Effects of Constant Magnetic Field to the Proliferation Rate of Human Fibroblasts Grown onto Different Substrates: Tissue Culture Polystyrene, Polyacrylamide Hydrogel and Ferrogels γ-Fe2O3 Magnetic Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Characterization of Magnetic Matrix
2.2. Iron Oxide Nanoparticles for the Embedding in Ferrogels
2.3. Synthesis of Ferrogels
2.4. Cell Proliferation Assay
2.5. Design of Experiments with Cell Cultures
3. Results
3.1. Properties of Nanoparticles and Ferrogels
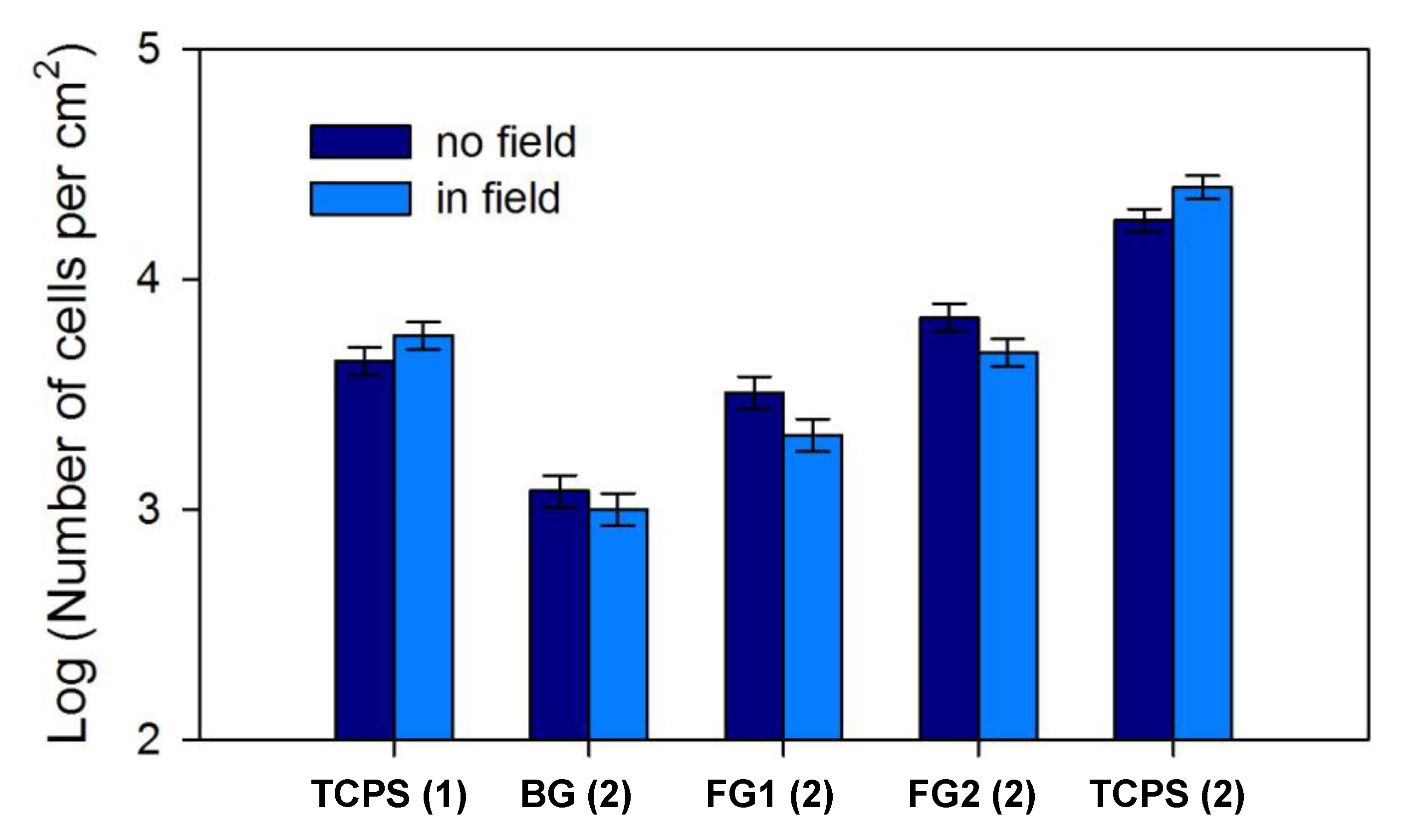
3.2. Cells Proliferation on TCPS and Ferrogells in a Magnetic Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Maoa, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Blyakhman, F.A.; Sokolov, S.Y.; Safronov, A.P.; Dinislamova, O.A.; Shklyar, T.F.; Zubarev, A.Y.; Kurlyandskaya, G.V. Ferrogels ultrasonography for biomedical applications. Sensors 2019, 19, 3959. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Roco, C.; Délérisa, A.; Spoerri, P.; Cezar, C.; Weaver, J.; Vandenburgh, H.; Mooney, D. Improved magnetic regulation of delivery profiles from ferrogels. Biomaterials 2018, 161, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Blyakhman, F.; Buznikov, N.; Sklyar, T.; Safronov, A.; Golubeva, E.; Svalov, A.; Sokolov, S.; Melnikov, G.; Orue, I.; Kurlyandskaya, G. Mechanical, electrical and magnetic properties of ferrogels with embedded iron oxide nanoparticles obtained by laser target evaporation: Focus on multifunctional biosensor applications. Sensors 2018, 18, 872. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Makarova, E.B.; Fadeyev, F.A.; Lugovets, D.V.; Safronov, A.P.; Shabadrov, P.A.; Shklyar, T.F.; Melnikov, G.Y.; Orue, I.; Kurlyandskaya, G.V. The contribution of magnetic nanoparticles to ferrogel biophysical properties. Nanomaterials 2019, 9, 232. [Google Scholar] [CrossRef]
- Safronov, A.P.; Beketov, I.V.; Komogortsev, S.V.; Kurlyandskaya, G.V.; Medvedev, A.I.; Leiman, D.V.; Larranaga, A.; Bhagat, S.M. Spherical magnetic nanoparticles fabricated by laser target evaporation. AIP Adv. 2013, 3, 052135. [Google Scholar] [CrossRef]
- Darton, N.J.; Ionescu, A.; Llandro, J. Magnetic Nanoparticles in Biosensing and Medicine; Cambridge University Press: Cambridge, UK, 2019; p. 279. [Google Scholar]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Novoselova, J.P.; Safronov, A.P.; Samatov, O.M.; Beketov, I.V.; Khurshid, H.; Nemati, Z.; Srikanth, H.; Denisova, T.P.; Andrade, R.; Kurlyandskaya, G.V. Laser target evaporation Fe2O3 Nanoparticles for water-based ferrofluids for biomedical applications. IEEE Trans. Magn. 2014, 50, 4600504. [Google Scholar] [CrossRef]
- Coïsson, M.; Barrera, G.; Appino, C.; Celegato, F.; Martino, L.; Safronov, A.P.; Kurlyandskaya, G.V.; Tiberto, P. Specific loss power measurements by calorimetric and thermal methods on γ-Fe2O3 nanoparticles for magnetic hyperthermia. J. Magn. Magn. Mater. 2019, 473, 403–409. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Novoselova, I.P.; Schupletsova, V.V.; Andrade, R.; Dunec, N.A.; Litvinova, L.S.; Safronov, A.P.; Yurova, K.A.; Kulesh, N.A.; Dzyuman, A.N.; et al. Nanoparticles for magnetic biosensing systems. J. Magn. Magn. Mater. 2017, 431, 249–254. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38–42. [Google Scholar] [CrossRef]
- Glaser, R. Biophysics; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Rusakov, V.; Raikher, Y. Magnetorelaxometry in the presence of a DC bias field of ferromagnetic nanoparticles bearing a viscoelastic corona. Sensors 2018, 18, 1661. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Gulisano, M.; Peruzzi, B.; Sgambati, E.; Gheri, G.; GheriBryk, S.; Vannucchi, S.; Polli, G.; Ruggiero, M. Effects of 0.2 T static magnetic field on human skin fibroblasts. Cancer Detect. Prev. 2003, 27, 327–332. [Google Scholar] [CrossRef]
- Lew, W.-Z.; Huang, Y.-C.; Huang, K.-Y.; Lin, C.-T.; Tsai, M.-T.; Huang, H.-M. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J. Tissue Eng. Regen. Med. 2018, 12, 19–29. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Chen, L.; Jiang, J.; Zhou, X.; Wang, M.; Fan, Y. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2029–2040. [Google Scholar] [CrossRef]
- Stolfa, S.; Skorvánek, M.; Stolfa, P.; Rosocha, J.; Vasko, G.; Sabo, J. Effects of static magnetic field and pulsed electromagnetic field on viability of human chondrocytes in vitro. Physiol. Res. 2007, 56 (Suppl. S1), S45–S49. [Google Scholar]
- Sullivan, K.; Balin, A.K.; Allen, R.G. Effects of static magnetic fields on the growth of various types of human cells. Bioelectromagnetics 2011, 32, 140–147. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, B.; Deng, J.; Freed, D.H.; Arora, R.C.; Tian, G. Inhibition of viability, proliferation, cytokines secretion, surface antigen expression, and adipogenic and osteogenic differentiation of adipose-derived stem cells by seven-day exposure to 0.5 T static magnetic fields. Stem Cells Int. 2016, 2016, 7168175. [Google Scholar] [CrossRef]
- Zafari, J.; Jouni, J.F.; Abdolmaleki, P.; Jalali, A.; Khodayar, M.J. Investigation on the effect of static magnetic field up to 30 mT on viability percent, proliferation rate and IC50 of HeLa and fibroblast cells. Electromagn. Biol. Med. 2015, 34, 216–220. [Google Scholar] [CrossRef]
- Raylman, R.R.; Clavo, A.C.; Wahl, R.L. Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics 1996, 17, 358–363. [Google Scholar] [CrossRef]
- Wiskirchen, J.; Grönewäller, E.; Heinzelmann, F.; Kehlbach, R.; Rodegerdts, E.; Wittau, M.; Rodemann, H.P.; Claussen, C.D.; Duda, S.H. Human fetal lung fibroblasts: In vitro study of repetitive magnetic field exposure at 0.2, 1.0, and 1.5 T. Radiology 2000, 215, 858–862. [Google Scholar] [CrossRef]
- Short, W.O.; Goodwill, L.; Taylor, C.W.; Job, C.; Arthur, M.E.; Cress, A.E. Alteration of human tumor cell adhesion by high-strength static magnetic fields. Investig. Radiol. 1992, 27, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Fang, X.; Sun, Y.; Bakhtina, A.; Ramakrishnan, A.; Sokolov, J.; Ulman, A.; Rafailovich, M. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small 2006, 2, 766–773. [Google Scholar] [CrossRef] [PubMed]
- O’Handley, R.C. Modern Magnetic Materials; John Wiley & Sons: New York, NY, USA, 1972. [Google Scholar]
- Terzian, T.V.; Shcherbinin, S.V.; Beketov, I.V.; Fernandez Armas, S.; Marcano Prieto, L.; Safronov, A.P.; Andrey, V.; Svalov, A.V.; Kurlyandskaya, G.V. Scanning electron microscopy for structural evaluation of metallic nanoparticles/polymer composites designed for high frequency applications. J. Int. Sci. Publ. Mater. Methods Technol. 2017, 11, 151–167. [Google Scholar]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Tscharnuter, W.W. Encyclopedia of Analytical Chemistry; John Wiley & Sons: New York, NY, USA, 2001; p. 5469. [Google Scholar]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: New York, NY, USA, 2010; p. 628. [Google Scholar]
- Liu, T.-Y.; Chan, T.-Y.; Wang, K.-S.; Tsou, H.-M. Influence of magnetic nanoparticle arrangement in ferrogels for tunable biomolecule diffusion. RSC Adv. 2015, 5, 90098–90102. [Google Scholar] [CrossRef]
- Denisova, T.P.; Simonova, E.V.; Kokorina, L.A.; Maximova, E.N.; Safronov, A.P.; Rommel, M.V.; Kurlyandskaya, G.V. Changes in morphotype in the population of E.coli in the presence of metal containing nanoparticles. J. Phys. Conf. Ser. 2019, 1389, 012074. [Google Scholar] [CrossRef]
- Romeo, S.; Sannino, A.; Scarfì, M.R.; Massa, R.; d’Angelo, R.; Zeni, O. Lack of effects on key cellular parameters of MRC-5 human lung fibroblasts exposed to 370 mT static magnetic field. Sci. Rep. 2016, 6, 19398. [Google Scholar] [CrossRef]
- Van Huizen, A.V.; Morton, J.M.; Kinsey, L.J.; Von Kannon, D.G.; Saad, M.A.; Birkholz, T.R.; Czajka, J.M.; Cyrus, J.; Barnes, F.S.; Beane, W.S. Weak magnetic fields alter stem cell-mediated growth. Sci. Adv. 2019, 5, 7201. [Google Scholar] [CrossRef]
- Shao, Y.; Fu, J. Integrated micro/nanoengineered functional biomaterials for cell mechanics and mechanobiology: A Materials Perspective. Adv. Mater. 2014, 26, 1494–1533. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Makarova, E.B.; Shabadrov, P.A.; Fadeyev, F.A.; Shklyar, T.F.; Safronov, A.P.; Komogortsev, S.V.; Kurlyandskaya, G.V. Magnetic nanoparticles as a strong contributor to the biocompatibility of ferrogels. Phys. Metals Metallogr. 2020, 121, 299–304. [Google Scholar] [CrossRef]
- Fievet, P. Donnan Potential. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Discher, A.; Janmey, P.; Wang, Y. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Cretu, A.; Castagnino, P.; Assoian, R. Studying the effects of matrix stiffness on cellular function using acrylamide-based hydrogels. J. Vis. Exp. 2010, 10, e2089. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering-Cells and Biomaterial; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Trappmann, A.; Gautrot, J.; Connelly, J.; Strange, D.; Li, Y.; Oyen, M.; Cohen Stuart, M.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef]
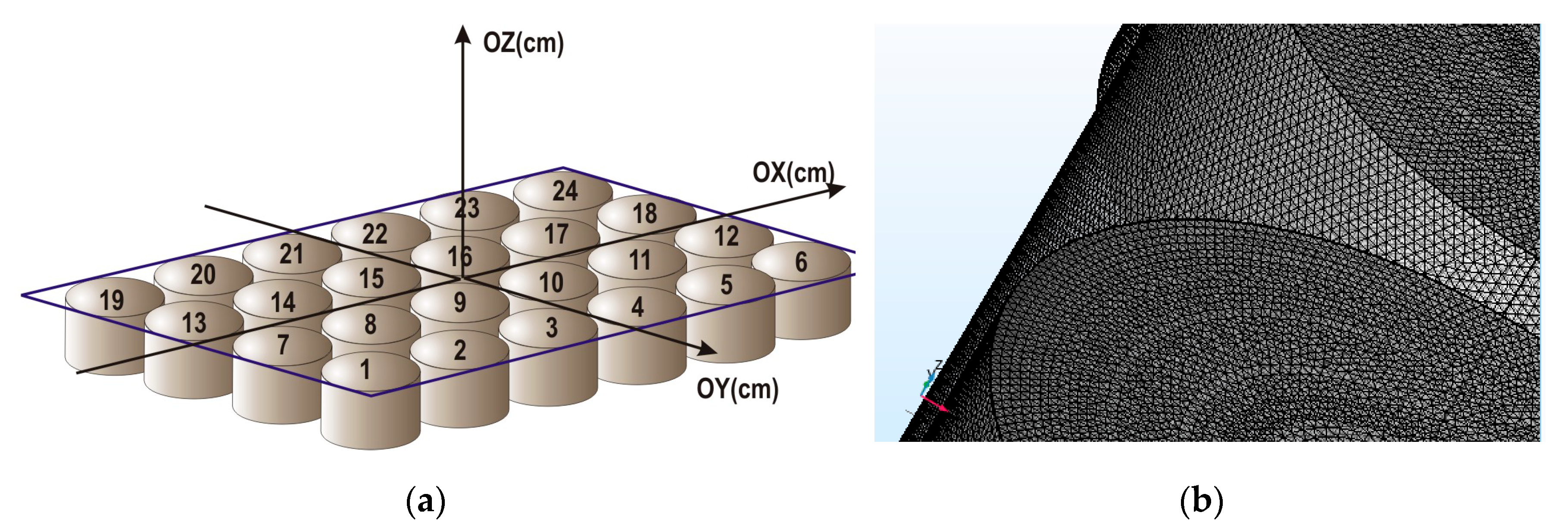
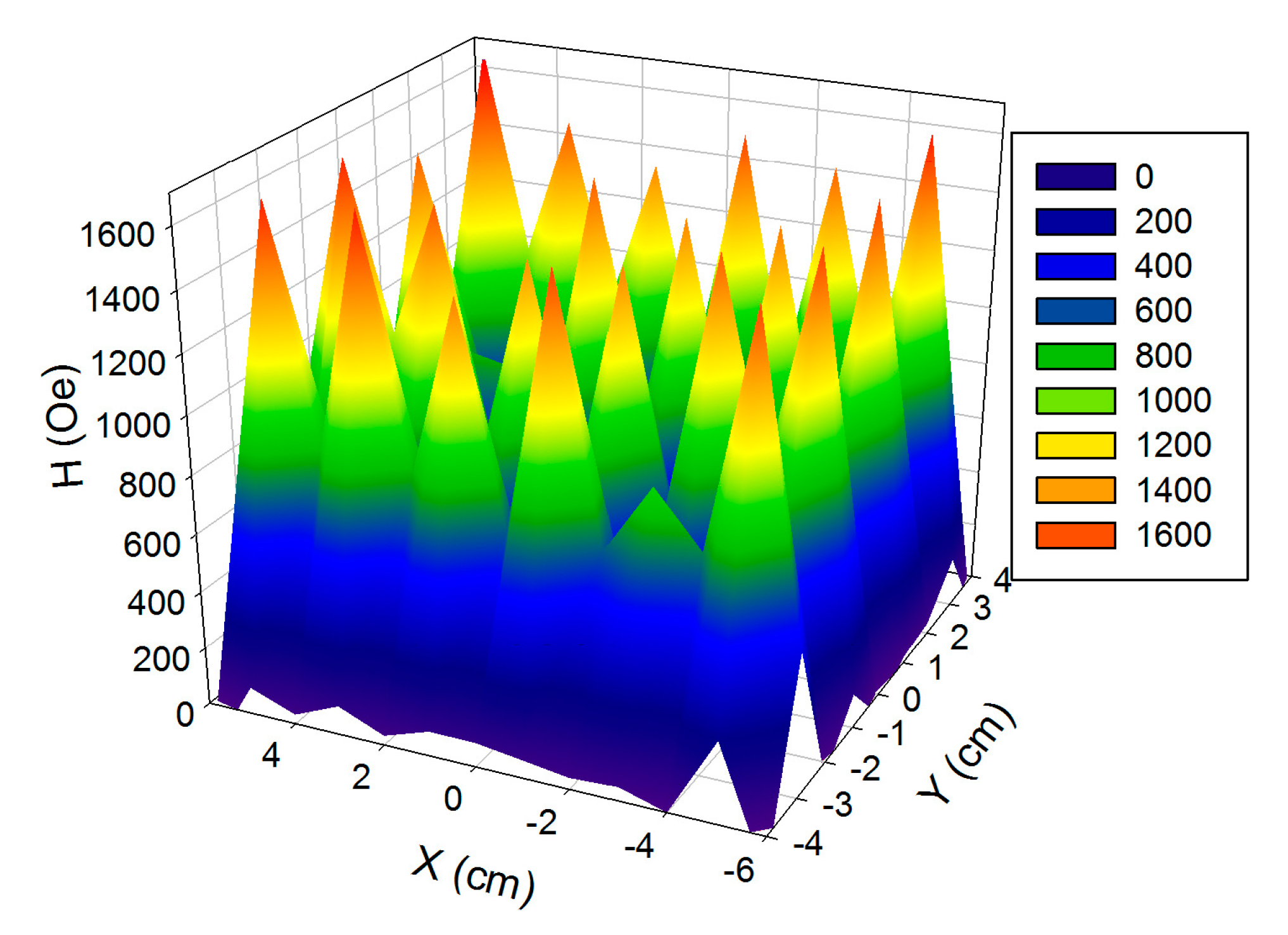


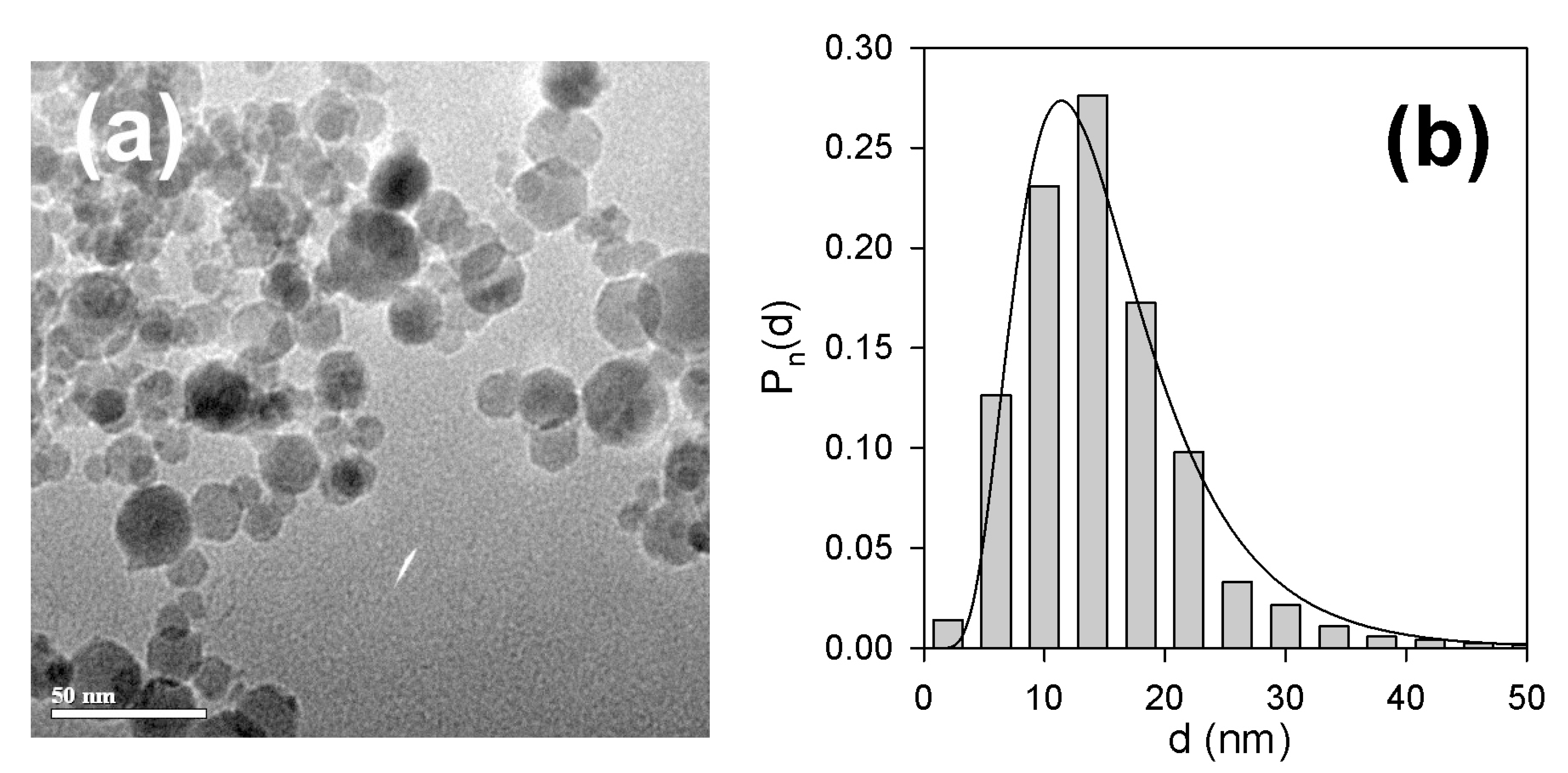
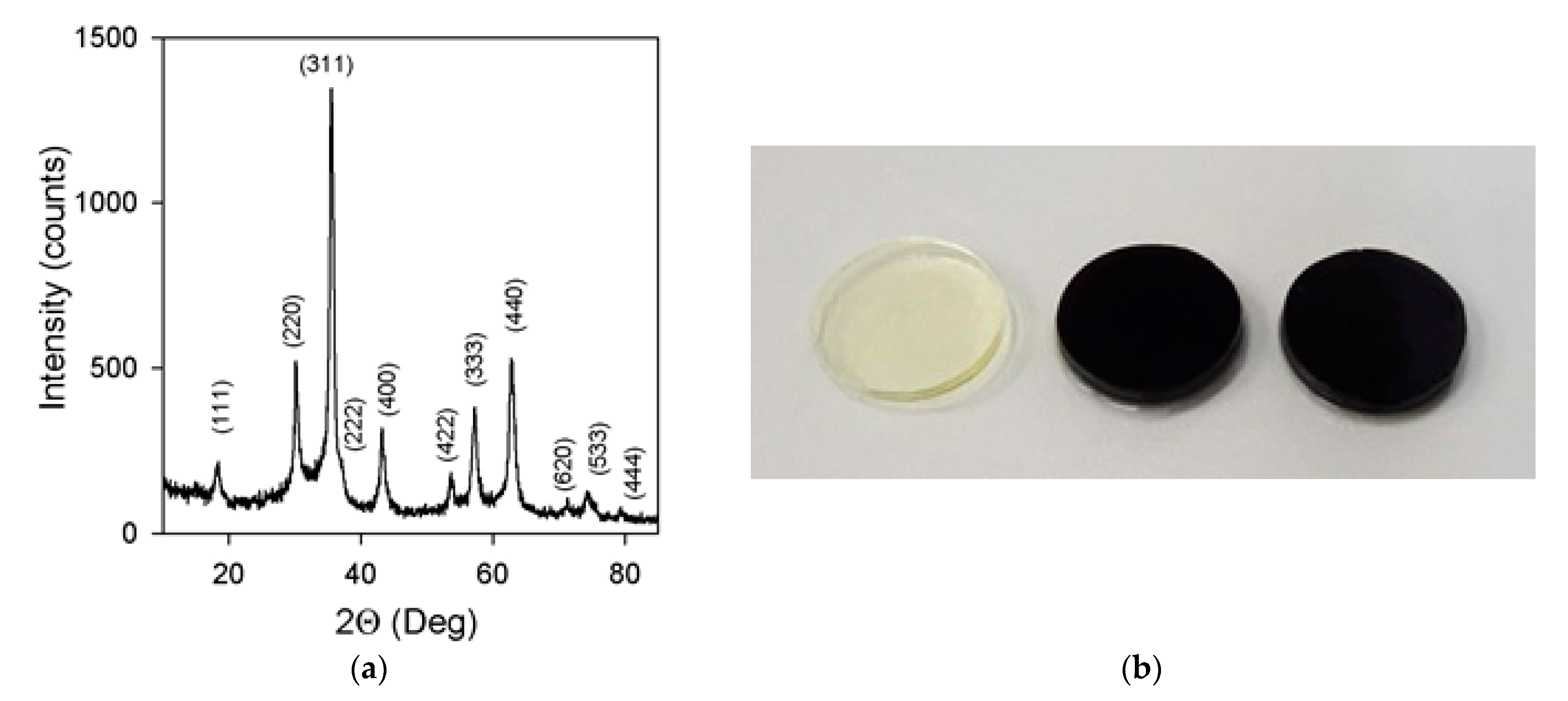
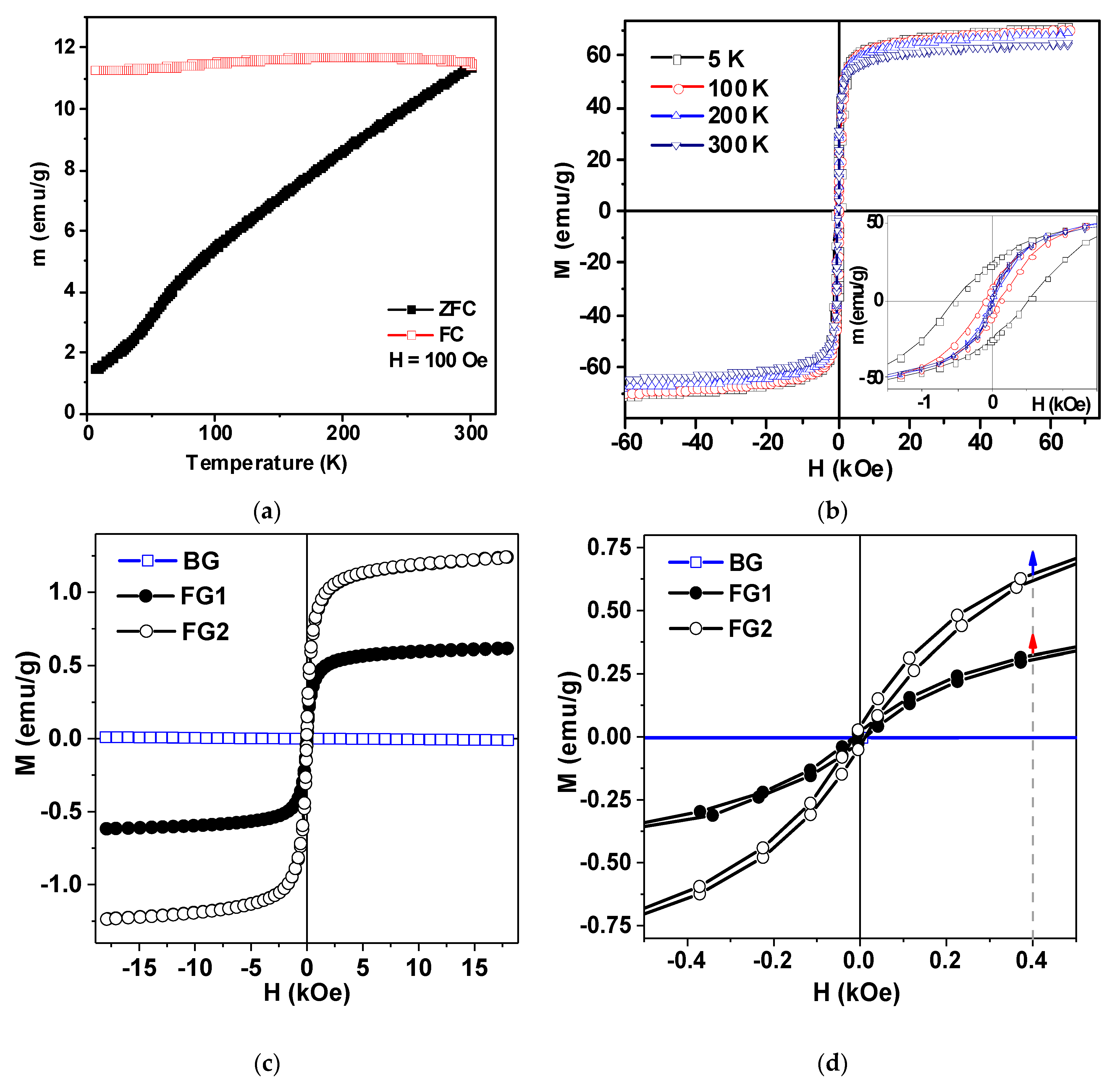

| Mark | Content of Mnps in the Reaction Mixture (wt. %) | Equilibrium Swelling Ratio in Water | Content of MNPs in the Gel (wt. %) | Equilibrium Swelling Ratio in 199 Salt Solution |
|---|---|---|---|---|
| BG | 0.0 | 11.8 ± 0.5 | 0.00 | 11.2 ± 0.4 |
| FG1 | 1.0 | 12.6 ± 0.6 | 0.63 | 11.5 ± 0.5 |
| FG2 | 2.0 | 13.2 ± 0.7 | 1.19 | 11.3 ± 0.5 |
| Fibroblasts Monolayer Density (cells/cm2) | |||
|---|---|---|---|
| Experimental Condition | 8 Central Wells | 16 Peripheral Wells | All 24 Wells |
| Magnetic field of 400 Oe | 5800 ± 500 | 5700 ± 400 | 5700 ± 400 |
| Control (absence of magnetic field) | 4500 ± 500 | 4300 ± 500 | 4400 ± 500 |
| Experimental Condition | BG (2) | FG1 (2) | FG2 (2) | TCPS (2) |
|---|---|---|---|---|
| Magnetic field of 400 Oe | 0.08 ± 0.03 | 0.16 ± 0.05 | 0.6 ± 0.1 | 2.5 ± 0.2 |
| Control (absence of magnetic field) | 0.08 ± 0.04 | 0.35 ± 0.07 * | 1.0 ± 0.1 * | 2.3 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blyakhman, F.A.; Melnikov, G.Y.; Makarova, E.B.; Fadeyev, F.A.; Sedneva-Lugovets, D.V.; Shabadrov, P.A.; Volchkov, S.O.; Mekhdieva, K.R.; Safronov, A.P.; Fernández Armas, S.; et al. Effects of Constant Magnetic Field to the Proliferation Rate of Human Fibroblasts Grown onto Different Substrates: Tissue Culture Polystyrene, Polyacrylamide Hydrogel and Ferrogels γ-Fe2O3 Magnetic Nanoparticles. Nanomaterials 2020, 10, 1697. https://doi.org/10.3390/nano10091697
Blyakhman FA, Melnikov GY, Makarova EB, Fadeyev FA, Sedneva-Lugovets DV, Shabadrov PA, Volchkov SO, Mekhdieva KR, Safronov AP, Fernández Armas S, et al. Effects of Constant Magnetic Field to the Proliferation Rate of Human Fibroblasts Grown onto Different Substrates: Tissue Culture Polystyrene, Polyacrylamide Hydrogel and Ferrogels γ-Fe2O3 Magnetic Nanoparticles. Nanomaterials. 2020; 10(9):1697. https://doi.org/10.3390/nano10091697
Chicago/Turabian StyleBlyakhman, Felix A., Grigory Yu. Melnikov, Emilia B. Makarova, Fedor A. Fadeyev, Daiana V. Sedneva-Lugovets, Pavel A. Shabadrov, Stanislav O. Volchkov, Kamiliya R. Mekhdieva, Alexander P. Safronov, Sergio Fernández Armas, and et al. 2020. "Effects of Constant Magnetic Field to the Proliferation Rate of Human Fibroblasts Grown onto Different Substrates: Tissue Culture Polystyrene, Polyacrylamide Hydrogel and Ferrogels γ-Fe2O3 Magnetic Nanoparticles" Nanomaterials 10, no. 9: 1697. https://doi.org/10.3390/nano10091697
APA StyleBlyakhman, F. A., Melnikov, G. Y., Makarova, E. B., Fadeyev, F. A., Sedneva-Lugovets, D. V., Shabadrov, P. A., Volchkov, S. O., Mekhdieva, K. R., Safronov, A. P., Fernández Armas, S., & Kurlyandskaya, G. V. (2020). Effects of Constant Magnetic Field to the Proliferation Rate of Human Fibroblasts Grown onto Different Substrates: Tissue Culture Polystyrene, Polyacrylamide Hydrogel and Ferrogels γ-Fe2O3 Magnetic Nanoparticles. Nanomaterials, 10(9), 1697. https://doi.org/10.3390/nano10091697







