Functionalization of Molybdenum Disulfide via Plasma Treatment and 3-Mercaptopropionic Acid for Gas Sensors
Abstract
1. Introduction
2. Materials and Methods
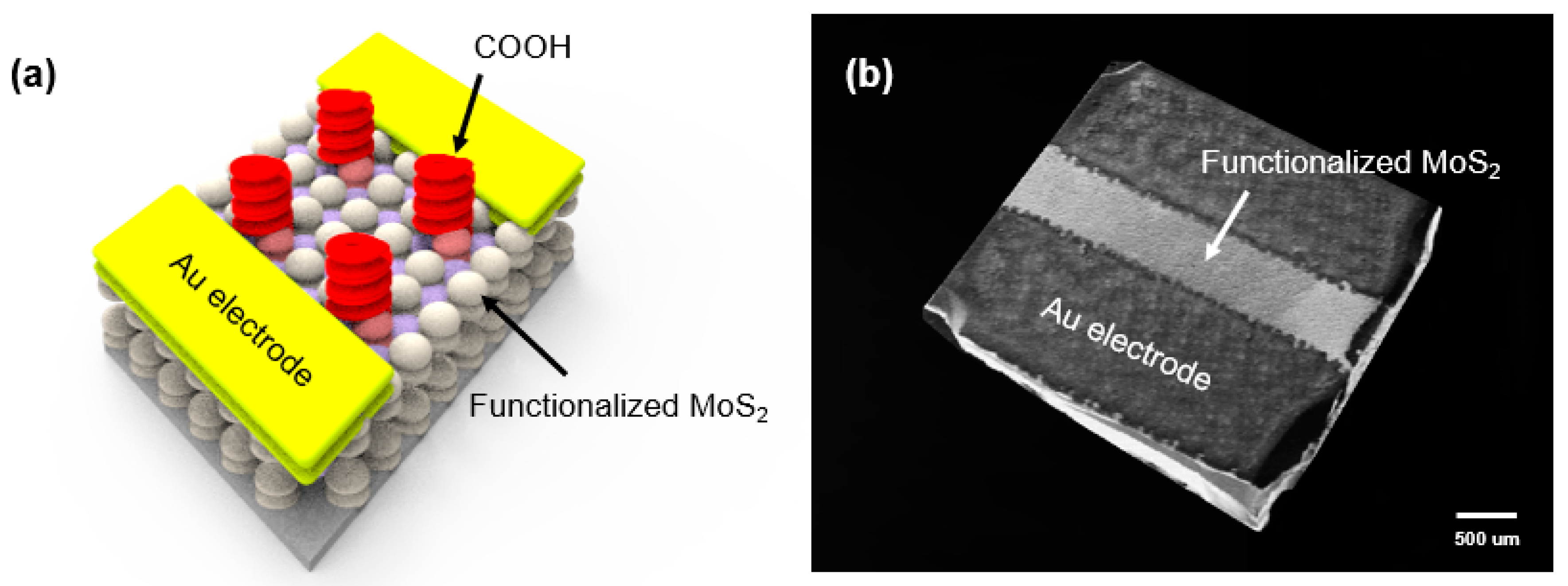
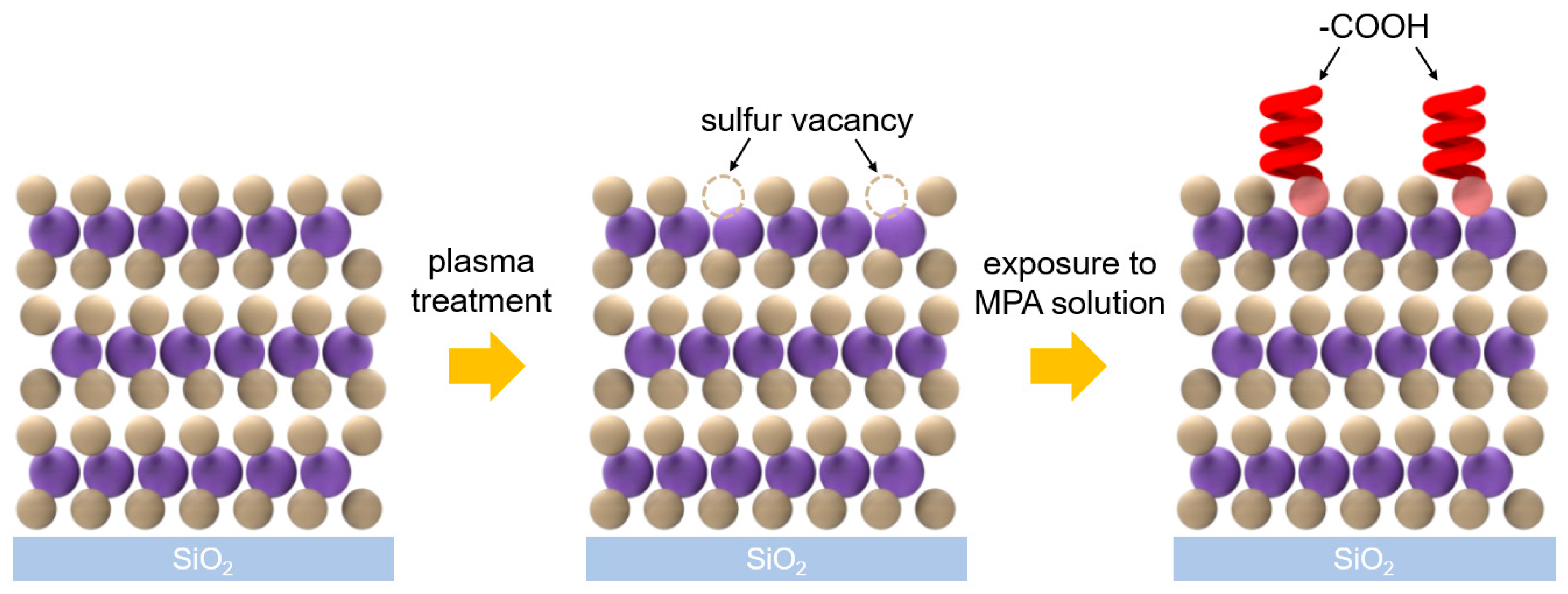
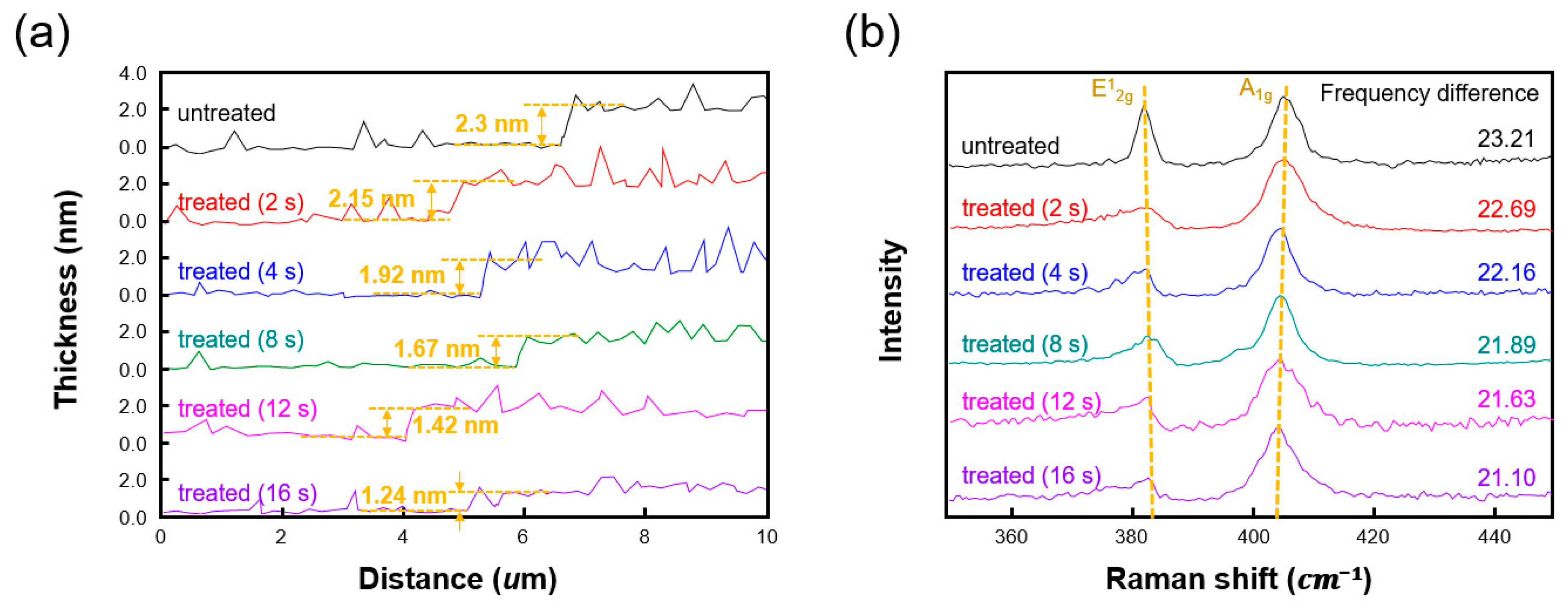
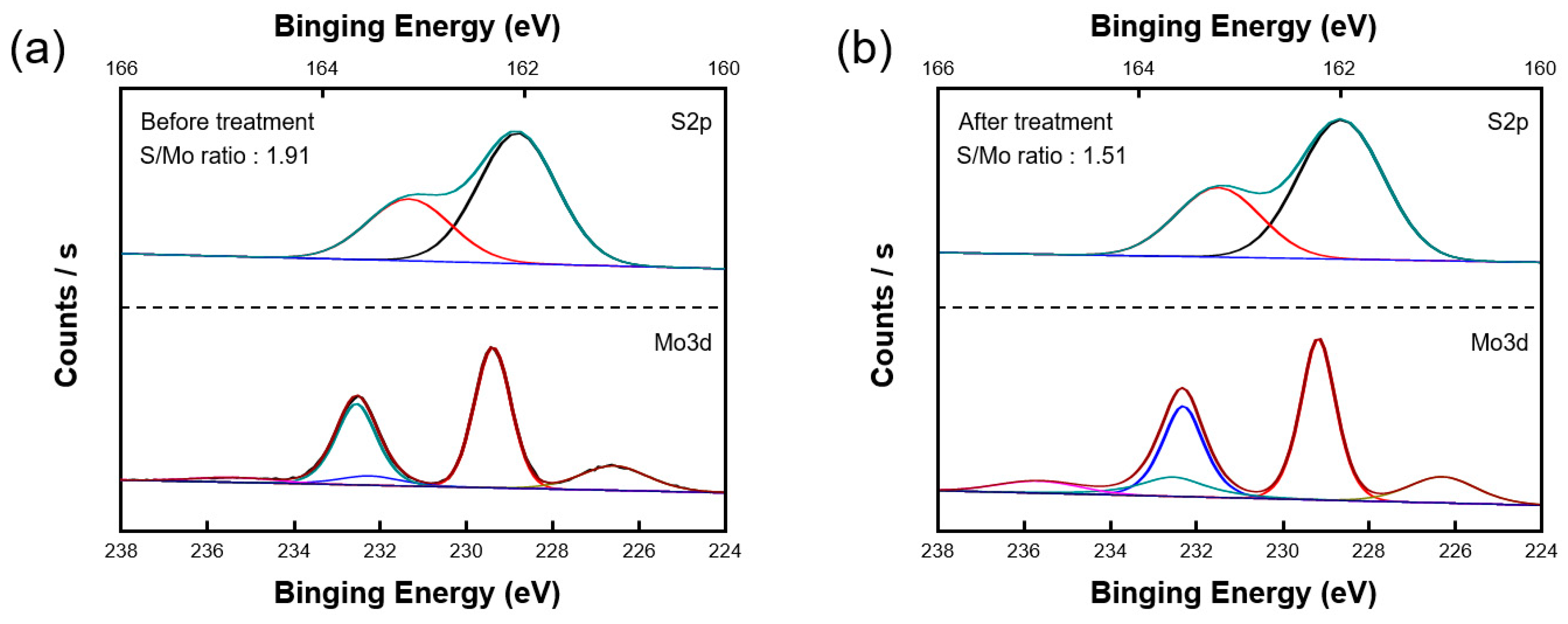
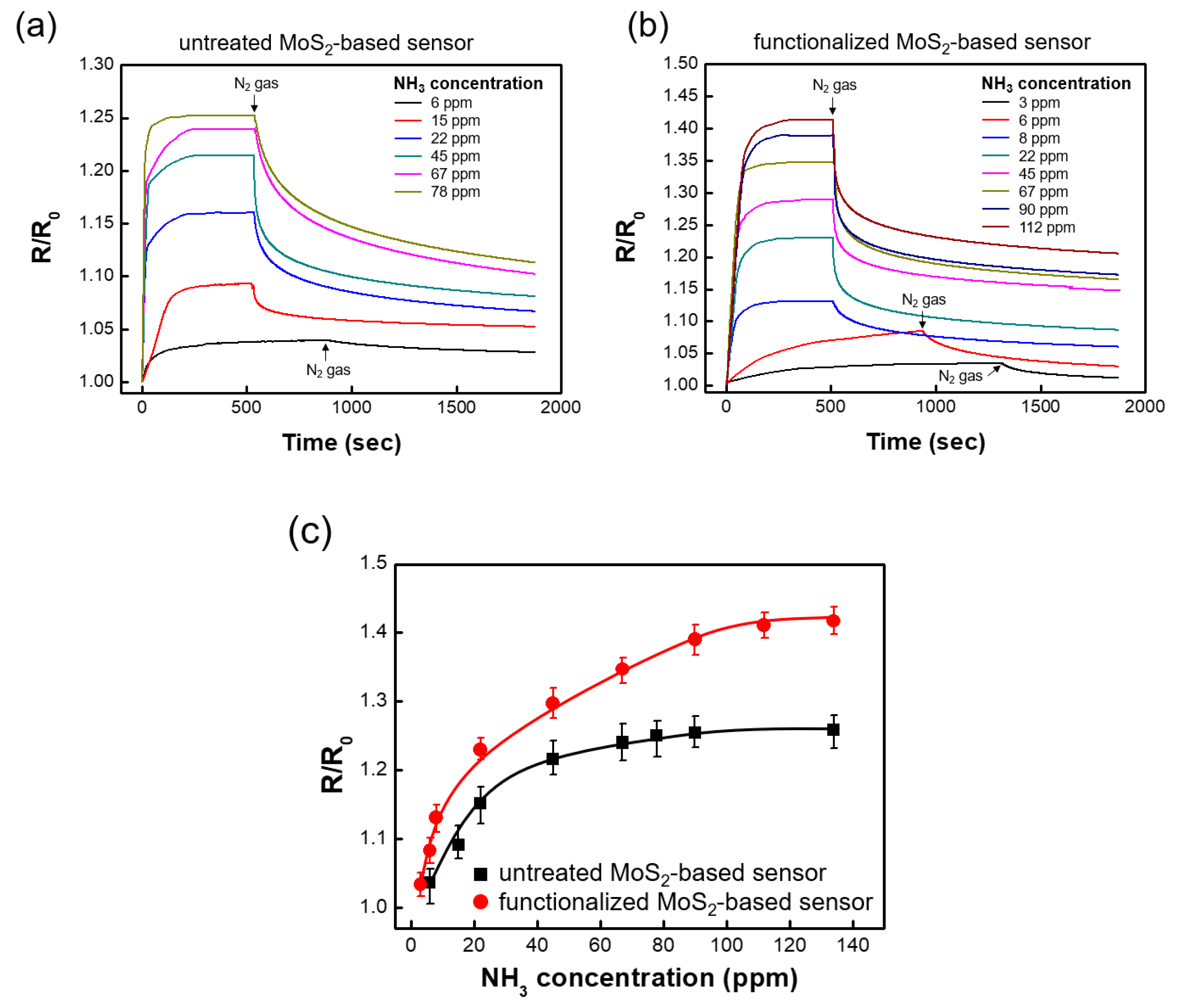
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.B.; Tang, T.T.; Girit, C.; Hao, Z.; Martin, M.C.; Zettl, A.; Crommie, M.F.; Shen, Y.R.; Wang, F. Direct observation of a widely tunable bandgap in bilayer graphene. Nature 2009, 459, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.H.; Yu, T.; Luo, Z.Q.; Wang, Y.Y.; Liu, L.; Wong, C.P.; Miao, J.M.; Huang, W.; Shen, Z.X. Probing charged impurities in suspended graphene using raman spectroscopy. ACS Nano 2009, 3, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; He, W.Y.; Chu, Z.D.; Liu, M.X.; Meng, L.; Dou, R.F.; Zhang, Y.F.; Liu, Z.F.; Nie, J.C.; He, L. Strain and curvature induced evolution of electronic band structures in twisted graphene bilayer. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.N.; Zhan, D.; Liu, L.; Suo, H.; Ni, Z.H.; Thuong, T.N.; Zhao, C.; Shen, Z.X. Thermal dynamics of graphene edges investigated by polarized raman spectroscopy. ACS Nano 2011, 5, 147–152. [Google Scholar] [CrossRef][Green Version]
- Lloyd, D.; Liu, X.H.; Boddeti, N.; Cantley, L.; Long, R.; Dunn, M.L.; Bunch, J.S. Adhesion, Stiffness, and Instability in Atomically Thin MoS2 Bubbles. Nano Lett. 2017, 17, 5329–5334. [Google Scholar] [CrossRef]
- Gamble, F.R.; Silbernagel, B.G. Anisotropy of the proton spin–lattice relaxation time in the superconducting intercalation complex TaS2(NH3): Structural and bonding implications. J. Chem. Phys. 1975, 63, 2544. [Google Scholar] [CrossRef]
- Clerc, F.; Battaglia, C.; Cercellier, H.; Monney, C.; Berger, H.; Despont, L.; Garnier, M.G.; Aebi, P. Fermi surface of layered compounds and bulk charge density wave systems. J. Phys.-Condens. Mat. 2007, 19, 355002. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.B.; Li, T.S.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Lembke, D.; Bertolazzi, S.; Kis, A. Single-Layer MoS2 electronics. Acc. Chem. Res. 2015, 48, 100–110. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Raybaud, P.; Hafner, J.; Kresse, G.; Kasztelan, S.; Toulhoat, H. Structure, energetics, and electronic properties of the surface of a promoted MoS2 catalyst: An ab initio local density functional study. J. Catal. 2000, 190, 128–143. [Google Scholar] [CrossRef]
- Pierucci, D.; Henck, H.; Ben Aziza, Z.; Naylor, C.H.; Balan, A.; Rault, J.E.; Silly, M.G.; Dappe, Y.J.; Bertran, F.; Le Fevre, P.; et al. Tunable doping in hydrogenated single layered molybdenum disulfide. ACS Nano 2017, 11, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.S.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.Y.; Yu, J.; Lv, F.T.; Yan, L.; Zheng, L.R.; Gu, Z.J.; Zhao, Y.L. Functionalized Nano-MoS2 with peroxidase catalytic and near-infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, D.; Blundo, E.; Felici, M.; Pettinari, G.; Liu, B.Q.; Yildrim, T.; Petroni, E.; Zhang, C.; Zhu, Y.; Sennato, S.; et al. Controlled micro/nanodome formation in proton-irradiated bulk transition-metal dichalcogenides. Adv. Mater 2019, 31, 1903795. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.H.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Choudhary, N.; Park, J.; Hwang, J.Y.; Choi, W. Growth of large-scale and thickness-modulated MoS2 nanosheets. ACS Appl. Mater. Int. 2014, 6, 21215–21222. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Shehzad, M.A.; Vikraman, D.; Khan, M.F.; Singh, J.; Choi, D.C.; Seo, Y.; Eom, J.; Lee, W.G.; Jung, J. Synthesis and characterization of large-area and continuous MoS2 atomic layers by RF magnetron sputtering. Nanoscale 2016, 8, 4340–4347. [Google Scholar] [CrossRef]
- Kang, K.; Xie, S.E.; Huang, L.J.; Han, Y.M.; Huang, P.Y.; Mak, K.F.; Kim, C.J.; Muller, D.; Park, J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 2015, 520, 656–660. [Google Scholar] [CrossRef]
- Tahir, M.N.; Zink, N.; Eberhardt, M.; Therese, H.A.; Kolb, U.; Theato, P.; Tremel, W. Overcoming the insolubility of molybdenum disulfide nanoparticles through a high degree of sidewall functionalization using polymeric chelating ligands. Angew. Chem. Int. Ed. 2006, 45, 4809–4815. [Google Scholar] [CrossRef]
- Chu, X.M.S.; Yousaf, A.; Li, D.O.; Tang, A.L.A.; Debnath, A.; Ma, D.; Green, A.A.; Santos, E.J.G.; Wang, Q.H. Direct covalent chemical functionalization of unmodified two-dimensional molybdenum disulfide. Chem. Mater. 2018, 30, 2112–2128. [Google Scholar] [CrossRef]
- Chen, X.; Berner, N.C.; Backes, C.; Duesberg, G.S.; McDonald, A.R. Functionalization of two-dimensional MoS2: On the reaction between MoS2 and organic thiols. Angew. Chem. Int. Ed. 2016, 55, 5803–5808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, B.Z.; Yang, Y.; He, Y.G. Facile approach to surface functionalized MoS2 nanosheets. Rsc Adv. 2014, 4, 32570–32578. [Google Scholar] [CrossRef]
- Nan, H.Y.; Wang, Z.L.; Wang, W.H.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.W.; Tan, P.H.; Miao, F.; Wang, X.R.; et al. Strong Photoluminescence Enhancement of MoS2 through Defect Engineering and Oxygen Bonding. Acs Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Makarova, M.; Okawa, Y.; Aono, M. Selective adsorption of thiol molecules at sulfur vacancies on MoS2(0001), followed by vacancy repair via S-C dissociation. J. Phys. Chem. C 2012, 116, 22411–22416. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.W. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Dai, Y.W.; Cao, Q.; Sun, Q.Q.; Ding, S.J.; Zhu, H.; Zhang, D.W. A two-dimensional semiconductor transistor with boosted gate control and sensing ability. Sci. Adv. 2017, 3, e1602246. [Google Scholar] [CrossRef]
- Kim, Y.; Song, J.G.; Park, Y.J.; Ryu, G.H.; Lee, S.J.; Kim, J.S.; Jeon, P.J.; Lee, C.W.; Woo, W.J.; Choi, T.; et al. Self-Limiting layer synthesis of transition metal dichalcogenides. Sci. Rep. UK 2016, 6, 18754. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.D.; Cui, N.Y.; McKinley, A. An XPS study of the surface modification of natural MoS2 following treatment in an RF-oxygen plasma. Appl. Surf. Sci. 1998, 134, 11–21. [Google Scholar] [CrossRef]
- Zhao, J.J.; Buldum, A.; Han, J.; Lu, J.P. Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar] [CrossRef]
- Andzelm, J.; Govind, N.; Maiti, A. Nanotube-based gas sensors—Role of structural defects. Chem. Phys. Lett. 2006, 421, 58–62. [Google Scholar] [CrossRef]
- Ham, S.W.; Hong, H.P.; Kim, J.H.; Min, S.J.; Min, N.K. Effect of oxygen plasma treatment on carbon nanotube-based sensors. J. Nanosci. Nanotechnol. 2014, 14, 8476–8481. [Google Scholar] [CrossRef] [PubMed]
- Dolui, K.; Rungger, I.; Sanvito, S. Origin of the n-type and p-type conductivity of MoS2 monolayers on a SiO2 substrate. Phys. Rev. B 2013, 87, 165402. [Google Scholar] [CrossRef]
- Watts, P.C.P.; Mureau, N.; Tang, Z.N.; Miyajima, Y.; Carey, J.D.; Silva, S.R.P. The importance of oxygen-containing defects on carbon nanotubes for the detection of polar and non-polar vapours through hydrogen bond formation. Nanotechnology 2007, 18, 175701. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, W.S.; Kim, D.K.; Han, J.-H.; Park, K.-B.; Ryu, S.C.; Min, N.K.; Kim, J.H. Functionalization of Molybdenum Disulfide via Plasma Treatment and 3-Mercaptopropionic Acid for Gas Sensors. Nanomaterials 2020, 10, 1860. https://doi.org/10.3390/nano10091860
Seo WS, Kim DK, Han J-H, Park K-B, Ryu SC, Min NK, Kim JH. Functionalization of Molybdenum Disulfide via Plasma Treatment and 3-Mercaptopropionic Acid for Gas Sensors. Nanomaterials. 2020; 10(9):1860. https://doi.org/10.3390/nano10091860
Chicago/Turabian StyleSeo, Won Seok, Dae Ki Kim, Ji-Hoon Han, Kang-Bak Park, Su Chak Ryu, Nam Ki Min, and Joon Hyub Kim. 2020. "Functionalization of Molybdenum Disulfide via Plasma Treatment and 3-Mercaptopropionic Acid for Gas Sensors" Nanomaterials 10, no. 9: 1860. https://doi.org/10.3390/nano10091860
APA StyleSeo, W. S., Kim, D. K., Han, J.-H., Park, K.-B., Ryu, S. C., Min, N. K., & Kim, J. H. (2020). Functionalization of Molybdenum Disulfide via Plasma Treatment and 3-Mercaptopropionic Acid for Gas Sensors. Nanomaterials, 10(9), 1860. https://doi.org/10.3390/nano10091860




