Photocatalytic Inactivation of Plant Pathogenic Bacteria Using TiO2 Nanoparticles Prepared Hydrothermally
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains
2.3. Synthesis
2.4. Methods
2.5. Photocatalytic Tests
2.6. Antibacterial Tests
3. Results
3.1. Structural Properties: Crystalline Structure, Specific Surface Area
3.2. Morphology and Size Distribution of TiO2 NPs
3.3. Optical Band Gap
3.4. Chemical Composition at the Near-Surface Region
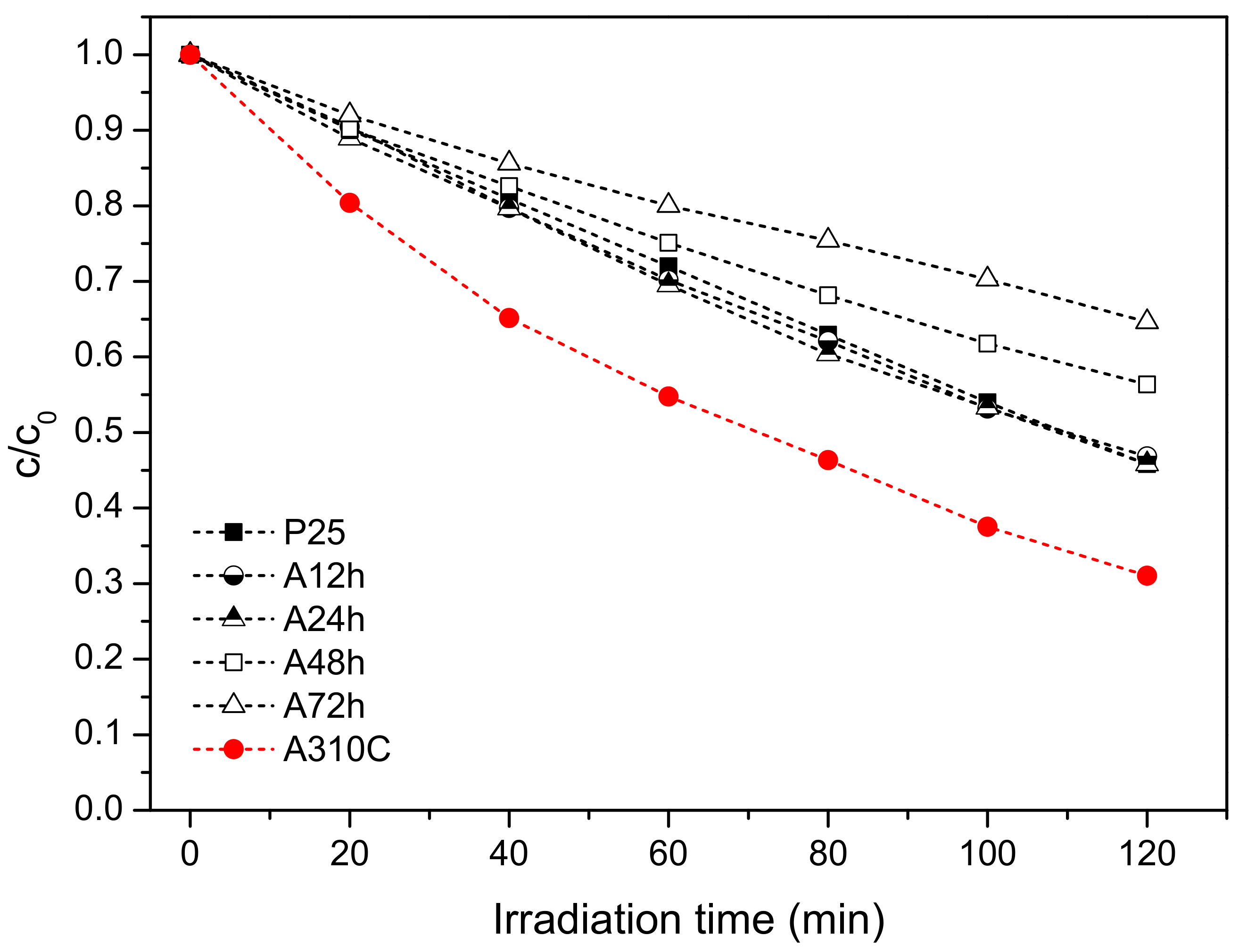
3.5. Photocatalytic Properties
3.6. Application of TiO2 NPs against Phytopathogens
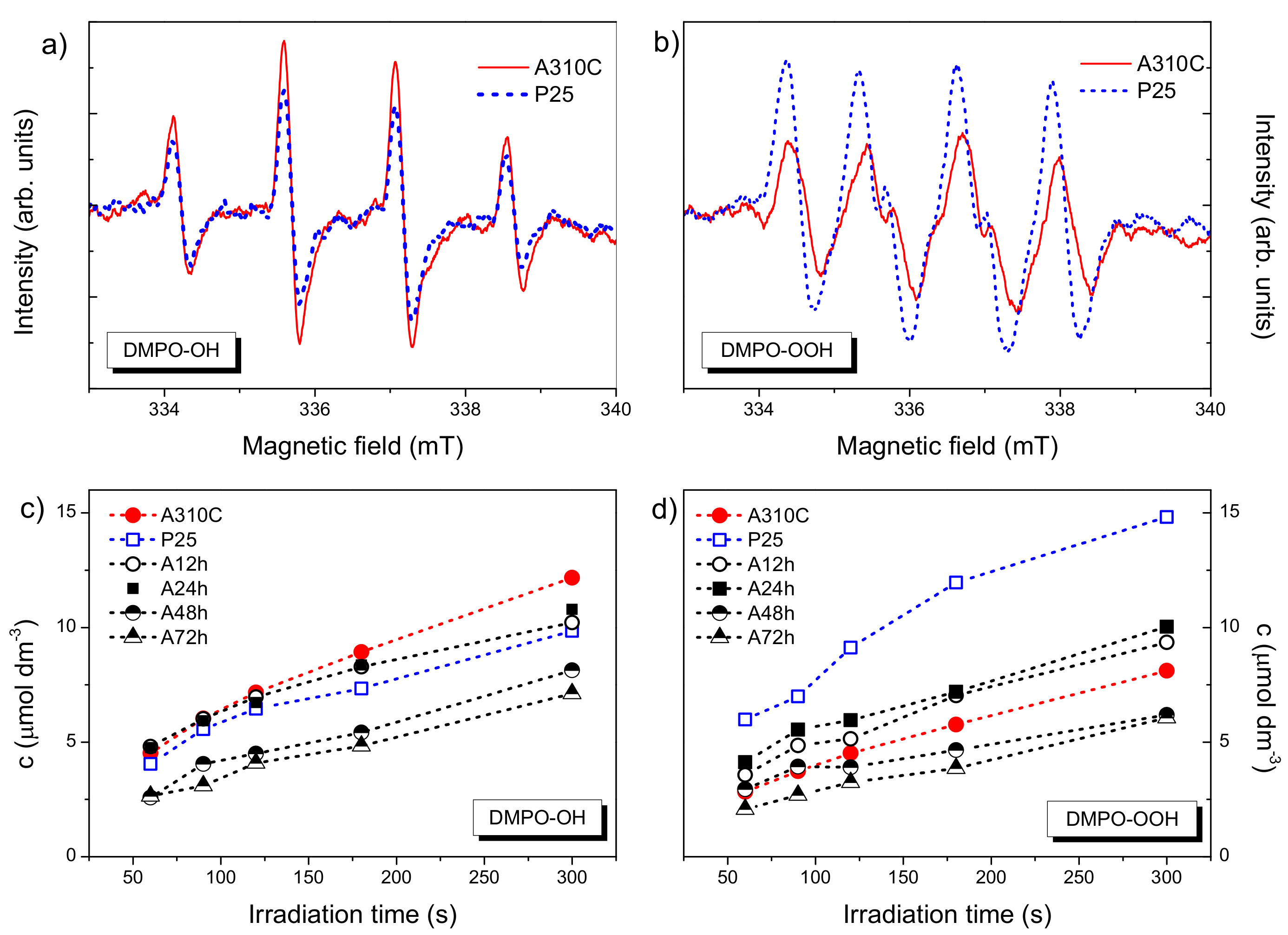
3.7. Main Reactive Oxygen Species-Formation Kinetics of OH● and O2●− Radicals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Ficke, A.; Aubertot, J.N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Sec. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Roberts, M.; Schimmelpfennig, D.; Ashley, E.; Livingston, M.; Ash, M.; Vasavada, U. The Value of Plant Disease Early-Warning Systems: A Case Study of USDA’s Soybean Rust Coordinated Framework; Economic Research Service/USDA: Department of Agriculture, Washington, DC, USA, 2006; p. 38. [Google Scholar]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. Rev. Sci. Tech. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Terashima, C.; Fujishima, A. Applications of photocatalytic titanium dioxide-based nanomaterials in sustainable agriculture. J. Photochem. Photobiol. C 2019, 40, 49–67. [Google Scholar] [CrossRef]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B 2013, 128, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, V.; Domínguez-Espíndola, R.B.; Casas-Flores, S.; Patrón-Soberano, O.A.; Camposeco-Solis, R.; Lee, S.W. Antifungal nanocomposites inspired by titanate nanotubes for complete inactivation of Botrytis cinerea isolated from tomato infection. ACS Appl. Mater. Interfaces 2016, 8, 31625–31637. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Baiju, K.V.; Shukla, S.; Biju, S.; Reddy, M.L.P.; Warrier, K.G.K. Hydrothermal processing of dye-adsorbing one-dimensional hydrogen titanate. Mater. Lett. 2009, 63, 923–926. [Google Scholar] [CrossRef]
- Lee, C.K.; Lin, K.S.; Wu, C.F.; Lyu, M.D.; Lo, C.C. Effects of synthesis temperature on the microstructures and basic dyes adsorption of titanate nanotubes. J. Hazard. Mater. 2008, 150, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Food Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sichel, C.; de Cara, M.; Tello, J.; Blanco, J.; Fernández-Ibáñez, P. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B 2007, 74, 152–160. [Google Scholar] [CrossRef]
- Yao, K.S.; Wang, D.Y.; Ho, W.Y.; Yan, J.J.; Tzeng, K.C. Photocatalytic bactericidal effect of TiO2 thin film on plant pathogens. Surf. Coat. Technol. 2007, 201, 6886–6888. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Li, Q.; Zhang, X.; Shang, J.K. Antifungal activity and mechanism of palladium-modified nitrogen-doped titanium oxide photocatalyst on agricultural pathogenic fungi Fusarium graminearum. ACS Appl. Mater. Interfaces 2013, 5, 10953–10959. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Acharya, K.; Biswas, A.; Jana, N.R. TiO2 nanoparticles co-doped with nitrogen and fluorine as visible-light-activated antifungal agents. ACS Appl. Nano Mater. 2020, 3, 2016–2025. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, M.; Liu, X.; Wang, X.; Liu, S.; Han, B.; Liu, B.; Shi, H. Copper modified Ti3+ self-doped TiO2 photocatalyst for highly efficient photodisinfection of five agricultural pathogenic fungus. Chem. Eng. J. (Amsterdam, Neth.) 2020, 387, 124171. [Google Scholar] [CrossRef]
- Liu, B.; Mu, L.; Zhang, J.; Han, X.; Shi, H. TiO2/Cu2(OH)2CO3 nanocomposite as efficient antimicrobials for inactivation of crop pathogens in agriculture. Mater. Sci. Eng. C 2020, 107, 110344. [Google Scholar] [CrossRef]
- Bonnett, R.; Brown, R.F.C.; Clark, V.M.; Sutherland, I.O.; Todd, A. Experiments towards the synthesis of corrins. Part II. The preparation and reactions of Δ-pyrroline 1-oxides. J. Chem. Soc. 1959, 2094–2102. [Google Scholar] [CrossRef]
- De Carolis, E.; Vella, A.; Vaccaro, L.; Torelli, R.; Spanu, T.; Fiori, B.; Posteraro, B.; Sanguinetti, M. Application of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. J. Infect. Dev. Countries 2014, 8, 1081–1088. [Google Scholar] [CrossRef]
- Kőrösi, L.; Prato, M.; Scarpellini, A.; Kovács, J.; Dömötör, D.; Kovács, T.; Papp, S. H2O2-assisted photocatalysis on flower-like rutile TiO2 nanostructures: Rapid dye degradation and inactivation of bacteria. Appl. Surf. Sci. 2016, 365, 171–179. [Google Scholar] [CrossRef]
- Kőrösi, L.; Bognár, B.; Horváth, M.; Schneider, G.; Kovács, J.; Scarpellini, A.; Castelli, A.; Colombo, M.; Prato, M. Hydrothermal evolution of PF-co-doped TiO2 nanoparticles and their antibacterial activity against carbapenem-resistant Klebsiella pneumoniae. Appl. Catal. B 2018, 231, 115–122. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Durães, L.; Matias, T.; Seabra, M.P.; Labrincha, J.A. Truncated tetragonal bipyramidal anatase nanocrystals formed without use of capping agents from the supercritical drying of a TiO2 sol. CrystEngComm 2016, 18, 164–176. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Specific Facets-Dominated Anatase TiO2: Fluorine-Mediated Synthesis and Photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar] [CrossRef]
- Kőrösi, L.; Bouderias, S.; Csepregi, K.; Bognár, B.; Teszlák, P.; Scarpellini, A.; Castelli, A.; Hideg, É.; Jakab, G. Nanostructured TiO2-induced photocatalytic stress enhances the antioxidant capacity and phenolic content in the leaves of Vitis vinifera on a genotype-dependent manner. J. Photochem. Photobiol. B 2019, 190, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Zhang, Y.; Hou, R.; Zhang, X.; Xue, C.; Wang, S.; Zhu, B.; Li, N.; Shao, G. Photogenerated Electron Transfer Process in Heterojunctions: In Situ Irradiation XPS. Small Methods 2020, 2000214. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Pieta, P.; Petr, A.; Kutner, W.; Dunsch, L. In situ ESR spectroscopic evidence of the spin-trapped superoxide radical, O2●−, electrochemically generated in DMSO at room temperature. Electrochim. Acta 2008, 53, 3412–3415. [Google Scholar] [CrossRef]
- Puddu, V.; Choi, H.; Dionysiouc, D.D.; Puma, G.L. TiO2 photocatalyst for indoor air remediation: Influence of crystallinity, crystal phase, and UV radiation intensity on trichloroethylene degradation. Appl. Catal. B 2010, 94, 211–218. [Google Scholar] [CrossRef]
- Lebedev, V.A.; Kozlov, D.A.; Kolesnik, I.V.; Poluboyarinov, A.S.; Becerikli, A.E.; Grünert, W.; Garshev, A.V. The amorphous phase in titania and its influence on photocatalytic properties. Appl. Catal. B 2016, 195, 39–47. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, The Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef]
- Wang, X.; Sø, L.; Su, R.; Wendt, S.; Hald, P.; Mamakhel, A.; Yang, C.; Huang, Y.; Iversen, B.B.; Besenbacher, F. The influence of crystallite size and crystallinity of anatase nanoparticles on the photo-degradation of phenol. J. Catal. 2014, 310, 100–108. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, R.; Qiao, P.; Xiao, L.; Fan, J. TiO2 nanoparticles with increased surface hydroxyl groups and their improved photocatalytic activity. Catal. Commun. 2012, 19, 96–99. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Vanneste, J.L. Fire Blight: The Disease and Its Causative Agent, Erwinia Amylovora, 1st ed.; CABI Publishing: Wallingford, UK, 2000; p. 370. [Google Scholar]
- Frutos, D. Bacterial diseases of walnut and hazelnut and genetic resources. J. Plant Pathol. 2010, 92, S79–S85. [Google Scholar]
- Galambos, A.; Zok, A.; Kuczmog, A.; Oláh, R.; Putnoky, P.; Ream, W.; Szegedi, E. Silencing Agrobacterium oncogenes in transgenic grapevine results in strain-specific crown gall resistance. Plant Cell Rep. 2013, 32, 1751–1757. [Google Scholar] [CrossRef][Green Version]
- Jones, R.K.; Benson, D.M. Diseases of Woody Ornamentals and Trees in Nurseries; APS Press: St. Paul, MN, USA, 2001; p. 482. [Google Scholar]
- Arts, I.S.; Gennaris, A.; Collet, J.F. Reducing systems protecting the bacterial cell envelope from oxidative damage. FEBS Lett. 2015, 589, 1559–1568. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.-P.; Van den Ende, W. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Trent, M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Venisse, J.-S.; Gullner, G.; Brisset, M.-N. Evidence for the Involvement of an Oxidative Stress in the Initiation of Infection of Pear by Erwinia amylovora. Plant Physiol. 2001, 125, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A. Agrobacterium infection and plant defense—Transformation success hangs by a thread. Front. Plant Sci. 2013, 4, 519. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling, J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Bouderias, S.; Teszlák, P.; Jakab, G.; Kőrösi, L. Age- and season-dependent pattern of flavonol glycosides in Cabernet Sauvignon grapevine leaves. Sci. Rep. 2020, 10, 14241. [Google Scholar] [CrossRef]
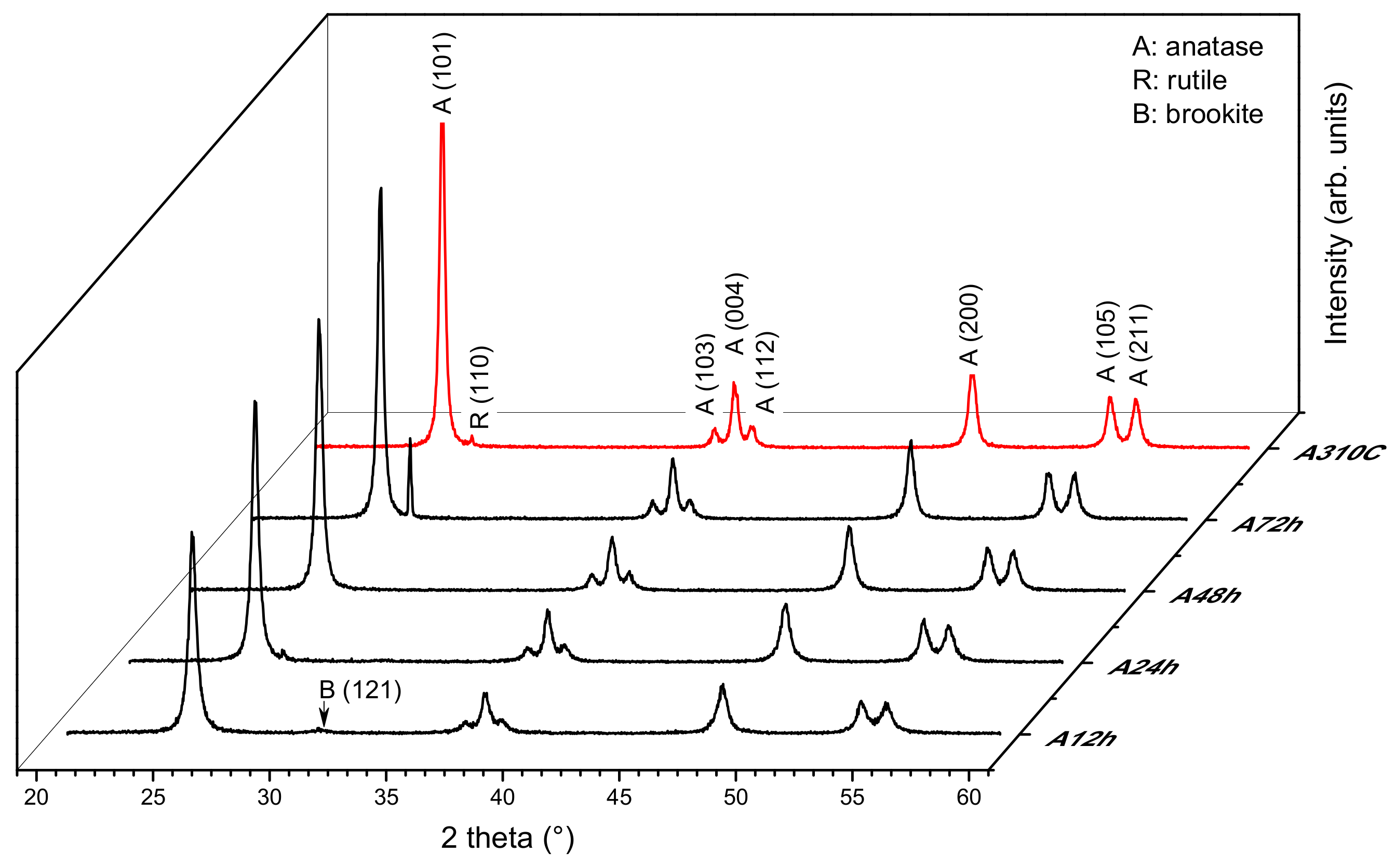
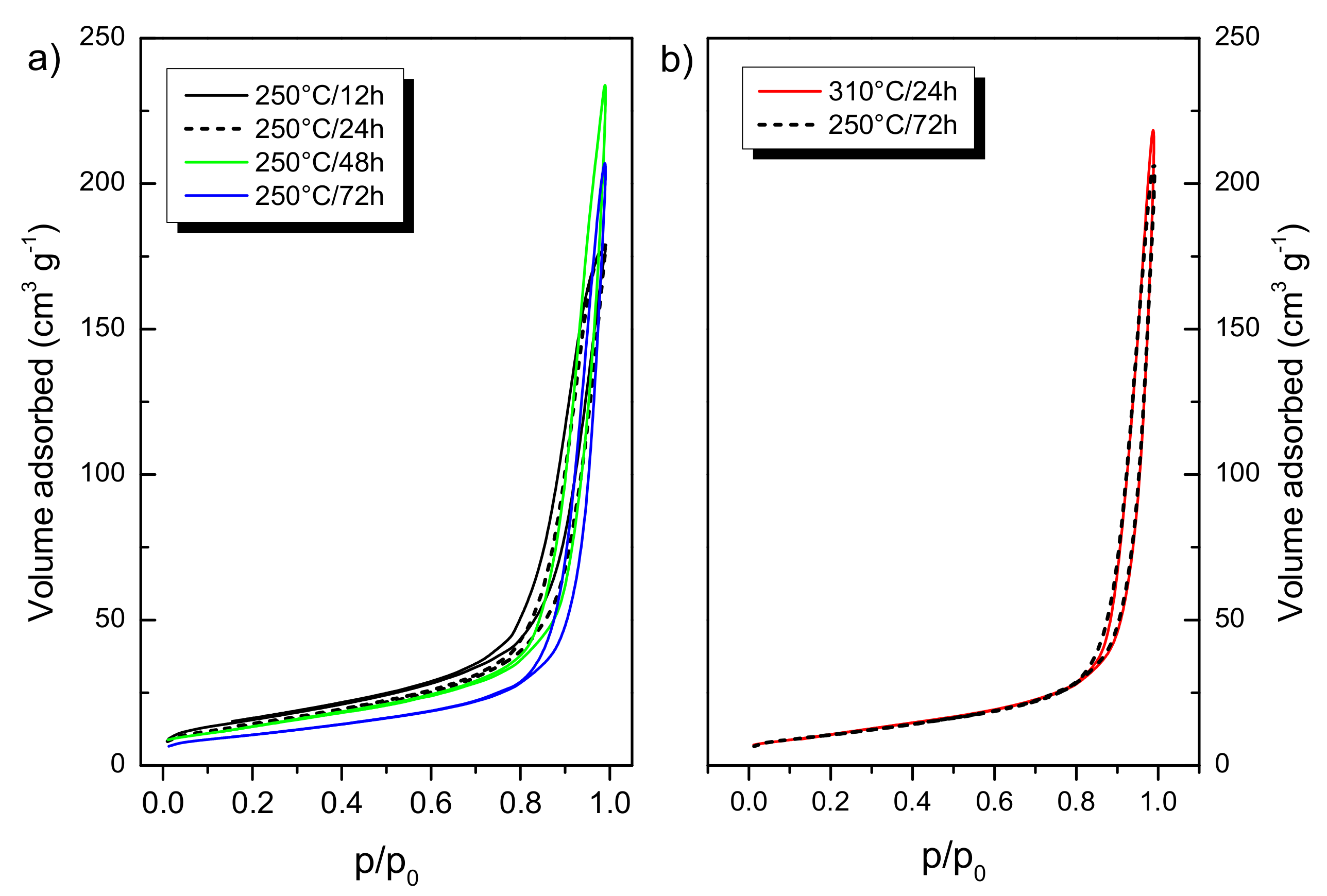

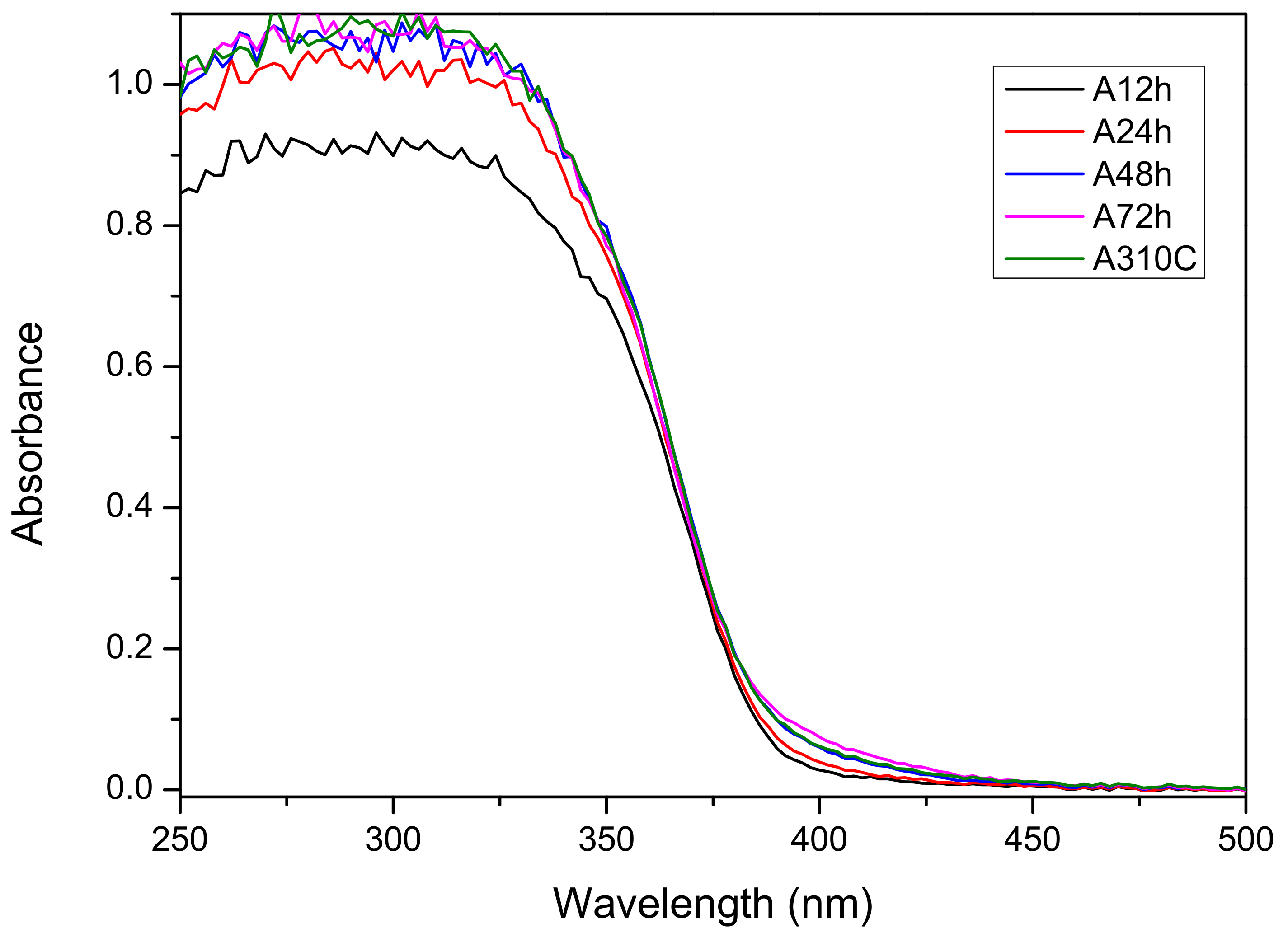
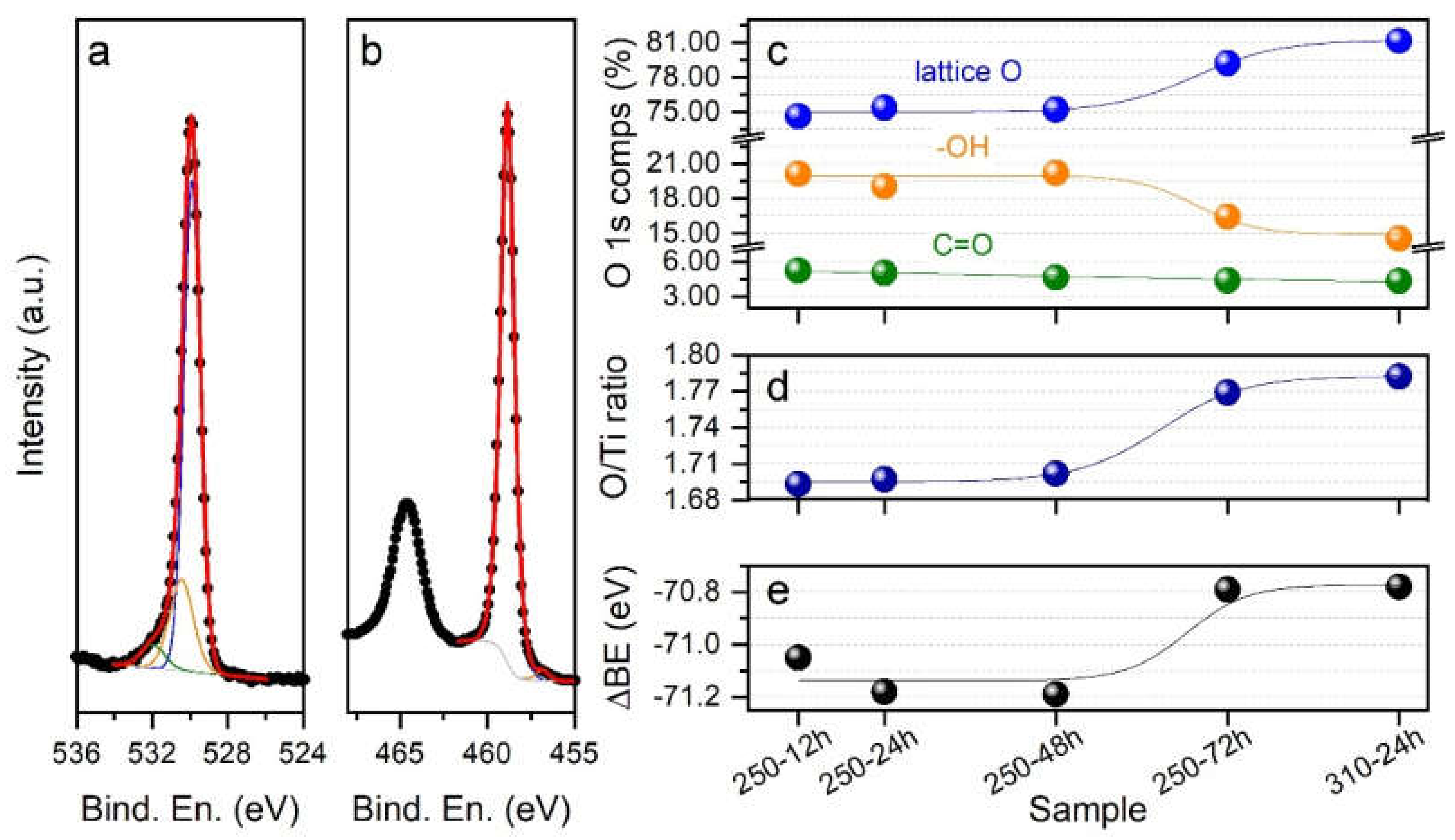

| Sample ID | Anatase (wt %) | Rutile (wt %) | Brookite (wt %) | Crystallinity (wt %) | Crystallite Size a (nm) | Specific Surface Area b (m2 g−1) |
|---|---|---|---|---|---|---|
| A12h | 75.5 | nd | trace | 75.5 | 29.7 | 56 |
| A24h | 86.1 | 0.6 | nd | 86.7 | 36.6 | 51 |
| A48h | 88.2 | nd | nd | 88.2 | 37.6 | 50 |
| A72h | 92.4 | 7.6 | nd | 99.6 | 48.7 | 38 |
| A310C | 94.1 | 0.7 | nd | 94.8 | 52.2 | 40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kőrösi, L.; Pertics, B.; Schneider, G.; Bognár, B.; Kovács, J.; Meynen, V.; Scarpellini, A.; Pasquale, L.; Prato, M. Photocatalytic Inactivation of Plant Pathogenic Bacteria Using TiO2 Nanoparticles Prepared Hydrothermally. Nanomaterials 2020, 10, 1730. https://doi.org/10.3390/nano10091730
Kőrösi L, Pertics B, Schneider G, Bognár B, Kovács J, Meynen V, Scarpellini A, Pasquale L, Prato M. Photocatalytic Inactivation of Plant Pathogenic Bacteria Using TiO2 Nanoparticles Prepared Hydrothermally. Nanomaterials. 2020; 10(9):1730. https://doi.org/10.3390/nano10091730
Chicago/Turabian StyleKőrösi, László, Botond Pertics, György Schneider, Balázs Bognár, János Kovács, Vera Meynen, Alice Scarpellini, Lea Pasquale, and Mirko Prato. 2020. "Photocatalytic Inactivation of Plant Pathogenic Bacteria Using TiO2 Nanoparticles Prepared Hydrothermally" Nanomaterials 10, no. 9: 1730. https://doi.org/10.3390/nano10091730
APA StyleKőrösi, L., Pertics, B., Schneider, G., Bognár, B., Kovács, J., Meynen, V., Scarpellini, A., Pasquale, L., & Prato, M. (2020). Photocatalytic Inactivation of Plant Pathogenic Bacteria Using TiO2 Nanoparticles Prepared Hydrothermally. Nanomaterials, 10(9), 1730. https://doi.org/10.3390/nano10091730







