Fluorescent, Prussian Blue-Based Biocompatible Nanoparticle System for Multimodal Imaging Contrast
Abstract
1. Introduction
2. Materials and Methods
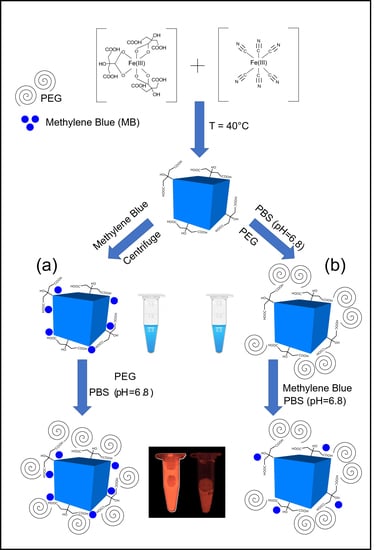
2.1. Synthesis of PBNPs
2.2. Fluorescent Labelling
- The synthesized PBNPs were labelled after centrifugation of “bare” PBNPs at 4 °C, using 21,130 rcf. Thereafter, the PEGylation process was performed and the solution was dialyzed against phosphate buffer saline solution (pH = 6.8; Ph.Eur. 8.).
- The PBNP-MB mixture was centrifuged at 21,130 rcf (Eppendorf 5424R centrifuge) at 4 °C. Then, the PBNP-MBs were PEGylated, dialyzed, and stored at 2–8 °C until further use.
2.3. PEGylation of PBNPs
2.4. DLS and Zeta Measurements
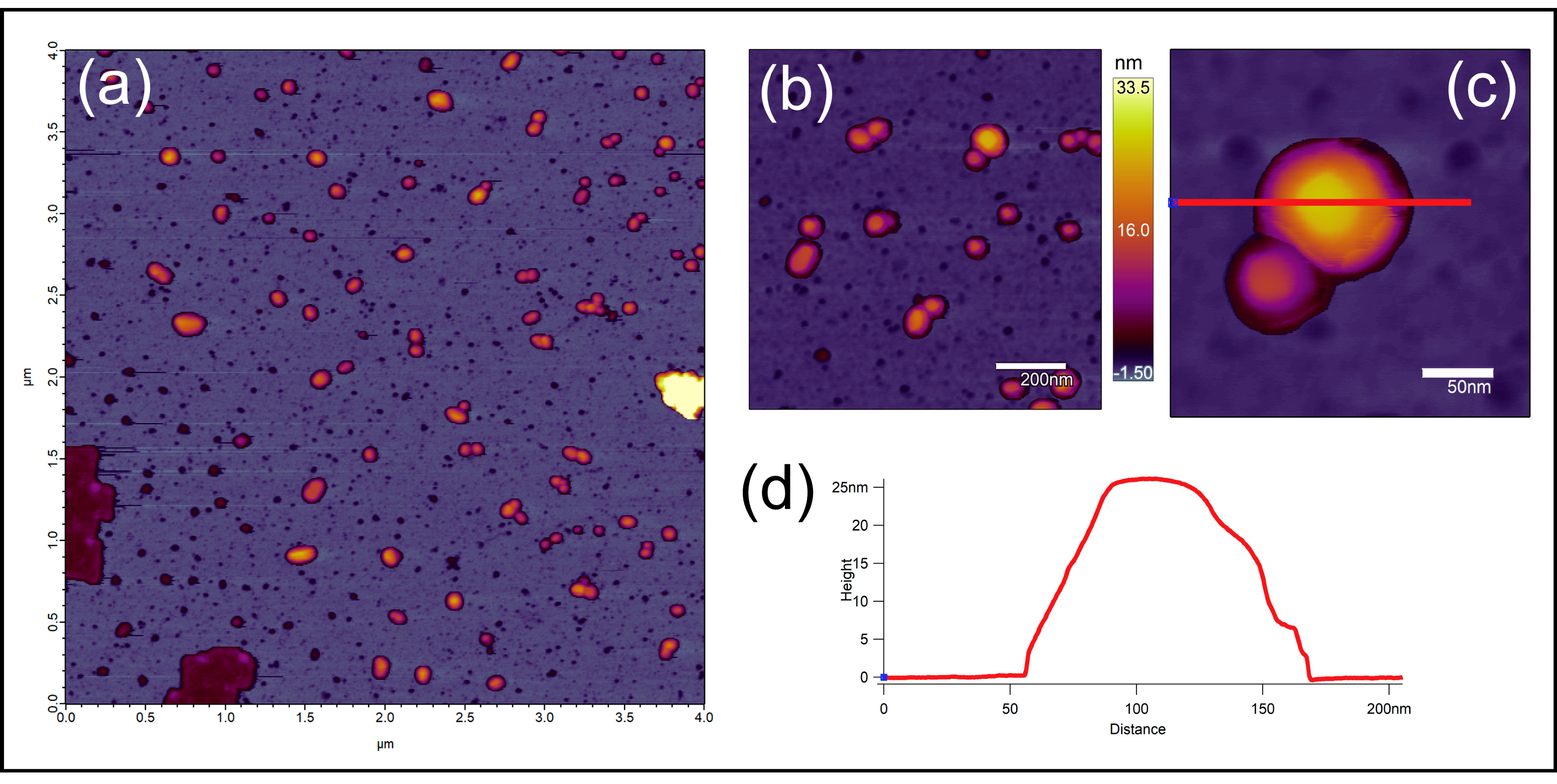
2.5. Atomic Force Microscopy (AFM)
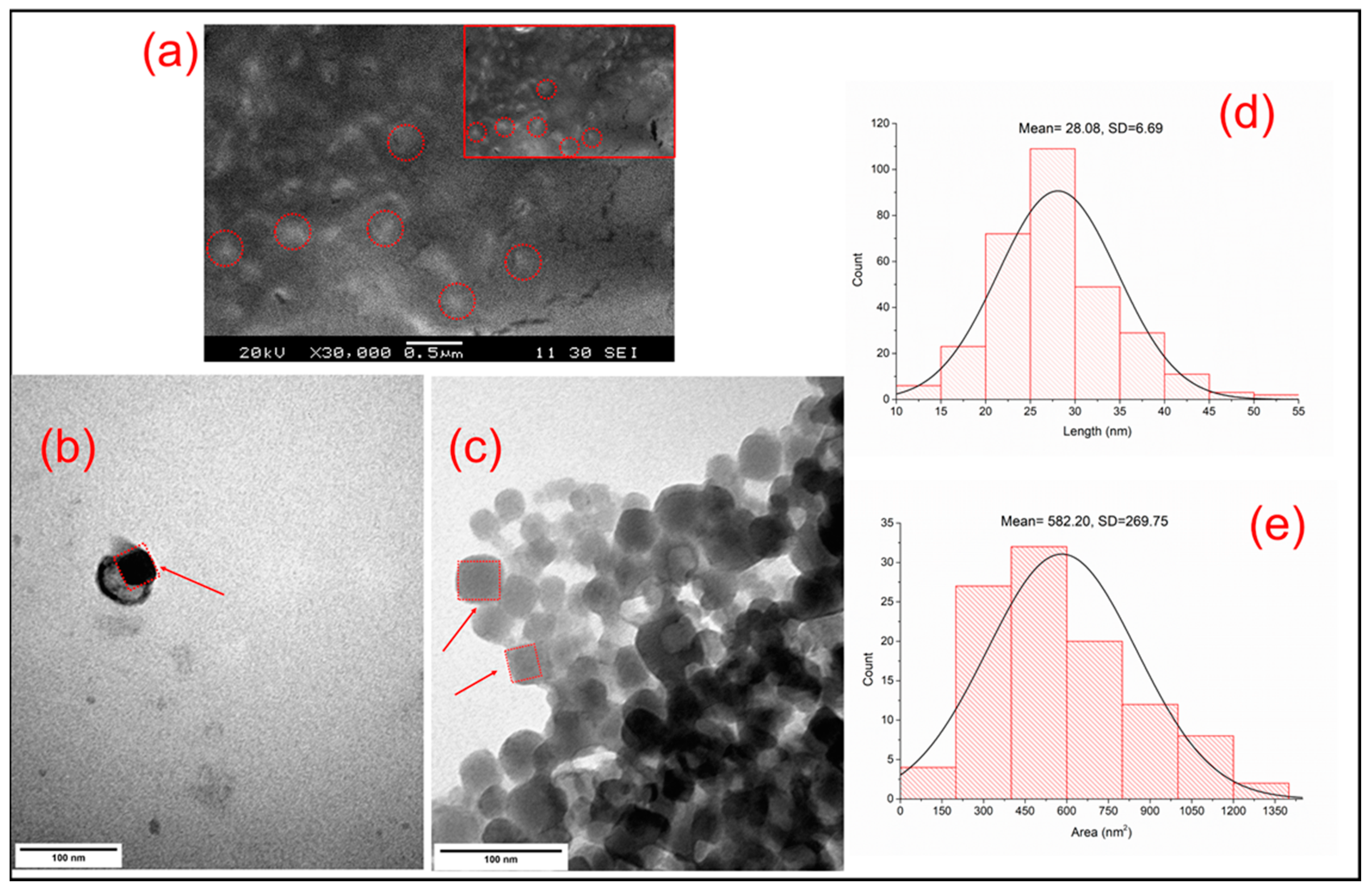
2.6. Scanning Electron Microscopy (SEM)
2.7. Transmission Electron Microscopy (TEM)
2.8. Animals
2.9. In Vivo Fluorescent Imaging
3. Results
3.1. Preformulation of PEGylated PBNPs
3.2. In Vitro Measurements
3.2.1. DLS and Zeta Potential of Fluorescently Labelled PBNPs
3.2.2. Atomic Force Microscopy (AFM)
3.2.3. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy
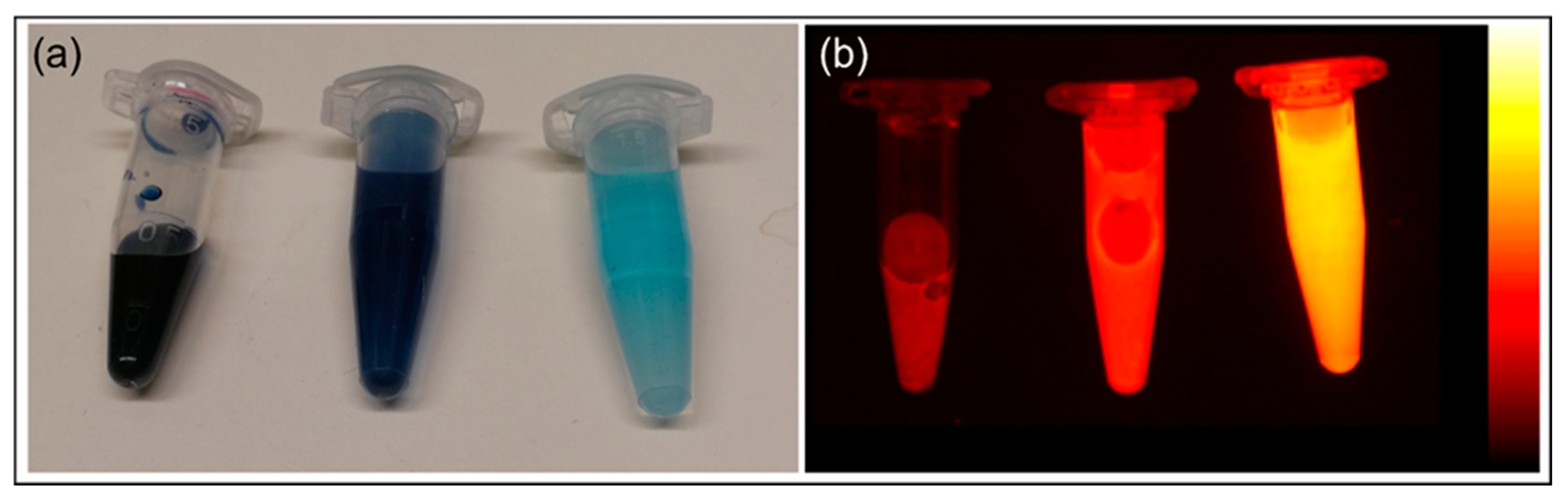
3.2.4. Measuring In Vitro Fluorescence
3.2.5. Stability Measurements of PBNP-MB@PEG Nanoparticles
3.3. In Vivo and Ex Vivo Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chance, B. Optical method. Annu. Rev. Biophys. Biophys. Chem. 1991, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Demos, S.G.; Gandour-Edwards, R.; Ramsamooj, R.; de Vere White, R. Near-infrared autofluorescence imaging for detection of cancer. J. Biomed. Opt. 2004, 9, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Van de Ven, S.M.; Elias, S.G.; Wiethoff, A.J.; Van der Voort, M.; Nielsen, T.; Brendel, B.; Van Beek, M. Diffuse optical tomography of the breast: Preliminary findings of a new prototype and comparison with magnetic resonance imaging. Eur. Radiol. 2009, 19, 1108. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Clinical implications of near-infrared fluorescence imaging in cancer. Future Oncol. 2009, 5, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Guidance for Industry, Investigators, and Reviewers. Exploratory IND Studies. 2006. Available online: https://www.fda.gov/media/72325/download (accessed on 24 August 2018).
- Xu, W.; Riikonen, J.; Nissinen, T.; Suvanto, M.; Rilla, K.; Li, B.; Lehto, V.P. Amine surface modifications and fluorescent labeling of thermally stabilized mesoporous silicon nanoparticles. J. Phys. Chem. C 2012, 116, 22307–22314. [Google Scholar] [CrossRef]
- Naeini, A.T.; Adeli, M.; Vossoughi, M. Poly (citric acid)-block-poly (ethylene glycol) copolymers—New biocompatible hybrid materials for nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 556–562. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Tang, Y.; Jiao, Z.; Xie, C.; Zhang, H.; Zhang, C. High MRI performance fluorescent mesoporous silica-coated magnetic nanoparticles for tracking neural progenitor cells in an ischemic mouse model. Nanoscale 2013, 5, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Ren, X.; Zhao, H.; Shirahata, N.; Sakka, Y.; Qin, W. Single-phased luminescent mesoporous nanoparticles for simultaneous cell imaging and anticancer drug delivery. Biomaterials 2011, 32, 7226–7233. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019; (No. WHO/MVP/EMP/IAU/2019.06); World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Wagner, M.; Suarez, E.R.; Theodoro, T.R.; Machado Filho, C.D.A.S.; Gama, M.F.M.; Tardivo, J.P.; Pinhal, M.A.S. Methylene blue photodynamic therapy in malignant melanoma decreases expression of proliferating cell nuclear antigen and heparanases. Clin. Exp. Dermatol. Exp. Dermatol. 2012, 37, 527–533. [Google Scholar] [CrossRef]
- Gupta, R.C. Handbook of Toxicology of Chemical Warfare Agents; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Szigeti, K.; Hegedűs, N.; Rácz, K.; Horváth, I.; Veres, D.S.; Szöllősi, D.; Kovács, N. Thallium labeled citrate-coated prussian blue nanoparticles as potential imaging agent. Contrast Media Mol. Imaging 2018, 2018. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Soehnlen, E.S.; Khitrin, A.; Basu, S.; Huang, S.D. Biocompatible Prussian blue nanoparticles: Preparation, stability, cytotoxicity, and potential use as an MRI contrast agent. Inorg. Chem. Commun. 2010, 13, 58–61. [Google Scholar] [CrossRef]
- Namazi, H.; Adeli, M. Dendrimers of citric acid and poly (ethylene glycol) as the new drug-delivery agents. Biomaterials 2005, 26, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Perrier, M.; Busson, M.; Massasso, G.; Long, J.; Boudousq, V.; Pouget, J.P.; Borras, M. 201 Tl+-labelled Prussian blue nanoparticles as contrast agents for SPECT scintigraphy. Nanoscale 2014, 6, 13425–13429. [Google Scholar] [CrossRef] [PubMed]
- Máthé, D.; Szigeti, K. Prussian Blue Based Nanoparticle as Multimodal Imaging Contrast Material. U.S. Patent Application No. 13/985,254, 15 February 2014. [Google Scholar]
- Cheng, L.; Gong, H.; Zhu, W.; Liu, J.; Wang, X.; Liu, G.; Liu, Z. PEGylated Prussian blue nanocubes as a theranostic agent for simultaneous cancer imaging and photothermal therapy. Biomaterials 2014, 35, 9844–9852. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Radiogardase Prussian Blue Insoluble Capsules. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021626s007lbl.pdf (accessed on 25 August 2008).
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Strauss, H.W. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Soehnlen, E.S.; Hao, J.; Griswold, M.; Flask, C.; Fan, X.; Huang, S.D. Dual purpose Prussian blue nanoparticles for cellular imaging and drug delivery: A new generation of T 1-weighted MRI contrast and small molecule delivery agents. J. Mater. Chem. 2010, 20, 5251–5259. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Guo, Z. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef]
- Penet, M.F.; Mikhaylova, M.; Li, C.; Krishnamachary, B.; Glunde, K.; Pathak, A.P.; Bhujwalla, Z.M. Applications of molecular MRI and optical imaging in cancer. Future Med. Chem. 2010, 2, 975–988. [Google Scholar] [CrossRef]
- National Institute of Biomedical Imaging and Engineering. 2016 Fact Sheet: Optical Imaging. Available online: https://www.nibib.nih.gov/sites/default/files/Optical%20Imaging%202016-english_FINAL.pdf (accessed on 20 July 2016).
- Dumont, M.F.; Yadavilli, S.; Sze, R.W.; Nazarian, J.; Fernandes, R. Manganese-containing Prussian blue nanoparticles for imaging of pediatric brain tumors. Int. J. Nanomed. 2014, 9, 2581. [Google Scholar] [CrossRef]
- Tu, C.; Nagao, R.; Louie, A.Y. Multimodal magnetic-resonance/optical-imaging contrast agent sensitive to NADH. Angew. Chem. Int. Ed. 2009, 48, 6547–6551. [Google Scholar] [CrossRef]
- Shcherbo, D.; Merzlyak, E.M.; Chepurnykh, T.V.; Fradkov, A.F.; Ermakova, G.V.; Solovieva, E.A.; Chudakov, D.M. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods 2007, 4, 741–746. [Google Scholar] [CrossRef]
- Kaplan, J.A. Essentials of Cardiac Anesthesia E-Book: A Volume in Essentials of Anesthesia and Critical Care; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Mondal, S.B.; Gao, S.; Zhu, N.; Liang, R.; Gruev, V.; Achilefu, S. Real-time fluorescence image-guided oncologic surgery. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2014; Volume 124, pp. 171–211. [Google Scholar] [CrossRef]
- Canard, J.M.; Letard, J.C.; Palazzo, L.; Penman, I.D.; Lennon, A.M. Gastrointestinal Endoscopy in Practice E-Book: Expert Consult: Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Shanks, G.D. Control and elimination of plasmodium vivax. In Advances in Parasitology; Academic Press: Cambridge, MA, USA, 2012; Volume 80, pp. 301–341. [Google Scholar] [CrossRef]
- Deng, T.; Li, J.S.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Preparation of near-IR fluorescent nanoparticles for fluorescence-anisotropy-based immunoagglutination assay in whole blood. Adv. Funct. Mater. 2006, 16, 2147–2155. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, D.; Du, J. Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 2014, 120, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, M.; Torrente-Rodríguez, R.M.; Kozell, A.; Liao, W.C.; Cecconello, A.; Campuzano, S.; Willner, I. Mimicking peroxidase activities with prussian blue nanoparticles and their cyanometalate structural analogues. Nano Lett. 2017, 17, 4958–4963. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, L.; Wang, Q.; Wu, M.; Xu, B.; Liu, X.; Liu, J. Toxicological evaluation of Prussian blue nanoparticles after short exposure of mice. Hum. Exp. Toxicol. 2016, 35, 1123–1132. [Google Scholar] [CrossRef]
- Zhang, X.; Rojas, J.C.; Gonzalez-Lima, F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox. Res. 2006, 9, 47–57. [Google Scholar] [CrossRef]
- He, X.; Wu, X.; Wang, K.; Shi, B.; Hai, L. Methylene blue-encapsulated phosphonate-terminated silica nanoparticles for simultaneous in vivo imaging and photodynamic therapy. Biomaterials 2009, 30, 5601–5609. [Google Scholar] [CrossRef]
- Seong, D.Y.; Kim, Y.J. Enhanced photodynamic therapy efficacy of methylene blue-loaded calcium phosphate nanoparticles. J. Photochem. Photobiol. B Biol. 2015, 146, 34–43. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.; Wang, X.; Zha, Z. Multifunctional phase-change hollow mesoporous Prussian blue nanoparticles as a NIR light responsive drug co-delivery system to overcome cancer therapeutic resistance. J. Mater. Chem. B 2017, 5, 7051–7058. [Google Scholar] [CrossRef]
- Jolivet, J.P.; Gzara, M.; Mazieres, J.; Lefebvre, J. Physicochemical study of aggregation in silver colloids. J. Colloid Interface Sci. 1985, 107, 429–441. [Google Scholar] [CrossRef]
- Siiman, O.; Bumm, L.A.; Callaghan, R.; Blatchford, C.G.; Kerker, M. Surface-enhanced Raman scattering by citrate on colloidal silver. J. Phys. Chem. 1983, 87, 1014–1023. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Plasma citrate homeostasis: How it is regulated; and Its physiological and clinical implications. An important, but neglected, relationship in medicine. HSOA J. Hum. Endocrinol. 2016, 1. [Google Scholar] [CrossRef]
- Granchi, D.; Baldini, N.; Ulivieri, F.M.; Caudarella, R. Role of citrate in pathophysiology and medical management of bone diseases. Nutrients 2019, 11, 2576. [Google Scholar] [CrossRef]
- Harris, D.C.; Bertolucci, M.D. Symmetry and Spectroscopy: An Introduction to Vibrational and Electronic Spectroscopy; Courier Corporation: Mineola, NY, USA, 1989. [Google Scholar]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Nelson Education: Scarborough, ON, Canada, 2013. [Google Scholar]
- Christian, G.D. Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Diao, S.; Blackburn, J.L.; Hong, G.; Antaris, A.L.; Chang, J.; Wu, J.Z.; Dai, H. Fluorescence imaging in vivo at wavelengths beyond 1500 nm. Angew. Chem. 2015, 127, 14971–14975. [Google Scholar] [CrossRef]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Wu, J.Z. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Long, J.; Guari, Y.; Guérin, C.; Larionova, J. Prussian blue type nanoparticles for biomedical applications. Dalton Trans. 2016, 45, 17581–17587. [Google Scholar] [CrossRef]
- Timchalk, C.; Creim, J.A.; Sukwarotwat, V.; Wiacek, R.; Addleman, R.S.; Fryxell, G.E.; Yantasee, W. In vitro and in vivo evaluation of a novel ferrocyanide functionalized nanopourous silica decorporation agent for cesium (Cs) in rats. Health Phys. 2010, 99, 420. [Google Scholar] [CrossRef]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef]
- Brady, C.E., III; DiPalma, J.A.; Morawski, S.G.; Santa Ana, C.A.; Fordtran, J.S. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology 1986, 90, 1914–1918. [Google Scholar] [CrossRef]
- Baumann, A.; Piel, I.; Hucke, F.; Sandmann, S.; Hetzel, T.; Schwarz, T. Pharmacokinetics, excretion, distribution, and metabolism of 60-kDa polyethylene glycol used in BAY 94-9027 in rats and its value for human prediction. Eur. J. Pharm. Sci. 2019, 130, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Perrier, M.; Gallud, A.; Ayadi, A.; Kennouche, S.; Porredon, C.; Gary-Bobo, M.; Basile, I. Investigation of cyano-bridged coordination nanoparticles Gd 3+/[Fe (CN) 6] 3−/d-mannitol as T 1-weighted MRI contrast agents. Nanoscale 2015, 7, 11899–11903. [Google Scholar] [CrossRef] [PubMed]
- Souris, J.S.; Lee, C.H.; Cheng, S.H.; Chen, C.T.; Yang, C.S.; Ja-an, A.H.; Lo, L.W. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials 2010, 31, 5564–5574. [Google Scholar] [CrossRef]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef]
- Liang, X.; Deng, Z.; Jing, L.; Li, X.; Dai, Z.; Li, C.; Huang, M. Prussian blue nanoparticles operate as a contrast agent for enhanced photoacoustic imaging. Chem. Commun. 2013, 49, 11029–11031. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forgách, L.; Hegedűs, N.; Horváth, I.; Kiss, B.; Kovács, N.; Varga, Z.; Jakab, G.; Kovács, T.; Padmanabhan, P.; Szigeti, K.; et al. Fluorescent, Prussian Blue-Based Biocompatible Nanoparticle System for Multimodal Imaging Contrast. Nanomaterials 2020, 10, 1732. https://doi.org/10.3390/nano10091732
Forgách L, Hegedűs N, Horváth I, Kiss B, Kovács N, Varga Z, Jakab G, Kovács T, Padmanabhan P, Szigeti K, et al. Fluorescent, Prussian Blue-Based Biocompatible Nanoparticle System for Multimodal Imaging Contrast. Nanomaterials. 2020; 10(9):1732. https://doi.org/10.3390/nano10091732
Chicago/Turabian StyleForgách, László, Nikolett Hegedűs, Ildikó Horváth, Bálint Kiss, Noémi Kovács, Zoltán Varga, Géza Jakab, Tibor Kovács, Parasuraman Padmanabhan, Krisztián Szigeti, and et al. 2020. "Fluorescent, Prussian Blue-Based Biocompatible Nanoparticle System for Multimodal Imaging Contrast" Nanomaterials 10, no. 9: 1732. https://doi.org/10.3390/nano10091732
APA StyleForgách, L., Hegedűs, N., Horváth, I., Kiss, B., Kovács, N., Varga, Z., Jakab, G., Kovács, T., Padmanabhan, P., Szigeti, K., & Máthé, D. (2020). Fluorescent, Prussian Blue-Based Biocompatible Nanoparticle System for Multimodal Imaging Contrast. Nanomaterials, 10(9), 1732. https://doi.org/10.3390/nano10091732