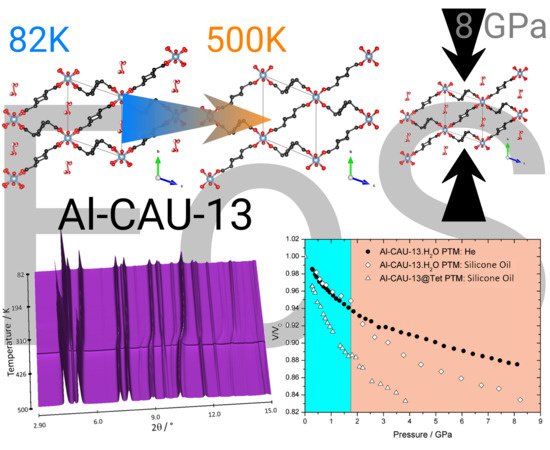
Influence of Thermal and Mechanical Stimuli on the Behavior of Al-CAU-13 Metal–Organic Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Synchrotron X-ray Powder Diffraction
2.2.1. In-Situ Thermodiffractometry
2.2.2. High Pressure In-Situ X-ray Diffraction
3. Results and Discussion
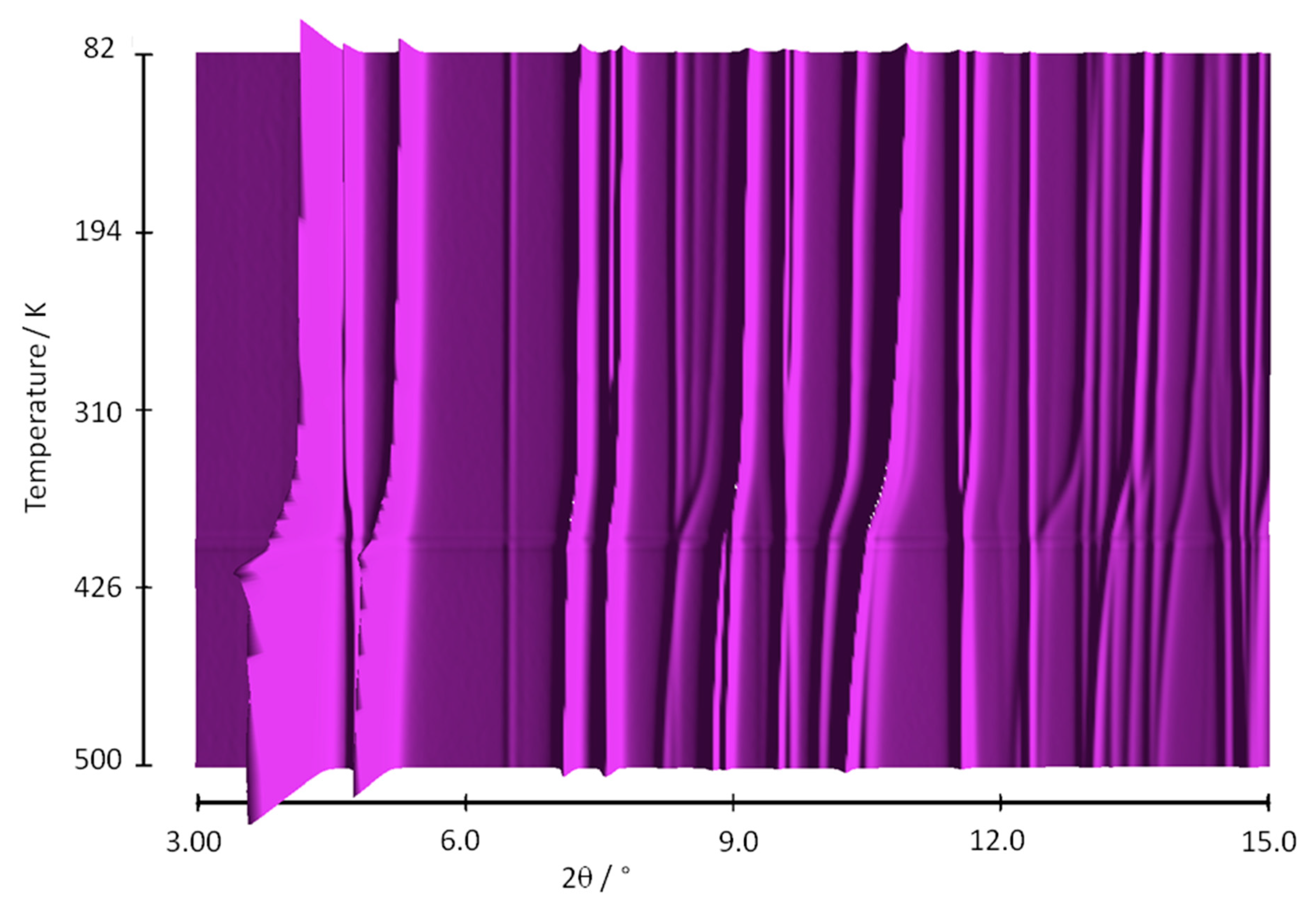
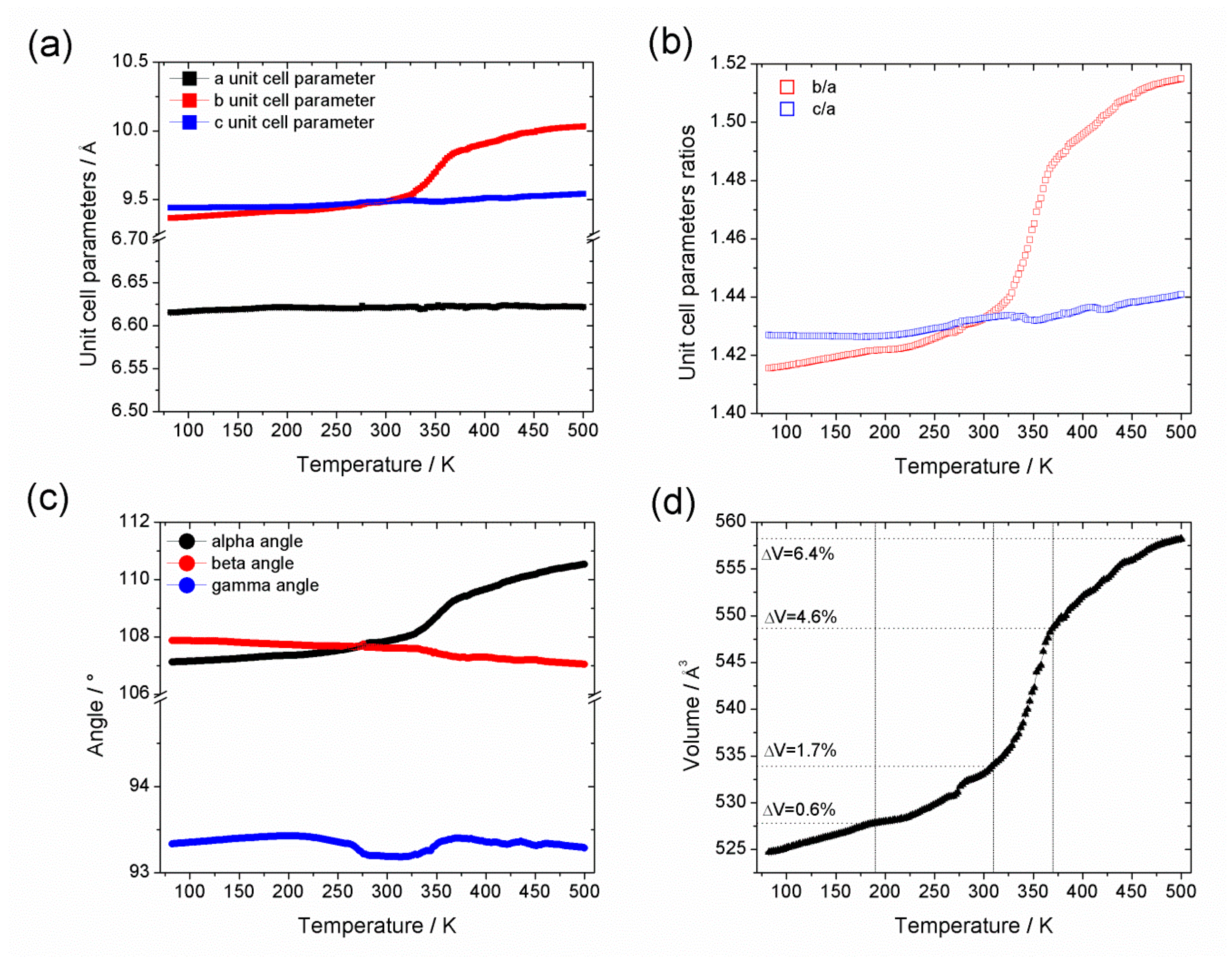
3.1. In Situ Synchrotron X-ray Thermodiffraction
3.2. In Situ Synchrotron High Pressure X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal–organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal-Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef]
- Chanut, N.; Ghoufi, A.; Coulet, M.-V.; Bourrelly, S.; Kuchta, B.; Maurin, G.; Llewellyn, P.L. Tailoring the separation properties of flexible metal-organic frameworks using mechanical pressure. Nat. Commun. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Maurin, G. Mechanical Control of the Kinetic Propylene/Propane Separation by Zeolitic Imidazolate Framework-8. Angew. Chem. Int. Ed. 2019, 58, 13734–13738. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-S.; Zhang, M.; Zou, R.; Xu, Q. Metal–Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Baxter, E.F.; Tian, T.; Bennett, T.D.; Slater, N.K.H.; Cheetham, A.K.; Fairen-Jimenez, D. Amorphous metal–organic frameworks for drug delivery. Chem. Commun. 2015, 51, 13878–13881. [Google Scholar] [CrossRef]
- Ma, L.; Evans, O.R.; Foxman, B.M.; Lin, W. Luminescent Lanthanide Coordination Polymers. Inorg. Chem. 1999, 38, 5837–5840. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330. [Google Scholar] [CrossRef]
- Weber, M.; Graniel, O.; Balme, S.; Miele, P.; Bechelany, M. On the Use of MOFs and ALD Layers as Nanomembranes for the Enhancement of Gas Sensors Selectivity. Nanomaterials 2019, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Calbo, J.; Golomb, M.J.; Walsh, A. Redox-active metal–organic frameworks for energy conversion and storage. J. Mater. Chem. A 2019, 7, 16571–16597. [Google Scholar] [CrossRef]
- Strauss, I.; Chakarova, K.; Mundstock, A.; Mihaylov, M.; Hadjiivanov, K.; Guschanski, N.; Caro, J. UiO-66 and UiO-66-NH2 based sensors: Dielectric and FTIR investigations on the effect of CO2 adsorption. Microporous Mesoporous Mater. 2020, 302, 110227. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353. [Google Scholar] [CrossRef]
- Wriedt, M.; Yakovenko, A.A.; Halder, G.J.; Prosvirin, A.V.; Dunbar, K.R.; Zhou, H.-C. Reversible Switching from Antiferro- to Ferromagnetic Behavior by Solvent-Mediated, Thermally-Induced Phase Transitions in a Trimorphic MOF-Based Magnetic Sponge System. J. Am. Chem. Soc. 2013, 135, 4040–4050. [Google Scholar] [CrossRef] [PubMed]
- Mínguez Espallargas, G.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Mileo, P.G.M.; Adil, K.; Davis, L.; Cadiau, A.; Belmabkhout, Y.; Aggarwal, H.; Maurin, G.; Eddaoudi, M.; Devautour-Vinot, S. Achieving Superprotonic Conduction with a 2D Fluorinated Metal–Organic Framework. J. Am. Chem. Soc. 2018, 140, 13156–13160. [Google Scholar] [CrossRef]
- Wang, S.; Wahiduzzaman, M.; Davis, L.; Tissot, A.; Shepard, W.; Marrot, J.; Martineau-Corcos, C.; Hamdane, D.; Maurin, G.; Devautour-Vinot, S.; et al. A robust zirconium amino acid metal-organic framework for proton conduction. Nat. Commun. 2018, 9, 4937. [Google Scholar] [CrossRef]
- Ye, Y.; Gong, L.; Xiang, S.; Zhang, Z.; Chen, B. Metal–Organic Frameworks as a Versatile Platform for Proton Conductors. Adv. Mater. 2020, 32, 1907090. [Google Scholar] [CrossRef]
- Lim, D.-W.; Kitagawa, H. Proton Transport in Metal–Organic Frameworks. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Vanduyfhuys, L.; Rogge, S.M.J.; Wieme, J.; Vandenbrande, S.; Maurin, G.; Waroquier, M.; Van Speybroeck, V. Thermodynamic insight into stimuli-responsive behaviour of soft porous crystals. Nat. Commun. 2018, 9, 204. [Google Scholar] [CrossRef]
- Chapman, K.W.; Halder, G.J.; Chupas, P.J. Guest-Dependent High Pressure Phenomena in a Nanoporous Metal−Organic Framework Material. J. Am. Chem. Soc. 2008, 130, 10524–10526. [Google Scholar] [CrossRef]
- Moggach, S.A.; Bennett, T.D.; Cheetham, A.K. The Effect of Pressure on ZIF-8: Increasing Pore Size with Pressure and the Formation of a High-Pressure Phase at 1.47 GPa. Angew. Chem. 2009, 121, 7221–7223. [Google Scholar] [CrossRef]
- Bennett, T.D.; Tan, J.-C.; Moggach, S.A.; Galvelis, R.; Mellot-Draznieks, C.; Reisner, B.A.; Thirumurugan, A.; Allan, D.R.; Cheetham, A.K. Mechanical Properties of Dense Zeolitic Imidazolate Frameworks (ZIFs): A High-Pressure X-ray Diffraction, Nanoindentation and Computational Study of the Zinc Framework Zn(Im)2, and its Lithium Boron Analogue, LiB(Im)4. Chem. Eur. J. 2010, 16, 10684–10690. [Google Scholar] [CrossRef]
- Tan, J.C.; Bennett, T.D.; Cheetham, A.K. Chemical structure, network topology, and porosity effects on the mechanical properties of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2010, 107, 9938–9943. [Google Scholar] [CrossRef]
- Tan, J.C.; Cheetham, A.K. Mechanical properties of hybrid inorganic–organic framework materials: Establishing fundamental structure–property relationships. Chem. Soc. Rev. 2011, 40, 1059. [Google Scholar] [CrossRef]
- Ghoufi, A.; Subercaze, A.; Ma, Q.; Yot, P.G.; Ke, Y.; Puente-Orench, I.; Devic, T.; Guillerm, V.; Zhong, C.; Serre, C.; et al. Comparative Guest, Thermal, and Mechanical Breathing of the Porous Metal Organic Framework MIL-53(Cr): A Computational Exploration Supported by Experiments. J. Phys. Chem. C 2012, 116, 13289–13295. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Boutin, A.; Fuchs, A.H.; Coudert, F.-X. Anisotropic Elastic Properties of Flexible Metal-Organic Frameworks: How Soft are Soft Porous Crystals? Phys. Rev. Lett. 2012, 109, 195502. [Google Scholar] [CrossRef]
- Yot, P.G.; Ma, Q.; Haines, J.; Yang, Q.; Ghoufi, A.; Devic, T.; Serre, C.; Dmitriev, V.; Férey, G.; Zhong, C.; et al. Large breathing of the MOF MIL-47(VIV) under mechanical pressure: A joint experimental–modelling exploration. Chem. Sci. 2012, 3, 1100. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Boutin, A.; Fuchs, A.H.; Coudert, F.-X. Metal–organic frameworks with wine-rack motif: What determines their flexibility and elastic properties? J. Chem. Phys. 2013, 138, 174703. [Google Scholar] [CrossRef]
- Yot, P.G.; Boudene, Z.; Macia, J.; Granier, D.; Vanduyfhuys, L.; Verstraelen, T.; Van Speybroeck, V.; Devic, T.; Serre, C.; Férey, G.; et al. Metal–organic frameworks as potential shock absorbers: The case of the highly flexible MIL-53(Al). Chem. Commun. 2014, 50, 9462–9464. [Google Scholar] [CrossRef] [PubMed]
- Yot, P.G.; Vanduyfhuys, L.; Alvarez, E.; Rodriguez, J.; Itié, J.-P.; Fabry, P.; Guillou, N.; Devic, T.; Beurroies, I.; Llewellyn, P.L.; et al. Mechanical energy storage performance of an aluminum fumarate metal–organic framework. Chem. Sci. 2016, 7, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Yot, P.G.; Yang, K.; Guillerm, V.; Ragon, F.; Dmitriev, V.; Parisiades, P.; Elkaïm, E.; Devic, T.; Horcajada, P.; Serre, C.; et al. Impact of the Metal Centre and Functionalization on the Mechanical Behaviour of MIL-53 Metal-Organic Frameworks: Impact of the Metal Centre and Functionalization on the Mechanical Behaviour of MIL-53 Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4424–4429. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wieme, J.; Alvarez, E.; Vanduyfhuys, L.; Itié, J.-P.; Fabry, P.; Van Speybroeck, V.; Serre, C.; Yot, P.G.; Maurin, G. Mechanical properties of a gallium fumarate metal–organic framework: A joint experimental-modelling exploration. J. Mater. Chem. A 2017, 5, 11047–11054. [Google Scholar] [CrossRef]
- Henke, S.; Wharmby, M.T.; Kieslich, G.; Hante, I.; Schneemann, A.; Wu, Y.; Daisenberger, D.; Cheetham, A.K. Pore closure in zeolitic imidazolate frameworks under mechanical pressure. Chem. Sci. 2018, 9, 1654–1660. [Google Scholar] [CrossRef]
- Krause, S.; Evans, J.D.; Bon, V.; Senkovska, I.; Ehrling, S.; Stoeck, U.; Yot, P.G.; Iacomi, P.; Llewellyn, P.; Maurin, G.; et al. Adsorption Contraction Mechanics: Understanding Breathing Energetics in Isoreticular Metal–Organic Frameworks. J. Phys. Chem. C 2018, 122, 19171–19179. [Google Scholar] [CrossRef]
- Wahiduzzaman, M.; Reimer, N.; Itié, J.-P.; Stock, N.; Maurin, G.; Yot, P.G. Mechanical-pressure induced response of the MOF Al-MIL-53-TDC. Polyhedron 2018, 155, 144–148. [Google Scholar] [CrossRef]
- Yot, P.G.; Wahiduzzaman, M.; Elkaim, E.; Fertey, P.; Fabry, P.; Serre, C.; Maurin, G. Modulation of the mechanical energy storage performance of the MIL-47(VIV) metal organic framework by ligand functionalization. Dalton Trans. 2019, 48, 1656–1661. [Google Scholar] [CrossRef]
- Krause, S.; Evans, J.D.; Bon, V.; Senkovska, I.; Iacomi, P.; Kolbe, F.; Ehrling, S.; Troschke, E.; Getzschmann, J.; Többens, D.M.; et al. Towards general network architecture design criteria for negative gas adsorption transitions in ultraporous frameworks. Nat. Commun. 2019, 10, 3632. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Bon, V.; Brunner, E.; Pöppl, A.; Kaskel, S. Unraveling Structure and Dynamics in Porous Frameworks via Advanced in Situ Characterization Techniques. Adv. Funct. Mater. 2020, 1907847. [Google Scholar] [CrossRef]
- Serre, C.; Bourrelly, S.; Vimont, A.; Ramsahye, N.A.; Maurin, G.; Llewellyn, P.L.; Daturi, M.; Filinchuk, Y.; Leynaud, O.; Barnes, P.; et al. An Explanation for the Very Large Breathing Effect of a Metal–Organic Framework during CO2 Adsorption. Adv. Mater. 2007, 19, 2246–2251. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Leubner, S.; Stäglich, R.; Franke, J.; Jacobsen, J.; Gosch, J.; Siegel, R.; Reinsch, H.; Maurin, G.; Yot, P.; Senker, J.; et al. Solvent Impact on the Framework Properties of Benchmark MOFs: Acetonitrile Based Synthesis of CAU-10, Ce-UiO-66 and Al-MIL-53. Chem. Eur. J. 2020. [Google Scholar] [CrossRef]
- Loiseau, T.; Mellot-Draznieks, C.; Muguerra, H.; Férey, G.; Haouas, M.; Taulelle, F. Hydrothermal synthesis and crystal structure of a new three-dimensional aluminum-organic framework MIL-69 with 2,6-naphthalenedicarboxylate (ndc), Al(OH)(ndc)·H2O. Comptes Rendus Chim. 2005, 8, 765–772. [Google Scholar] [CrossRef]
- Alvarez, E.; Guillou, N.; Martineau, C.; Bueken, B.; Van de Voorde, B.; Le Guillouzer, C.; Fabry, P.; Nouar, F.; Taulelle, F.; de Vos, D.; et al. The Structure of the Aluminum Fumarate Metal-Organic Framework A520. Angew. Chem. Int. Ed. 2015, 54, 3664–3668. [Google Scholar] [CrossRef]
- Tschense, C.B.L.; Reimer, N.; Hsu, C.-W.; Reinsch, H.; Siegel, R.; Chen, W.-J.; Lin, C.-H.; Cadiau, A.; Serre, C.; Senker, J.; et al. New Group 13 MIL-53 Derivates based on 2,5-Thiophenedicarboxylic Acid: New Group 13 MIL-53 Derivates based on 2,5-Thiophenedicarboxylic Acid. Z. Anorg. Allg. Chem. 2017, 643, 1600–1608. [Google Scholar] [CrossRef]
- Millange, F.; Walton, R.I. MIL-53 and its Isoreticular Analogues: A Review of the Chemistry and Structure of a Prototypical Flexible Metal-Organic Framework. Isr. J. Chem. 2018, 58, 1019–1035. [Google Scholar] [CrossRef]
- Liu, Y.; Her, J.-H.; Dailly, A.; Ramirez-Cuesta, A.J.; Neumann, D.A.; Brown, C.M. Reversible Structural Transition in MIL-53 with Large Temperature Hysteresis. J. Am. Chem. Soc. 2008, 130, 11813–11818. [Google Scholar] [CrossRef]
- Kolokolov, D.I.; Jobic, H.; Stepanov, A.G.; Plazanet, M.; Zbiri, M.; Ollivier, J.; Guillerm, V.; Devic, T.; Serre, C.; Férey, G. Comparison of the dynamics of MIL-53(Cr) and MIL-47(V) frameworks using neutron scattering and DFT methods. Eur. Phys. J. Spec. Top. 2010, 189, 263–271. [Google Scholar] [CrossRef]
- Ghoufi, A.; Benhamed, K.; Boukli-Hacene, L.; Maurin, G. Electrically Induced Breathing of the MIL-53(Cr) Metal–Organic Framework. ACS Cent. Sci. 2017, 3, 394–398. [Google Scholar] [CrossRef]
- Dürholt, J.P.; Jahromi, B.F.; Schmid, R. Tuning the Electric Field Response of MOFs by Rotatable Dipolar Linkers. ACS Cent. Sci. 2019, 5, 1440–1448. [Google Scholar] [CrossRef]
- Bueken, B.; Vermoortele, F.; Cliffe, M.J.; Wharmby, M.T.; Foucher, D.; Wieme, J.; Vanduyfhuys, L.; Martineau, C.; Stock, N.; Taulelle, F.; et al. A Breathing Zirconium Metal-Organic Framework with Reversible Loss of Crystallinity by Correlated Nanodomain Formation. Chem. Eur. J. 2016, 22, 3264–3267. [Google Scholar] [CrossRef]
- Niekiel, F.; Ackermann, M.; Guerrier, P.; Rothkirch, A.; Stock, N. Aluminum-1,4-cyclohexanedicarboxylates: High-Throughput and Temperature-Dependent in Situ EDXRD Studies. Inorg. Chem. 2013, 52, 8699–8705. [Google Scholar] [CrossRef]
- Niekiel, F.; Lannoeye, J.; Reinsch, H.; Munn, A.S.; Heerwig, A.; Zizak, I.; Kaskel, S.; Walton, R.I.; de Vos, D.; Llewellyn, P.; et al. Conformation-Controlled Sorption Properties and Breathing of the Aliphatic Al-MOF [Al(OH)(CDC)]. Inorg. Chem. 2014, 53, 4610–4620. [Google Scholar] [CrossRef]
- Reinsch, H.; Benecke, J.; Etter, M.; Heidenreich, N.; Stock, N. Combined in- and ex situ studies of pyrazine adsorption into the aliphatic MOF Al-CAU-13: Structures, dynamics and correlations. Dalton Trans. 2017, 46, 1397–1405. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Boutin, A.; Coudert, F.-X. Prediction of flexibility of metal–organic frameworks CAU-13 and NOTT-300 by first principles molecular simulations. Chem. Commun. 2014, 50, 5867. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J. Appl. Cryst. 1991, 24, 987–993. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Cryst. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Filik, J.; Ashton, A.W.; Chang, P.C.Y.; Chater, P.A.; Day, S.J.; Drakopoulos, M.; Gerring, M.W.; Hart, M.L.; Magdysyuk, O.V.; Michalik, S.; et al. Processing two-dimensional X-ray diffraction and small-angle scattering data in DAWN 2. J. Appl. Cryst. 2017, 50, 959–966. [Google Scholar] [CrossRef]
- Syassen, K. Ruby under pressure. High Press. Res. 2008, 28, 75–126. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Dewaele, A. Equations of state of MgO, Au, Pt, NaCl-B1, and NaCl-B2: Internally consistent high-temperature pressure scales. High Press. Res. 2007, 27, 431–446. [Google Scholar] [CrossRef]
- Dewaele, A.; Belonoshko, A.B.; Garbarino, G.; Occelli, F.; Bouvier, P.; Hanfland, M.; Mezouar, M. High-pressure—High-temperature equation of state of KCl and KBr. Phys. Rev. B 2012, 85, 214105. [Google Scholar] [CrossRef]
- Berman, R.G. Internally-consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO-Fe3O2-Al2O3-SiO2-TiO2-H2O-CO2. J. Petrol. 1988, 29, 445–522. [Google Scholar] [CrossRef]
- Serra-Crespo, P.; Dikhtiarenko, A.; Stavitski, E.; Juan-Alcañiz, J.; Kapteijn, F.; Coudert, F.-X.; Gascon, J. Experimental evidence of negative linear compressibility in the MIL-53 metal–organic framework family. CrystEngComm 2015, 17, 276–280. [Google Scholar] [CrossRef]
- Haines, J.; Cambon, O.; Levelut, C.; Santoro, M.; Gorelli, F.; Garbarino, G. Deactivation of Pressure-Induced Amorphization in Silicalite SiO2 by Insertion of Guest Species. J. Am. Chem. Soc. 2010, 132, 8860–8861. [Google Scholar] [CrossRef]
- Vinet, P.; Ferrante, J.; Rose, J.H.; Smith, J.R. Compressibility of solids. J. Geophys. Res. 1987, 92, 9319. [Google Scholar] [CrossRef]
- Graham, A.J.; Allan, D.R.; Muszkiewicz, A.; Morrison, C.A.; Moggach, S.A. The Effect of High Pressure on MOF-5: Guest-Induced Modification of Pore Size and Content at High Pressure. Angew. Chem. Int. Ed. 2011, 50, 11138–11141. [Google Scholar] [CrossRef]
- Dissegna, S.; Vervoorts, P.; Hobday, C.L.; Düren, T.; Daisenberger, D.; Smith, A.J.; Fischer, R.A.; Kieslich, G. Tuning the Mechanical Response of Metal–Organic Frameworks by Defect Engineering. J. Am. Chem. Soc. 2018, 140, 11581–11584. [Google Scholar] [CrossRef]
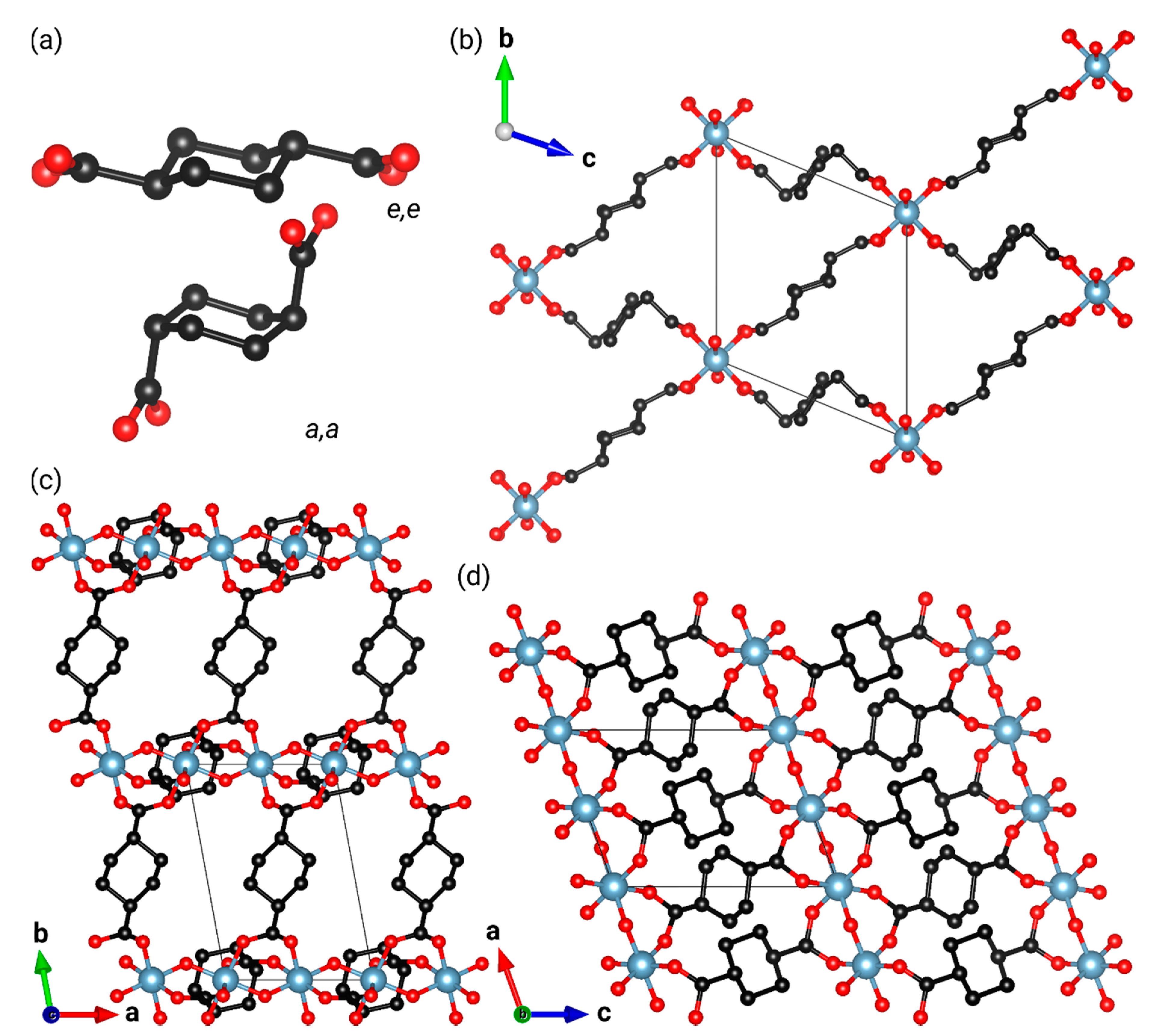
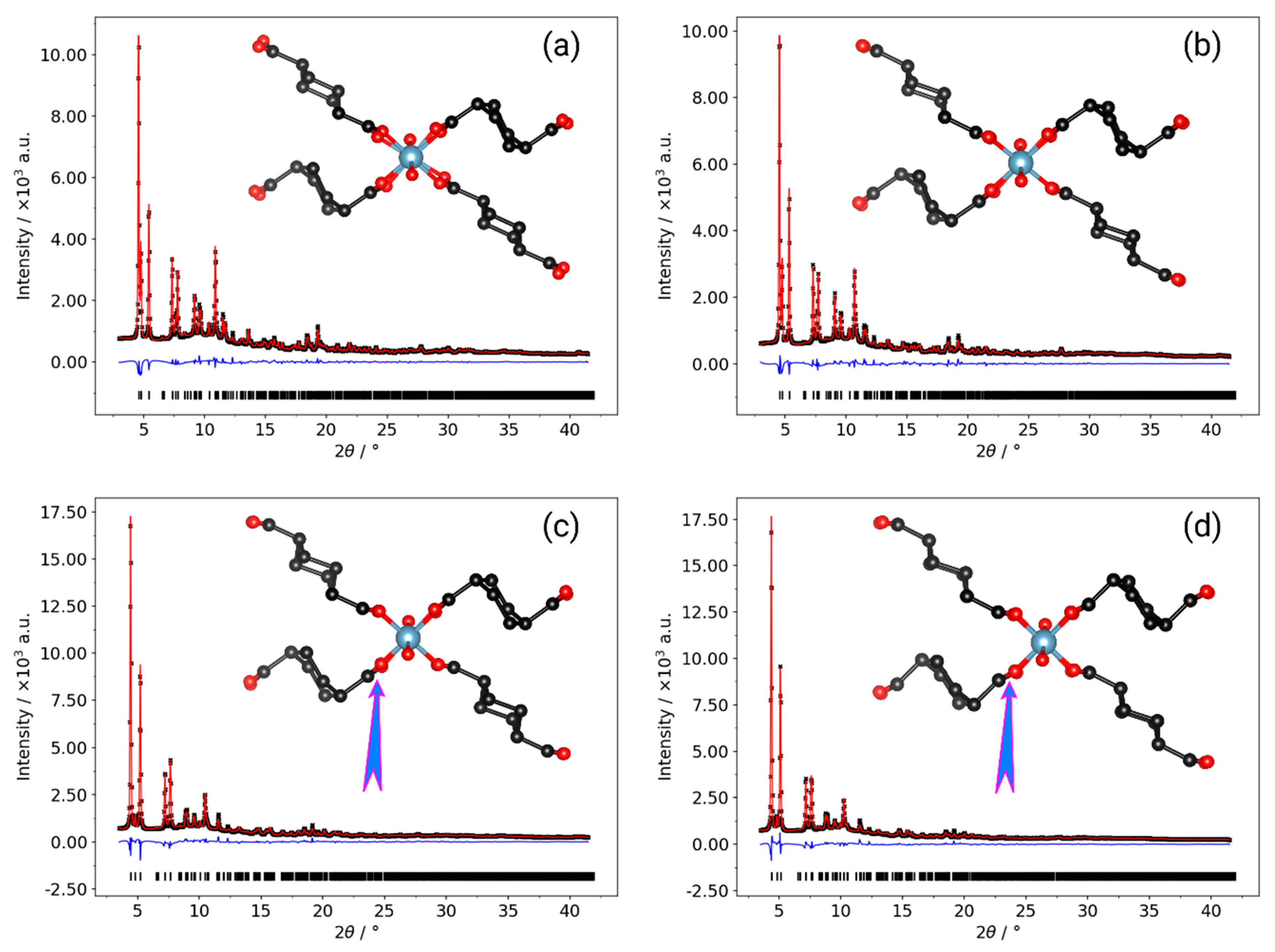
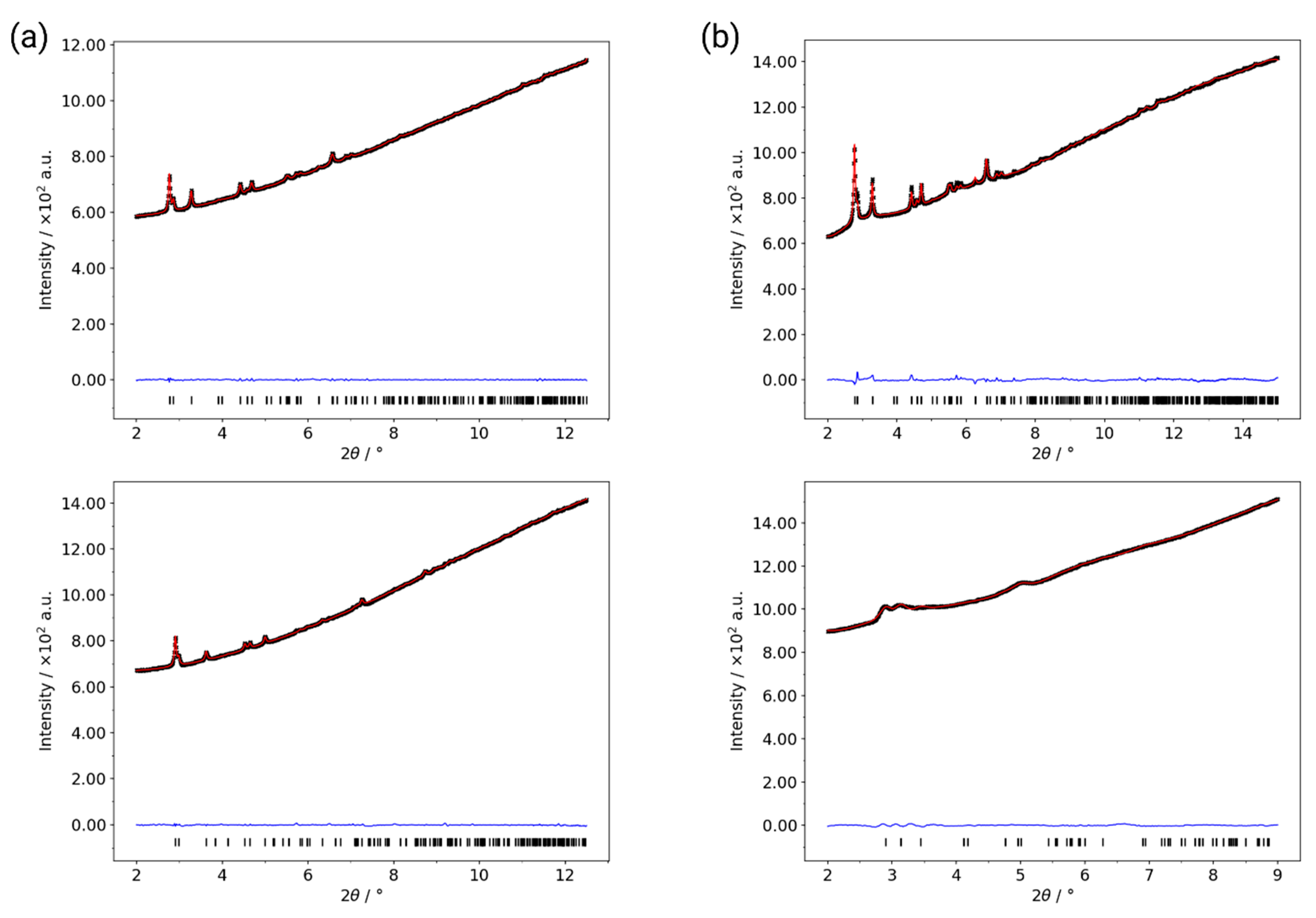
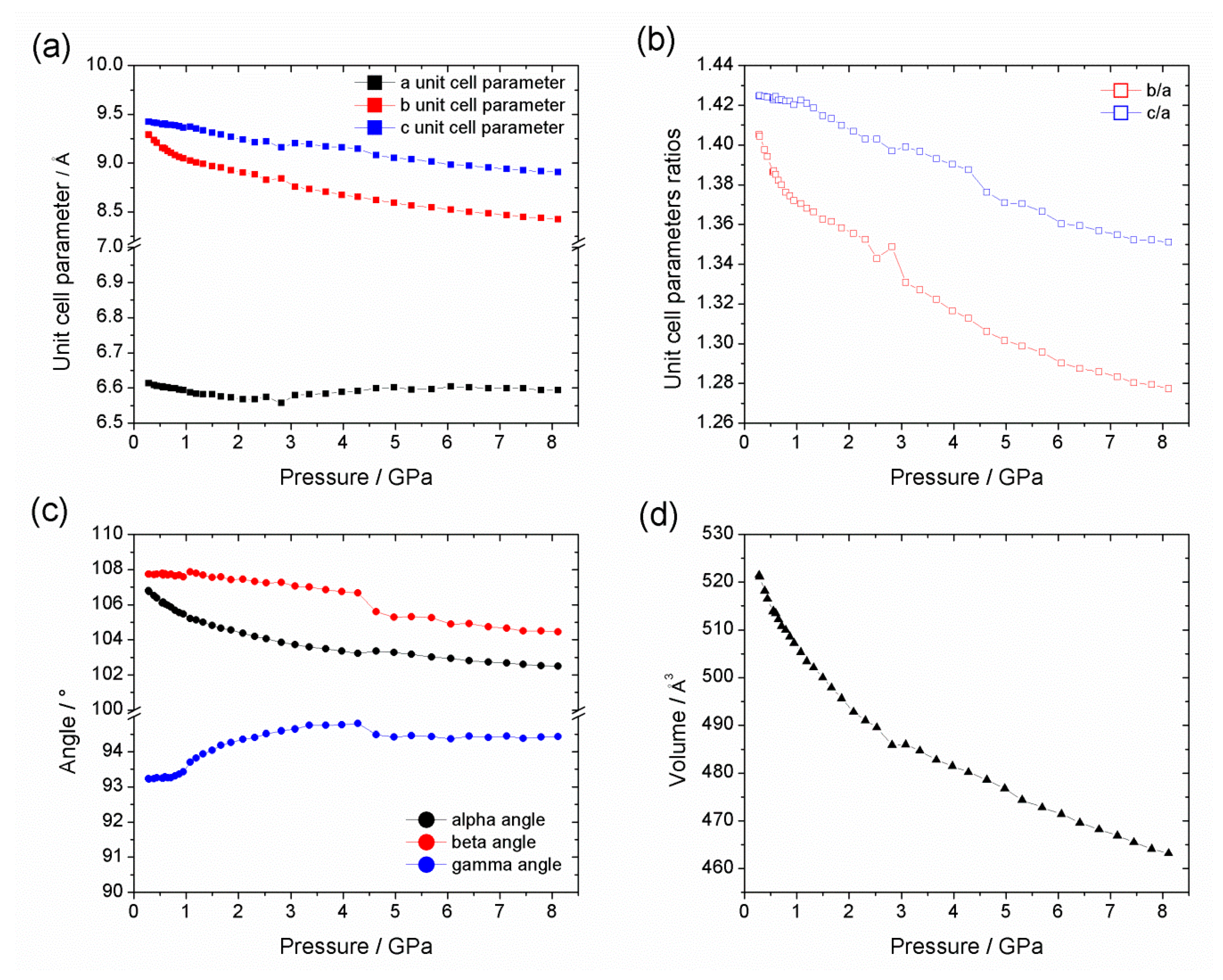
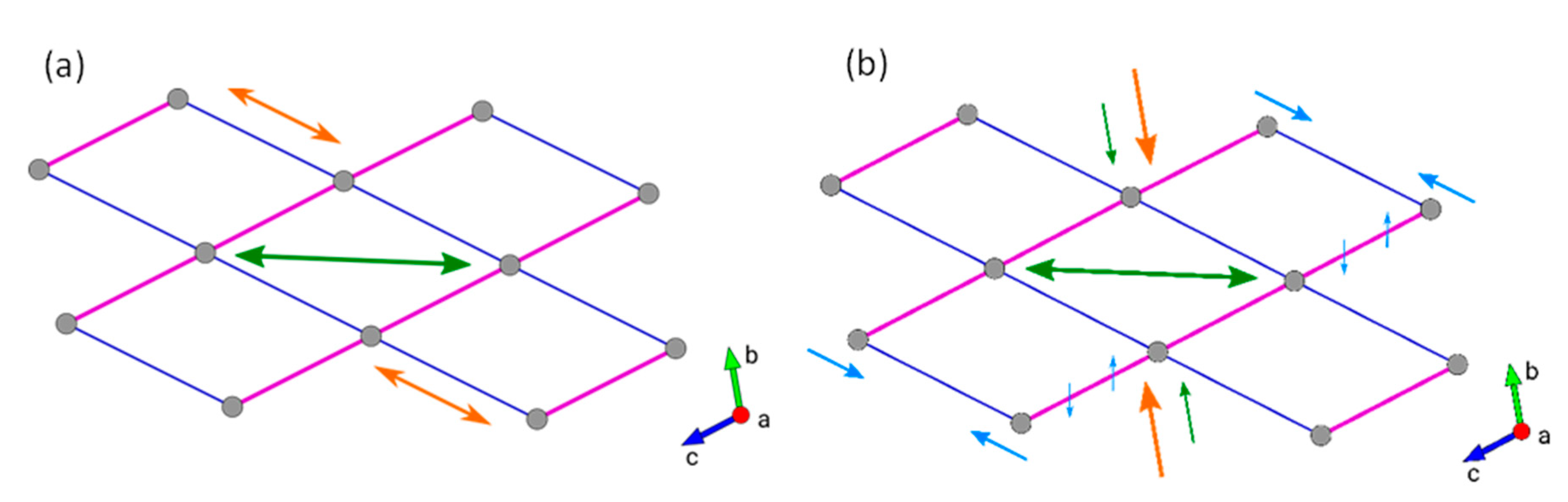
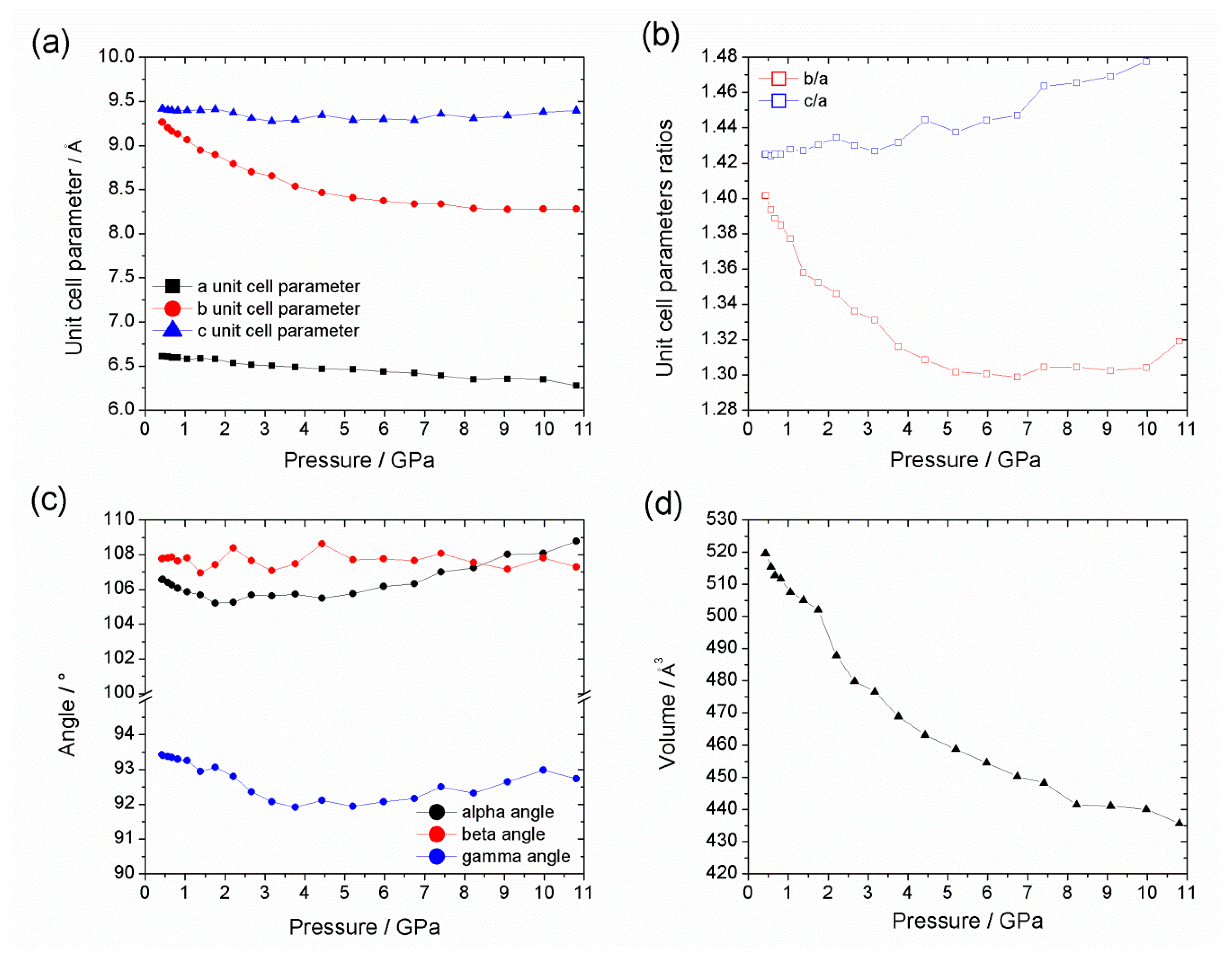
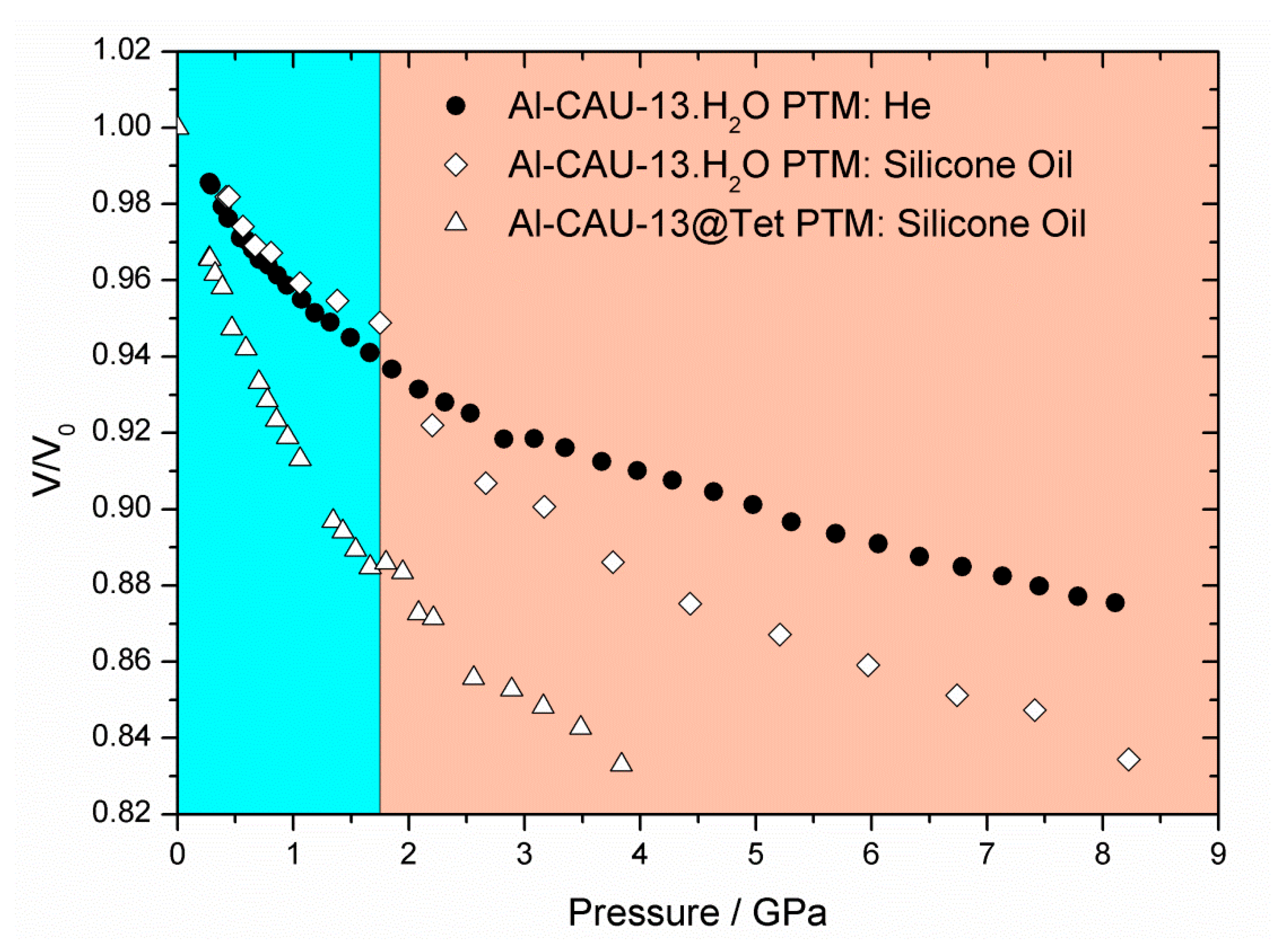
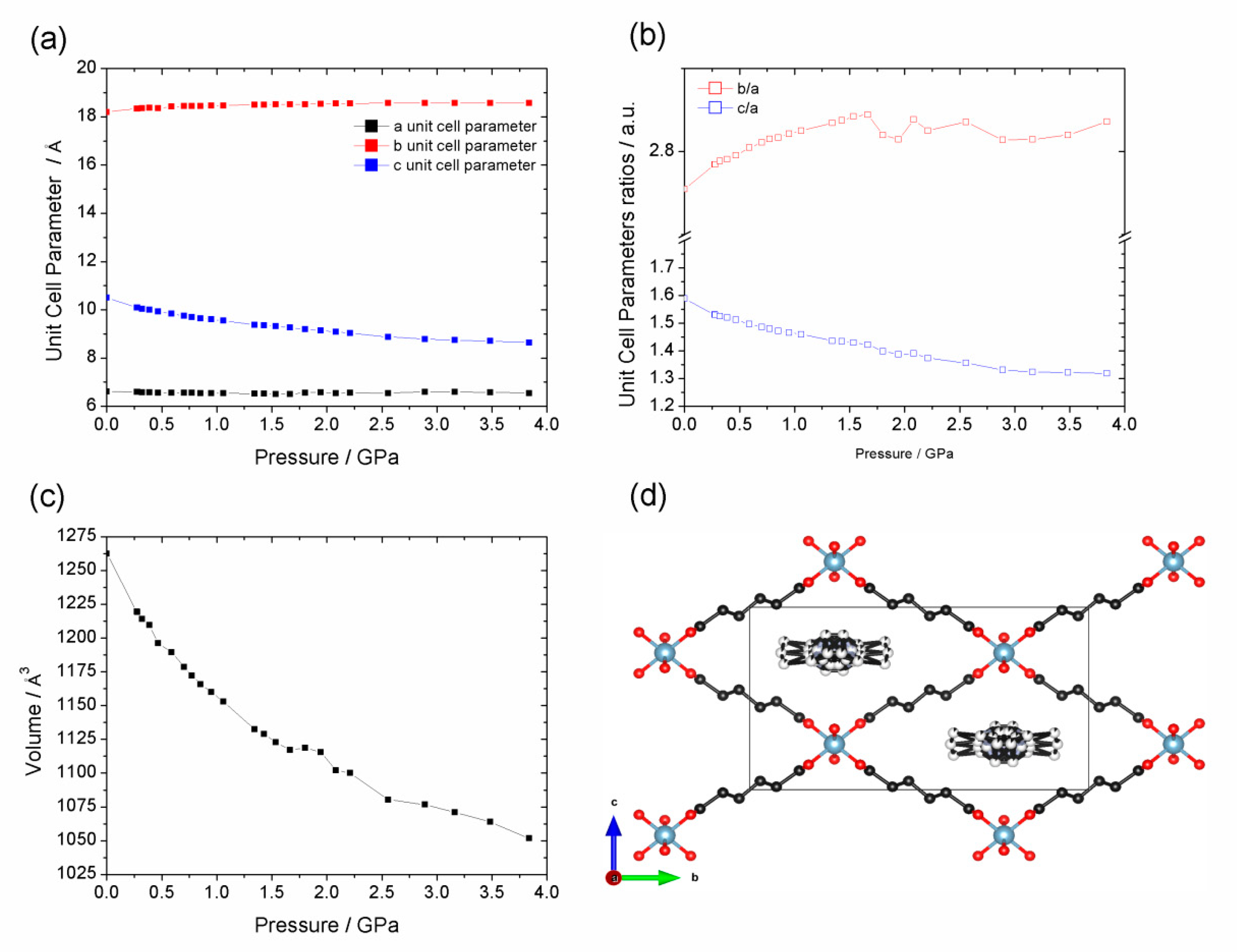
| Al-CAU-13·H2O | Al-CAU-13 | |||
|---|---|---|---|---|
| Formula | [Al(OH)(C8H10O4)] 1.47H2O | [Al(OH)(C8H10O4)] 1.29H2O | [Al(OH)(C8H10O4)] | |
| Temp./K | 82 | 316 | 367 | 500 |
| Space Group | ||||
| Z | 2 | 2 | 2 | 2 |
| a/Å | 6.6177(4) | 6.6197(6) | 6.6252(8) | 6.6207(12) |
| b/Å | 9.3651(7) | 9.5103(10) | 9.8233(15) | 10.027(2) |
| c/Å | 9.4371(6) | 9.4896(8) | 9.4899(11) | 9.5375(15) |
| α/° | 107.097(4) | 107.980(6) | 109.229(8) | 110.492(10) |
| β/° | 107.915(6) | 107.636(10) | 107.355(12) | 107.054(18) |
| γ/° | 93.336(3) | 93.161(9) | 93.407(10) | 93.335(13) |
| V/Å3 | 524.76(7) | 534.21(10) | 548.03(14) | 557.78(19) |
| Rwp | 2.77 | 3.23 | 3.62 | 3.33 |
| RBragg | 2.55 | 2.21 | 3.01 | 1.87 |
| GoF * | 27.308 | 29.470 | 34.287 | 31.875 |
| Temperature Range (K) | V0/Å3 | α0/× 10−5 K−1 | α1/× 10−8 K−2 | |
|---|---|---|---|---|
| Al-CAU-13·H2O | 84–190 | 524.7s(1) at 84 K | 5.1(1) | 9.8(1) |
| 190–316 | 533.1(1) at 298 K | 15.7(3) | 118.1(1) | |
| Al-CAU-13 | 378–500 | 549.8(1) at 380 K | 20.5(3) | −126.3(1) |
| Pressure Range (GPa) | V0/Å3 | K0/GPa | K’ | |
|---|---|---|---|---|
| Al-CAU-13·H2O (He) | 0.28–8.11 | 521.16 | 25.08 | 17.49 |
| Al-CAU-13·H2O (Sil. Oil) | 0.42–1.75 | 525.64 | 24.55 | 17.49 |
| 1.75–7.41 | 547.49 | 8.93 | 14.01 | |
| Al-CAU-13@Tet (Sil. Oil) | 0.27–3.84 | 1262.54 | 6.19 | 13.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wharmby, M.T.; Niekiel, F.; Benecke, J.; Waitschat, S.; Reinsch, H.; Daisenberger, D.; Stock, N.; Yot, P.G. Influence of Thermal and Mechanical Stimuli on the Behavior of Al-CAU-13 Metal–Organic Framework. Nanomaterials 2020, 10, 1698. https://doi.org/10.3390/nano10091698
Wharmby MT, Niekiel F, Benecke J, Waitschat S, Reinsch H, Daisenberger D, Stock N, Yot PG. Influence of Thermal and Mechanical Stimuli on the Behavior of Al-CAU-13 Metal–Organic Framework. Nanomaterials. 2020; 10(9):1698. https://doi.org/10.3390/nano10091698
Chicago/Turabian StyleWharmby, Michael T., Felicitas Niekiel, Jannik Benecke, Steve Waitschat, Helge Reinsch, Dominik Daisenberger, Norbert Stock, and Pascal G. Yot. 2020. "Influence of Thermal and Mechanical Stimuli on the Behavior of Al-CAU-13 Metal–Organic Framework" Nanomaterials 10, no. 9: 1698. https://doi.org/10.3390/nano10091698
APA StyleWharmby, M. T., Niekiel, F., Benecke, J., Waitschat, S., Reinsch, H., Daisenberger, D., Stock, N., & Yot, P. G. (2020). Influence of Thermal and Mechanical Stimuli on the Behavior of Al-CAU-13 Metal–Organic Framework. Nanomaterials, 10(9), 1698. https://doi.org/10.3390/nano10091698