In Situ Observation of Electron-Beam-Induced Formation of Nano-Structures in PbTe
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystal Growth and Preparation of TEM Specimens
2.2. TEM
3. Results
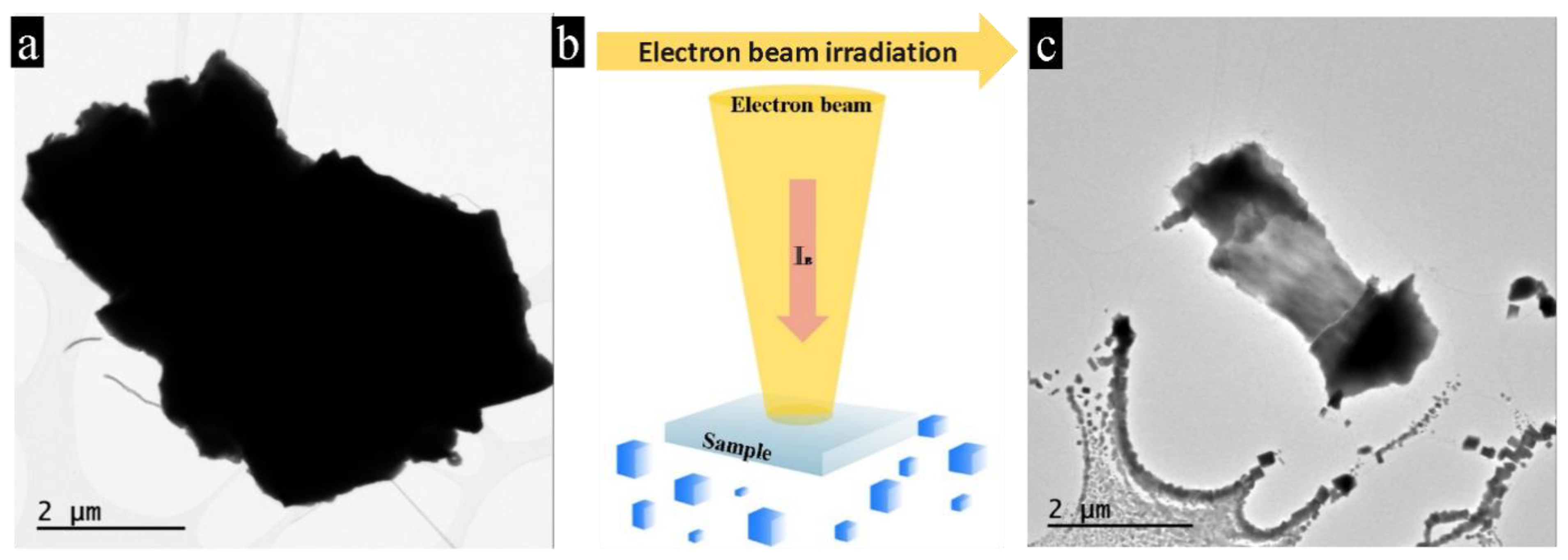
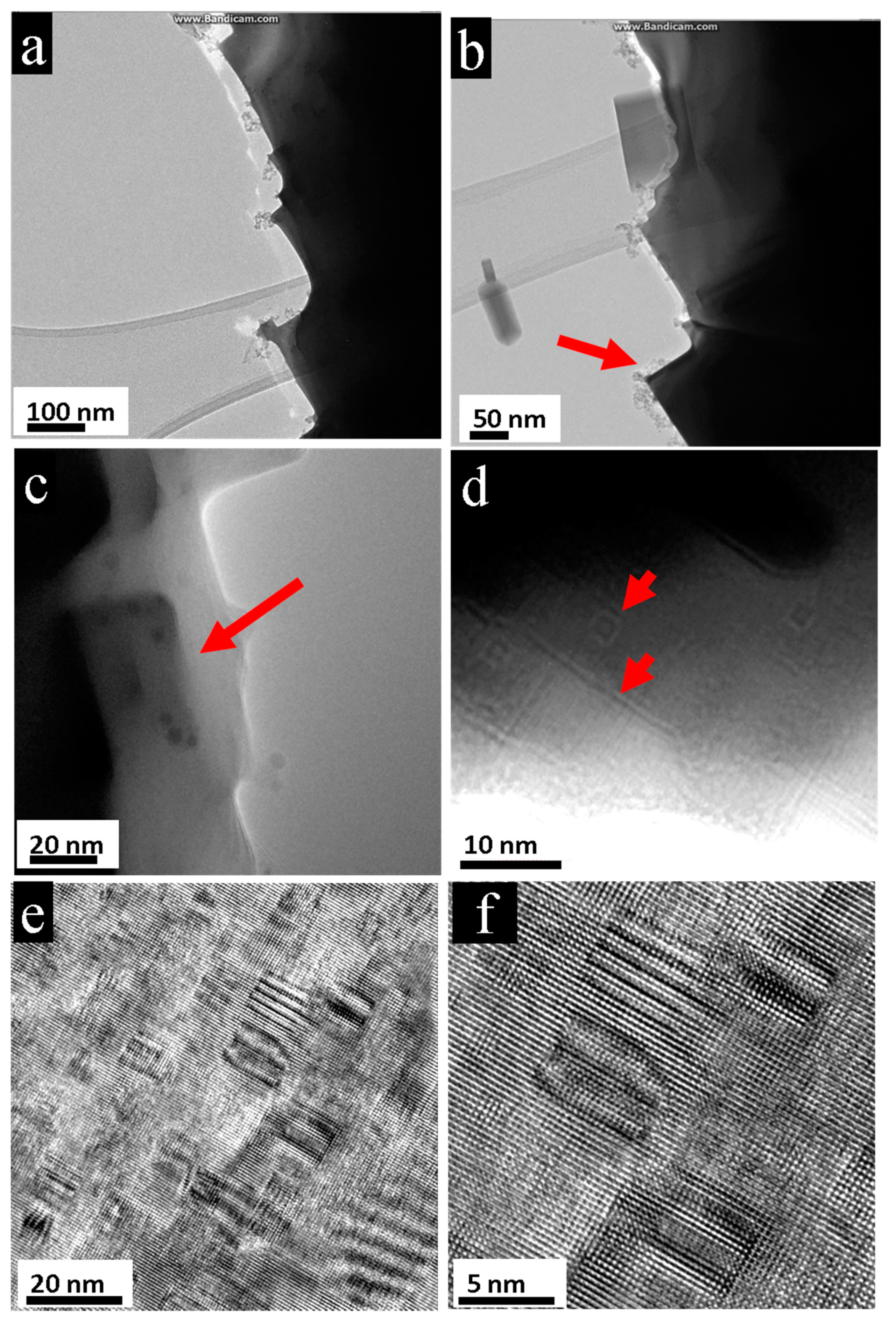
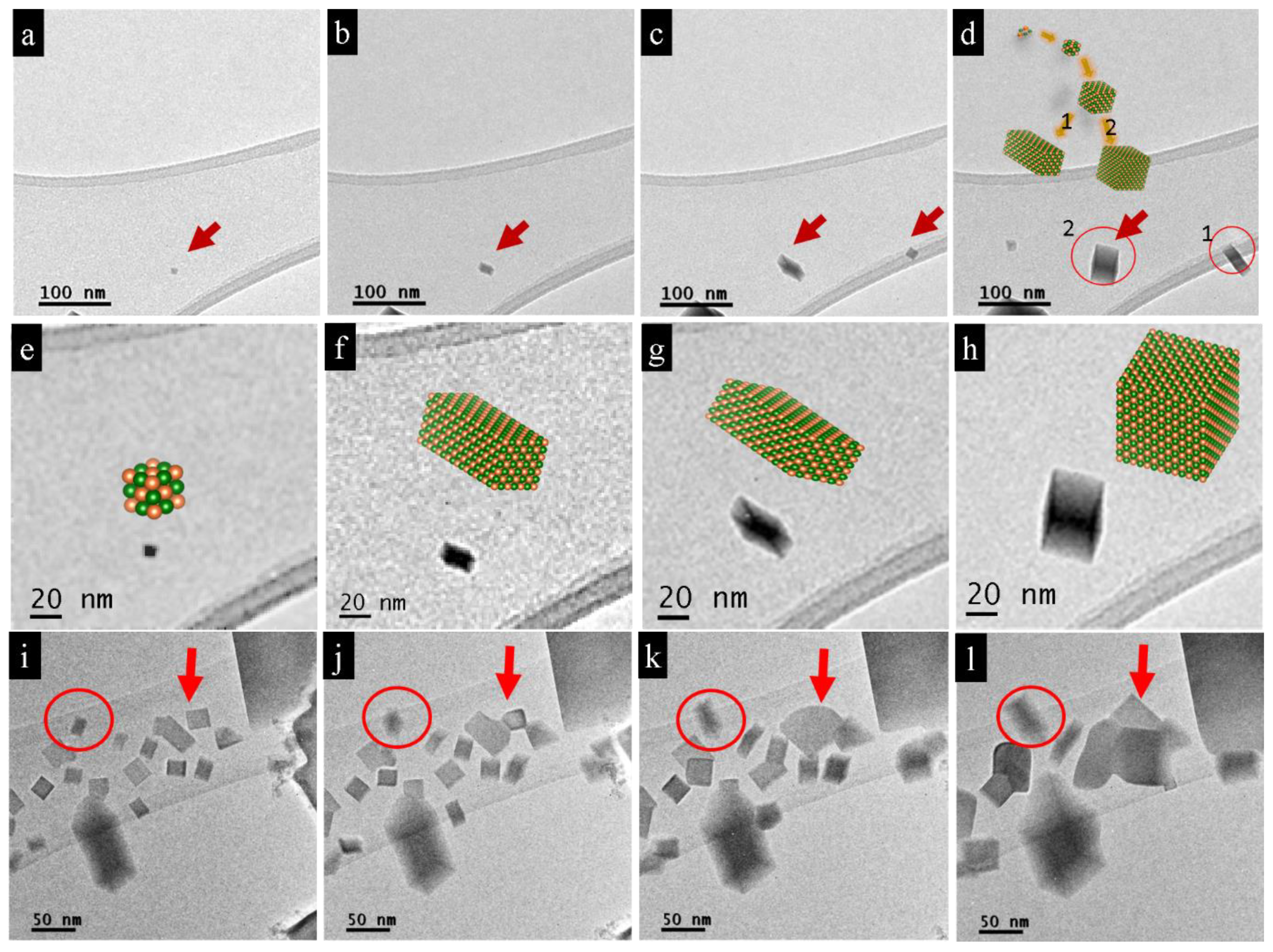
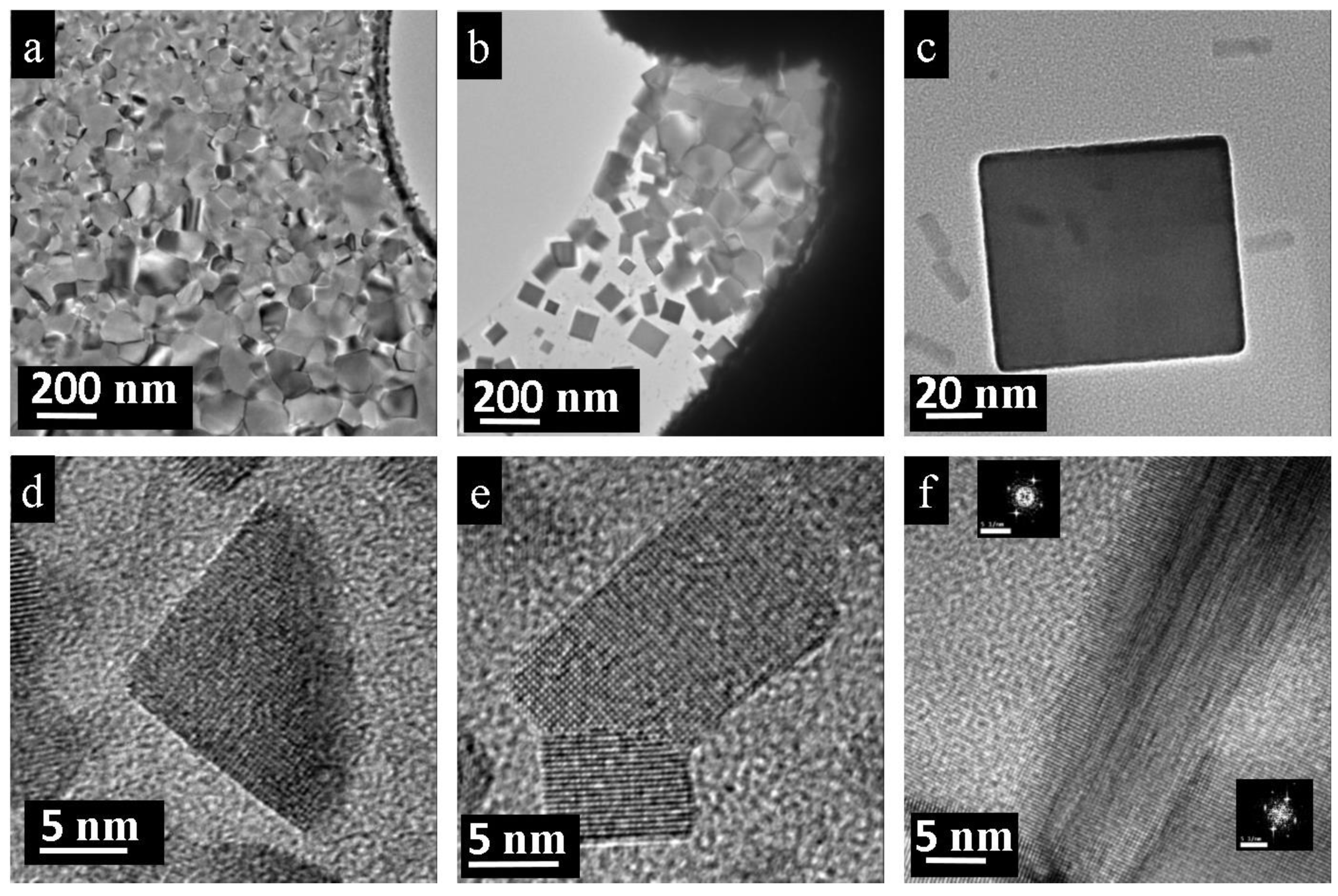
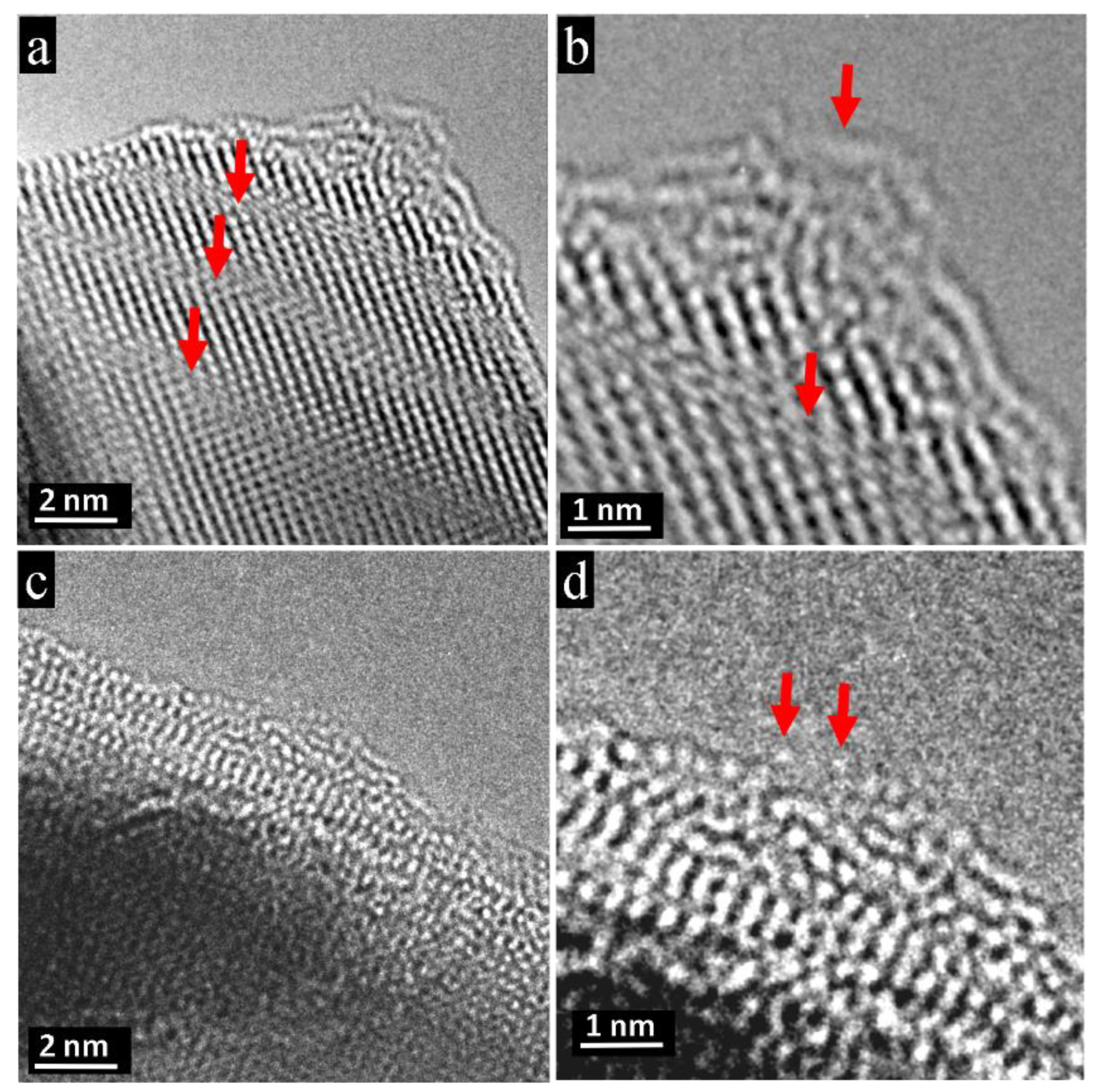
3.1. Nano-Recrystallization of Powdered Single Crystal of PbTe under Electron-Beam Conditions
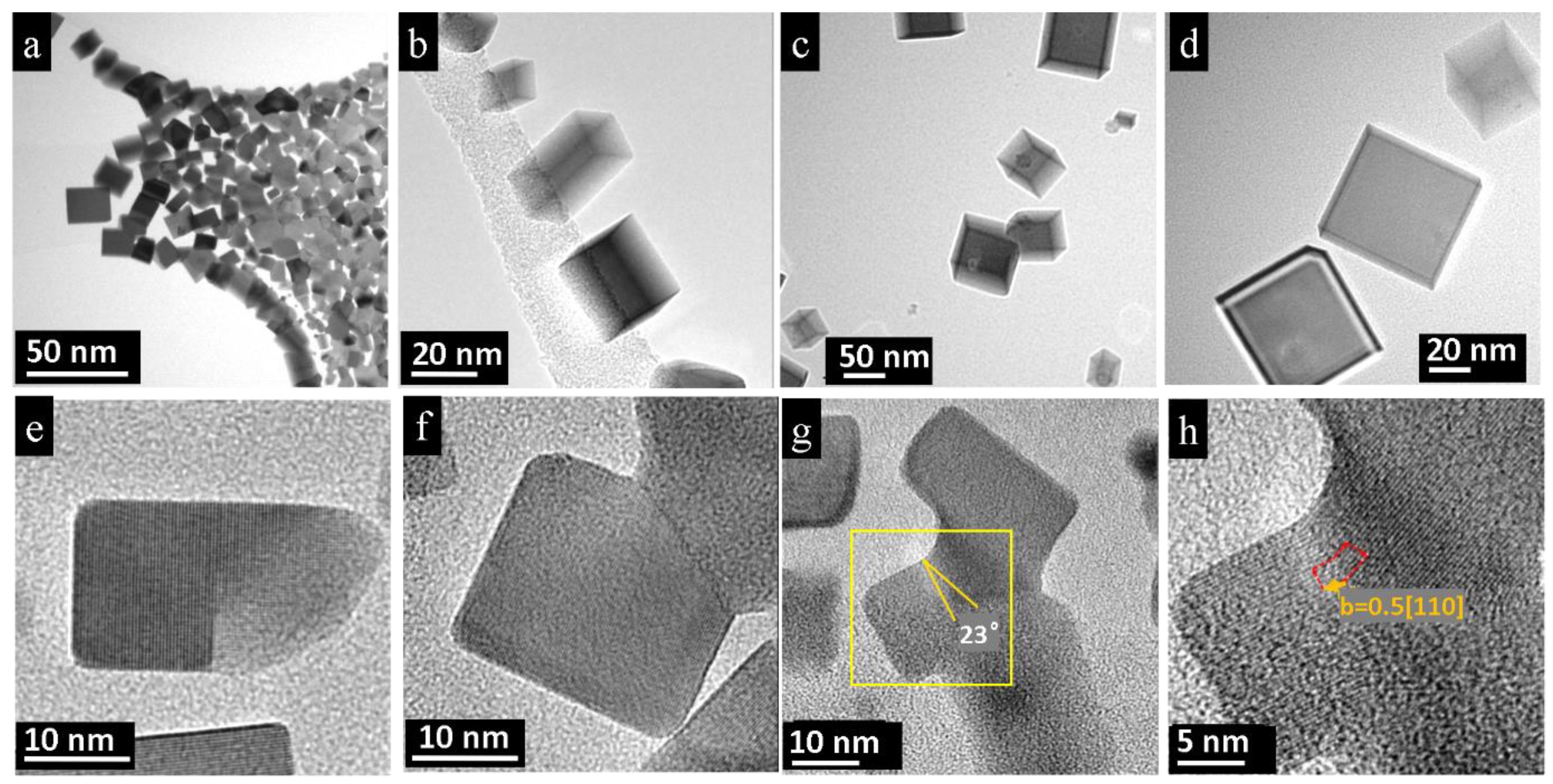
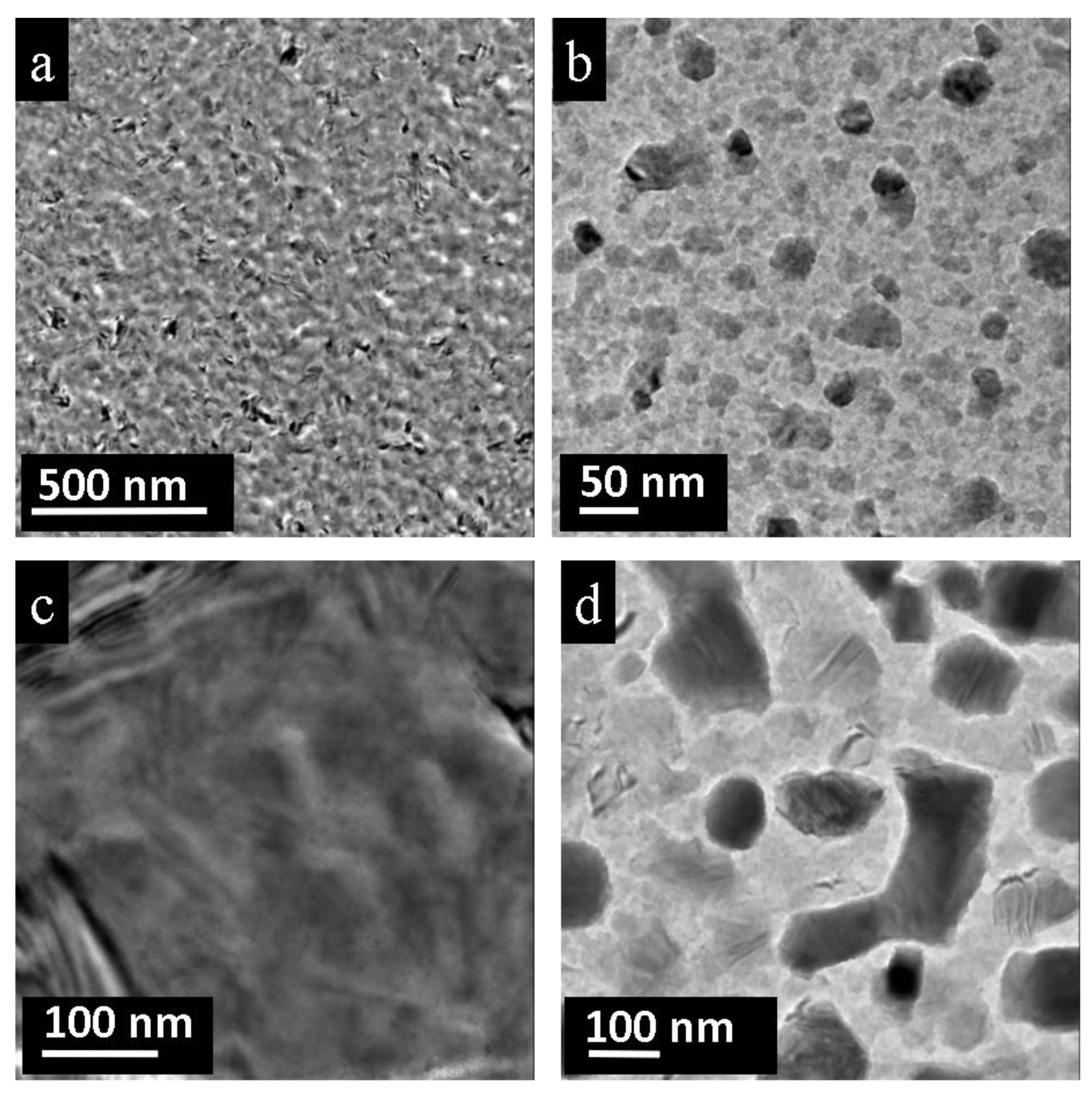
3.2. Nano-Recrystallization of Spark-Plasma Sintered Polycrystalline PbTe
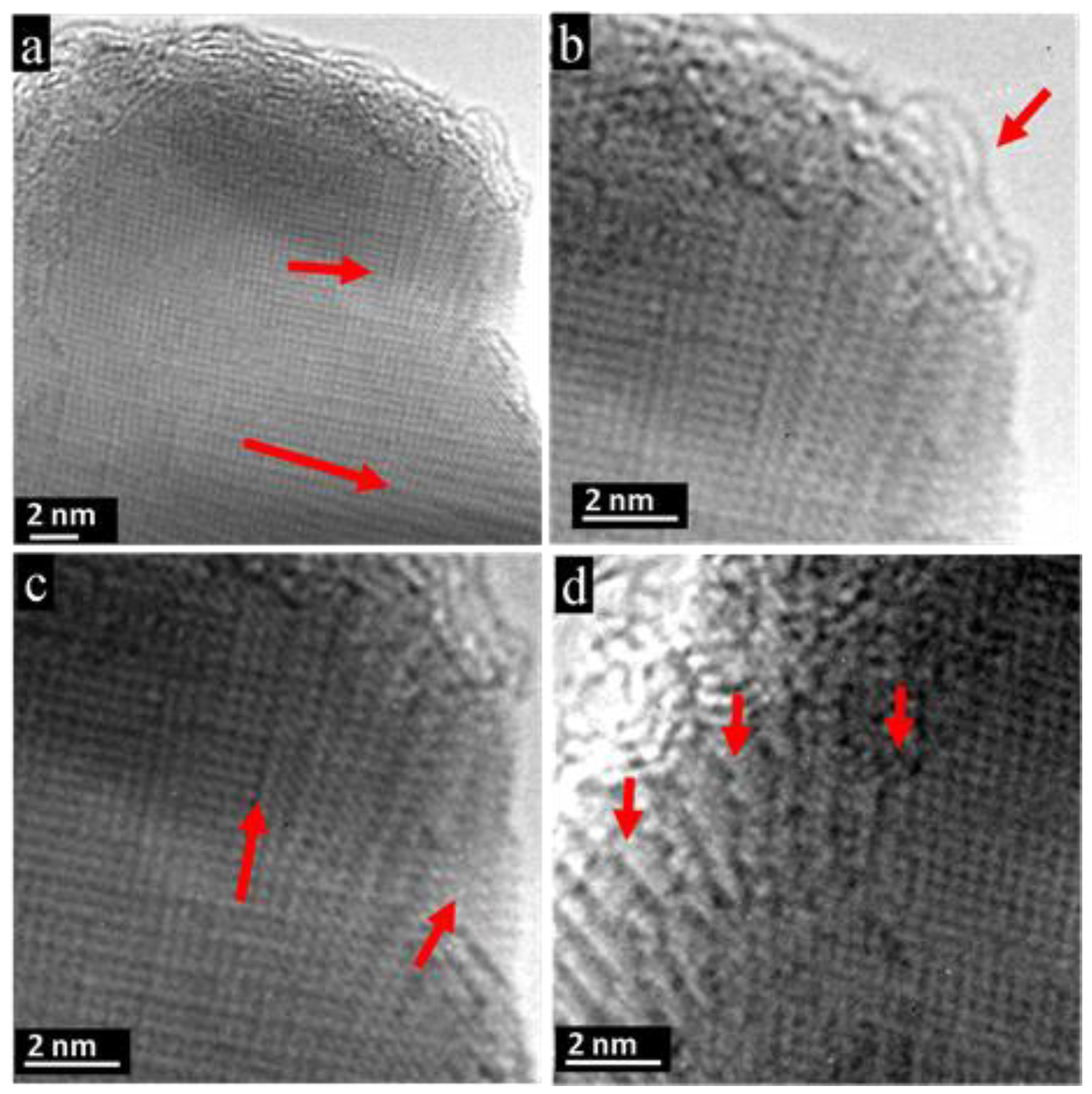
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hicks, L.; Dresselhaus, M.S. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. 1993, B47, 12727. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Dresselhaus, M.; Dresselhaus, G.; Fleurial, J.-P.; Caillat, T. Recent developments in thermoelectric materials. Int. Mater. Rev. 2003, 48, 45. [Google Scholar] [CrossRef]
- Gelbstein, Y.; Davidow, Y.; Leshem, E.; Pinshow, O.; Moisa, S. Significant lattice thermal conductivity reduction following phase separation of the highly efficient GexPb1−xTe thermoelectric alloys. Phys. Status Solidi B 2014, 251, 1431–1437. [Google Scholar] [CrossRef]
- Ben-Ayoun, D.; Sadia, Y.; Gelbstein, Y. High temperature thermoelectric properties evolution of Pb1−xSnxTe based alloys. J. Alloys Compd. 2017, 722, 33–38. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, R.; Liao, J.; Zhang, Q.; Xia, X.; Wang, C.; Huang, H.; Chu, J.; Gu, M.; Zhu, T.; et al. High-efficiency half-Heusler thermoelectric modules enabled by self-propagating synthesis and topologic structure optimization. Energy Environ. Sci. 2019, 12, 3390. [Google Scholar] [CrossRef]
- Rull-Bravo, M.; Moure, A.; Fernández, J.F.; Martín-González, M. Skutterudites as thermoelectric materials: Revisited. RSC Adv. 2017, 5, 41653. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 554. [Google Scholar] [CrossRef]
- Khasimsaheb, B.; Neeleshwar, S.; Srikanth, M.; Bathula, S.; Gahtori, B.; Srivsatava, A.K.; Dhar, A.; Sankarakumar, A.; Panigrahi, B.K.; Bhattacharya, S.; et al. Thermoelectric properties of spark plasma sintered lead telluride nanocubes. J. Mater. Res. 2015, 30, 2638. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, I.; Bachmatiuk, A.; Bezugly, V.; Kunstmann, J.; Gemming, T.; Liu, Z.; Cuniberti, G.; Rümmeli, M. Electron-beam induced synthesis of nanostructures: A review. Nanoscale 2016, 8, 11340. [Google Scholar] [CrossRef]
- Scanlon, W.W. Precipitation of Te and Pb in PbTe Crystals. Phys. Rev. 1962, 126, 509. [Google Scholar] [CrossRef]
- Muehlberg, M.; Hesse, D. TEM precipitation studies in Te-rich as-grown PbTe single crystals. Phys. Stat. Sol. 1983, 76, 513. [Google Scholar] [CrossRef]
- Breitsameter, B.; Hartmann, W.; Lowe, H. Chemical lap polishing of PbTe and Pb1−xSnxTe crystals. Krist. Technol. 1980, 15, 497. [Google Scholar] [CrossRef]
- Gille, P.; Muehlberg, M.; Parthier, L.; Rudolph, P. Crystal growth of PbTe and (Pb, Sn)Te by the Bridgman method and by THM. Cryst. Res. Technol. 1984, 19, 881. [Google Scholar] [CrossRef]
- Anderson, P.M.; Hirth, J.P.; Lothe, J. Theory of Dislocations; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Evers, W.H.; Goris, B.; Bals, S.; Casavola, M.; De Graaf, J.; Van Roij, R.; Dijkstra, M.; Vanmaekelbergh, D. Low-Dimensional Semiconductor Superlattices Formed by Geometric Control over Nanocrystal Attachment. Nano Lett. 2012, 13, 2317. [Google Scholar] [CrossRef]
- Sear, R.P. Nucleation: Theory and applications to protein solutions and colloidal suspensions. J. Phys. Condens. Matter 2007, 19, 033101. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef]
- Kirchner, H.O.K. Coarsening of grain-boundary precipitates. Metall. Trans. 1971, 2, 2861–2864. [Google Scholar] [CrossRef]
- Zelenina, I.; Simon, P.; Veremchuk, I.; Bobnar, M.; Wang, X.; Muehlberg, M.; Lu, W.; Liebscher, C.; Grin, Y. Structural complexity of PbTe single crystals. Unpublished work.
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.-I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, W.S.; Zhao, L.D.; Zhou, M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Stöber, D.; Hiltmann, B.; Böttner, H.; Schelb, S.; Bachem, K.-H.; Binnewies, M. Chemical Transport Reactions during Crystal Growth of PbTe and PbSe via Vapour Phase Influenced by AgI. J. Cryst. Growth 1992, 121, 656–664. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelenina, I.; Veremchuk, I.; Grin, Y.; Simon, P. In Situ Observation of Electron-Beam-Induced Formation of Nano-Structures in PbTe. Nanomaterials 2021, 11, 163. https://doi.org/10.3390/nano11010163
Zelenina I, Veremchuk I, Grin Y, Simon P. In Situ Observation of Electron-Beam-Induced Formation of Nano-Structures in PbTe. Nanomaterials. 2021; 11(1):163. https://doi.org/10.3390/nano11010163
Chicago/Turabian StyleZelenina, Iryna, Igor Veremchuk, Yuri Grin, and Paul Simon. 2021. "In Situ Observation of Electron-Beam-Induced Formation of Nano-Structures in PbTe" Nanomaterials 11, no. 1: 163. https://doi.org/10.3390/nano11010163
APA StyleZelenina, I., Veremchuk, I., Grin, Y., & Simon, P. (2021). In Situ Observation of Electron-Beam-Induced Formation of Nano-Structures in PbTe. Nanomaterials, 11(1), 163. https://doi.org/10.3390/nano11010163



