Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review
Abstract
1. Graphene and Nanodiamond—The Opposite Limits of Carbon at the Nanoscale
2. Graphene—Diamond Phase Transformations at Nanoscale
2.1. First Principle Calculations and Modeling
| System | Evidence | References |
|---|---|---|
| Diamane | Atomistic first principles computations | [41] |
| F-diamane | HRTEM, EELS | [42] |
| H-diamane | Optical absorption, XRD | [43] |
| LA10 phase | DFT | [44] |
| Graphene Arch-Bridge | First principle calculations | [45] |
| Diaphite | DFT, HRTEM | [46,47,48] |
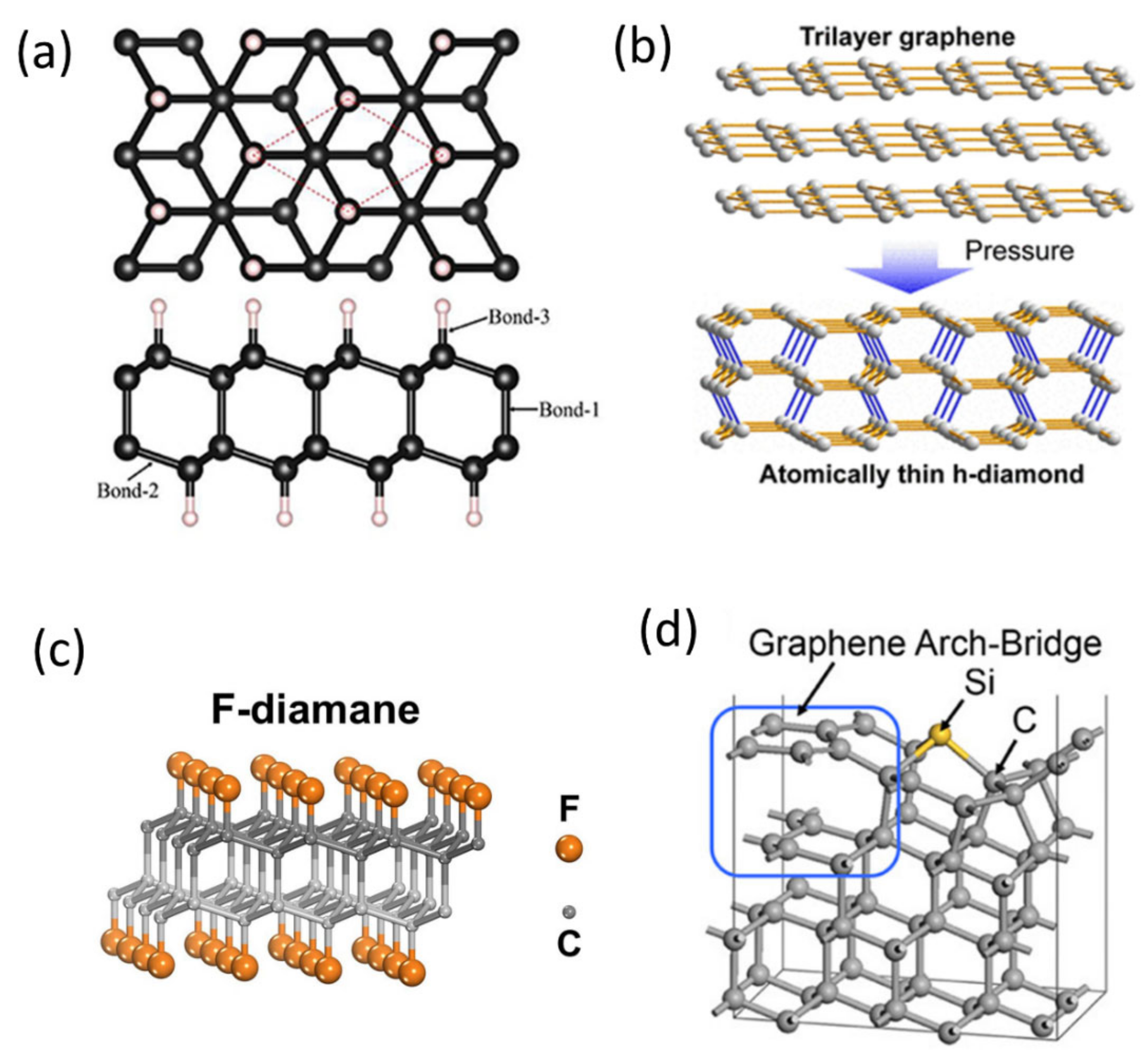
2.2. Experimental Demonstration and Growth Mechanism
3. Graphene—Diamond Sensors and Biosensors
3.1. Electrochemical Sensors
3.2. Biosensors
4. Graphene—Diamond Interfaces and Heterojunctions
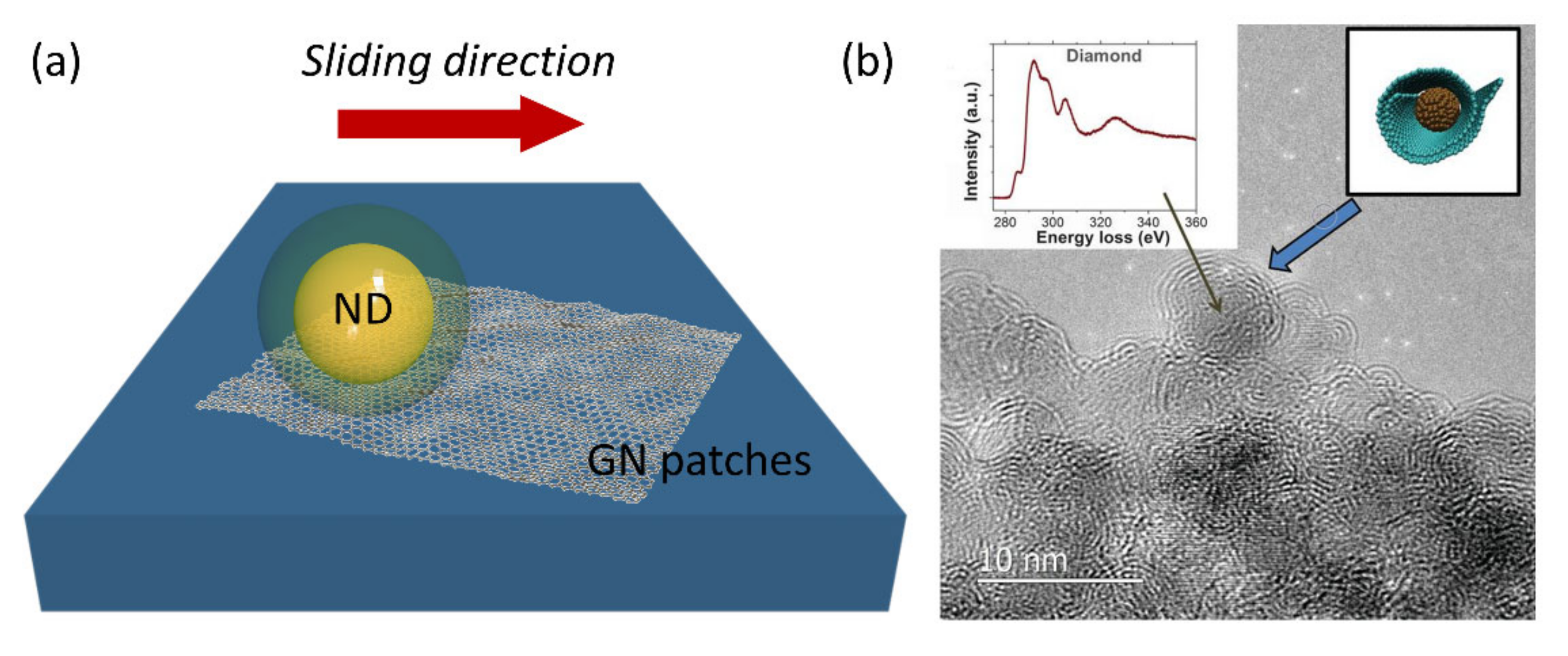
4.1. Friction, Tribology, and Mechanical Properties
4.2. Nanoelectronic and Spintronic Platforms
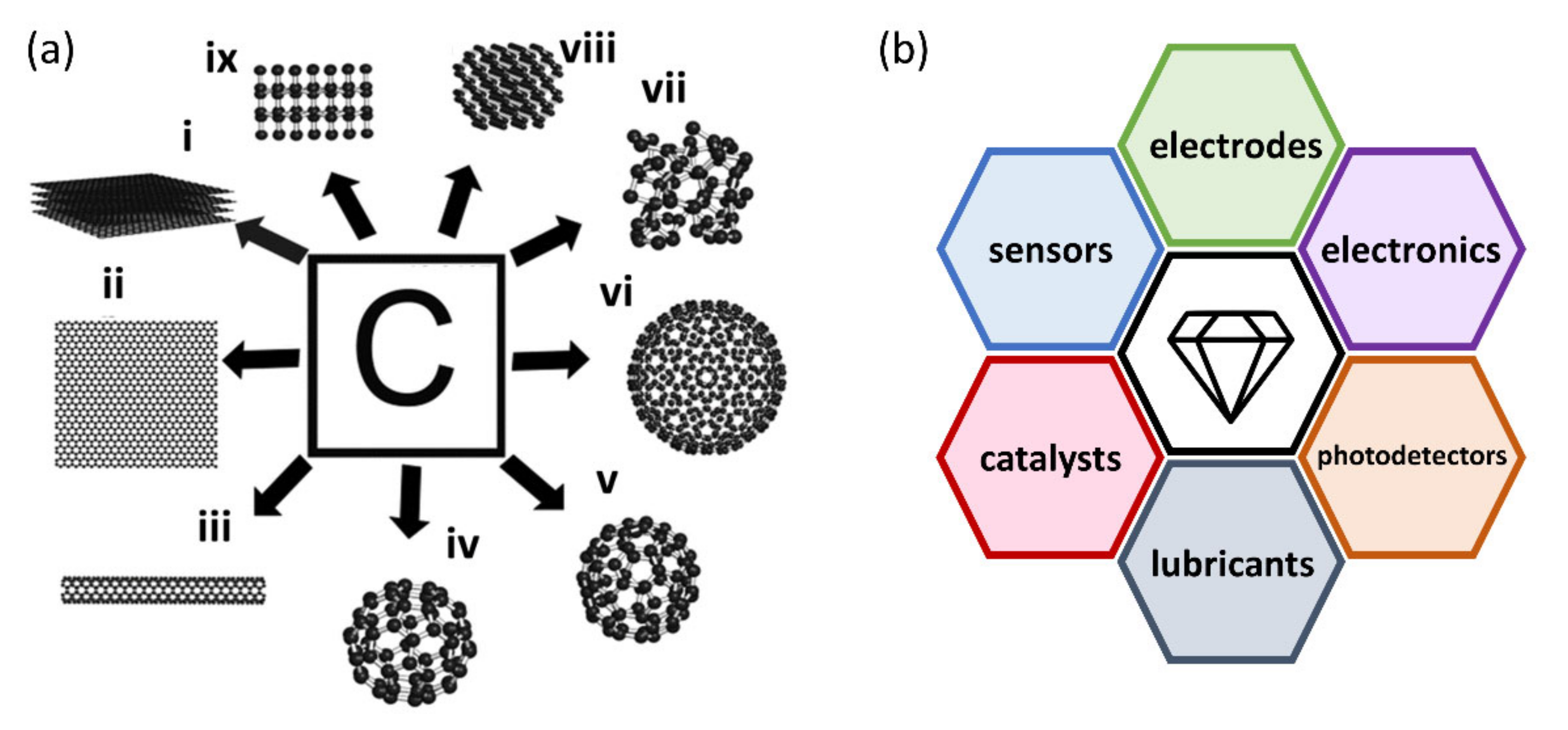
5. Other Applications
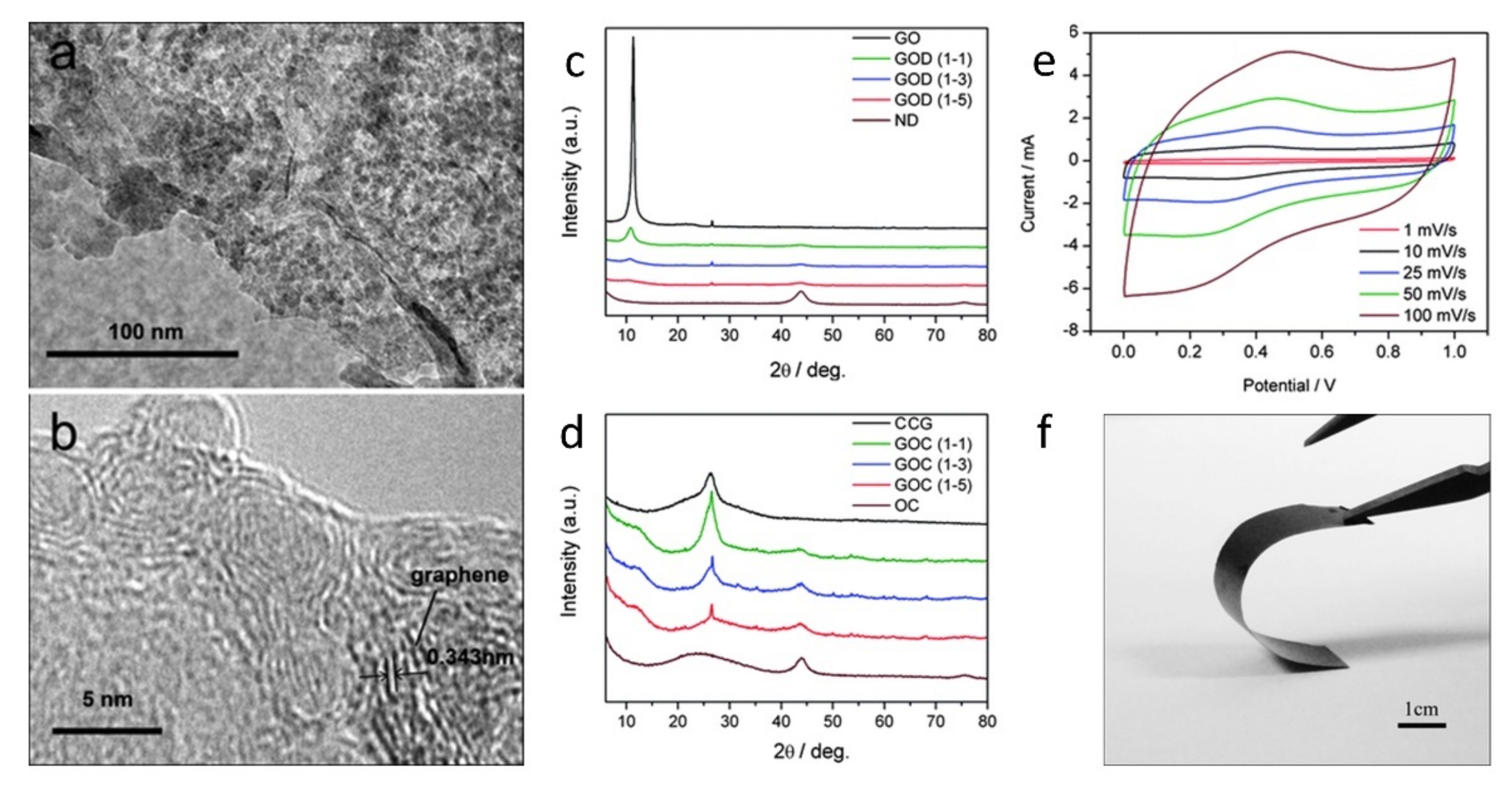
5.1. Energy Storage—Supercapacitors
5.2. Detectors and Light Sources
5.3. Materials’ Processing Technologies
5.4. Catalysis
5.5. Generation of Extreme Environments
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 0D: 1D: 2D, 3D | zero-, one-, two-, three-dimensional |
| AFM | atomic force microscopy |
| BD | boron-doped, e.g., BDND—boron-doped nanodiamond |
| CVD | chemical vapor deposition |
| DFT | density functional theory |
| EDCL | electrochemical double-layer capacitance |
| EFE | electron field emission |
| FET | field-effect transistor |
| GC | glassy carbon |
| GO | graphene oxide |
| GN | graphene |
| HPHT | high-pressure high-temperature |
| HRTEM | high-resolution transmission electron microscopy |
| ML | multi-layer, e.g., MLGN—multi-layer graphene |
| ND | nanodiamond |
| NV | nitrogen-vacancy |
| ORR | oxygen reduced reaction |
| PCD | polycrystalline diamond compact |
| RGO | reduced graphene oxide |
| TBG | twisted bilayer graphene |
| UNCD | ultra-nanocrystalline diamond |
| UV | ultraviolet |
| vdW | van der Waals |
| XRD | X-ray diffraction |
References
- Iwasaki, T.; Muruganathan, M.; Mizuta, H. Impacts of Channel Constriction Dimensions of Graphene Single-Carrier Transistors on the Coulomb Diamond Characteristics. In Proceedings of the 2015 Silicon Nanoelectronics Workshop (SNW), Kyoto, Japan, 14–15 June 2015; ISBN 978-1-4673-7604-4. [Google Scholar]
- Abendroth, J.M.; Stemer, D.M.; Bloom, B.P.; Roy, P.; Naaman, R.; Waldeck, D.H.; Weiss, P.S.; Mondal, P.C. Spin Selectivity in Photoinduced Charge-Transfer Mediated by Chiral Molecules. ACS Nano 2019, 13, 4928–4946. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Ye, C.; Wang, G.; Tian, H.; Zhu, C.; He, P.; Ding, G.; Xie, X.; Liu, Y.; et al. C3N—A 2D Crystalline, Hole-Free, Tunable-Narrow-Bandgap Semiconductor with Ferromagnetic Properties. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Xu, A.; Wang, G.; Li, Y.; Dong, H.; Yang, S.; He, P.; Ding, G. Carbon-Based Quantum Dots with Solid-State Photoluminescent: Mechanism, Implementation, and Application. Small 2020, 16, 1–31. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Bolotin, K.I. Electronic transport in graphene: Towards high mobility. In Graphene – Properties, Preparation, Characterization and Devices; Skákalová, V., Kaiser, A.B., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2014; pp. 199–227. [Google Scholar] [CrossRef]
- Tomanek, D. Guide through the Nanocarbon Jungle; Morgan & Claypool Publishers: San Rafael, CA, USA, 2014; ISBN 9781627052733. [Google Scholar]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Gupta, S.; Joshi, P.; Narayan, J. Electron mobility modulation in graphene oxide by controlling carbon melt lifetime. Carbon 2020, 170, 327–337. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, S.; Zhou, P.; Sun, Q.; Wang, P.; Wan, L.; Li, J.; Chen, L.; Wang, X.; Ding, S.; et al. Evolution of the band-gap and optical properties of graphene oxide with controllable reduction level. Carbon 2013, 62, 157–164. [Google Scholar] [CrossRef]
- Acik, M.; Chabal, Y.J. A Review on Reducing Graphene Oxide for Band Gap Engineering. J. Mater. Sci. Res. 2012, 2, 101–112. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Ahn, E.C. 2D materials for spintronic devices. NPJ 2D Mater. Appl. 2020, 4, 17. [Google Scholar] [CrossRef]
- Kang, S.; Lee, D.; Kim, J.; Capasso, A.; Kang, H.S.; Park, J.W.; Lee, C.H.; Lee, G.H. 2D semiconducting materials for electronic and optoelectronic applications: Potential and challenge. 2D Mater. 2020, 7, 02203. [Google Scholar] [CrossRef]
- Wei, W.; Yang, S.; Wang, G.; Zhang, T.; Pan, W.; Cai, Z.; Yang, Y.; Zheng, L.; He, P.; Wang, L.; et al. Bandgap engineering of two-dimensional C3N bilayers. Nat. Electron. 2021, 4, 486–494. [Google Scholar] [CrossRef]
- Xu, J.; Mahmood, J.; Dou, Y.; Dou, S.; Li, F.; Dai, L.; Baek, J.B. 2D Frameworks of C2N and C3N as New Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Kumar, P.; Vahidzadeh, E.; Thakur, U.K.; Kar, P.; Alam, K.M.; Goswami, A.; Mahdi, N.; Cui, K.; Bernard, G.M.; Michaelis, V.K.; et al. C3N5: A Low Bandgap Semiconductor Containing an Azo-Linked Carbon Nitride Framework for Photocatalytic, Photovoltaic and Adsorbent Applications. J. Am. Chem. Soc. 2019, 141, 5415–5436. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Lee, E.K.; Jung, M.; Shin, D.; Jeon, I.Y.; Jung, S.M.; Choi, H.J.; Seo, J.M.; Bae, S.Y.; Sohn, S.D.; et al. Nitrogenated holey two-dimensional structures. Nat. Commun. 2015, 6, 4–10. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Detonation synthesis ultradispersed diamonds: Properties and applications. Russ. Chem. Rev. 2001, 70, 607–626. [Google Scholar] [CrossRef]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A high impact nanomaterial. Curr. Opin. Solid State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Shenderova, O.A.; McGuire, G.E. Science and engineering of nanodiamond particle surfaces for biological applications (Review). Biointerphases 2015, 10, 30802. [Google Scholar] [CrossRef]
- Fokin, A.A.; Schreiner, P.R. Band gap tuning in nanodiamonds: First principle computational studies. Mol. Phys. 2009, 107, 823–830. [Google Scholar] [CrossRef]
- Pernot, J.; Tavares, C.; Gheeraert, E.; Bustarret, E.; Katagiri, M.; Koizumi, S. Hall electron mobility in diamond. Appl. Phys. Lett. 2006, 89, 9–11. [Google Scholar] [CrossRef]
- Wort, C.J.H.; Balmer, R.S. Diamond as an electronic material. Mater. Today 2008, 11, 22–28. [Google Scholar] [CrossRef]
- Ikeda, T.; Teii, K.; Casiraghi, C.; Robertson, J.; Ferrari, A.C. Effect of the s p2 carbon phase on n -type conduction in nanodiamond films. J. Appl. Phys. 2008, 104. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, G.Z.; Song, X.R.; Feng, F.P.; Zhu, W.; Lou, L.R.; Wang, J.F.; Wang, H.; Bao, P.F. Energy transfer from a single nitrogen-vacancy center in nanodiamond to a graphene monolayer. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Bray, K.; Cheung, L.; Hossain, K.R.; Aharonovich, I.; Valenzuela, S.M.; Shimoni, O. Versatile multicolor nanodiamond probes for intracellular imaging and targeted labeling. J. Mater. Chem. B 2018, 6, 3078–3084. [Google Scholar] [CrossRef]
- Rondin, L.; Tetienne, J.-P.; Hingant, T.; Roch, J.-F.; Maletinsky, P.; Jacques, V. Magnetometry with nitrogen-vacancy defects in diamond. Rep. Prog. Phys. 2014, 77, 056503. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Chernozatonskii, L.A.; Yakobson, B.I.; Sorokin, P.B. Phase Diagram of Quasi-Two-Dimensional Carbon, From Graphene to Diamond. Nano Lett. 2014, 14, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Antipina, L.Y.; Sorokin, P.B. Converting Chemically Functionalized Few-Layer Graphene to Diamond Films: A Computational Study. J. Phys. Chem. C 2015, 119, 2828–2836. [Google Scholar] [CrossRef]
- Belenkov, E.A.; Greshnyakov, V.A. Diamond-like phases prepared from graphene layers. Phys. Solid State 2015, 57, 205–212. [Google Scholar] [CrossRef]
- Geng, P.; Branicio, P.S. Atomistic insights on the pressure-induced multi-layer graphene to diamond-like structure transformation. Carbon 2021, 175, 243–253. [Google Scholar] [CrossRef]
- Alekseev, N.I. Matrix Synthesis of Graphene on a Diamond Surface and Its Simulation. Russ. J. Phys. Chem. A 2018, 92, 1369–1374. [Google Scholar] [CrossRef]
- Xu, M.J.; Zhang, Y.Z.; Zhang, J.; Lu, J.Y.; Qian, B.J.; Lu, D.J.; Zhang, Y.F.; Wang, L.; Chen, X.S.; Shigekawa, H. Spontaneous formation of graphene-like stripes on high-index diamond C(331) surface. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, M.M.; Fasolino, A. Minimal graphene thickness for wear protection of diamond. AIP Adv. 2015, 5. [Google Scholar] [CrossRef]
- Cellini, F.; Lavini, F.; Cao, T.F.; de Heer, W.; Berger, C.; Bongiorno, A.; Riedo, E. Epitaxial two-layer graphene under pressure: Diamene stiffer than Diamond. FlatChem 2018, 10, 8–13. [Google Scholar] [CrossRef]
- Erohin, S.V.; Ruan, Q.Y.; Sorokin, P.B.; Yakobson, B.I. Nano-Thermodynamics of Chemically Induced Graphene-Diamond Transformation. Small 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Momeni, K. Mechanochemistry of Stable Diamane and Atomically Thin Diamond Films Synthesis from Bi- and Multilayer Graphene: A Computational Study. J. Phys. Chem. C 2019, 123, 15751–15760. [Google Scholar] [CrossRef]
- Bakharev, P.V.; Huang, M.; Saxena, M.; Lee, S.W.; Joo, S.H.; Park, S.O.; Dong, J.C.; Camacho-Mojica, D.C.; Jin, S.; Kwon, Y.; et al. Chemically induced transformation of chemical vapour deposition grown bilayer graphene into fluorinated single-layer diamond. Nat. Nanotechnol. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Zhang, L.; Chen, Y.; Yin, K.; Wang, C.; Tzeng, Y.K.; Lin, Y.; Dong, H.; Liu, Z.; Tse, J.S.; et al. Synthesis of Atomically Thin Hexagonal Diamond with Compression. Nano Lett. 2020, 20, 5916–5921. [Google Scholar] [CrossRef] [PubMed]
- Greshnyakov, V.A.; Belenkov, E.A. Structure and properties of diamond-like phase obtained from tetragonal graphene layers. Lett. Mater. 2016, 6, 159–162. [Google Scholar] [CrossRef]
- Bai, S.D.; Xu, J.X.; Wang, Y.; Zhang, Q.; Tsuruda, T.; Higuchi, Y.; Ozawa, N.; Adachi, K.; Martin, J.M.; Kubo, M. Generation of “Graphene Arch-Bridge” on a Diamond Surface by Si Doping: A First-Principles Computational Study. J. Phys. Chem. C 2020, 124, 26379–26386. [Google Scholar] [CrossRef]
- Nemeth, P.; McColl, K.; Smith, R.L.; Murri, M.; Garvie, L.A.J.; Alvaro, M.; Pecz, B.; Jones, A.P.; Cora, F.; Salzmann, C.G.; et al. Diamond-Graphene Composite Nanostructures. Nano Lett. 2020, 20, 3611–3619. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Sumant, A.V. Macroscale superlubricity enabled by graphene nanoscroll formation. Science 2015, 348, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Radosinski, L.; Formalik, F.; Olejniczak, A.; Radosz, A. Diaphite, a new type of surface with mixed sp 2 -sp 3 hybridization for adsorption and functionalization. Appl. Surf. Sci. 2017, 404, 154–161. [Google Scholar] [CrossRef]
- Gu, C.Z.; Li, W.X.; Xu, J.; Xu, S.C.; Lu, C.; Xu, L.F.; Li, J.J.; Zhang, S.B. Graphene grown out of diamond. Appl. Phys. Lett. 2016, 109. [Google Scholar] [CrossRef]
- Lu, C.; Yang, H.X.; Xu, J.; Xu, L.F.; Chshiev, M.; Zhang, S.B.; Gu, C.Z. Spontaneous formation of graphene on diamond (111) driven by B- doping induced surface reconstruction. Carbon 2017, 115, 388–393. [Google Scholar] [CrossRef]
- Okada, S. Formation of graphene nanostructures on diamond nanowire surfaces. Chem. Phys. Lett. 2009, 483, 128–132. [Google Scholar] [CrossRef]
- Ileri, N.; Goldman, N. Graphene and nano-diamond synthesis in expansions of molten liquid carbon. J. Chem. Phys. 2014, 141. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Li, D.F.; Yin, Y.; Li, S.C.; Ding, G.Q.; Zhou, H.B.; Zhang, G. The important role of strain on phonon hydrodynamics in diamond-like bi-layer graphene. Nanotechnology 2020, 31. [Google Scholar] [CrossRef]
- Liu, J.; Muinos, H.V.; Nordlund, K.; Djurabekova, F. Structural properties of protective diamond-like-carbon thin films grown on multilayer graphene. J. Phys. Condens. Matter 2019, 31. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Holz, T.; Santos, N.F.; Ferro, M.C.; Martins, M.A.; Fernandes, A.J.S.; Silva, R.F.; Costa, F.M. Simultaneous CVD synthesis of graphene-diamond hybrid films. Carbon 2016, 98, 99–105. [Google Scholar] [CrossRef]
- Ueda, K.; Aichi, S.; Asano, H. Direct formation of graphene layers on diamond by high-temperature annealing with a Cu catalyst. Diam. Relat. Mater. 2016, 63, 148–152. [Google Scholar] [CrossRef]
- Cooil, S.P.; Song, F.; Williams, G.T.; Roberts, O.R.; Langstaff, D.P.; Jorgensen, B.; Hoydalsvik, K.; Breiby, D.W.; Wahlstrom, E.; Evans, D.A.; et al. Iron-mediated growth of epitaxial graphene on SiC and diamond. Carbon 2012, 50, 5099–5105. [Google Scholar] [CrossRef]
- Cooil, S.P.; Wells, J.W.; Hu, D.; Niu, Y.R.; Zakharov, A.A.; Bianchi, M.; Evans, D.A. Controlling the growth of epitaxial graphene on metalized diamond (111) surface. Appl. Phys. Lett. 2015, 107. [Google Scholar] [CrossRef]
- Garcia, J.M.; He, R.; Jiang, M.P.; Kim, P.; Pfeiffer, L.N.; Pinczuk, A. Multi layer graphene grown by precipitation upon cooling of nickel on diamond. Carbon 2011, 49, 1006–1012. [Google Scholar] [CrossRef]
- Kanada, S.; Nagai, M.; Ito, S.; Matsumoto, T.; Ogura, M.; Takeuchi, D.; Yamasaki, S.; Inokuma, T.; Tokuda, N. Fabrication of graphene on atomically flat diamond (111) surfaces using nickel as a catalyst. Diam. Relat. Mater. 2017, 75, 105–109. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Narayanan, B.; Sankaranarayanan, S.; Yan, Z.; Balandin, A.A.; Zinovev, A.; Rosenmann, D.; Sumant, A.V. Metal-induced rapid transformation of diamond into single and multilayer graphene on wafer scale. Nat. Commun. 2016, 7, 12099. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Le Normand, F. Surface transformations of carbon (graphene, graphite, diamond, carbide), deposited on polycrystalline nickel by hot filaments chemical vapour deposition. Thin Solid Films 2011, 519, 4426–4428. [Google Scholar] [CrossRef]
- Tsubota, T.; Morioka, A.; Osoekawa, Y.; Nakao, M. Direct Synthesis of Graphene Layer Covered Micro Channel on Diamond Surface Using Ni Wire. J. Nanosci. Nanotechnol. 2018, 18, 4418–4422. [Google Scholar] [CrossRef]
- Zou, H.H.; Bai, H.; Yu, J.H.; Wang, Y.; Liao, Q.L.; Nishimura, K.; Zeng, L.M.; Jiang, N. Architecting graphene nanowalls on diamond powder surface. Compos. Part B Eng. 2015, 73, 57–60. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.H.; Wang, Y.; Song, Y.Z.; Bai, H.; Jiang, N. Single crystalline 3C-SiC nanowires grown on the diamond surface with the assistance of graphene. J. Cryst. Growth 2015, 420, 6–10. [Google Scholar] [CrossRef]
- Li, D.S.; Zou, W.; Jiang, W.G.; Peng, X.Y.; Song, S.L.; Qin, Q.H.; Xue, J.M. Quasi homoepitaxial growth of modified diamond: Nickel-substrate catalytic multilayer graphene transforming to diamond. Ceram. Int. 2020, 46, 10885–10892. [Google Scholar] [CrossRef]
- Hussain, H.A.; Akman, N.; Ozdogan, C. Investigation of the mono vacancy effects on the structural, electronic and magnetic properties of graphene hexagonal-boron nitride in-plane hybrid embracing diamond shaped graphene island. Solid State Sci. 2020, 108. [Google Scholar] [CrossRef]
- Zkria, A.; Haque, A.; Egiza, M.; Abubakr, E.; Murasawa, K.; Yoshitake, T.; Narayan, J. Laser-induced structure transition of diamond-like carbon coated on cemented carbide and formation of reduced graphene oxide. MRS Commun. 2019, 9, 910–915. [Google Scholar] [CrossRef]
- Hembram, K.; Lee, S.; Im, H.; Ju, H.; Jeong, S.H.; Lee, J.K. The surface hybridization of diamond with vertical graphene: A new route to diamond electronics. Mater. Horiz. 2020, 7, 470–476. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Yao, M.G.; Dong, J.J.; Hu, K.; Liu, R.; Gong, C.; Wang, Y.; Liu, B.B. Direct Conversion of Graphene Aerogel into Low-Density Diamond Aerogel Composed of Ultrasmall Nanocrystals. J. Phys. Chem. C 2018, 122, 13193–13198. [Google Scholar] [CrossRef]
- Filonenko, V.P.; Zibrov, I.P.; Davydov, V.A.; Trenikhin, M. V Phase Formation in B-C-N System at High Pressures: Structure and Characteristics of Hetero-Graphene and Hetero-Diamond Particles. Izv. Vyss. Uchebnykh. Zaved. Khimiya Khimicheskaya Tekhnol. 2015, 58, 37. [Google Scholar]
- Fukuda, M.; Islam, M.S.; Sekine, Y.; Shinmei, T.; Lindoy, L.F.; Hayami, S. Crystallization of Diamond from Graphene Oxide Nanosheets by a High Temperature and High Pressure Method. ChemistrySelect 2021, 6, 3399–3402. [Google Scholar] [CrossRef]
- Ogawa, S.; Yamada, T.; Ishizduka, S.; Yoshigoe, A.; Hasegawa, M.; Teraoka, Y.; Takakuwa, Y. Vacuum Annealing Formation of Graphene on Diamond C(111) Surfaces Studied by Real-Time Photoelectron Spectroscopy. Jpn. J. Appl. Phys. 2012, 51. [Google Scholar] [CrossRef]
- Tokuda, N.; Fukui, M.; Makino, T.; Takeuchi, D.; Yamsaki, S.; Inokuma, T. Formation of Graphene-on-Diamond Structure by Graphitization of Atomically Flat Diamond (111) Surface. Jpn. J. Appl. Phys. 2013, 52. [Google Scholar] [CrossRef]
- Zhao, F.; Afandi, A.; Jackman, R.B. Graphene diamond-like carbon films heterostructure. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Tzeng, Y.H.; Chang, C.C. Graphene Induced Diamond Nucleation on Tungsten. IEEE Open J. Nanotechnol. 2020, 1, 117–127. [Google Scholar] [CrossRef]
- Tinchev, S.S. Surface modification of diamond-like carbon films to graphene under low energy ion beam irradiation. Appl. Surf. Sci. 2012, 258, 2931–2934. [Google Scholar] [CrossRef]
- Tinchev, S.; Valcheva, E.; Petrova, E. Low temperature crystallization of diamond-like carbon films to graphene. Appl. Surf. Sci. 2013, 280, 512–517. [Google Scholar] [CrossRef]
- Huang, Y.X.; Hara, A.; Terashima, C.; Fujishima, A.; Takai, M. Protein adsorption behavior on reduced graphene oxide and boron-doped diamond investigated by electrochemical impedance spectroscopy. Carbon 2019, 152, 354–362. [Google Scholar] [CrossRef]
- Hu, J.P.; Wisetsuwannaphum, S.; Foord, J.S. Glutamate biosensors based on diamond and graphene platforms. Faraday Discuss. 2014, 172, 457–472. [Google Scholar] [CrossRef]
- Cui, N.Y.; Guo, P.; Yuan, Q.L.; Ye, C.; Yang, M.Y.; Yang, M.H.; Chee, K.W.A.; Wang, F.; Fu, L.; Wei, Q.P.; et al. Single-Step Formation of Ni Nanoparticle-Modified Graphene-Diamond Hybrid Electrodes for Electrochemical Glucose Detection. Sensors 2019, 19, 2979. [Google Scholar] [CrossRef]
- Liu, A.P.; Xu, T.; Ren, Q.H.; Yuan, M.; Dong, W.J.; Tang, W.H. Graphene modulated 2D assembly of plasmonic gold nanostructure on diamond-like carbon substrate for surface-enhanced Raman scattering. Electrochem. Commun. 2012, 25, 74–78. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, Z.Q.; Chen, C.K.; Ma, W.C.; Han, S.J.; Li, X.; Lu, S.H.; Hu, X.J. High efficient oxygen reduced reaction electrodes by constructing vertical graphene sheets on separated papillary granules formed nanocrystalline diamond films. Carbon 2020, 168, 536–545. [Google Scholar] [CrossRef]
- Dettlaff, A.; Jakobczyk, P.; Ficek, M.; Wilk, B.; Szal, M.; Wojta, J.; Ossowski, T.; Bogdanowicz, R. Electrochemical determination of nitroaromatic explosives at boron-doped diamond/graphene nanowall electrodes: 2,4,6-trinitrotoluene and 2,4,6-trinitroanisole in liquid effluents. J. Hazard. Mater. 2020, 387. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Y.; Zhang, H.Y.; Ye, C.; Yuan, Q.L.; Chee, K.W.A.; Su, W.T.; Yu, A.M.; Yu, J.H.; Lin, C.T.; Dai, D.; et al. Electrochemical Enantiomer Recognition Based on sp(3)-to-sp(2) Converted Regenerative Graphene/Diamond Electrode. Nanomaterials 2018, 8, 1050. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.; Spataru, T.; Calderon-Moreno, J.M.; Osiceanu, P.; Preda, L.; Spataru, N. Anodic Voltammetry of Epinephrine at Graphene-Modified Conductive Diamond Electrodes and Its Analytical Application. J. Electrochem. Soc. 2018, 165, B523–B529. [Google Scholar] [CrossRef]
- Negrea, S.; Diaconu, L.A.; Nicorescu, V.; Motoc, S.; Orha, C.; Manea, F. Graphene Oxide Electroreduced onto Boron-Doped Diamond and Electrodecorated with Silver (Ag/GO/BDD) Electrode for Tetracycline Detection in Aqueous Solution. Nanomaterials 2021, 11, 1566. [Google Scholar] [CrossRef]
- Pei, J.X.; Yu, X.; Zhang, Z.Q.; Zhang, J.; Wei, S.B.; Boukherroub, R. In-situ graphene modified self-supported boron-doped diamond electrode for Pb(II) electrochemical detection in seawater. Appl. Surf. Sci. 2020, 527. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Idris, A.O.; Umukoro, E.H.; Babalola, J.O.; Arotiba, O.A. Electrochemical Detection of 2,4-Dichlorophenol on a Ternary Composite Electrode of Diamond, Graphene, and Polyaniline. ChemElectroChem 2017, 4, 1074–1080. [Google Scholar] [CrossRef]
- Peng, X.L.; Yuan, W.; Zou, J.X.; Wang, B.; Hu, W.Y.; Xiong, Y. Nitrogen-incorporated ultrananocrystalline diamond/multilayer graphene composite carbon films: Synthesis and electrochemical performances. Electrochim. Acta 2017, 257, 504–509. [Google Scholar] [CrossRef]
- Pop, A.; Lung, S.; Orha, C.; Manea, F. Silver/graphene-modified Boron Doped Diamond Electrode for Selective Detection of Carbaryl and Paraquat from Water. Int. J. Electrochem. Sci. 2018, 13, 2651–2660. [Google Scholar] [CrossRef]
- Pop, A.; Manea, F.; Flueras, A.; Schoonman, J. Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode. Sensors 2017, 17, 2033. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Poh, H.L.; Sofer, Z.; Pumera, M. Boron-doped graphene and boron-doped diamond electrodes: Detection of biomarkers and resistance to fouling. Analyst 2013, 138, 4885–4891. [Google Scholar] [CrossRef]
- Yuan, Q.L.; Liu, Y.; Ye, C.; Sun, H.Y.; Dai, D.; Wei, Q.P.; Lai, G.S.; Wu, T.Z.; Yu, A.M.; Fu, L.; et al. Highly stable and regenerative graphene-diamond hybrid electrochemical biosensor for fouling target dopamine detection. Biosens. Bioelectron. 2018, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff, A.; Jakobczyk, P.; Sobaszek, M.; Ficek, M.; Dec, B.; Luczkiewicz, A.; Szala, M.; Wojtas, J.; Ossowski, T.; Bogdanowicz, R. Electrochemical Detection of 4,4′,5,5′-Tetranitro-1H,1′ H-2,2′-Biimidazole on Boron-Doped Diamond/Graphene Nanowall Electrodes. IEEE Sens. J. 2020, 20, 9637–9643. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Ma, W.C.; Han, S.J.; Chen, C.K.; Fan, D.; Li, X.; Hu, X.J. Microstructure and electrochemical properties of nanocrystalline diamond and graphene hybridized films. J. Appl. Phys. 2020, 127. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy SERS: Surface-enhanced Raman spectroscopy Raman scattering: Inelastic scattering of a photon from a molecule in which the frequency change precisely matches the difference in vibrational energy levels. Annu. Rev. Anal. Chem 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.J.; Wang, T.; Long, Y.J.; Ni, J.R. Synergetic antibacterial activity of reduced graphene oxide and boron doped diamond anode in three dimensional electrochemical oxidation system. Sci. Rep. 2015, 5, 10388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, L.; Yang, W.T.; Li, M.; Guo, P.; Zhao, B.; Yang, L.F.; Fang, L.L.; Sun, B.; Jia, Y. The Flexible Lubrication Performance of Graphene Used in Diamond Interface as a Solid Lubricant: First-Principles Calculations. Nanomaterials 2019, 9, 1784. [Google Scholar] [CrossRef]
- Hu, W.; Li, Z.Y.; Yang, J.L. Diamond as an inert substrate of graphene. J. Chem. Phys. 2013, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.N.; Larsson, K. First principle study of the attachment of graphene onto non-doped and doped diamond (111). Diam. Relat. Mater. 2016, 66, 52–60. [Google Scholar] [CrossRef]
- Ma, Y.D.; Dai, Y.; Guo, M.; Huang, B.B. Graphene-diamond interface: Gap opening and electronic spin injection. Phys. Rev. B 2012, 85. [Google Scholar] [CrossRef]
- Bouchet, M.I.D.; Martin, J.M.; Avila, J.; Kano, M.; Yoshida, K.; Tsuruda, T.; Bai, S.; Higuchi, Y.; Ozawa, N.; Kubo, M.; et al. Diamond-like carbon coating under oleic acid lubrication: Evidence for graphene oxide formation in superlow friction. Sci. Rep. 2017, 7, 46394. [Google Scholar] [CrossRef] [PubMed]
- Sealy, C. Diamond puts a new shine on friction-free graphene. Nano Today 2015, 10, 412–413. [Google Scholar] [CrossRef]
- Yi, S.; Chen, X.C.; Li, J.J.; Liu, Y.F.; Ding, S.L.; Luo, J.B. Macroscale superlubricity of Si-doped diamond-like carbon film enabled by graphene oxide as additives. Carbon 2021, 176, 358–366. [Google Scholar] [CrossRef]
- Liu, Y.H.; Chen, L.; Jiang, B.Z.; Liu, Y.Q.; Zhang, B.; Xiao, C.; Zhang, J.Y.; Qian, L.M. Origin of low friction in hydrogenated diamond-like carbon films due to graphene nanoscroll formation depending on sliding mode: Unidirection and reciprocation. Carbon 2021, 173, 696–704. [Google Scholar] [CrossRef]
- Chen, S.L.; Shen, B.; Chen, Y.S.; Sun, F.H. Synergistic friction-reducing and anti-wear behaviors of graphene with micro- and nano-crystalline diamond films. Diam. Relat. Mater. 2017, 73, 25–32. [Google Scholar] [CrossRef]
- Chen, S.L.; Shen, B.; Sun, F.H. The influence of normal load on the tribological performance of electrophoretic deposition prepared graphene coating on micro-crystalline diamond surface. Diam. Relat. Mater. 2017, 76, 50–57. [Google Scholar] [CrossRef]
- Shen, B.; Chen, S.L.; Chen, Y.S.; Sun, F.H. Enhancement on the tribological performance of diamond films by utilizing graphene coating as a solid lubricant. Surf. Coat. Technol. 2017, 311, 35–45. [Google Scholar] [CrossRef]
- Shen, B.; Chen, S.L.; Huang, Z.W.; Ji, Z.; Lin, Q.; Zhang, Z.N. Elucidating the atomic mechanism of the lubricity of graphene on the diamond substrate. Appl. Surf. Sci. 2020, 504. [Google Scholar] [CrossRef]
- Zhang, J.; Osloub, E.; Siddiqui, F.; Zhang, W.X.; Ragab, T.; Basaran, C. Anisotropy of Graphene Nanoflake Diamond Interface Frictional Properties. Materials 2019, 12, 1425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Peng, Y.; Tang, X.Q.; Xu, Q.; Liu, B.; Bai, L.C. Lubrication Performance of Hydrogenated Graphene on Diamond-Like Carbon Films Based on Molecular Dynamics Simulation. Tribol. Lett. 2021, 69. [Google Scholar] [CrossRef]
- Zhao, S.; Larsson, K. First Principle Study of the Attachment of Graphene onto Different Terminated Diamond (111) Surfaces. Adv. Condens. Matter Phys. 2019, 2019. [Google Scholar] [CrossRef]
- Zhang, L.L.; Pu, J.B.; Wang, L.P.; Xue, Q.J. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Machado, A.S.; Maroudas, D.; Muniz, A.R. Tunable mechanical properties of diamond superlattices generated by interlayer bonding in twisted bilayer graphene. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Rysaeva, L.K.; Lisovenko, D.S.; Gorodtsov, V.A.; Baimova, J.A. Stability, elastic properties and deformation behavior of graphene-based diamond-like phases. Comput. Mater. Sci. 2020, 172. [Google Scholar] [CrossRef]
- Yin, N.; Zhang, Z.N.; Zhang, J.Y. Frictional Contact Between the Diamond Tip and Graphene Step Edges. Tribol. Lett. 2019, 67. [Google Scholar] [CrossRef]
- Zhao, F.; Nguyen, T.T.; Golsharifi, M.; Amakubo, S.; Loh, K.P.; Jackman, R.B. Electronic properties of graphene-single crystal diamond heterostructures. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Zhao, F.; Nguyen, T.T.; Golsharifi, M.; Amakubo, S.; Loh, K.P.; Jackman, R.B. Electronic properties of graphene-single crystal diamond heterostructures (vol 114, 053709, 2013). J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Wang, Y.; Jaiswal, M.; Lin, M.; Saha, S.; Ozyilmaz, B.; Loh, K.P. Electronic Properties of Nanodiamond Decorated Graphene. ACS Nano 2012, 6, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Chen, C.K.; Lu, S.H. High mobility n-type conductive ultrananocrystalline diamond and graphene nanoribbon hybridized carbon films. Carbon 2016, 98, 671–680. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Ficek, M.; Sobaszek, M.; Nosek, A.; Golunski, L.; Karczewski, J.; Jaramillo-Botero, A.; Goddard, W.A.; Bockrath, M.; Ossowski, T. Growth and Isolation of Large Area Boron-Doped Nanocrystalline Diamond Sheets: A Route toward Diamond-on-Graphene Heterojunction. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Selli, D.; Baburin, I.; Leoni, S.; Zhu, Z.; Tomanek, D.; Seifert, G. Theoretical investigation of the electronic structure and quantum transport in the graphene-C(111) diamond surface system. J. Phys. Condens. Matter 2013, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, A.H.; Schirmann, E.; Kovi, K.K. Fluorinated graphene oxide, nanocrystalline diamond multilayer thin films for optical and electromagnetic limiting applications. Emerg. Mater. 2021, 4, 525–530. [Google Scholar] [CrossRef]
- Konabe, S.; Cuong, N.T.; Otani, M.; Okada, S. High-Efficiency Photoelectric Conversion in Graphene-Diamond Hybrid Structures: Model and First-Principles Calculations. Appl. Phys. Express 2013, 6. [Google Scholar] [CrossRef]
- Luo, B.R.; Yuan, A.H.; Yang, S.; Han, L.Q.; Guan, R.; Duan, J.X.; Wang, C.; Dong, L.; Zhang, B.; Li, D.J. Synthesis of Diamond-like Carbon as a Dielectric Platform for Graphene Field Effect Transistors. ACS Appl. Nano Mater. 2021, 4, 1385–1393. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Lin, Y.M.; Bol, A.A.; Jenkins, K.A.; Xia, F.N.; Farmer, D.B.; Zhu, Y.; Avouris, P. High-frequency, scaled graphene transistors on diamond-like carbon. Nature 2011, 472, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Konabe, S.; Shiomi, J.; Yamamoto, T.; Maruyama, S.; Okada, S. Graphene-diamond hybrid structure as spin-polarized conducting wire with thermally efficient heat sinks. Appl. Phys. Lett. 2012, 100. [Google Scholar] [CrossRef]
- Tzeng, Y.H.; Chen, Y.R.; Li, P.Y.; Chang, C.C.; Chu, Y.C. NV Center Charge State Controlled Graphene-on-Diamond Field Effect Transistor Actions With Multi-Wavelength Optical Inputs. IEEE Open J. Nanotechnol. 2020, 1, 18–24. [Google Scholar] [CrossRef]
- Yu, J.; Liu, G.X.; Sumant, A.V.; Goyal, V.; Balandin, A.A. Graphene-on-Diamond Devices with Increased Current-Carrying Capacity: Carbon sp(2)-on-sp(3) Technology. Nano Lett. 2012, 12, 1603–1608. [Google Scholar] [CrossRef]
- Loh, G.C.; Teo, E.H.T.; Tay, B.K. Compounded effect of vacancy on interfacial thermal transport in diamond-graphene nanostructures. Diam. Relat. Mater. 2011, 20, 1137–1142. [Google Scholar] [CrossRef]
- Mirabedini, P.S.; Debnath, B.; Neupane, M.R.; Greaney, P.A.; Birdwell, A.G.; Ruzmetov, D.; Crawford, K.G.; Shah, P.; Weil, J.; Ivanov, T.G. Structural and electronic properties of 2D (graphene, hBN)/H-terminated diamond (100) heterostructures. Appl. Phys. Lett. 2020, 117. [Google Scholar] [CrossRef]
- Muniz, A.R.; Maroudas, D. Opening and tuning of band gap by the formation of diamond superlattices in twisted bilayer graphene. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef]
- Ueda, K.; Aichi, S.; Asano, H. Photo-controllable memristive behavior of graphene/diamond heterojunctions. Appl. Phys. Lett. 2016, 108. [Google Scholar] [CrossRef]
- Ueda, K.; Mizuno, Y.; Asano, H. Multibit optoelectronic memory using graphene/diamond (carbon sp(2)-sp(3)) heterojunctions and its arithmetic functions. Appl. Phys. Lett. 2020, 117. [Google Scholar] [CrossRef]
- Banerjee, D.; Sankaran, K.J.; Deshmukh, S.; Ficek, M.; Bhattacharya, G.; Ryl, J.; Phase, D.M.; Gupta, M.; Bogdanowicz, R.; Lin, I.N.; et al. 3D Hierarchical Boron-Doped Diamond-Multilayered Graphene Nanowalls as an Efficient Supercapacitor Electrode. J. Phys. Chem. C 2019, 123, 15458–15466. [Google Scholar] [CrossRef]
- Tzeng, Y.H.; Chen, W.L.; Wu, C.H.; Lo, J.Y.; Li, C.Y. The synthesis of graphene nanowalls on a diamond film on a silicon substrate by direct-current plasma chemical vapor deposition. Carbon 2013, 53, 120–129. [Google Scholar] [CrossRef]
- Cui, D.D.; Li, H.J.; Li, M.J.; Li, C.P.; Qian, L.R.; Zhou, B.Z.; Yang, B.H. Boron-Doped Graphene Directly Grown on Boron-Doped Diamond for High-Voltage Aqueous Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 1526. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Wu, Q.O.; Xu, Y.X.; Bai, H.; Li, C.; Shi, G.Q. Highly conductive and flexible mesoporous graphitic films prepared by graphitizing the composites of graphene oxide and nanodiamond. J. Mater. Chem. 2011, 21, 7154–7160. [Google Scholar] [CrossRef]
- Wang, Q.; Plylahan, N.; Shelke, M.V.; Devarapalli, R.R.; Li, M.S.; Subramanian, P.; Djenizian, T.; Boukherroub, R.; Szunerits, S. Nanodiamond particles/reduced graphene oxide composites as efficient supercapacitor electrodes. Carbon 2014, 68, 175–184. [Google Scholar] [CrossRef]
- Benfante, A.; Tomada, A.; Carini, G.A. IEEE Novel diamond X-ray detectors with patterned reduced graphene oxide contacts. In Proceedings of the 2015 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), San Diego, CA, USA, 31 October–7 November 2015; IEEE: Piscataway, NJ, USA, 2015. ISBN 1095-7863. [Google Scholar]
- Brenneis, A.; Gaudreau, L.; Seifert, M.; Karl, H.; Brandt, M.S.; Huebl, H.; Garrido, J.A.; Koppens, F.H.L.; Holleitner, A.W. Ultrafast electronic readout of diamond nitrogen-vacancy centres coupled to graphene. Nat. Nanotechnol. 2015, 10, 135–139. [Google Scholar] [CrossRef]
- Qiao, Y.; Qi, T.; Liu, J.; He, Z.Y.; Yu, S.W.; Shen, Y.Y.; Hei, H.J. synthesis of graphene/diamond double-layered structure for improving electron field emission properties. Surf. Rev. Lett. 2016, 23. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Chang, T.H.; Bikkarolla, S.K.; Roy, S.S.; Papakonstantinou, P.; Drijkoningen, S.; Pobedinskas, P.; Van Bael, M.K.; Tai, N.H.; Lin, I.N.; et al. Growth, structural and plasma illumination properties of nanocrystalline diamond-decorated graphene nanoflakes. RSC Adv. 2016, 6, 63178–63184. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Ficek, M.; Kunuku, S.; Panda, K.; Yeh, C.J.; Park, J.Y.; Sawczak, M.; Michalowski, P.P.; Leou, K.C.; Bogdanowicz, R.; et al. Self-organized multi-layered graphene-boron-doped diamond hybrid nanowalls for high-performance electron emission devices. Nanoscale 2018, 10, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, K.J.; Yeh, C.J.; Drijkoningen, S.; Pobedinskas, P.; Van Bael, M.K.; Leou, K.C.; Lin, I.N.; Haenen, K. Enhancement of plasma illumination characteristics of few-layer graphene-diamond nanorods hybrid. Nanotechnology 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.F.; Zubets, U.; Carvalho, A.F.; Fernandes, A.J.S.; Pereira, L.; Costa, F.M. Tuning the field emission of graphene-diamond hybrids by pulsed methane flow CVD. Carbon 2017, 122, 726–736. [Google Scholar] [CrossRef]
- Varshney, D.; Rao, C.V.; Guinel, M.J.F.; Ishikawa, Y.; Weiner, B.R.; Morell, G. Free standing graphene-diamond hybrid films and their electron emission properties. J. Appl. Phys. 2011, 110. [Google Scholar] [CrossRef]
- Yamada, T.; Masuzawa, T.; Mimura, H.; Okano, K. Field emission spectroscopy measurements of graphene/n-type diamond heterojunction. Appl. Phys. Lett. 2019, 114. [Google Scholar] [CrossRef]
- Yamada, T.; Masuzawa, T.; Neo, Y.; Mimura, H.; Ogawa, S.; Takakuwa, Y.; Okano, K. Field emission from n-type diamond NEA surface and graphene/n-type diamond junction. In Proceedings of the 30th International Vacuum Nanoelectronics Conference (IVNC), Regensburg, Germany, 10–14 July 2017; Langer, C., Lawrowski, R., Eds.; IEEE: Piscataway, NJ, USA, 2017; pp. 20–21, ISBN 2164-2370. [Google Scholar]
- Yang, J.; Tang, L.L.; Luo, W.; Feng, S.L.; Leng, C.Q.; Shi, H.F.; Wei, X.Z. Interface Engineering of a Silicon/Graphene Heterojunction Photodetector via a Diamond-Like Carbon Interlayer. ACS Appl. Mater. Interfaces 2021, 13, 4692–4702. [Google Scholar] [CrossRef] [PubMed]
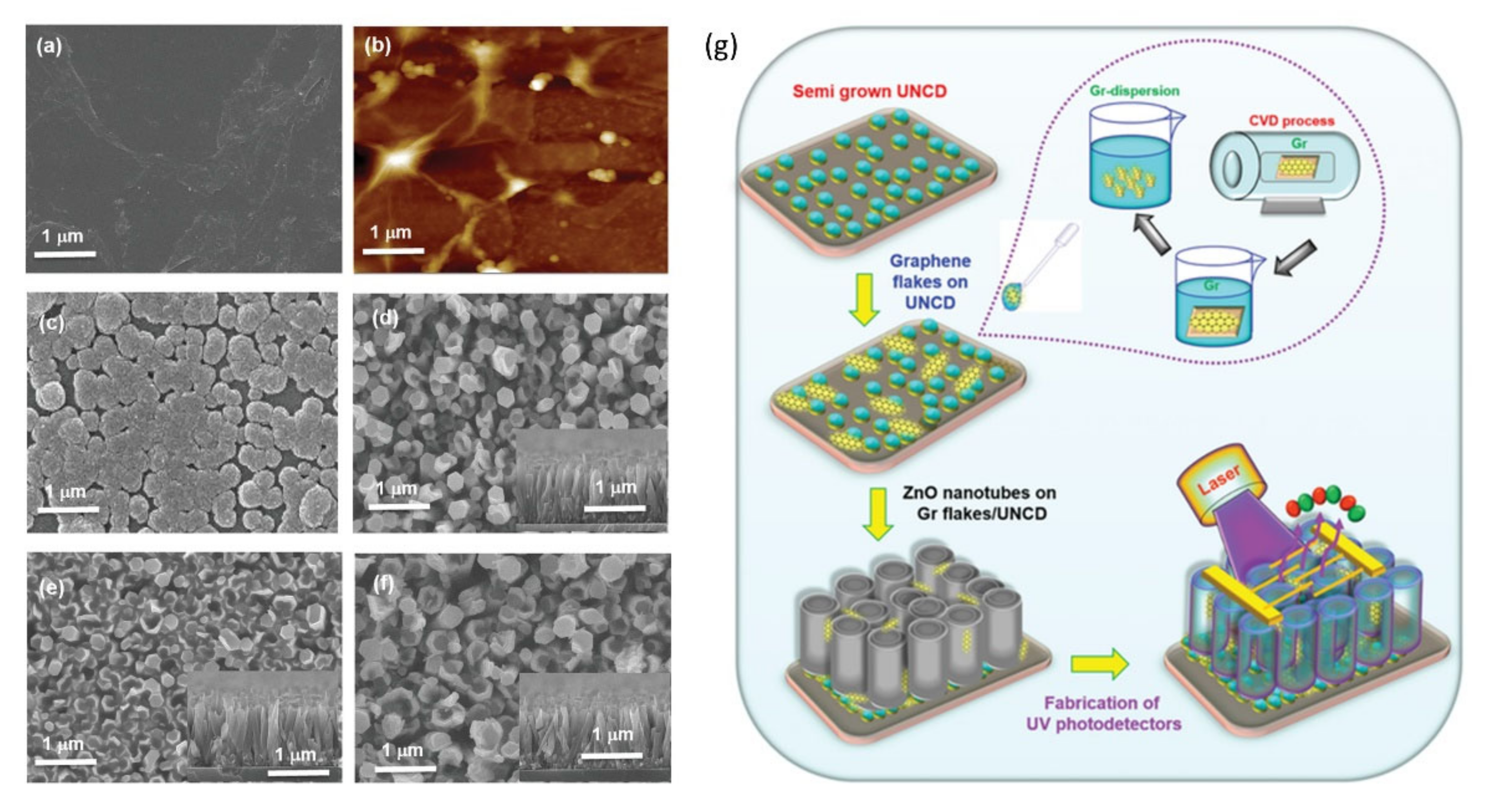
- Huang, B.R.; Saravanan, A.; Lu, H.C. Structural Engineering of Dispersed Graphene Flakes into ZnO Nanotubes on Discontinues Ultra-Nanocrystalline Diamond Substrates for High-Performance Photodetector with Excellent UV Light to Dark Current Ratios. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Wei, M.S.; Yao, K.Y.; Liu, Y.M.; Yang, C.; Zang, X.N.; Lin, L.W. A Solar-Blind UV Detector Based on Graphene-Microcrystalline Diamond Heterojunctions. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.Y.; Yang, C.; Zang, X.N.; Feng, F.; Lin, L.W. Carbon SP2-SP3 Technology: Graphene-on-Diamond Thin Film UV Detector. In Proceedings of the 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; IEEE: Piscataway, NJ, USA, 2014. ISBN 978-1-4799-3509-3. [Google Scholar]
- Wieghold, S.; Li, J.; Simon, P.; Krause, M.; Avlasevich, Y.; Li, C.; Garrido, J.A.; Heiz, U.; Samori, P.; Mullen, K.; et al. Photoresponse of supramolecular self-assembled networks on graphene-diamond interfaces. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Huang, J.; Xi, Y.F.; Zhou, X.Y.; Tang, K.; Xia, Y.B.; Lu, Y.C.; Wang, L.J. CVD diamond film detectors for a particles with a new electrode structure of reduced graphene oxide/Au. Mater. Sci. Semicond. Process. 2019, 91, 260–266. [Google Scholar] [CrossRef]
- Chen, Z.R.; Ma, D.J.; Wang, S.M.; Dai, W.H.; Li, S.Q.; Zhu, Y.Q.; Liu, B.C. Effects of graphene addition on mechanical properties of polycrystalline diamond compact. Ceram. Int. 2020, 46, 11255–11260. [Google Scholar] [CrossRef]
- Chu, B.; Shi, Y.F.; Samuel, J. Mitigation of chemical wear by graphene platelets during diamond cutting of steel. Carbon 2016, 108, 61–71. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.H.; Chen, S.Y.; Wu, L.X.; Zhuo, D.X.; Cheng, X.Y. Green fabrication of graphene oxide/epoxy nanocomposite and its application in diamond abrasive tools. Compos. Part B Eng. 2019, 177. [Google Scholar] [CrossRef]
- Duan, D.Z.; Ma, Y.S.; Ding, J.J.; Chang, Z.D.; Liu, H.B.; Xu, L.; Jiang, Z.D. Effect of multilayer graphene addition on performance of brazed diamond drill bits with Ni-Cr alloy and its mechanism. Ceram. Int. 2020, 46, 16684–16692. [Google Scholar] [CrossRef]
- Duan, D.Z.; Li, C.S.; Sun, L.; Liu, Y.P.; Fang, X.D.; Lin, Q.J.; Jiang, Z.D. Microstructure and performance of brazed diamonds with Ni-Cr plus multilayer graphene composite alloy. J. Alloys Compd. 2020, 816. [Google Scholar] [CrossRef]
- Smith, P.J.; Chu, B.; Singh, E.; Chow, P.; Samuel, J.; Koratkar, N. Graphene oxide colloidal suspensions mitigate carbon diffusion during diamond turning of steel. J. Manuf. Process. 2015, 17, 41–47. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Ridha, S.; Sardar, S.; Estelle, P.; Kumar, A.; Pendyala, R. Rheological behavior of stabilized diamond-graphene nanoplatelets hybrid nanosuspensions in mineral oil. J. Mol. Liq. 2021, 328. [Google Scholar] [CrossRef]
- Tzeng, Y.H.; Yeh, S.P.; Fang, W.C.; Chu, Y.C. Nitrogen-incorporated ultrananocrystalline diamond and multi-layer-graphene-like hybrid carbon films. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, F.X.; Zhuang, K.Y.; Chen, D.Q.; Chen, G.H. Composites of epoxy/graphene-modified-diamond filler show enhanced thermal conductivity and high electrical insulation. RSC Adv. 2017, 7, 40761–40766. [Google Scholar] [CrossRef]
- Saw, W.P.S.; Mariatti, M. Properties of synthetic diamond and graphene nanoplatelet-filled epoxy thin film composites for electronic applications. J. Mater. Sci. Electron. 2012, 23, 817–824. [Google Scholar] [CrossRef]
- Bittencourt, R.A.; Costa, G.D.; Lima, A.T.D.; Lima, I.C.D. Cluster formation and non-metal-to-metal transition in a diamond-shaped graphene-like lattice. AIP Adv. 2021, 11. [Google Scholar] [CrossRef]
- Yu, J.H.; Qian, R.; Jiang, P.K. Enhanced Thermal Conductivity for PVDF Composites with a Hybrid Functionalized Graphene Sheet-Nanodiamond Filler. Fibers Polym. 2013, 14, 1317–1323. [Google Scholar] [CrossRef]
- Shul’zhenko, A.A.; Jaworska, L.; Gargin, V.G.; Sokolov, A.N.; Nikolenko, A.S.; Strelchuk, V. V Dry mixing of diamond and n-layered graphene powders substantially different in density and particle size. High Press. Res. 2018, 38, 53–61. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, N.C.; Ju, F.S. Enhanced Growth Rate of Chemical Vapor Deposition Diamond Coating Motivated by Graphene Oxide. Coatings 2021, 11, 559. [Google Scholar] [CrossRef]
- Lowe, S.E.; Shi, G.; Zhang, Y.B.; Qin, J.D.; Wang, S.J.; Uijtendaal, A.; Sun, J.Q.; Jiang, L.X.; Jiang, S.Y.; Qi, D.C.; et al. Scalable Production of Graphene Oxide Using a 3D-Printed Packed-Bed Electrochemical Reactor with a Boron-Doped Diamond Electrode. ACS Appl. Nano Mater. 2019, 2, 867–878. [Google Scholar] [CrossRef]
- Lan, G.J.; Qiu, Y.Y.; Fan, J.T.; Wang, X.L.; Tang, H.D.; Han, W.F.; Liu, H.Z.; Liu, H.Y.; Song, S.; Li, Y. Defective graphene@ diamond hybrid nanocarbon material as an effective and stable metal-free catalyst for acetylene hydrochlorination. Chem. Commun. 2019, 55, 1430–1433. [Google Scholar] [CrossRef]
- Roldan, L.; Benito, A.M.; Garcia-Bordeje, E. Self-assembled graphene aerogel and nanodiamond hybrids as high performance catalysts in oxidative propane dehydrogenation. J. Mater. Chem. A 2015, 3, 24379–24388. [Google Scholar] [CrossRef]
- Zang, J.B.; Wang, Y.H.; Bian, L.Y.; Zhang, J.H.; Meng, F.W.; Zhao, Y.L.; Lu, R.; Qu, X.H.; Ren, S.B. Graphene growth on nanodiamond as a support for a Pt electrocatalyst in methanol electro-oxidation. Carbon 2012, 50, 3032–3038. [Google Scholar] [CrossRef]
- Kayal, A.; Chandra, A. Infrared Spectral and Dynamical Properties of Water Confined in Nanobubbles at Hybrid Interfaces of Diamond and Graphene: A Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 23455–23462. [Google Scholar] [CrossRef]
- Lim, C.; Sorkin, A.; Bao, Q.L.; Li, A.; Zhang, K.; Nesladek, M.; Loh, K.P. A hydrothermal anvil made of graphene nanobubbles on diamond. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
| System | Application | References |
|---|---|---|
| RGO/BDND | Protein sensor | [79] |
| Diamond/GN nanoplatelets/Pt nanoparticle hybrid | L-Glutamate electrochemical sensor | [80] |
| Ni nanoparticle-modified GN-diamond hybrid | Glucose sensor | [81] |
| Plasmonic gold nanostructure/diamond-like film | SERS sensor | [82] |
| Vertical GN sheets/separated papillary granules on ND film | ORR electrode | [83] |
| BD diamond/GN nanowalls electrode | Detection of explosives | [84] |
| GN/diamond electrode | Enantiomer recognition | [85] |
| GN/BDND electrode | Epinephrine detection | [86] |
| GO/BD-diamond electrode | Tetracycline detection | [87] |
| GN/BD-diamond electrode | Electrochemical sensing of trace Pb2+ in seawater | [88] |
| diamond, GN, and polyaniline/GC electrode | Electrochemical sensing of 2,4-dichlorophenol | [89] |
| N-doped UNCD/MLGN film | Electrochemical sensor (Ag+) | [90] |
| (Ag)GN-modified BD-diamond electrode | Electrochemical sensor of pesticides | [91,92] |
| GN/BD-diamond electrode | Electrochemical sensor of biomarkers | [93] |
| Few-layer GN/HPHT diamond electrode | Dopamine detection | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vejpravová, J. Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review. Nanomaterials 2021, 11, 2469. https://doi.org/10.3390/nano11102469
Vejpravová J. Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review. Nanomaterials. 2021; 11(10):2469. https://doi.org/10.3390/nano11102469
Chicago/Turabian StyleVejpravová, Jana. 2021. "Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review" Nanomaterials 11, no. 10: 2469. https://doi.org/10.3390/nano11102469
APA StyleVejpravová, J. (2021). Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review. Nanomaterials, 11(10), 2469. https://doi.org/10.3390/nano11102469







