Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
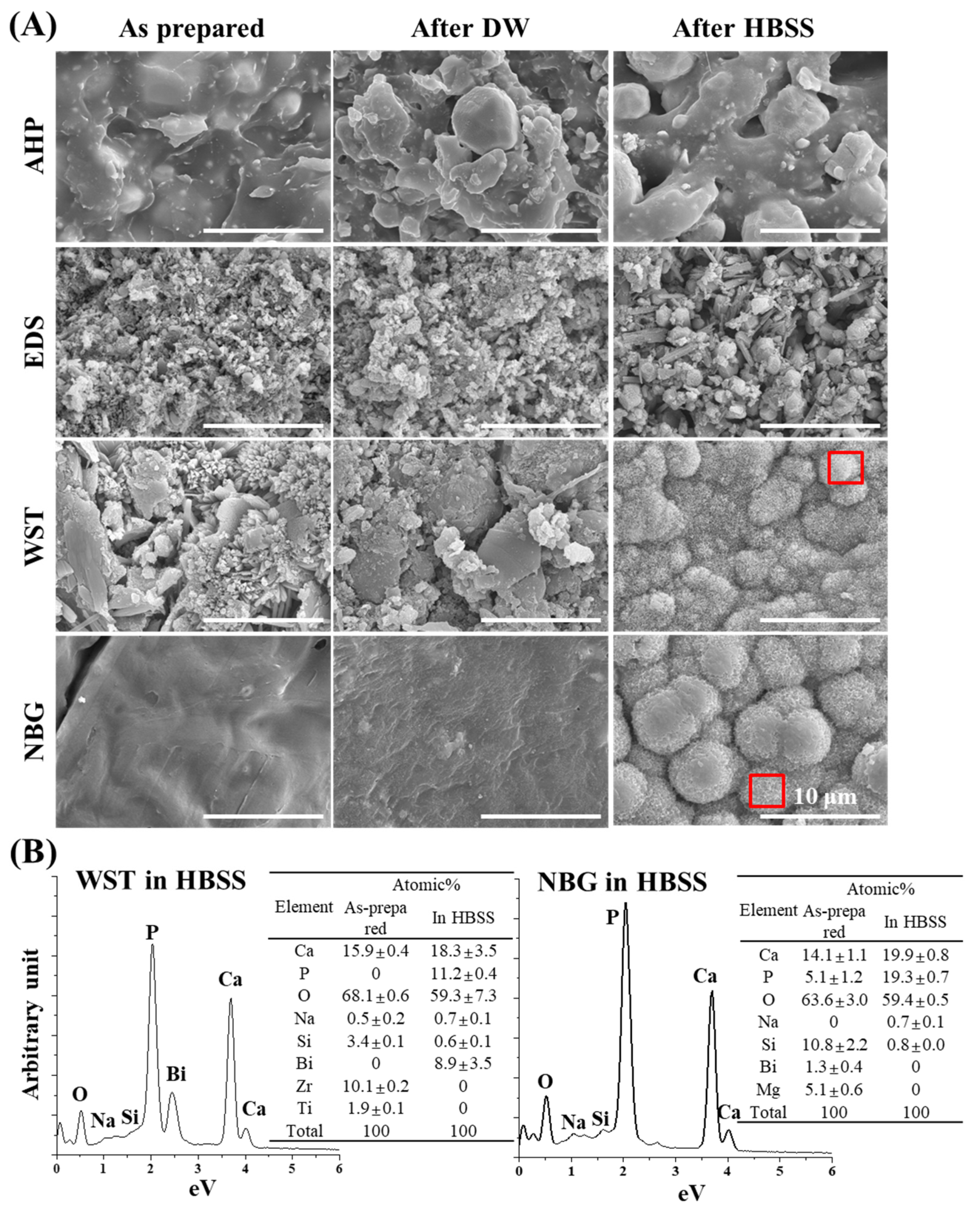
2.2. Surface Morphology and Elemental Composition Analyses
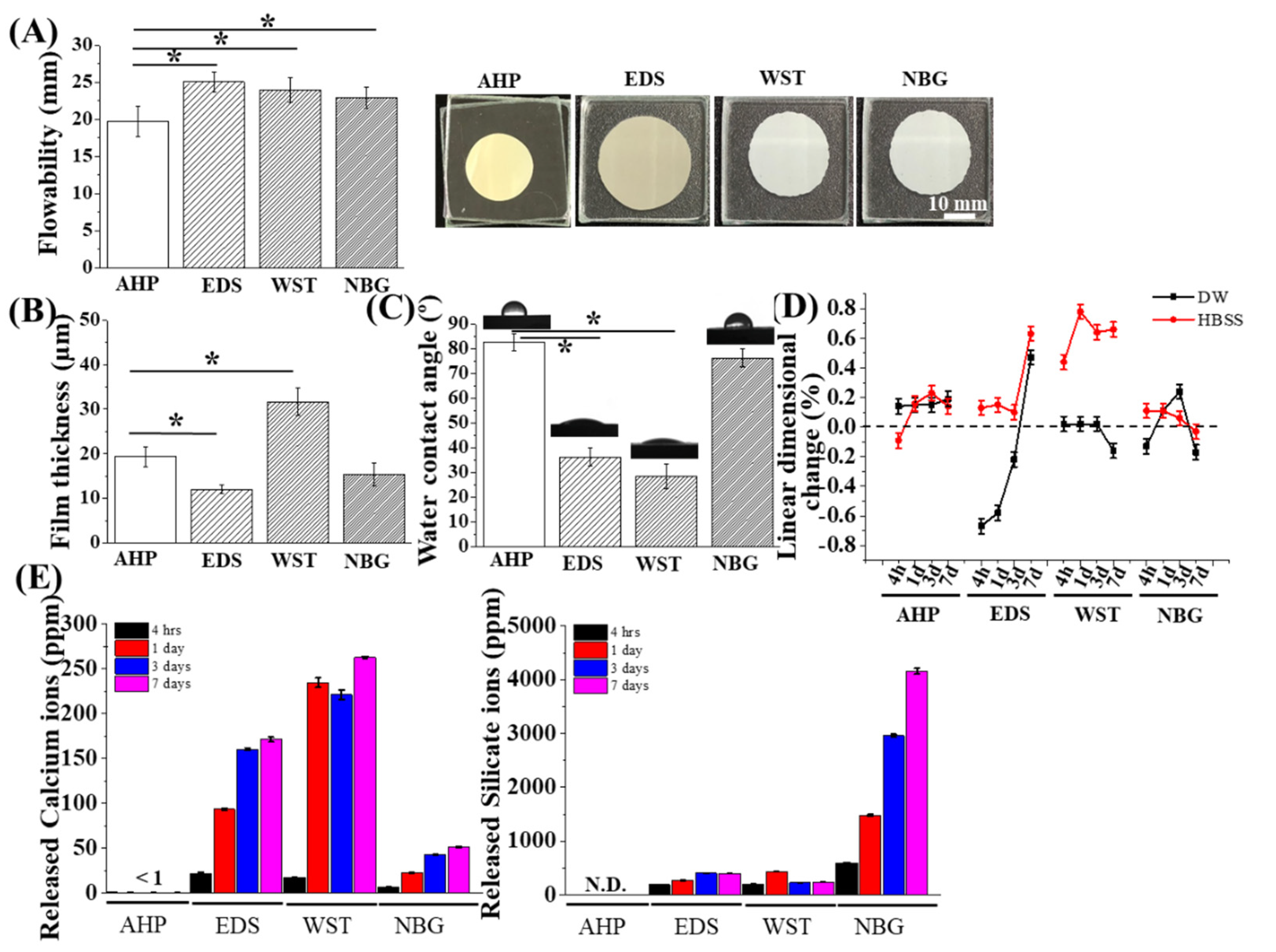
2.3. Ion Releasing Profiling
2.4. Film Thickness Measurement
2.5. Flow Distance Measurement
2.6. Linear Dimensional Change after Setting
2.7. Water Contact Angle (WCA) Analysis
2.8. Preparation of Sealer Extract Media
2.9. Isolation and Culture of Human Periodontal Ligament Stem Cells (hPDLSCs)
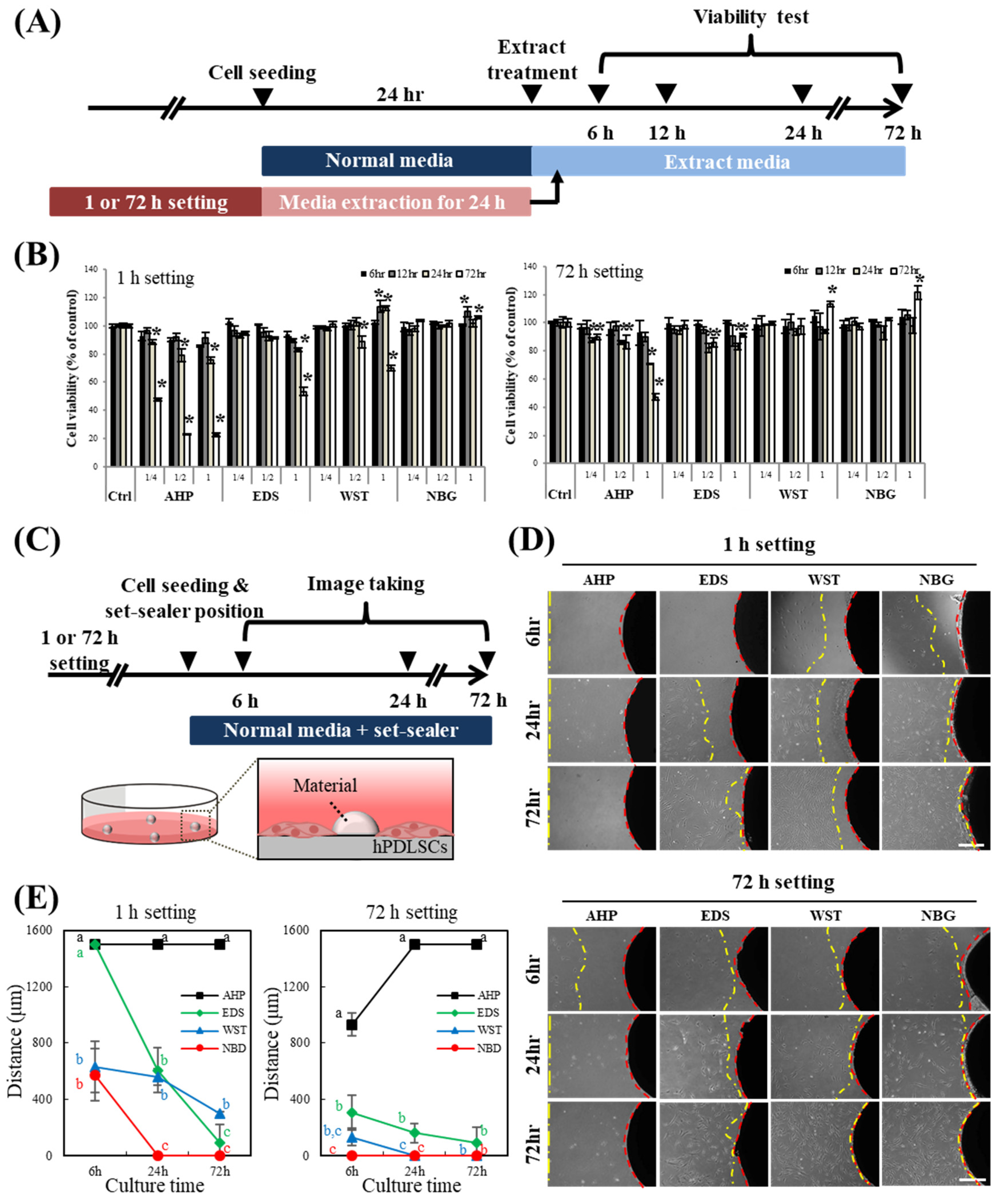
2.10. Cell Viability Assay
2.11. Direct Contact Assay
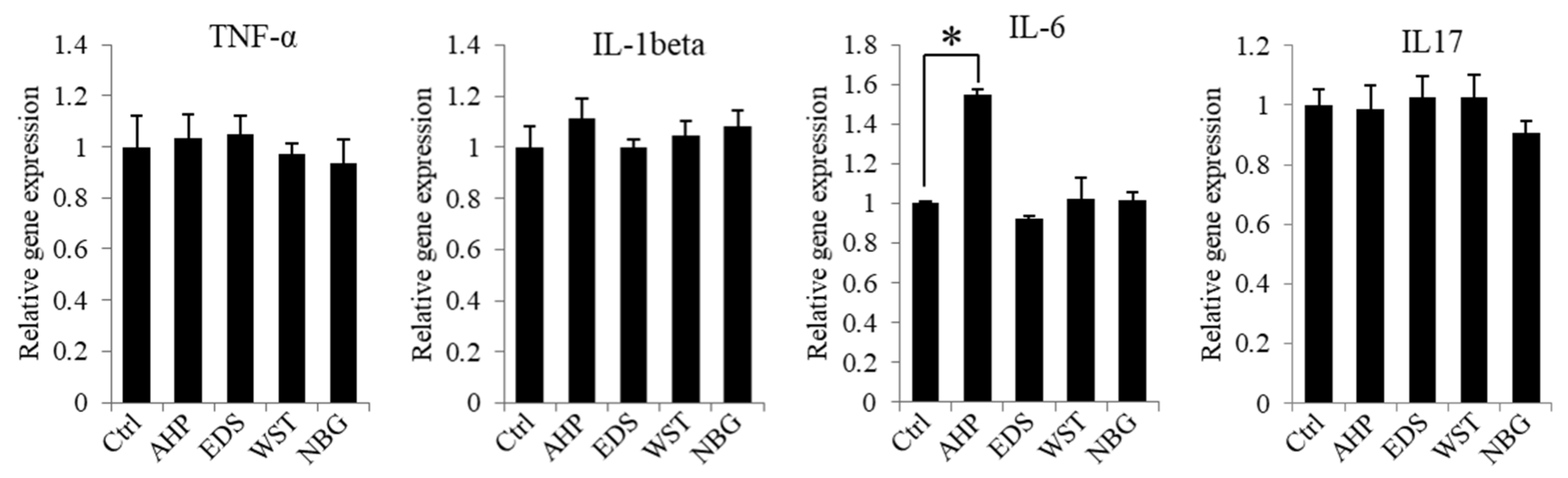
2.12. Inflammatory Gene Expression
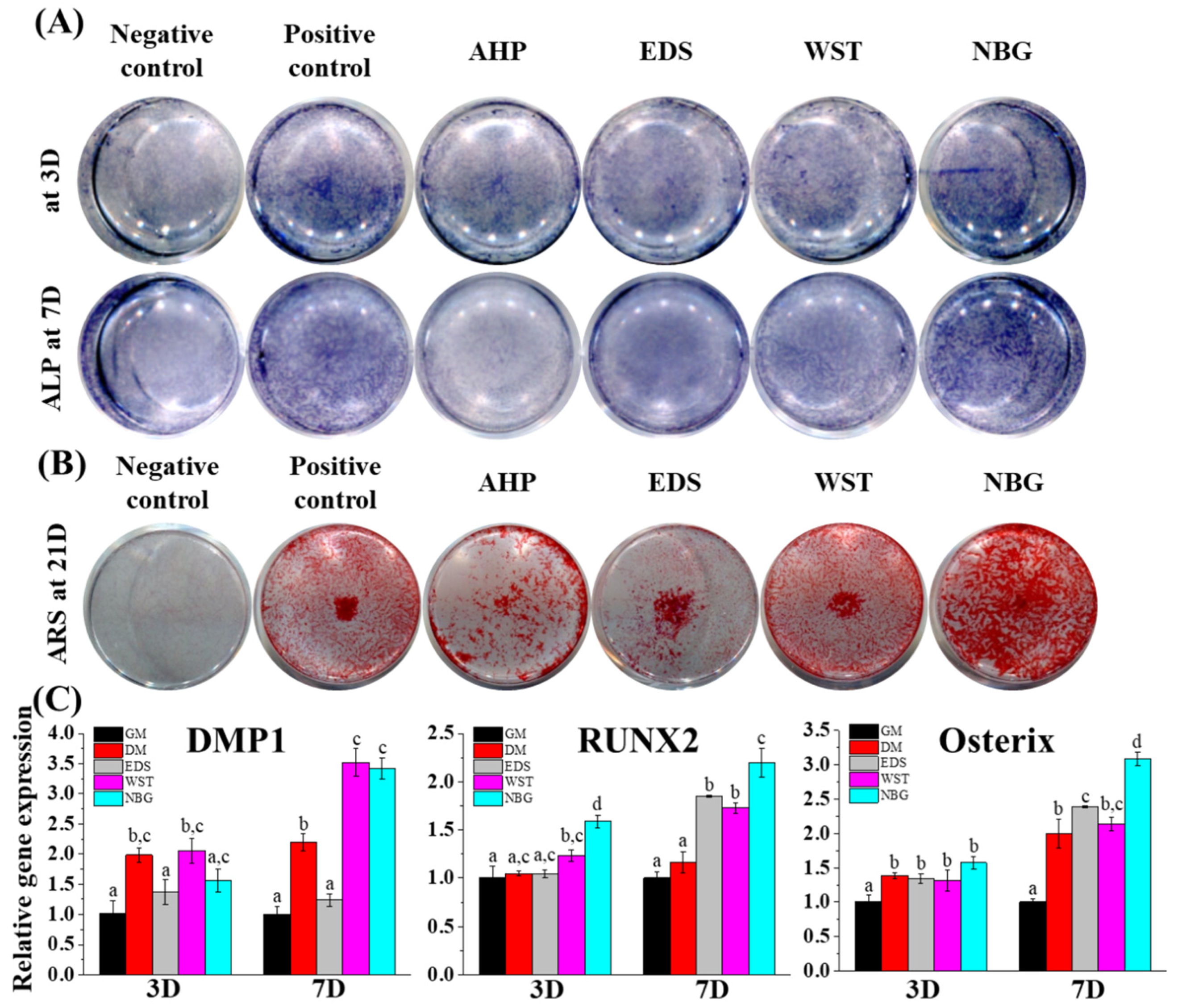
2.13. Osteogenic Differentiation Assay
2.14. Angiogenesis Assay
2.15. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Bioactive Root Canal Sealers
3.2. Cytocompatibility of hPDLSCs to Bioactive Root Canal Sealers
3.3. Osteogenic Differentiation Capacity of Bioactive Root Canal Sealers
3.4. Angiogenic Capacity of Bioactive Root Canal Sealers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.-B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10, 2041731418819263. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.M.; Berman, L.H. Cohen’s Pathways of the Pulp Expert Consult; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- ISO. ISO 6876:2012 Dentistry—Root Canal Sealing Materials; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Grossman, I.; Oliet, S.; Del Rio, E. Endodontic Practice, 11th ed.; Lea and Fabringer: Philadelphia, PA, USA, 1988; pp. 145–155. [Google Scholar]
- Carvalho-Junior, J.R.; Correr-Sobrinho, L.; Correr, A.B.; Sinhoreti, M.A.; Consani, S.; Sousa-Neto, M.D. Solubility and dimensional change after setting of root canal sealers: A proposal for smaller dimensions of test samples. J. Endod. 2007, 33, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Marin-Bauza, G.A.; Rached-Junior, F.J.; Souza-Gabriel, A.E.; Sousa-Neto, M.D.; Miranda, C.E.; Silva-Sousa, Y.T. Physicochemical properties of methacrylate resin-based root canal sealers. J. Endod. 2010, 36, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.V.; Silva-Sousa, Y.T.C.; Rosa, R.; Mazzi-Chaves, J.F.; Lopes, F.C.; Steier, L.; Sousa-Neto, M.D. Evaluation of the physicochemical properties of silicone- and epoxy resin-based root canal sealers. Braz. Oral Res. 2017, 31, e72. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.H.R.; Gomes, L.C.L.; França, M.C.M.; Bittencourt, T.S.; Valera, M.C.; Camargo, S.E.A.; Bottino, M.C. Incorporating N-acetylcysteine and tricalcium phosphate into epoxy resin-based sealer improved its biocompatibility and adhesiveness to radicular dentine. Dent. Mater. 2019, 35, 1750–1756. [Google Scholar] [CrossRef]
- Baras, B.H.; Sun, J.; Melo, M.A.S.; Tay, F.R.; Oates, T.W.; Zhang, K.; Weir, M.D.; Xu, H.H.K. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent. Mater. 2019, 35, 1479–1489. [Google Scholar] [CrossRef]
- Almeida, L.H.S.; Moraes, R.R.; Morgental, R.D.; Cava, S.S.; Rosa, W.L.O.; Rodrigues, P.; Ribeiro, A.S.; Só, M.; Pappen, F.G. Synthesis of silver-containing calcium aluminate particles and their effects on a MTA-based endodontic sealer. Dent. Mater. 2018, 34, e214–e223. [Google Scholar] [CrossRef]
- Seung, J.; Weir, M.D.; Melo, M.A.S.; Romberg, E.; Nosrat, A.; Xu, H.H.K.; Tordik, P.A. A Modified Resin Sealer: Physical and Antibacterial Properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef]
- AlShwaimi, E.; Bogari, D.; Ajaj, R.; Al-Shahrani, S.; Almas, K.; Majeed, A. Antimicrobial Effectiveness of Root Canal Sealers against Enterococcus faecalis: A Systematic Review. J. Endod. 2016, 42, 1588–1597. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Weir, M.D.; Xie, X.; Bai, Y.; Xu, H.H.K. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent. Mater. 2019, 35, 1117–1129. [Google Scholar] [CrossRef]
- Monteiro, J.C.; Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; de Souza Balbinot, G.; Samuel, S.M.W.; Makeeva, I.; Collares, F.M.; Sauro, S. Halloysite nanotubes loaded with alkyl trimethyl ammonium bromide as antibacterial agent for root canal sealers. Dent. Mater. 2019, 35, 789–796. [Google Scholar] [CrossRef]
- Singh, R.K.; Knowles, J.C.; Kim, H.-W. Advances in nanoparticle development for improved therapeutics delivery: Nanoscale topographical aspect. J. Tissue Eng. 2019, 10, 2041731419877528. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Duarte, M.A.H.; Camilleri, J. Calcium silicate-based sealers: Assessment of physicochemical properties, porosity and hydration. Dent. Mater. 2016, 32, e30–e40. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, U.; Hagglund, B.; Sundqvist, G.; Wing, K. Factors affecting the long-term results of endodontic treatment. J. Endod. 1990, 16, 498–504. [Google Scholar] [CrossRef]
- Seltzer, S.; Bender, I.B.; Turkenkopf, S. Factors Affecting Successful Repair after Root Canal Therapy. J. Am. Dent. Assoc. 1963, 67, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, M.A.; White, R.R.; Walton, R.E. Determining the optimal obturation length: A meta-analysis of literature. J. Endod. 2005, 31, 271–274. [Google Scholar] [CrossRef]
- Ricucci, D.; Rocas, I.N.; Alves, F.R.; Loghin, S.; Siqueira, J.F., Jr. Apically Extruded Sealers: Fate and Influence on Treatment Outcome. J. Endod. 2016, 42, 243–249. [Google Scholar] [CrossRef]
- Jun, S.-K.; Lee, J.-H.; Lee, H.-H. The Biomineralization of a Bioactive Glass-Incorporated Light-Curable Pulp Capping Material Using Human Dental Pulp Stem Cells. Biomed. Res. Int. 2017, 2017, 2495282. [Google Scholar] [CrossRef]
- Dashnyam, K.; Buitrago, J.O.; Bold, T.; Mandakhbayar, N.; Perez, R.A.; Knowles, J.C.; Lee, J.-H.; Kim, H.-W. Angiogenesis-promoted bone repair with silicate-shelled hydrogel fiber scaffolds. Biomater. Sci. 2019, 7, 5221–5231. [Google Scholar] [CrossRef]
- Jun, S.-K.; Yoon, J.-Y.; Mahapatra, C.; Park, J.H.; Kim, H.-W.; Kim, H.-R.; Lee, J.-H.; Lee, H.-H. Ceria-incorporated MTA for accelerating odontoblastic differentiation via ROS downregulation. Dent. Mater. 2019, 35, 1291–1299. [Google Scholar] [CrossRef]
- Moon, H.-J.; Lee, J.-H.; Kim, J.-H.; Knowles, J.C.; Cho, Y.-B.; Shin, D.-H.; Lee, H.-H.; Kim, H.-W. Reformulated mineral trioxide aggregate components and the assessments for use as future dental regenerative cements. J. Tissue Eng. 2018, 9, 2041731418807396. [Google Scholar] [CrossRef] [PubMed]
- Dashnyam, K.; Jin, G.-Z.; Kim, J.-H.; Perez, R.; Jang, J.-H.; Kim, H.-W. Promoting angiogenesis with mesoporous microcarriers through a synergistic action of delivered silicon ion and VEGF. Biomaterials 2017, 116, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; El-Fiqi, A.; Mandakhbayar, N.; Lee, H.-H.; Kim, H.-W. Drug/ion co-delivery multi-functional nanocarrier to regenerate infected tissue defect. Biomaterials 2017, 142, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.-W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Boldbaatar, K.; Dashnyam, K.; Knowles, J.C.; Lee, H.-H.; Lee, J.-H.; Kim, H.-W. Dual-ion delivery for synergistic angiogenesis and bactericidal capacity with silica-based microsphere. Acta Biomater. 2019, 83, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.-H.; Kim, H.-W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin–Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K.; Kawala, B.; Gredes, T. Trace Elements in Living Systems: From Beneficial to Toxic Effects. Biomed. Res. Int. 2017, 2017, 8297814. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, S.; Lee, S.; Kim, H.-C.; Kim, E. In Vitro Comparison of Biocompatibility of Calcium Silicate-Based Root Canal Sealers. Materials 2019, 12, 2411. [Google Scholar] [CrossRef]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive Glass-Based Endodontic Sealer as a Promising Root Canal Filling Material without Semisolid Core Materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Yin, C.; Okamoto, R.; Kondo, M.; Tanaka, T.; Hattori, H.; Tanaka, M.; Sato, H.; Iino, S.; Koshiro, Y. Electrospinning of Block and Graft Type Silicone Modified Polyurethane Nanofibers. Nanomaterials 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Labban, N.; Wayu, M.B.; Steele, C.M.; Munoz, T.S.; Pollock, J.A.; Case, W.S.; Leopold, M.C. First Generation Amperometric Biosensing of Galactose with Xerogel-Carbon Nanotube Layer-By-Layer Assemblies. Nanomaterials 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Bardi, G. Metallic Nanoparticles: General Research Approaches to Immunological Characterization. Nanomaterials 2018, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Yoon, J.Y.; Dev Patel, K.; Ma, L.; Lee, H.H.; Kim, J.; Lee, J.H.; Shin, J. Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth. Nanomaterials 2020, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Joo, J.; Kwon, E.J.; Skalak, M.; Hussain, S.; She, Z.G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Self-Sealing Porous Silicon-Calcium Silicate Core-Shell Nanoparticles for Targeted siRNA Delivery to the Injured Brain. Adv. Mater. 2016, 28, 7962–7969. [Google Scholar] [CrossRef] [PubMed]
- Del Carpio-Perochena, A.; Kishen, A.; Shrestha, A.; Bramante, C.M. Antibacterial Properties Associated with Chitosan Nanoparticle Treatment on Root Dentin and 2 Types of Endodontic Sealers. J. Endod. 2015, 41, 1353–1358. [Google Scholar] [CrossRef]
- ISO. ISO 3107:2011 Dentistry—Zinc Oxide/Eugenol Cements and Zinc Oxide/Non-Eugenol Cements; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Abedian, Z.; Jenabian, N.; Moghadamnia, A.A.; Zabihi, E.; Pourbagher, R.; Hossein-Nataj, H.; Mohamadnia-Afrouzi, M. A comparative study on immunophenotypic characterization and osteogenic differentiation of human Mesenchymal stromal cells derived from periodontal ligament and gingiva. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Kayahan, M.B.; Nekoofar, M.H.; Kazandag, M.; Canpolat, C.; Malkondu, O.; Kaptan, F.; Dummer, P.M. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int. Endod. J. 2009, 42, 1004–1014. [Google Scholar] [CrossRef]
- Schweikle, M.; Bjørnøy, S.H.; van Helvoort, A.T.J.; Haugen, H.J.; Sikorski, P.; Tiainen, H. Stabilisation of amorphous calcium phosphate in polyethylene glycol hydrogels. Acta Biomater. 2019, 90, 132–145. [Google Scholar] [CrossRef]
- Kim, D.-A.; Lee, J.-H.; Jun, S.-K.; Kim, H.-W.; Eltohamy, M.; Lee, H.-H. Sol–gel-derived bioactive glass nanoparticle-incorporated glass ionomer cement with or without chitosan for enhanced mechanical and biomineralization properties. Dent. Mater. 2017, 33, 805–817. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Mandakhbayar, N.; Jo, S.B.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Nanotherapeutics for regeneration of degenerated tissue infected by bacteria through the multiple delivery of bioactive ions and growth factor with antibacterial/angiogenic and osteogenic/odontogenic capacity. Bioact. Mater. 2020, 6, 123–136. [Google Scholar] [CrossRef]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 2017, 50 (Suppl. 2), e120–e136. [Google Scholar] [CrossRef] [PubMed]
- Hezi-Yamit, A.; Sullivan, C.; Wong, J.; David, L.; Chen, M.; Cheng, P.; Shumaker, D.; Wilcox, J.N.; Udipi, K. Impact of polymer hydrophilicity on biocompatibility: Implication for DES polymer design. J. Biomed. Mater. Res. Part A 2009, 90, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.F.; Gomes, B.P.; Ferraz, C.C.; Souza-Filho, F.J.; Zaia, A.A. Filling of artificial lateral canals and microleakage and flow of five endodontic sealers. Int. Endod. J. 2007, 40, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Nunes, V.H.; Silva, R.G.; Alfredo, E.; Sousa-Neto, M.D.; Silva-Sousa, Y.T. Adhesion of Epiphany and AH Plus sealers to human root dentin treated with different solutions. Braz. Dent. J. 2008, 19, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Lin, F.-H.; Wang, W.-H.; Ritchie, H.H.; Lan, W.-H.; Lin1, C.-P. Effects of EDTA on the Hydration Mechanism of Mineral Trioxide Aggregate. J. Dent. Res. 2007, 86, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Nagas, E.; Cehreli, Z.C.; Uyanik, M.O.; Durmaz, V.; Vallittu, P.K.; Lassila, L.V. Bond strength of mineral trioxide aggregate to root dentin after exposure to different irrigation solutions. Dent. Traumatol. 2014, 30, 246–249. [Google Scholar] [CrossRef]
- Savadkouhi, S.T.; Fazlyab, M. Discoloration Potential of Endodontic Sealers: A Brief Review. Iran. Endod. J. 2016, 11, 250–254. [Google Scholar]
- Krastl, G.; Allgayer, N.; Lenherr, P.; Filippi, A.; Taneja, P.; Weiger, R. Tooth discoloration induced by endodontic materials: A literature review. Dent. Traumatol. 2013, 29, 2–7. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; Tayyar, M.; Darmani, H. Cytotoxicity evaluation of various resin based root canal sealers. Int. Endod. J. 2010, 43, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Leyhausen, G.; Heil, J.; Reifferscheid, G.; Waldmann, P.; Geurtsen, W. Genotoxicity and Cytotoxicity of the Epoxy Resin-Based Root Canal Sealer AH Plus. J. Endod. 1999, 25, 109–113. [Google Scholar] [CrossRef]
- Miletic, I.; Devcic, N.; Anic, I.; Borcic, J.; Karlovic, Z.; Osmak, M. The cytotoxicity of RoekoSeal and AH plus compared during different setting periods. J. Endod. 2005, 31, 307–309. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Prati, C. Properties of a novel polysiloxane-guttapercha calcium silicate-bioglass-containing root canal sealer. Dent. Mater. 2016, 32, e113–e126. [Google Scholar] [CrossRef] [PubMed]
- Collado-Gonzalez, M.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Lozano, A.; Forner, L.; Llena, C.; Rodriguez-Lozano, F.J. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int. Endod. J. 2017, 50, 875–884. [Google Scholar] [CrossRef]
- Kebudi Benezra, M.; Schembri Wismayer, P.; Camilleri, J. Interfacial Characteristics and Cytocompatibility of Hydraulic Sealer Cements. J. Endod. 2018, 44, 1007–1017. [Google Scholar] [CrossRef]
- de Pablo, O.V.; Estevez, R.; Peix Sanchez, M.; Heilborn, C.; Cohenca, N. Root anatomy and canal configuration of the permanent mandibular first molar: A systematic review. J. Endod. 2010, 36, 1919–1931. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]

| Product | Code | Manufacturer | Lot Number | Composition (wt%) * |
|---|---|---|---|---|
| AH Plus | AHP | Dentsply | 1910000640 | Component A: epoxyresin (25–50%), calcium tungstate, zirconium oxide, aerosol, iron oxide |
| Component B: adamantane amine, N,N-dibenzil -5-oxanonane, TCD-diamine, calcium tungstate, zirconium oxide, aerosol | ||||
| Endoseal MTA | EDS | Maruchi | CD191207C | Calcium silicates (dicalcium silicate), tricalcium aluminates, calcium aluminoferrite, calcium sulfates, bismuth oixide, zirconium oxide (66.5%), thickening agent |
| Well-Root ST | WST | Vericom | WR9N6100 | Calcium silicate compound, calcium sulfate dehydrate, calcium sodium phosphosilicate, zirconium oxide, titanium oxide, thickening agents |
| Nishika-BG | NBG | Nippon shika yakuhin | H7T | Component A: fatty acid, bismuth subcarbonate, silicon dioxide |
| Component B: magnesium oxide, purified water, calcium silicate glass, silicon dioxide |
| Gene Name | Sequence (5′ → 3′) |
|---|---|
| GAPDH_Fwd | CCAGAACATCATCCCTGCCTCT |
| GAPDH_Rev | GACGCCTGCTTCACCACCTT |
| TNF-alpha_Fwd | CGTGGAGCTGGCCGAGGAG |
| TNF-alpha_Rev | AGGAAGGAGAAGAGGCTGAGGAAC |
| IL-6_Fwd | GGTGTTGCCTGCTGCCTTCC |
| IL-6_Rev | GTTCTGAAGAGGTGAGTGGCTGTC |
| IL-1beta_Fwd | TGGCTTATTACAGTGGCAATGAGGATG |
| IL-1beta_Rev | TGTAGTGGTGGTCGGAGATTCGTAG |
| DMP-1_Fwd | CAGGAAGAGGTGGTGAGTGAGT |
| DMP-1_Rev | TGGATTCGCTGTCTGCTTGCT |
| RUNX2_Fwd | TCCAGACCAGCAGCACTCCATA |
| RUNX2_Rev | TCCATCAGCGTCAACACCATCA |
| OSX_Fwd | CAGCAGCTAAACTTGGAAGGA |
| OSX_Rev | TGCTTTCGCTTGTCTGAGTC |
| IL17_Fwd | TCAACCCGATTGTCCACCAT |
| IL17_Rev | GAGTTTAGTCCGAAATGAGGCTG |
| VEGF_Fwd | CAAAAACGAAAGCGCAAGAAA |
| VEGF_Rev | GCGGGCACCAACGTACAC |
| PDGFBB_Fwd | CTGGCATGCAAGTGTGAGAC |
| PDGFBB_Rev | AATGGTCACCCGAGTTTGG |
| bFGF_Fwd | GGCTTCTTCCTGCGCATCCA |
| bFGF_Rev | GCTCTTAGCAGACATTGGAAGA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.-J.; Dev Patel, K.; Campbell Knowles, J.; Lee, J.-H.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. https://doi.org/10.3390/nano10091750
Jo SB, Kim HK, Lee HN, Kim Y-J, Dev Patel K, Campbell Knowles J, Lee J-H, Song M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials. 2020; 10(9):1750. https://doi.org/10.3390/nano10091750
Chicago/Turabian StyleJo, Seung Bin, Hyun Kyung Kim, Hae Nim Lee, Yu-Jin Kim, Kapil Dev Patel, Jonathan Campbell Knowles, Jung-Hwan Lee, and Minju Song. 2020. "Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro" Nanomaterials 10, no. 9: 1750. https://doi.org/10.3390/nano10091750
APA StyleJo, S. B., Kim, H. K., Lee, H. N., Kim, Y.-J., Dev Patel, K., Campbell Knowles, J., Lee, J.-H., & Song, M. (2020). Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials, 10(9), 1750. https://doi.org/10.3390/nano10091750