Mixed Mercaptocarboxylic Acid Shells Provide Stable Dispersions of InPZnS/ZnSe/ZnS Multishell Quantum Dots in Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
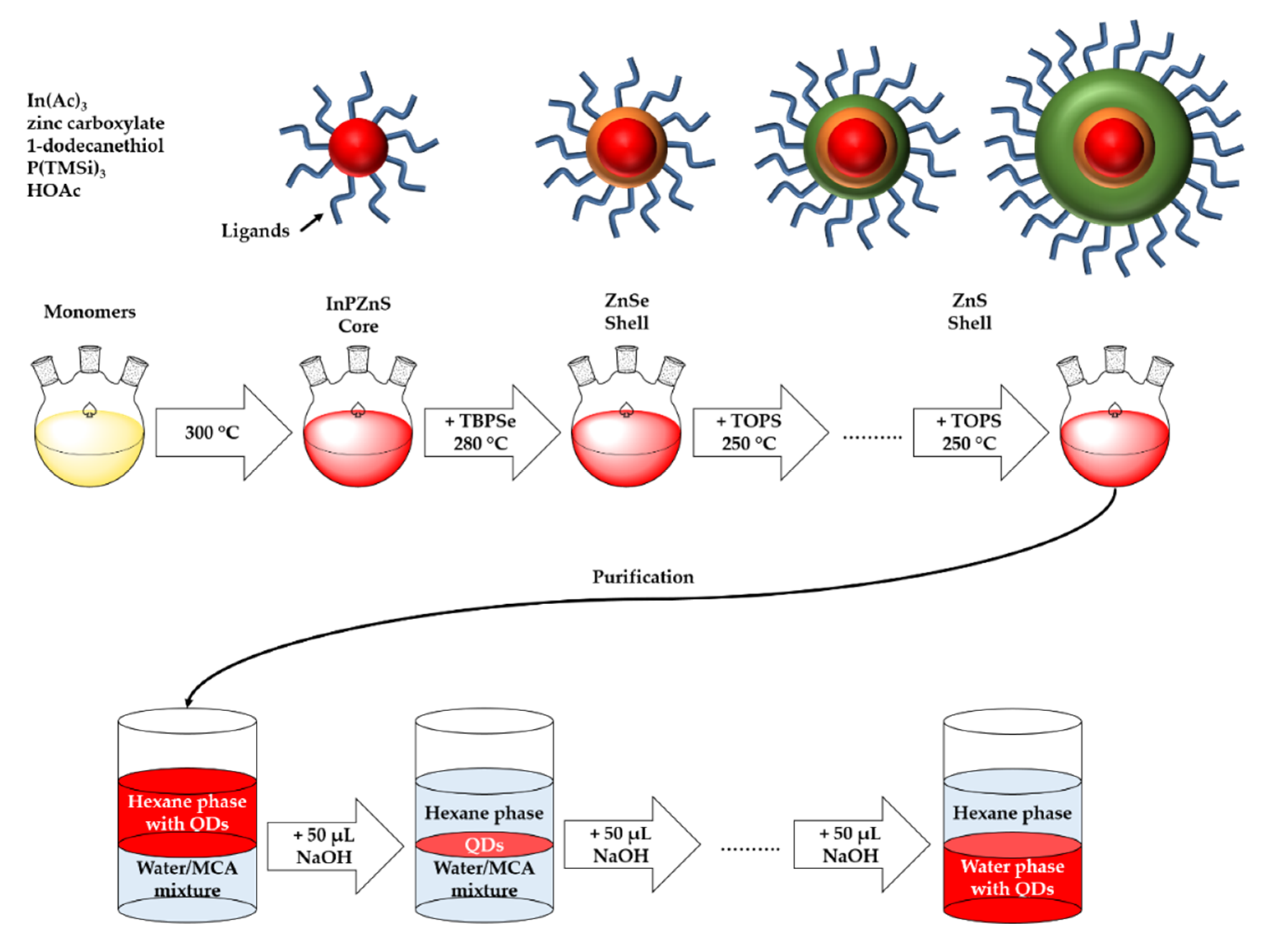
2.2. Synthesis
2.2.1. Precursors
2.2.2. InPZnS Multi Shell QDs
2.2.3. Ligand Exchange
2.3. Measurements
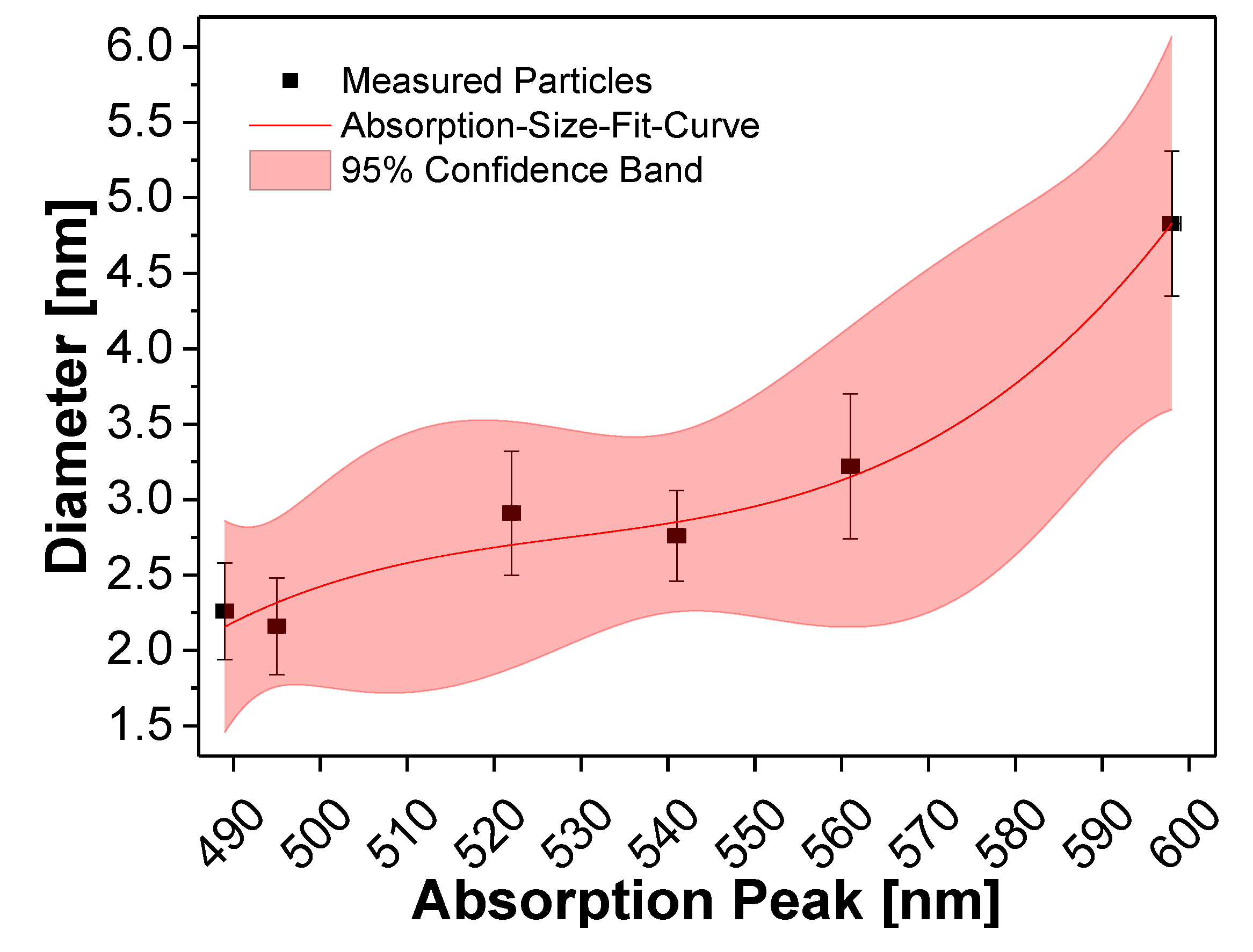
2.4. Calculation of Core Sizes and Amounts of Shell Precursor
3. Results
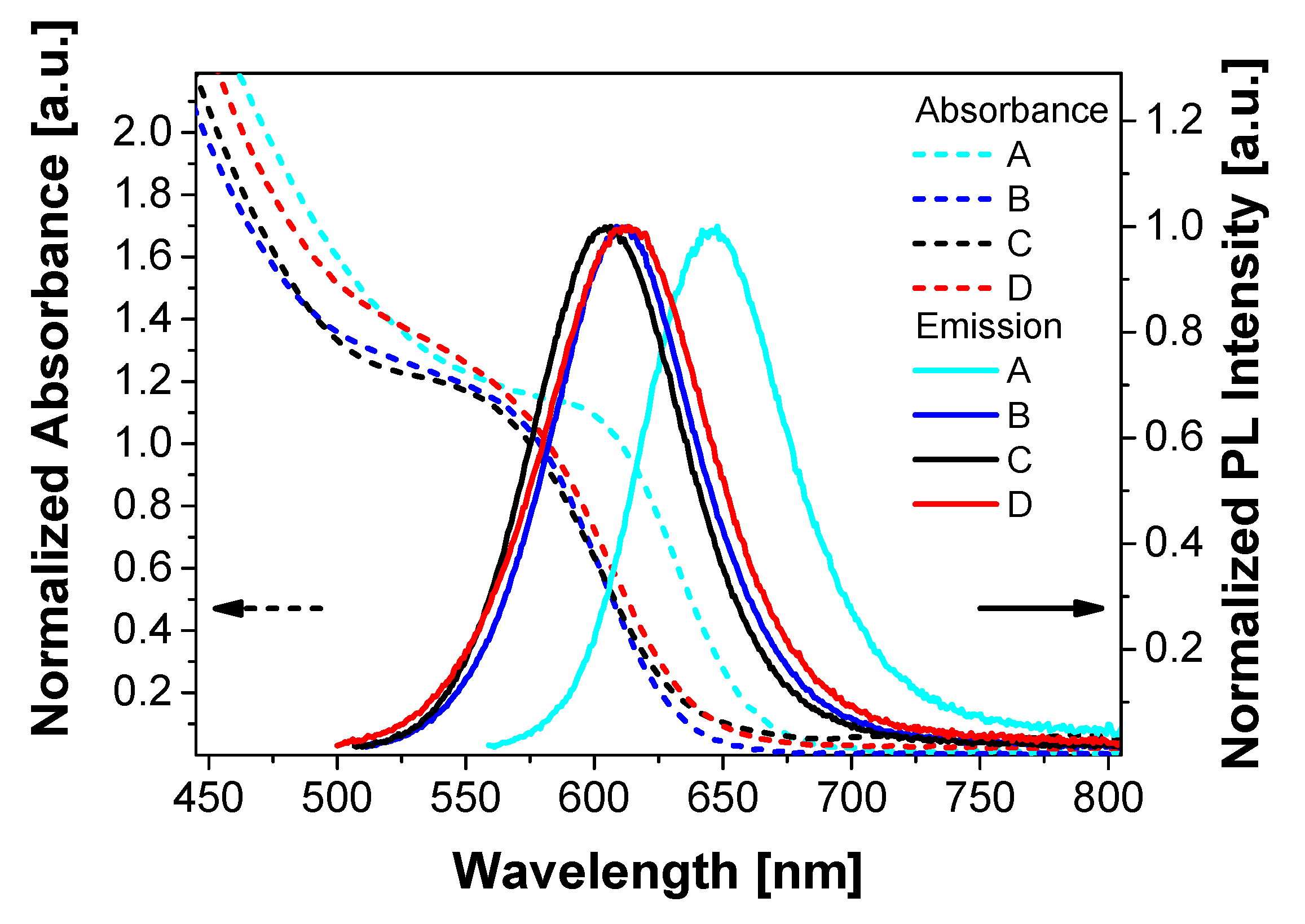
3.1. QD Synthesis and Characterization
3.2. Ligand Exchange
3.2.1. Phase Transfer of QDs with Different Shell Thicknesses
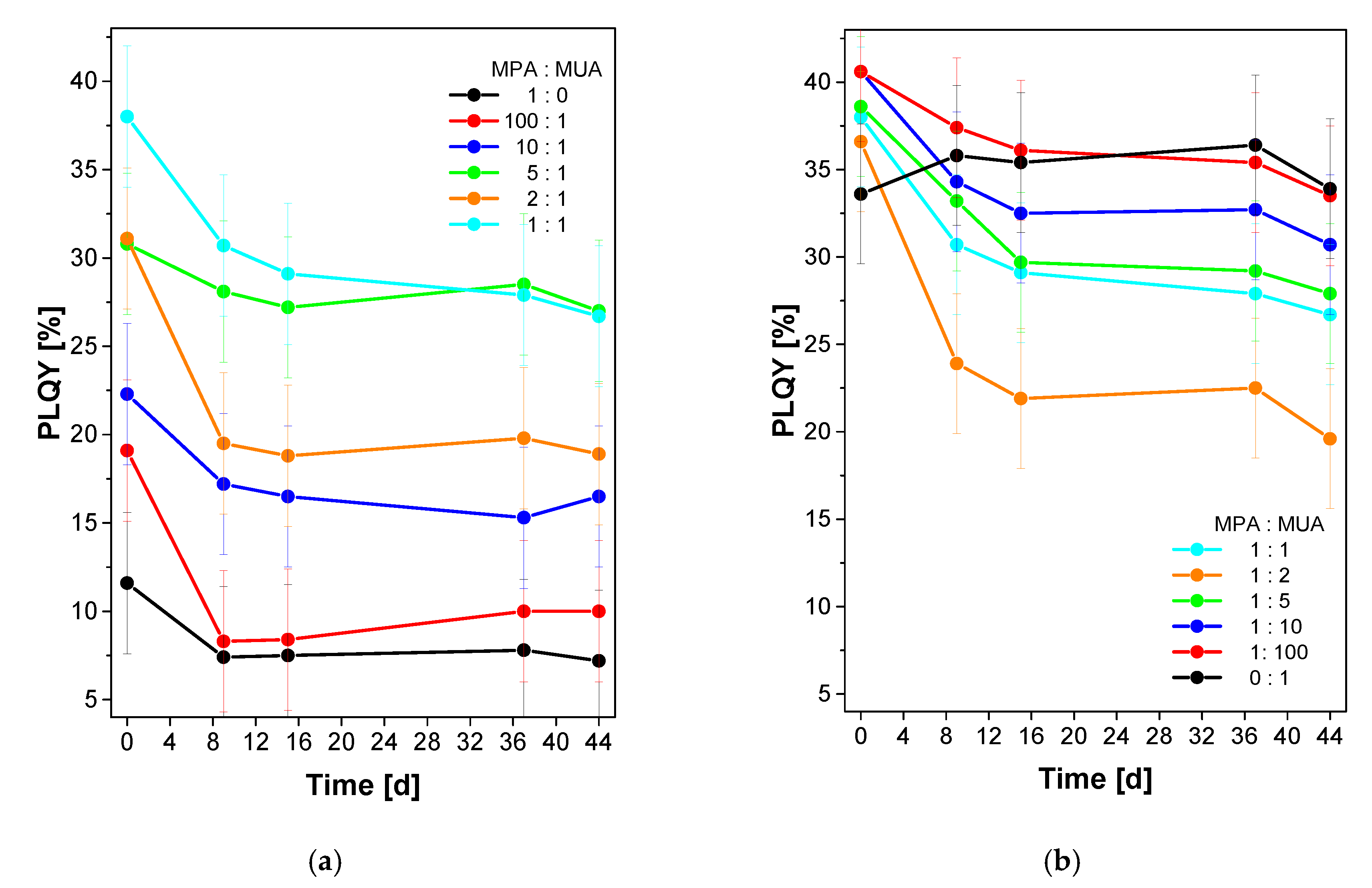
3.2.2. Phase Transfer of QDs Using Mixtures of MPA and MUA
3.2.3. Long Term Stability Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
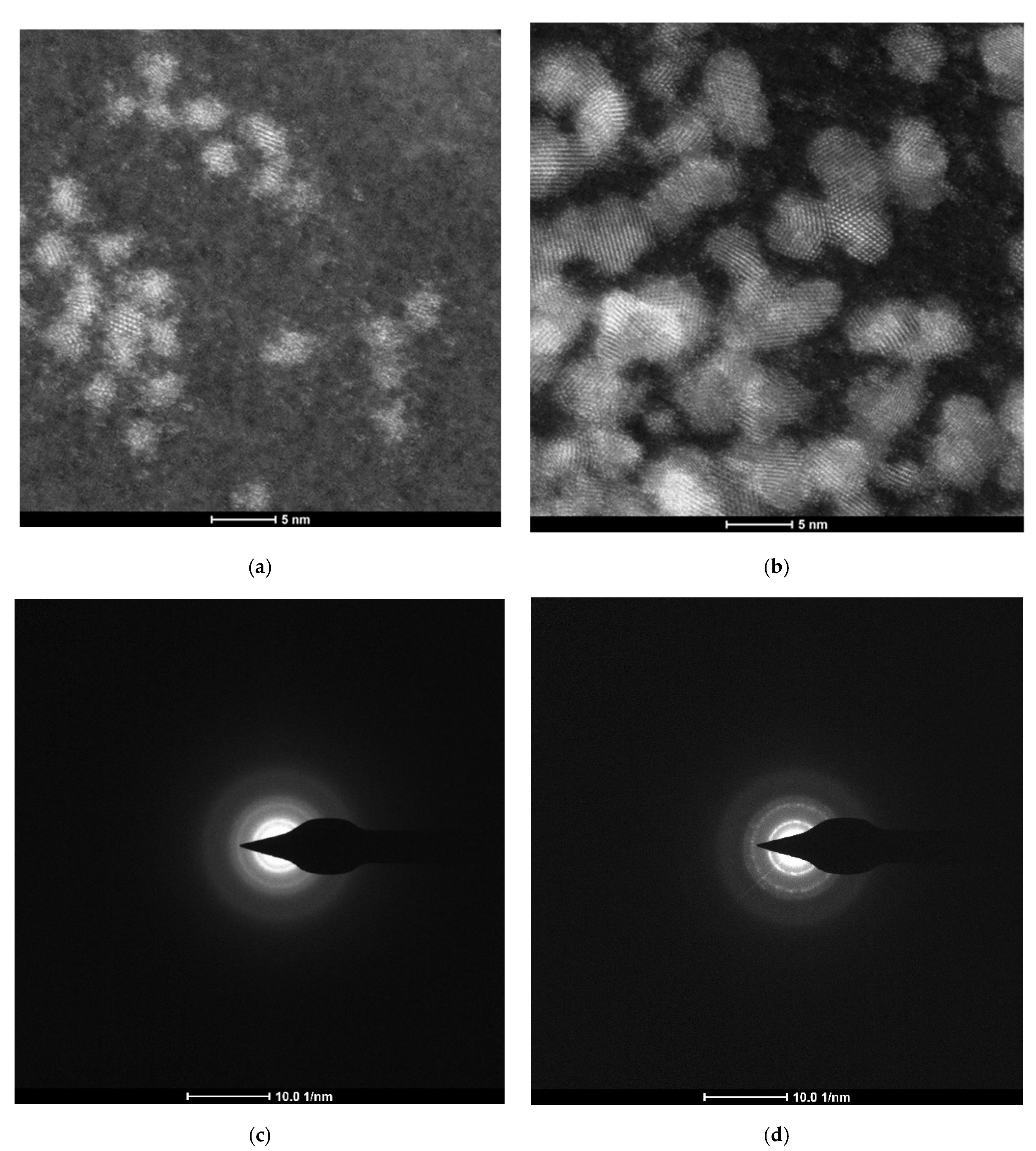
Appendix B
Appendix C
| d(hkl) [Å] | hkl | Structure |
|---|---|---|
| 3.3 | (111) | InP zinc blende |
| 2.0 | (220) | InP zinc blende |
| 1.7 | (222) (311) | InP zinc blende |
Appendix D
| d(hkl) [Å] | hkl | Structure |
|---|---|---|
| 3.1 | (111) | ZnS zinc blende |
| 3.4 | (111) | InP zinc blende |
| 1.9 | (220) | ZnS zinc blende |
| 2.0 | (220) | InP zinc blende |
| 1.6 | (311) | ZnS zinc blende |
| 1.7 | (222) (311) | InP zinc blende |
Appendix E
| Sample | 1st Exciton Absorption Wavelength [nm] | Diameter [nm] |
|---|---|---|
| E | 495 | 2.16 ± 0.32 |
| F | 489 | 2.26 ± 0.32 |
| G | 522 | 2.91 ± 0.41 |
| H | 541 | 2.76 ± 0.30 |
| I | 561 | 3.22 ± 0.48 |
| J | 598 | 4.83 ± 0.48 |
Appendix F
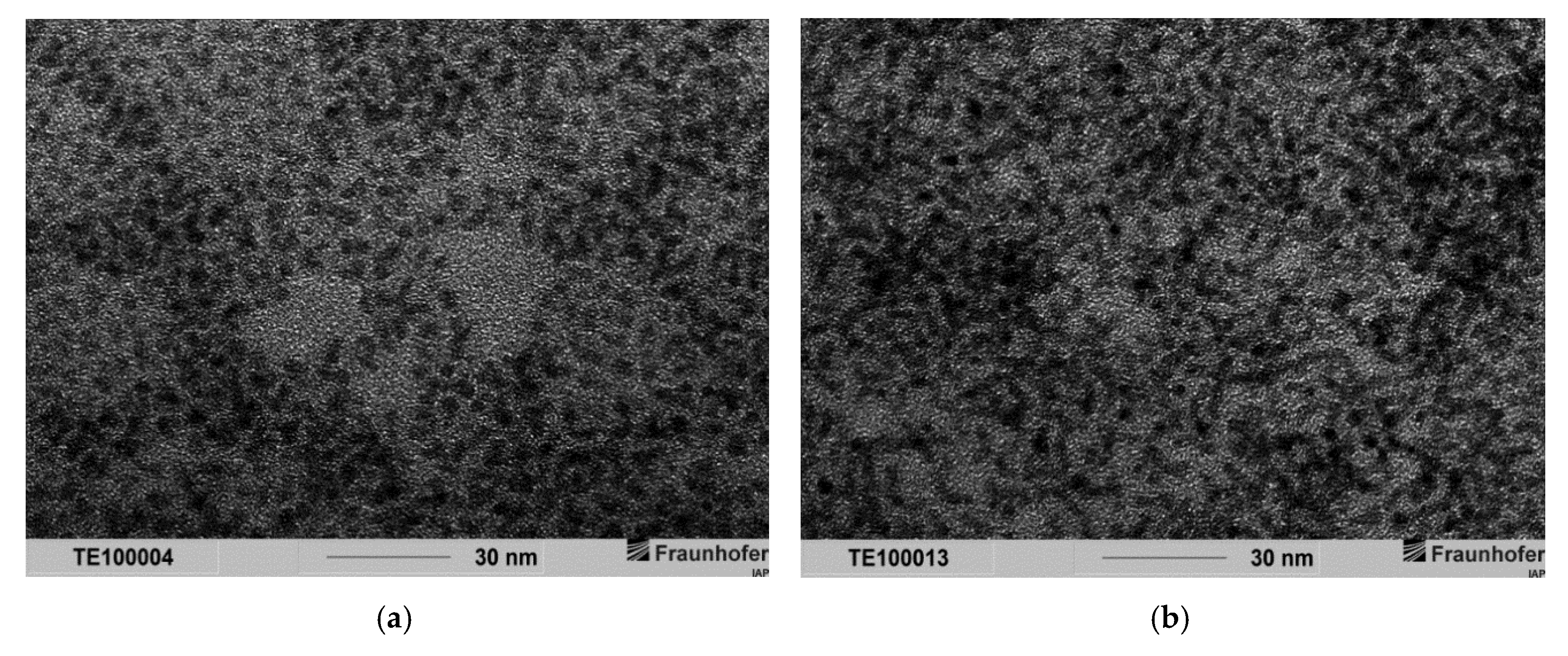
Appendix G
| QD Sample Name | Ligand | PLQY [%] | Emission Peak Wavelength [nm] | FWHM [nm] |
|---|---|---|---|---|
| A | MPA | 13 | 649 | 72 |
| MUA | 19 | 649 | 73 | |
| B | MPA | 27 | 619 | 70 |
| MUA | 45 | 620 | 72 | |
| C | MPA | 18 | 618 | 66 |
| MUA | 44 | 610 | 71 | |
| D | MPA | 24 | 615 | 78 |
| MUA | 39 | 614 | 76 |
Appendix H
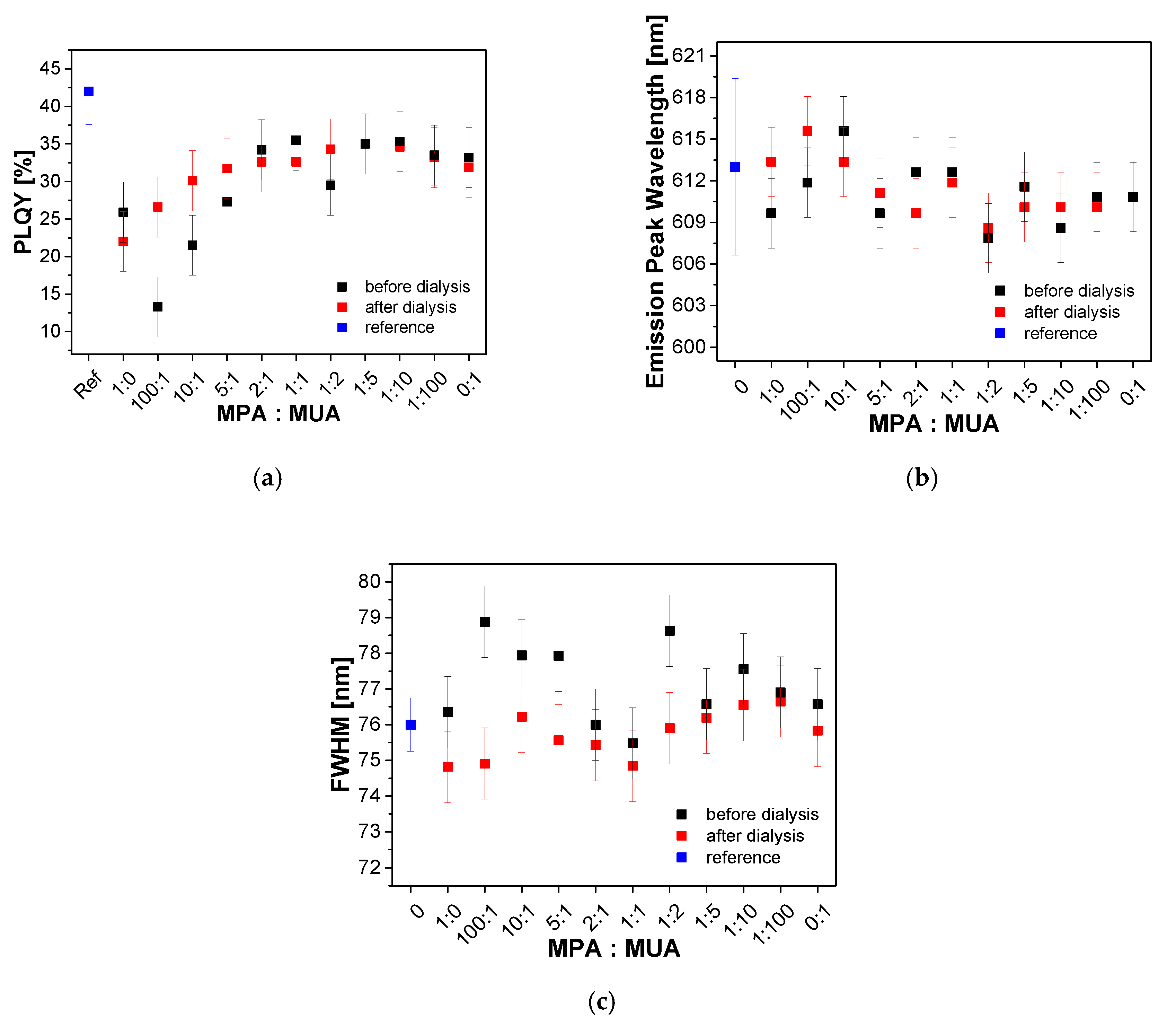
Appendix I
| Reference Sample | MPA:MUA Ratio | PLQY [%] | Emission Peak Wavelength [nm] | FWHM [nm] |
|---|---|---|---|---|
| C | 1:0 | 12 | 613 | 70 |
| 100:1 | 12 | 618 | 73 | |
| 10:1 | 18 | 617 | 71 | |
| 5:1 | 20 | 614 | 72 | |
| 2:1 | 24 | 615 | 71 | |
| 1:1 | 43 | 610 | 69 | |
| 1:2 | 43 | 615 | 69 | |
| 1:5 | 44 | 610 | 70 | |
| 1:10 | 45 | 610 | 70 | |
| 1:100 | 44 | 608 | 70 | |
| 0:1 | 44 | 607 | 70 | |
| D | 1:0 | 26 | 610 | 76 |
| 100:1 | 13 | 612 | 79 | |
| 10:1 | 32 | 616 | 78 | |
| 5:1 | 27 | 610 | 78 | |
| 2:1 | 34 | 613 | 76 | |
| 1:1 | 36 | 613 | 75 | |
| 1:2 | 30 | 608 | 79 | |
| 1:5 | 35 | 612 | 77 | |
| 1:10 | 35 | 609 | 78 | |
| 1:100 | 34 | 611 | 77 | |
| 0:1 | 33 | 611 | 77 |
Appendix J
| Reference Sample | MPA:MUA Ratio | PLQY [%] | Emission Peak Wavelength [nm] | FWHM [nm] |
|---|---|---|---|---|
| C | 1:0 | 11 | 618 | 67 |
| 100:1 | 19 | 618 | 66 | |
| 10:1 | 22 | 619 | 65 | |
| 5:1 | 31 | 610 | 68 | |
| 2:1 | 31 | 610 | 68 | |
| 1:1 | 38 | 614 | 68 | |
| 1:2 | 37 | 616 | 69 | |
| 1:5 | 39 | 613 | 70 | |
| 1:10 | 41 | 610 | 71 | |
| 1:100 | 41 | 608 | 72 | |
| 0:1 | 34 | 610 | 71 | |
| D | 1:0 | 22 | 613 | 75 |
| 100:1 | 27 | 616 | 75 | |
| 10:1 | 30 | 613 | 76 | |
| 5:1 | 32 | 611 | 76 | |
| 2:1 | 33 | 610 | 75 | |
| 1:1 | 33 | 612 | 75 | |
| 1:2 | 34 | 609 | 76 | |
| 1:5 | 35 | 610 | 76 | |
| 1:10 | 35 | 610 | 77 | |
| 1:100 | 33 | 610 | 77 | |
| 0:1 | 32 | 611 | 76 |
Appendix K
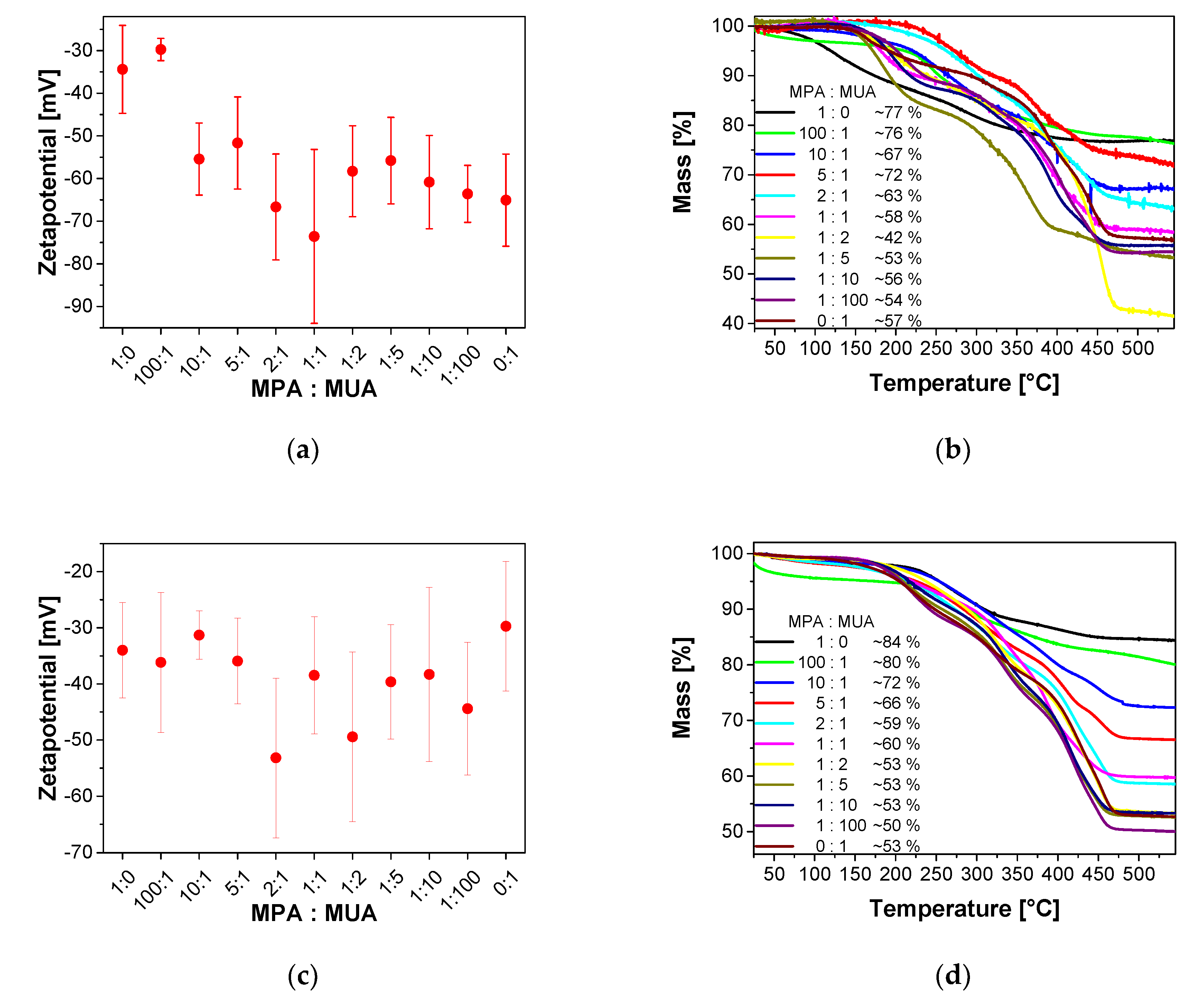
Appendix L
References
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodríguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS Core−Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Reiss, P.; Protière, M.; Li, L. Core/Shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Xu, S.; Shen, H.; Zhou, C.; Yuan, H.; Liu, C.; Wang, H.; Ma, L.; Li, L.S. Effect of Shell Thickness on the Optical Properties in CdSe/CdS/Zn 0.5 Cd 0.5 S/ZnS and CdSe/CdS/ZnxCd1−xS/ZnS Core/Multishell Nanocrystals. J. Phys. Chem. C 2011, 115, 20876–20881. [Google Scholar] [CrossRef]
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core–shell quantum dots: Properties and applications. J. Alloy Compd. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Stein, J.L.; Mader, E.A.; Cossairt, B.M. Luminescent InP Quantum Dots with Tunable Emission by Post-Synthetic Modification with Lewis Acids. J. Phys. Chem. Lett. 2016, 7, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, C.; Kumar, M.; Kumari, R.; Srivastava, R.; Sharma, S.N. Enhanced luminescence efficiency of wet chemical route synthesized InP-based quantum dots by a novel method: Probing the humidity sensing properties. J. Lumin. 2018, 198, 108–116. [Google Scholar] [CrossRef]
- Brown, R.P.; Gallagher, M.J.; Fairbrother, D.H.; Rosenzweig, Z. Synthesis and Degradation of Cadmium-Free InP and InPZn/ZnS Quantum Dots in Solution. Langmuir 2018, 34, 13924–13934. [Google Scholar] [CrossRef]
- Kim, Y.; Ippen, C.; Greco, T.; Lee, J.; Oh, M.S.; Han, C.J.; Wedel, A.; Kim, J. Increased shell thickness in indium phosphide multishell quantum dots leading to efficiency and stability enhancement in light-emitting diodes. Opt. Mater. Express 2014, 4, 1436. [Google Scholar] [CrossRef]
- Kim, Y.; Ippen, C.; Fischer, B.; Lange, A.; Wedel, A. Efficiency enhancement of InP-based inverted QD-LEDs by incorporation of a polyethylenimine modified Al: ZnO layer. J. Soc. Inf. Disp. 2015, 23, 377–383. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Cao, W.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.; Holloway, P.H.; Qian, L. High-efficiency light-emitting devices based on quantum dots with tailored nanostructures. Nat. Photon. 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Crisp, R.W.; Pach, G.F.; Kurley, J.M.; France, R.M.; Reese, M.O.; Nanayakkara, S.U.; MacLeod, B.A.; Talapin, D.V.; Beard, M.C.; Luther, J.M. Tandem Solar Cells from Solution-Processed CdTe and PbS Quantum Dots Using a ZnTe-ZnO Tunnel Junction. Nano Lett. 2017, 17, 1020–1027. [Google Scholar] [CrossRef]
- Bruns, O.T.; Bischof, T.S.; Harris, D.K.; Franke, D.; Shi, Y.; Riedemann, L.; Bartelt, A.; Jaworski, F.B.; Carr, J.A.; Rowlands, C.J.; et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017, 1, 0056. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.-Y. Synthetic strategies and biomedical applications of I–III–VI ternary quantum dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. Weinh. 2017, 29, 1604847. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Lin, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Boopathyraja, A. Structural and optical properties of Mg doped ZnS quantum dots and biological applications. Superlattices Microstruct. 2018, 113, 236–243. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, Y.; Park, J.C.; Oh, M.H.; Jeon, D.Y.; Nam, Y.S. Highly luminescent, off-stoichiometric CuxInyS2/ZnS quantum dots for near-infrared fluorescence bio-imaging. RSC Adv. 2015, 5, 43449–43455. [Google Scholar] [CrossRef]
- Rogach, A.L.; Franzl, T.; Klar, T.A.; Feldmann, J.; Gaponik, N.; Lesnyak, V.; Shavel, A.; Eychmüller, A.; Rakovich, Y.P.; Donegan, J.F. Aqueous Synthesis of Thiol-Capped CdTe Nanocrystals: State-of-the-Art. J. Phys. Chem. C 2007, 111, 14628–14637. [Google Scholar] [CrossRef]
- Musaev, O.R.; Wrobel, J.M.; Wieliczka, D.M.; Dusevich, V.; Kruger, M.B. Formation of InP nanoparticles by laser ablation in an aqueous environment. Phys. E Low-Dimens. Syst. Nanostructures 2008, 40, 3147–3150. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Clapp, A. Overview of stabilizing ligands for biocompatible quantum dot nanocrystals. Sensors 2011, 11, 11036–11055. [Google Scholar] [CrossRef]
- Allocca, M.; Mattera, L.; Bauduin, A.; Miedziak, B.; Moros, M.; Trizio, L.; de Tino, A.; Reiss, P.; Ambrosone, A.; Tortiglione, C. An Integrated Multilevel Analysis Profiling Biosafety and Toxicity Induced by Indium- and Cadmium-Based Quantum Dots In Vivo. Environ. Sci. Technol. 2019, 53, 3938–3947. [Google Scholar] [CrossRef]
- Mattera, L.; Bhuckory, S.; Wegner, K.D.; Qiu, X.; Agnese, F.; Lincheneau, C.; Senden, T.; Djurado, D.; Charbonnière, L.J.; Hildebrandt, N.; et al. Compact quantum dot-antibody conjugates for FRET immunoassays with subnanomolar detection limits. Nanoscale 2016, 8, 11275–11283. [Google Scholar] [CrossRef]
- Kalinowska, D.; Drozd, M.; Grabowska-Jadach, I.; Pietrzak, M.; Dybko, A.; Malinowska, E.; Brzózka, Z. The influence of selected ω-mercaptocarboxylate ligands on physicochemical properties and biological activity of Cd-free, zinc-copper-indium sulfide colloidal nanocrystals. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 583–592. [Google Scholar] [CrossRef]
- Xie, R.; Battaglia, D.; Peng, X. Colloidal InP nanocrystals as efficient emitters covering blue to near-infrared. J. Am. Chem. Soc. 2007, 129, 15432–15433. [Google Scholar] [CrossRef] [PubMed]
- Tamang, S.; Beaune, G.; Texier, I.; Reiss, P. Aqueous phase transfer of InP/ZnS nanocrystals conserving fluorescence and high colloidal stability. ACS Nano 2011, 5, 9392–9402. [Google Scholar] [CrossRef]
- Brunetti, V.; Chibli, H.; Fiammengo, R.; Galeone, A.; Malvindi, M.A.; Vecchio, G.; Cingolani, R.; Nadeau, J.L.; Pompa, P.P. InP/ZnS as a safer alternative to CdSe/ZnS core/shell quantum dots: In Vitro and In Vivo toxicity assessment. Nanoscale 2013, 5, 307–317. [Google Scholar] [CrossRef]
- Beaune, G.; Tamang, S.; Bernardin, A.; Bayle-Guillemaud, P.; Fenel, D.; Schoehn, G.; Vinet, F.; Reiss, P.; Texier, I. Luminescence of polyethylene glycol coated CdSeTe/ZnS and InP/ZnS nanoparticles in the presence of copper cations. Chemphyschem 2011, 12, 2247–2254. [Google Scholar] [CrossRef]
- Xia, C.; Meeldijk, J.D.; Gerritsen, H.C.; de Mello Donega, C. Highly Luminescent Water-Dispersible NIR-Emitting Wurtzite CuInS2/ZnS Core/Shell Colloidal Quantum Dots. Chem. Mater. 2017, 29, 4940–4951. [Google Scholar] [CrossRef]
- Di Corato, R.; Quarta, A.; Piacenza, P.; Ragusa, A.; Figuerola, A.; Buonsanti, R.; Cingolani, R.; Manna, L.; Pellegrino, T. Water solubilization of hydrophobic nanocrystals by means of poly(maleic anhydride-alt-1-octadecene). J. Mater. Chem. 2008, 18, 1991. [Google Scholar] [CrossRef]
- Pellegrino, T.; Manna, L.; Kudera, S.; Liedl, T.; Koktysh, D.; Rogach, A.L.; Keller, S.; Rädler, J.; Natile, G.; Parak, W.J. Hydrophobic Nanocrystals Coated with an Amphiphilic Polymer Shell: A General Route to Water Soluble Nanocrystals. Nano Lett. 2004, 4, 703–707. [Google Scholar] [CrossRef]
- Huang, L.; Liao, M.; Chen, S.; Demillo, V.G.; Dupre, S.A.; Zhu, X.; Publicover, N.G.; Hunter, K.W. A polymer encapsulation approach to prepare zwitterion-like, biocompatible quantum dots with wide pH and ionic stability. J. Nanopart. Res. 2014, 16, 2555. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and Properties of Biocompatible Water-Soluble Silica-Coated CdSe/ZnS Semiconductor Quantum Dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Hutter, E.M.; Pietra, F.; van Dijk-Moes, R.J.A.; Mitoraj, D.; Meeldijk, J.D.; de Mello Donegá, C.; Vanmaekelbergh, D. Method to Incorporate Anisotropic Semiconductor Nanocrystals of All Shapes in an Ultrathin and Uniform Silica Shell. Chem. Mater. 2014, 26, 1905–1911. [Google Scholar] [CrossRef]
- Clift, M.J.D.; Varet, J.; Hankin, S.M.; Brownlee, B.; Davidson, A.M.; Brandenberger, C.; Rothen-Rutishauser, B.; Brown, D.M.; Stone, V. Quantum dot cytotoxicity In Vitro: An investigation into the cytotoxic effects of a series of different surface chemistries and their core/shell materials. Nanotoxicology 2011, 5, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Kong, L.; Xue, Y.; Tang, M. Threshold Dose of Three Types of Quantum Dots (QDs) Induces Oxidative Stress Triggers DNA Damage and Apoptosis in Mouse Fibroblast L929 Cells. Int. J. Environ. Res. Public Health 2015, 12, 13435–13454. [Google Scholar] [CrossRef]
- Ayupova, D.; Dobhal, G.; Laufersky, G.; Nann, T.; Goreham, R.V. An In Vitro Investigation of Cytotoxic Effects of InP/Zns Quantum Dots with Different Surface Chemistries. Nanomaterials 2019, 9, 135. [Google Scholar] [CrossRef]
- Yaghini, E.; Turner, H.; Pilling, A.; Naasani, I.; MacRobert, A.J. In Vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model. Nanomedicine 2018, 14, 2644–2655. [Google Scholar] [CrossRef]
- Ippen, C.; Greco, T.; Kim, Y.; Pries, C.; Kim, J.; Oh, M.S.; Han, C.J.; Wedel, A. Color tuning of indium phosphide quantum dots for cadmium-free quantum dot light-emitting devices with high efficiency and color saturation. J. Soc. Inf. Disp. 2015, 23, 285–293. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Talapin, D.V. Experimental and Theoretical Studies on the Formation of Highly Luminescent II-VI, III-V and Core-Shell Semiconductor Nanocrystals. Ph.D. Thesis, Staats- und Universitätsbibliothek Carl von Ossietzky, Hamburg, Germany, 2002. [Google Scholar]
- Ippen, C. Indium Phosphide and Zinc Selenide Quantum Dots for Light-Emitting Devices: Relationships between Surface Structure and Device Performance. Ph.D. Thesis, University of Potsdam, Potsdam, Germany, 2014. [Google Scholar]
- Sugii, K.; Koizumi, H.; Kubota, E. Precision lattice parameter measurements on doped indium phosphide single crystals. JEM 1983, 12, 701–712. [Google Scholar] [CrossRef]
- Nitta, E.; Kimata, M.; Hoshino, M.; Echigo, T.; Hamasaki, S.; Nishida, N.; Shimizu, M.; Akasaka, T. Crystal chemistry of ZnS minerals formed as high-temperature volcanic sublimates: Matraite identical with sphalerite. J. Mineral. Petrol. Sci. 2008, 103, 145–151. [Google Scholar] [CrossRef][Green Version]
- Koh, S.; Eom, T.; Kim, W.D.; Lee, K.; Lee, D.; Lee, Y.K.; Kim, H.; Bae, W.K.; Lee, D.C. Zinc–Phosphorus Complex Working as an Atomic Valve for Colloidal Growth of Monodisperse Indium Phosphide Quantum Dots. Chem. Mater. 2017, 29, 6346–6355. [Google Scholar] [CrossRef]
- Gray, V.; Xia, P.; Huang, Z.; Moses, E.; Fast, A.; Fishman, D.A.; Vullev, V.I.; Abrahamsson, M.; Moth-Poulsen, K.; Lee Tang, M. CdS/ZnS core-shell nanocrystal photosensitizers for visible to UV upconversion. Chem. Sci. 2017, 8, 5488–5496. [Google Scholar] [CrossRef]
- Lao, P.D.; Guo, Y.; Siu, G.G.; Shen, S.C. Optical-phonon behavior in Zn1-xMnxSe: Zinc-blende and wurtzite structures. Phys. Rev. B Condens. Matter 1993, 48, 11701–11704. [Google Scholar] [CrossRef]
- Yim, W.M. Solid Solutions in the Pseudobinary (III-V)-(II-VI) Systems and Their Optical Energy Gaps. J. Chem. Phys. 1969, 40, 2617–2623. [Google Scholar] [CrossRef]
- Noh, M.; Kim, T.; Lee, H.; Kim, C.-K.; Joo, S.-W.; Lee, K. Fluorescence quenching caused by aggregation of water-soluble CdSe quantum dots. Colloids Surf. A Physicochem. Eng. Asp. 2010, 359, 39–44. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Dai, X.; Yao, Z.; Lv, L.; Jin, Y.; Peng, X. Stoichiometry-Controlled InP-Based Quantum Dots: Synthesis, Photoluminescence, and Electroluminescence. J. Am. Chem. Soc. 2019, 141, 6448–6452. [Google Scholar] [CrossRef]
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Pong, B.-K.; Trout, B.L.; Lee, J.-Y. Modified ligand-exchange for efficient solubilization of CdSe/ZnS quantum dots in water: A procedure guided by computational studies. Langmuir 2008, 24, 5270–5276. [Google Scholar] [CrossRef] [PubMed]
- Tohgha, U.; Varga, K.; Balaz, M. Achiral CdSe quantum dots exhibit optical activity in the visible region upon post-synthetic ligand exchange with D- or L-cysteine. Chem. Commun. (Camb) 2013, 49, 1844–1846. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Goodman, M.D.; Chen, Y.; Cai, M.; Shinar, J.; Lin, Z. A simple biphasic route to water soluble dithiocarbamate functionalized quantum dots. J. Mater. Chem. 2008, 18, 3270. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, X. A general and reversible phase transfer strategy enabling nucleotides modified high-quality water-soluble nanocrystals. Chem. Commun. (Camb) 2012, 48, 5718–5720. [Google Scholar] [CrossRef]
- Wang, M.; Felorzabihi, N.; Guerin, G.; Haley, J.C.; Scholes, G.D.; Winnik, M.A. Water-Soluble CdSe Quantum Dots Passivated by a Multidentate Diblock Copolymer. Macromolecules 2007, 40, 6377–6384. [Google Scholar] [CrossRef]
- Pries, C. Überführung von InP—Basierten Quantenpunkten in Eine Wässrige Phase. Master’s Thesis, University of Potsdam, Potsdam, Germany, 2016. [Google Scholar]
- Clarke, M.T.; Viscomi, F.N.; Chamberlain, T.W.; Hondow, N.; Adawi, A.M.; Sturge, J.; Erwin, S.C.; Bouillard, J.-S.G.; Tamang, S.; Stasiuk, G.J. Synthesis of super bright indium phosphide colloidal quantum dots through thermal diffusion. Commun. Chem. 2019, 2, 36. [Google Scholar] [CrossRef]
- Breus, V.V.; Heyes, C.D.; Nienhaus, G.U. Quenching of CdSe−ZnS Core−Shell Quantum Dot Luminescence by Water-Soluble Thiolated Ligands. J. Phys. Chem. C 2007, 111, 18589–18594. [Google Scholar] [CrossRef]

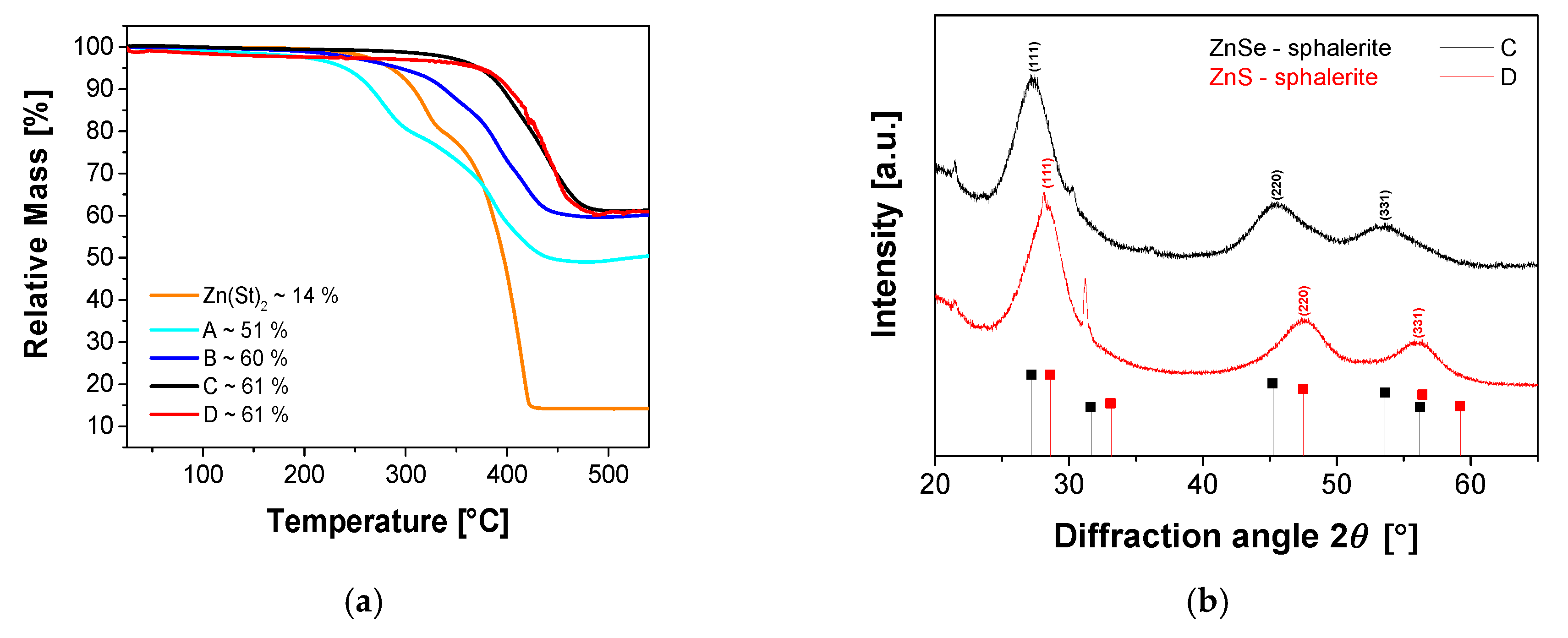

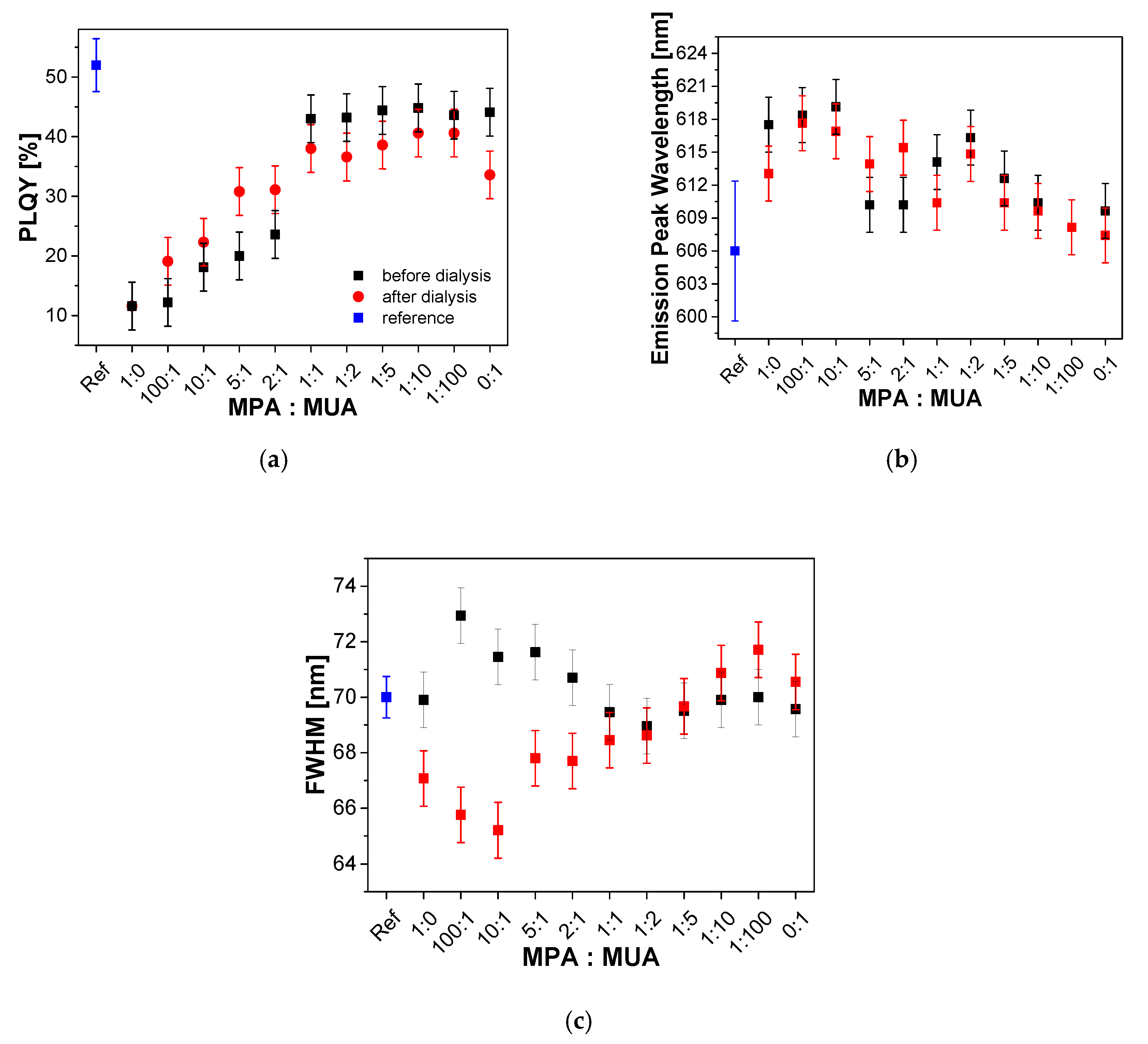
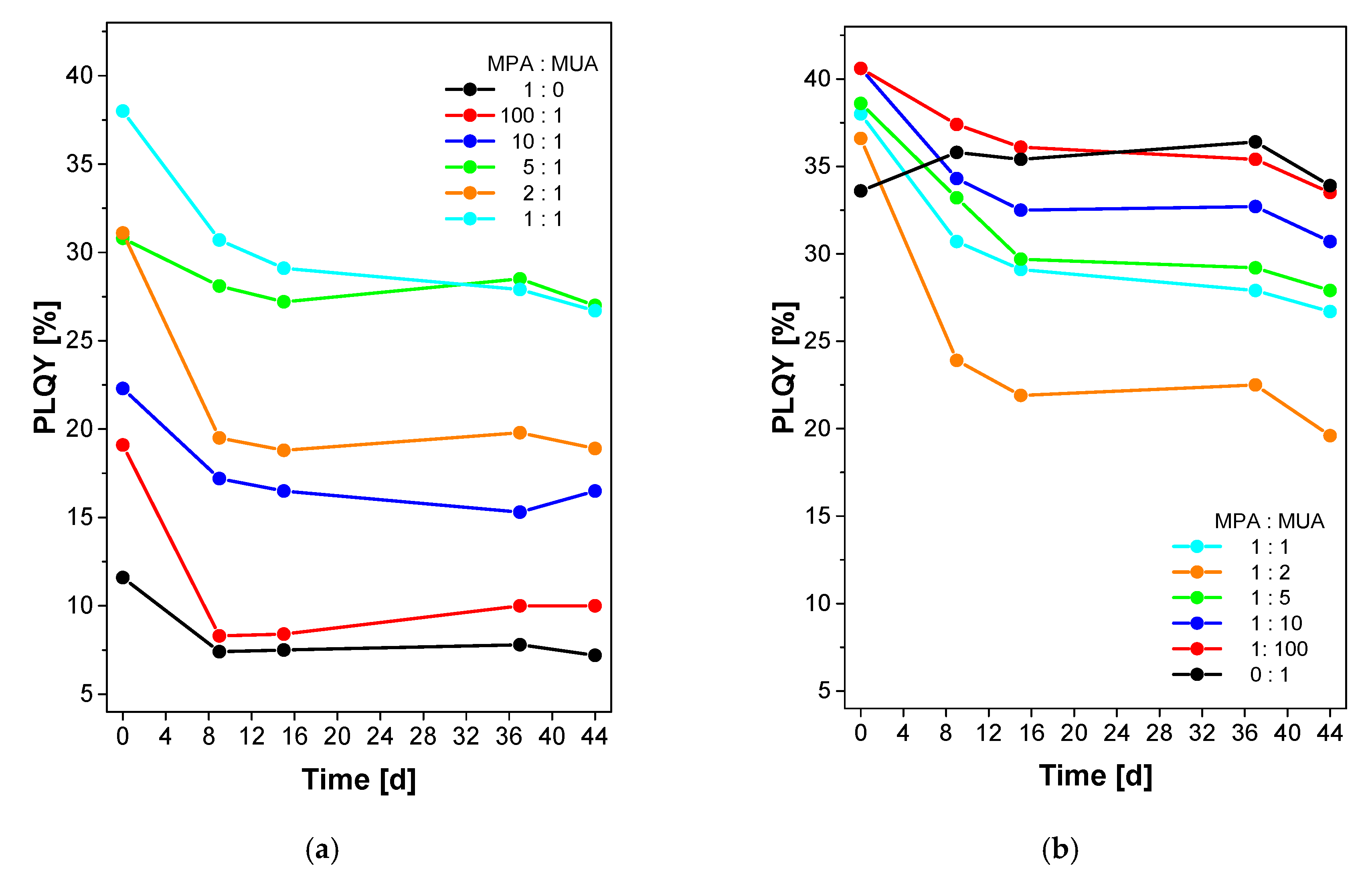
| Sample Name | In(Ac)3 [g, (mmol)] | Zn(St)2 [g, (mmol)] | Zn(Oct)2 [g, (mmol)] | 1-Dodecanethiol [mL, (mmol)] | HOAc [mL, (mmol)] | P(TMSi)3 [mL, (mmol)] |
|---|---|---|---|---|---|---|
| A | 0.58 (2.00) | 2.53 (4.00) | - | 0.24 (1.00) | 0.30 (5.25) | 2.00 (2.00) |
| B | 0.73 (2.50) | 3.16 (5.00) | - | 0.30 (1.25) | 0.30 (5.25) | 2.50 (2.50) |
| C | 0.73 (2.50) | 3.16 (5.00) | - | 0.30 (1.25) | 0.48 (8.39) | 2.50 (2.50) |
| D | 1.17 (4.00) | 5.60 (8.00) | - | 0.48 (2.00) | 0.30 (5.25) | 3.00 (3.00) |
| E | 0.29 (1.00) | - | 0.70 (2.00) | 0.12 (0.50) | 0.00 (0.00) | 1.00 (1.00) |
| F | 0.29 (1.00) | - | 0.70 (2.00) | 0.12 (0.50) | 0.02 (0.35) | 1.00 (1.00) |
| G | 0.29 (1.00) | - | 0.70 (2.00) | 0.12 (0.50) | 0.03 (0.52) | 1.00 (1.00) |
| H | 0.29 (1.00) | - | 0.70 (2.00) | 0.12 (0.50) | 0.05 (0.87) | 1.00 (1.00) |
| I | 0.29 (1.00) | - | 0.70 (2.00) | 0.12 (0.50) | 0.09 (1.57) | 1.00 (1.00) |
| J | 0.29 (1.00) | - | 0.70 (2.00) | 0.12 (0.50) | 0.30 (5.25) | 1.00 (1.00) |
| Sample Name | TBPSe [mL, (mmol)] | Zn(St)2 [g, (mmol)] |
|---|---|---|
| A | 0.10 (0.20) | 0.00 (0.00) |
| B | 0.28 (0.56) | 3.00 (4.74) |
| C | 0.42 (0.84) | 4.00 (6.33) |
| D | 0.58 (1.06) | 3.00 (4.74) |
| Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | TOPS [mL, (mmol)] | Zn(St)2 [g, (mmol)] | TOPS [mL, (mmol)] | Zn(St)2 [g, (mmol)] | TOPS [mL, (mmol)] | Zn(St)2 [g, (mmol)] | TOPS [mL, (mmol)] | Zn(St)2 [g, (mmol)] | TOPS [mL, (mmol)] | Zn(St)2 [g, (mmol)] |
| A | 0.31 (0.62) | 0.00 (0.00) | - | - | - | - | - | - | - | - |
| B | 1.02 (2.04) | 0.00 (0.00) | 1.34 (2.68) | 0.00 (0.00) | - | - | - | - | - | - |
| C | 1.69 (3.38) | 0.00 (0.00) | 2.41 (4.81) | 6.00 (9.49) | 5.28 (10.56) | 0.00 (0.00) | - | - | - | - |
| D | 2.20 (4.40) | 6.00 (9.49) | 3.98 (7.96) | 3.02 (4.77) | 3.98 (7.96) | 16.30 (25.78) | 15.42 (30.84) | 0.00 (0.00) | 34.14 (68.28) | 16.30 (25.78) |
| MPA/MUA Molar Ratio | MPA [µL, (mmol)] | MUA [mg, (mmol)] |
|---|---|---|
| 1:0 | 100 (1.15) | 0.0 (0.00) |
| 100:1 | 99 (1.14) | 2.5 (0.01) |
| 10:1 | 91 (1.05) | 22.8 (0.10) |
| 5:1 | 83 (0.96) | 41.8 (0.19) |
| 2:1 | 67 (0.77) | 83.5 (0.38) |
| 1:1 | 50 (0.57) | 125.3 (0.57) |
| 1:2 | 33 (0.38) | 167.1 (0.77) |
| 1:5 | 17 (0.19) | 208.8 (0.96) |
| 1:10 | 9 (0.10) | 227.8 (1.05) |
| 1:100 | 1 (0.01) | 248.1 (1.14) |
| 0:1 | 0 (0.00) | 250.6 (1.15) |
| QD Sample Name | ZnSe Shell Thickness [ML] | ZnS Shell Thickness [ML] | Total Diameter [nm] | First Exciton Absorption Wavelength [nm] | Emission Peak Wavelength [nm] | PLQY [%] | FWHM [nm] |
|---|---|---|---|---|---|---|---|
| A | 0.2 (+0.0; −0.2) | 0.6 (+1.0; −0.2) | 5.8 (+0.9; −1.8) | 610 | 648 | 31 | 67 |
| B | 0.3 (±0.1) | 1.8 (±0.6) | 4.7 (±1.6) | 579 | 609 | 60 | 68 |
| C | 0.5 (±0.2) | 4.9 (±1.6) | 7.0 (±2.2) | 575 | 606 | 52 | 70 |
| D | 0.4 (±0.1) | 9.5 (+3.3; −3.2) | 9.4 (±3.1) | 582 | 613 | 42 | 76 |
| E | - | - | 2.2 (±0.3) | 495 | 535 | 38 | 55 |
| F | - | - | 2.3 (±0.3) | 489 | 536 | 52 | 59 |
| G | - | - | 2.9 (±0.4) | 522 | 547 | 48 | 61 |
| H | - | - | 2.8 (±0.3) | 541 | 570 | 58 | 63 |
| I | - | - | 3.2 (±0.5) | 561 | 586 | 52 | 65 |
| J | - | - | 4.8 (±0.5) | 598 | 628 | 17 | 72 |
| Atomic Content (Normalized to in Atomic Content) [a.u.] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| QD Sample | In | P | Zn | S | Se | C | H | O | ZnS: InP |
| C | 1.00 | 0.61 | 3.60 | 2.71 | 0.66 | 24.43 | 49.20 | 3.20 | 4.54 |
| D | 1.00 | 0.61 | 13.31 | 11.92 | 0.32 | 66.18 | 133.67 | 8.94 | 15.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyne, B.; Arlt, K.; Geßner, A.; Richter, A.F.; Döblinger, M.; Feldmann, J.; Taubert, A.; Wedel, A. Mixed Mercaptocarboxylic Acid Shells Provide Stable Dispersions of InPZnS/ZnSe/ZnS Multishell Quantum Dots in Aqueous Media. Nanomaterials 2020, 10, 1858. https://doi.org/10.3390/nano10091858
Heyne B, Arlt K, Geßner A, Richter AF, Döblinger M, Feldmann J, Taubert A, Wedel A. Mixed Mercaptocarboxylic Acid Shells Provide Stable Dispersions of InPZnS/ZnSe/ZnS Multishell Quantum Dots in Aqueous Media. Nanomaterials. 2020; 10(9):1858. https://doi.org/10.3390/nano10091858
Chicago/Turabian StyleHeyne, Benjamin, Kristin Arlt, André Geßner, Alexander F. Richter, Markus Döblinger, Jochen Feldmann, Andreas Taubert, and Armin Wedel. 2020. "Mixed Mercaptocarboxylic Acid Shells Provide Stable Dispersions of InPZnS/ZnSe/ZnS Multishell Quantum Dots in Aqueous Media" Nanomaterials 10, no. 9: 1858. https://doi.org/10.3390/nano10091858
APA StyleHeyne, B., Arlt, K., Geßner, A., Richter, A. F., Döblinger, M., Feldmann, J., Taubert, A., & Wedel, A. (2020). Mixed Mercaptocarboxylic Acid Shells Provide Stable Dispersions of InPZnS/ZnSe/ZnS Multishell Quantum Dots in Aqueous Media. Nanomaterials, 10(9), 1858. https://doi.org/10.3390/nano10091858






