun, J.-Y.; Han, C.-H.; Wu, Z.-W.; Li, Y.-L.; Xiong, X.-Q. Novel electrochemical sensor based on functionalized graphene for simultaneous determination of adenine and guanine in DNA. Colloids Surf. B Biointerfaces 2011, 82, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh Tabrizi, M.; Shamsipur, M. A label-free electrochemical DNA biosensor based on covalent immobilization of salmonella DNA sequences on the nanoporous glassy carbon electrode. Biosens. Bioelectron. 2015, 69, 100–105. [Google Scholar] [CrossRef]
- Benvidi, A.; Tezerjani, M.D.; Jahanbani, S.; Mazloum Ardakani, M.; Moshtaghioun, S.M. Comparison of impedimetric detection of DNA hybridization on the various biosensors based on modified glassy carbon electrodes with PANHS and nanomaterials of RGO and MWCNTs. Talanta 2016, 147, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Yola, M.L.; Qureshi, M.S.; Solak, A.O.; Atar, N.; Üstündaǧ, Z. A novel impedimetric biosensor based on graphene oxide/gold nanoplatform for detection of DNA arrays. Sens. Actuators B Chem. 2013, 188, 1201–1211. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, E.C. Highly selective, reusable electrochemical impedimetric DNA sensors based on carbon nanotube/polymer composite electrode without surface modification. Biosens. Bioelectron. 2018, 118, 16–22. [Google Scholar] [CrossRef]
- Li, J.; Lee, E.C. Carbon nanotube/polymer composite electrodes for flexible, attachable electrochemical DNA sensors. Biosens. Bioelectron. 2015, 71, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yang, T.; Li, X.; Jiao, K. Fabrication of DNA/graphene/polyaniline nanocomplex for label-free voltammetric detection of DNA hybridization. Talanta 2012, 88, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Xu, M. A sensitive DNA biosensor fabricated with gold nanoparticles/ploy (p-aminobenzoic acid)/carbon nanotubes modified electrode. Colloids Surf. B Biointerfaces 2010, 75, 179–185. [Google Scholar] [CrossRef]
- Niu, S.; Zhao, M.; Hu, L.; Zhang, S. Carbon nanotube-enhanced DNA biosensor for DNA hybridization detection using rutin-Mn as electrochemical indicator. Sens. Actuators B Chem. 2008, 135, 200–205. [Google Scholar] [CrossRef]
- Miao, X.; Guo, X.; Xiao, Z.; Ling, L. Electrochemical molecular beacon biosensor for sequence-specific recognition of double-stranded DNA. Biosens. Bioelectron. 2014, 59, 54–57. [Google Scholar] [CrossRef]
- Liu, J.; Rinzler, L.C.; Dai, L.S.; Hafner, M.; Bradley, Q.; Boul, S.M.; Lu, F.J.; Iverson, U.; Shelimov; Huffman; et al. Fullerene Pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef]
- Jagadish, K.; Srikantaswamy, S.; Byrappa, K.; Shruthi, L.; Abhilash, M.R. Dispersion of multiwall carbon nanotubes in organic solvents through hydrothermal supercritical condition. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Bell, M.C.; Suni, I.I. Impedance biosensor for peanut protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Kawde, A.-N.; Wang, J. Aptamer biosensor for label-free impedance spectroscopy detection of proteins based on recognition-induced switching of the surface charge. Chem. Commun. 2005, 4267–4269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Murphy, A.; O’Riordan, A.; O’Connell, I. Equivalent Impedance Models for Electrochemical Nanosensor-Based Integrated System Design. Sensors 2021, 21, 3259. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanal. An Int. J. Devoted to Fundam. Pract. Asp. Electroanal. 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Hajisadeghian, E.; Golestaneh, M. Electrochemical determination of acetaminophen at the surface of a glassy carbon electrode modified with multi-walled carbon nanotube. J. Chil. Chem. Soc. 2013, 58, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Qi, B.-P.; Wang, B.-S.; Bao, L.; Hu, H.; Pang, D.-W.; Zhang, Z.-L.; Tang, B. An efficient edge-functionalization method to tune the photoluminescence of graphene quantum dots. Nanoscale 2015, 7, 5969–5973. [Google Scholar] [CrossRef]
- Shankar, A.; Mittal, J.; Jagota, A. Binding between DNA and carbon nanotubes strongly depends upon sequence and chirality. Langmuir 2014, 30, 3176–3183. [Google Scholar] [CrossRef]
- Shapter, J.G.; Sibley, A.J.; Shearer, C.J.; Andersson, G.G.; Ellis, A.V.; Gibson, C.T.; Yu, L.; Quinton, J.S.; Fenati, R. Adsorption and Desorption of Single-Stranded DNA from Single-Walled Carbon Nanotubes. Chem. An Asian J. 2017, 12, 1625–1634. [Google Scholar] [CrossRef]
- Star, A.; Joiner, C.S.; Gabriel, J.-C.P.; Valcke, C.; Tu, E.; Niemann, J. Label-free detection of DNA hybridization using carbon nanotube network field-effect transistors. Proc. Natl. Acad. Sci. USA 2006, 103, 921–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Johnson, J.K. Simulation of adsorption of DNA on carbon nanotubes. J. Am. Chem. Soc. 2007, 129, 10438–10445. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Liu, B.; Salgado, S.; Maheshwari, V.; Liu, J. DNA adsorbed on graphene and graphene oxide: Fundamental interactions, de-sorption and applications. Curr. Opin. Colloid Interface Sci. 2016, 26, 41–49. [Google Scholar] [CrossRef]
| Type | Name | Sequence |
|---|---|---|
| Probe DNA | P | 5′-GTG TTG TCT CCT AGG TTG GCT CTG-3′ |
| Complementary target DNA | T1 | 5′-CAG AGC CAA CCT AGG AGA CAA CAC-3′ |
| One base-non-complementary target DNA | T2 | 5′-CAG AGC CAA CCT CGG AGA CAA CAC-3′ |
| Non-complementary target DNA | T3 | 5′-ATA TCG ACC TTG GCC GAG ACG GTG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.; Jiang, H.; Lee, E.-C. Physical Surface Modification of Carbon-Nanotube/Polydimethylsiloxane Composite Electrodes for High-Sensitivity DNA Detection. Nanomaterials 2021, 11, 2661. https://doi.org/10.3390/nano11102661
Moon J, Jiang H, Lee E-C. Physical Surface Modification of Carbon-Nanotube/Polydimethylsiloxane Composite Electrodes for High-Sensitivity DNA Detection. Nanomaterials. 2021; 11(10):2661. https://doi.org/10.3390/nano11102661
Chicago/Turabian StyleMoon, Junga, Huaide Jiang, and Eun-Cheol Lee. 2021. "Physical Surface Modification of Carbon-Nanotube/Polydimethylsiloxane Composite Electrodes for High-Sensitivity DNA Detection" Nanomaterials 11, no. 10: 2661. https://doi.org/10.3390/nano11102661
APA StyleMoon, J., Jiang, H., & Lee, E.-C. (2021). Physical Surface Modification of Carbon-Nanotube/Polydimethylsiloxane Composite Electrodes for High-Sensitivity DNA Detection. Nanomaterials, 11(10), 2661. https://doi.org/10.3390/nano11102661





