Facile Fabrication of CeO2/Electrochemically Reduced Graphene Oxide Nanocomposites for Vanillin Detection in Commercial Food Products
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Apparatus
2.3. Synthesis of CeO2 Nanoparticles
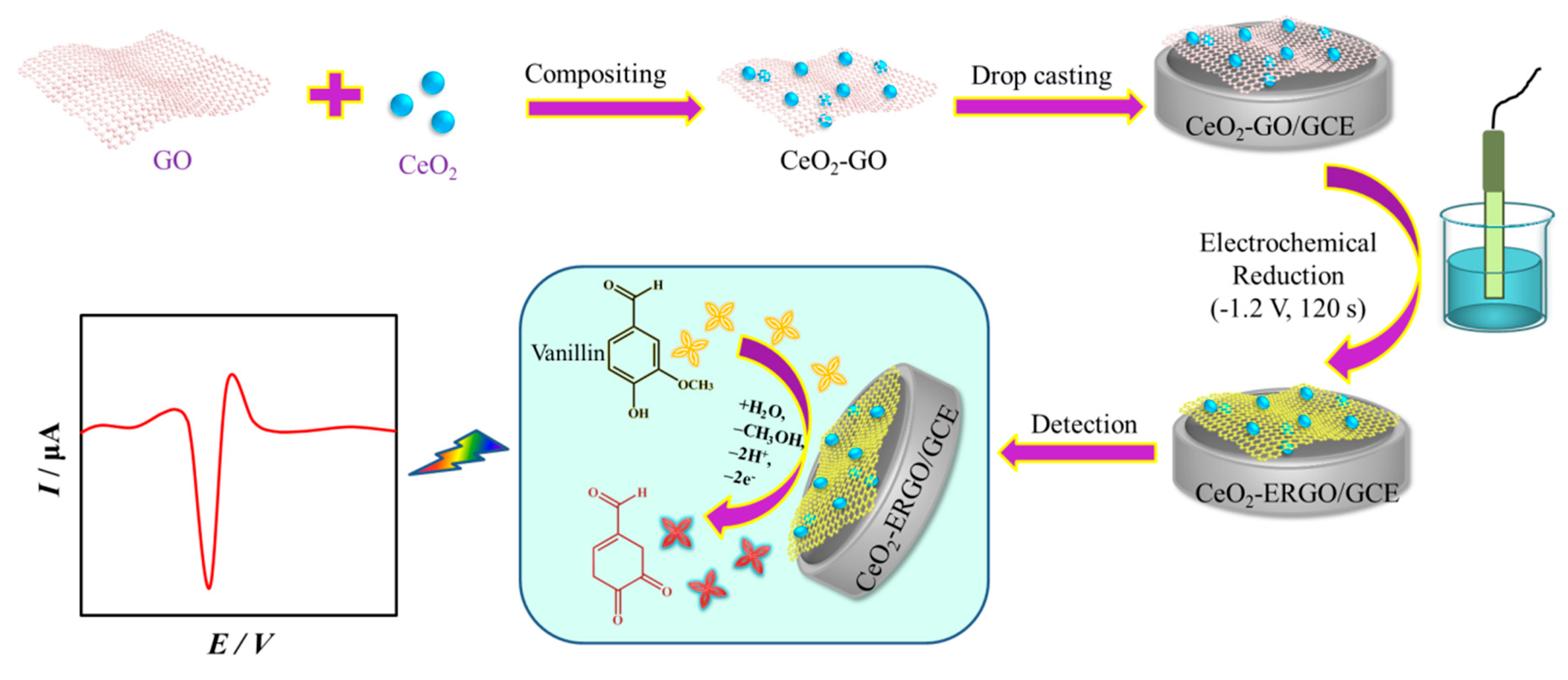
2.4. Preparation of GO and CeO2-GO Composite
2.5. Fabrication of Modified Electrodes
2.6. Electrochemical Measurement
3. Results and Discussion
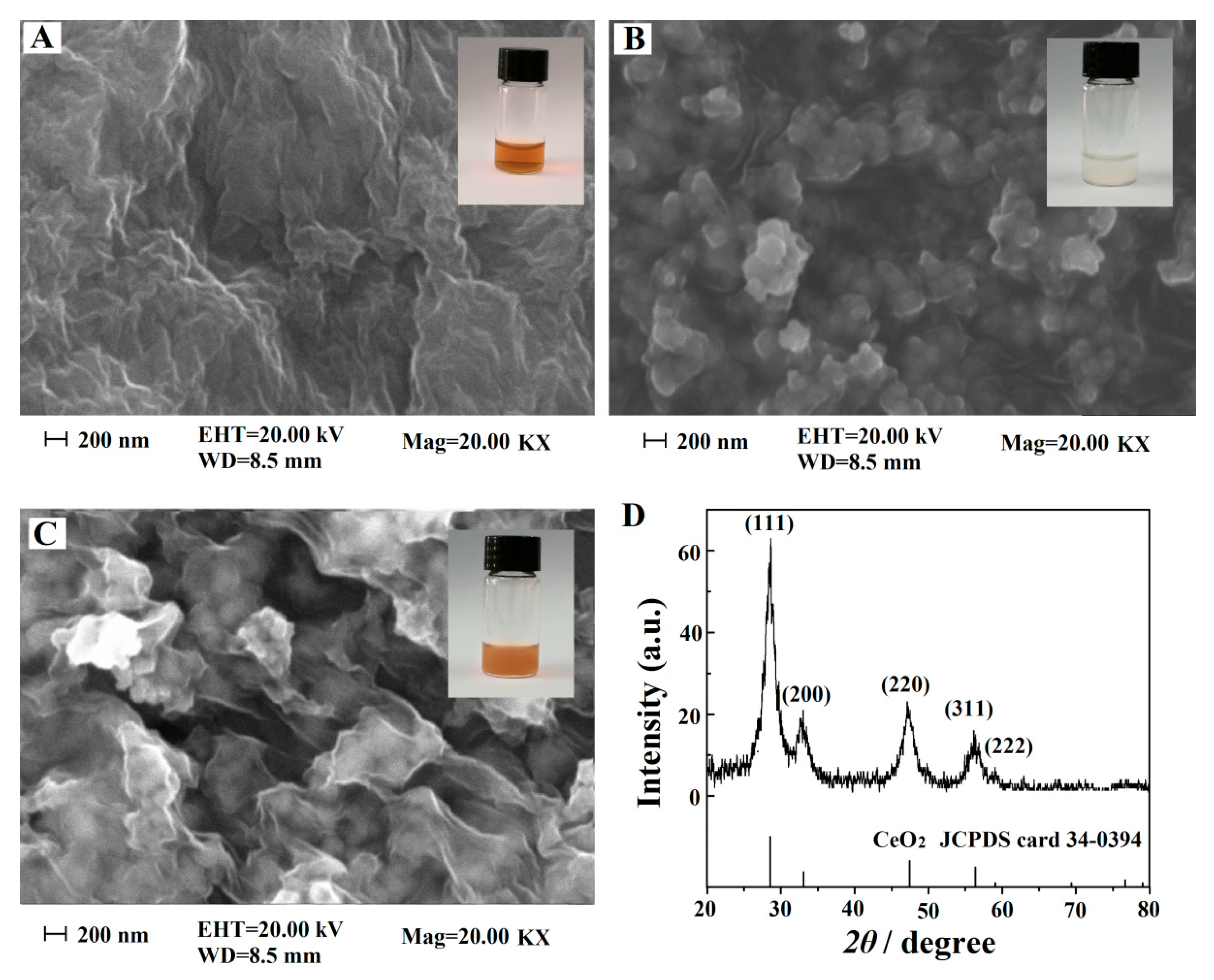
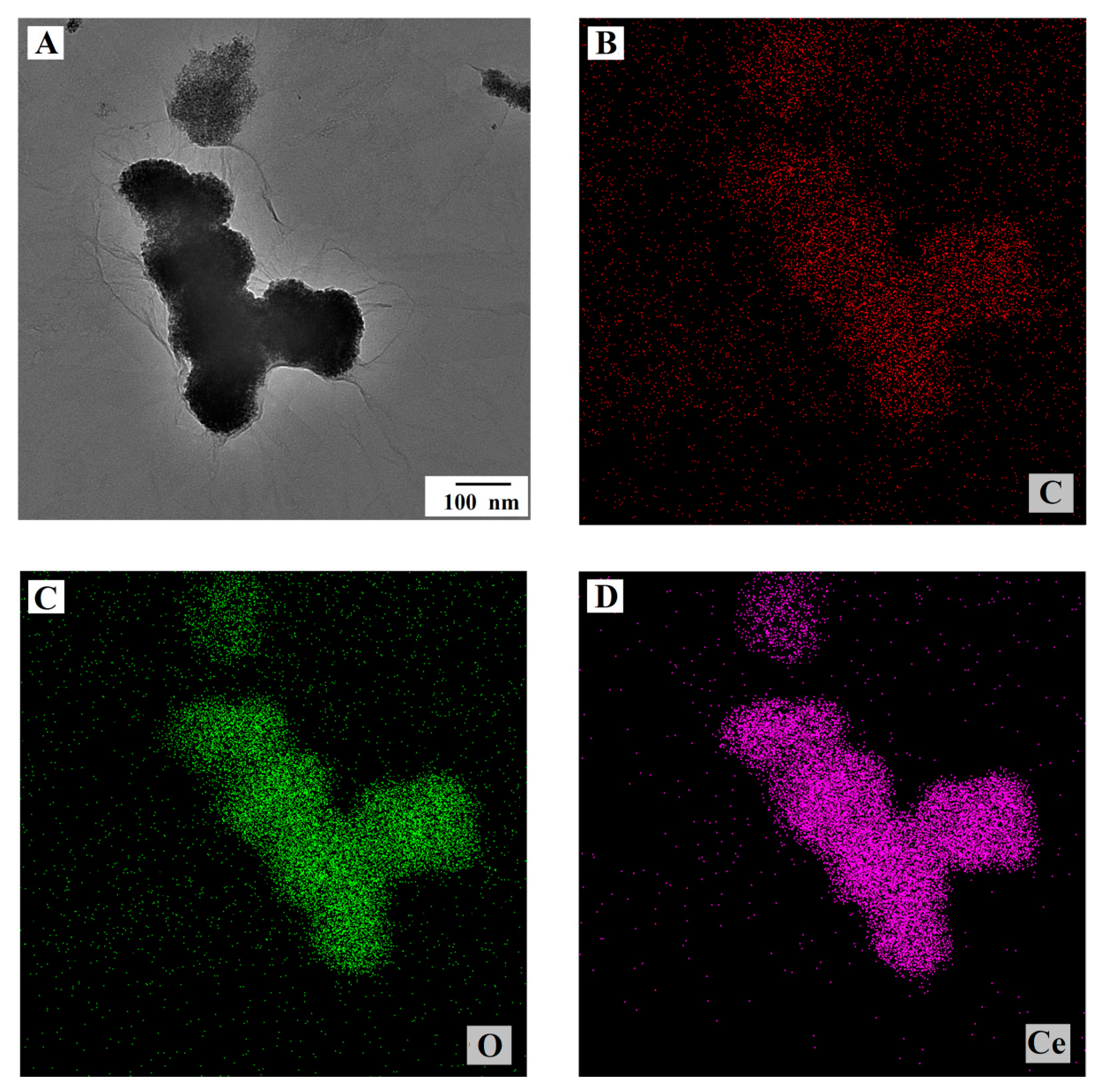
3.1. Morphological and Structural Characterizations
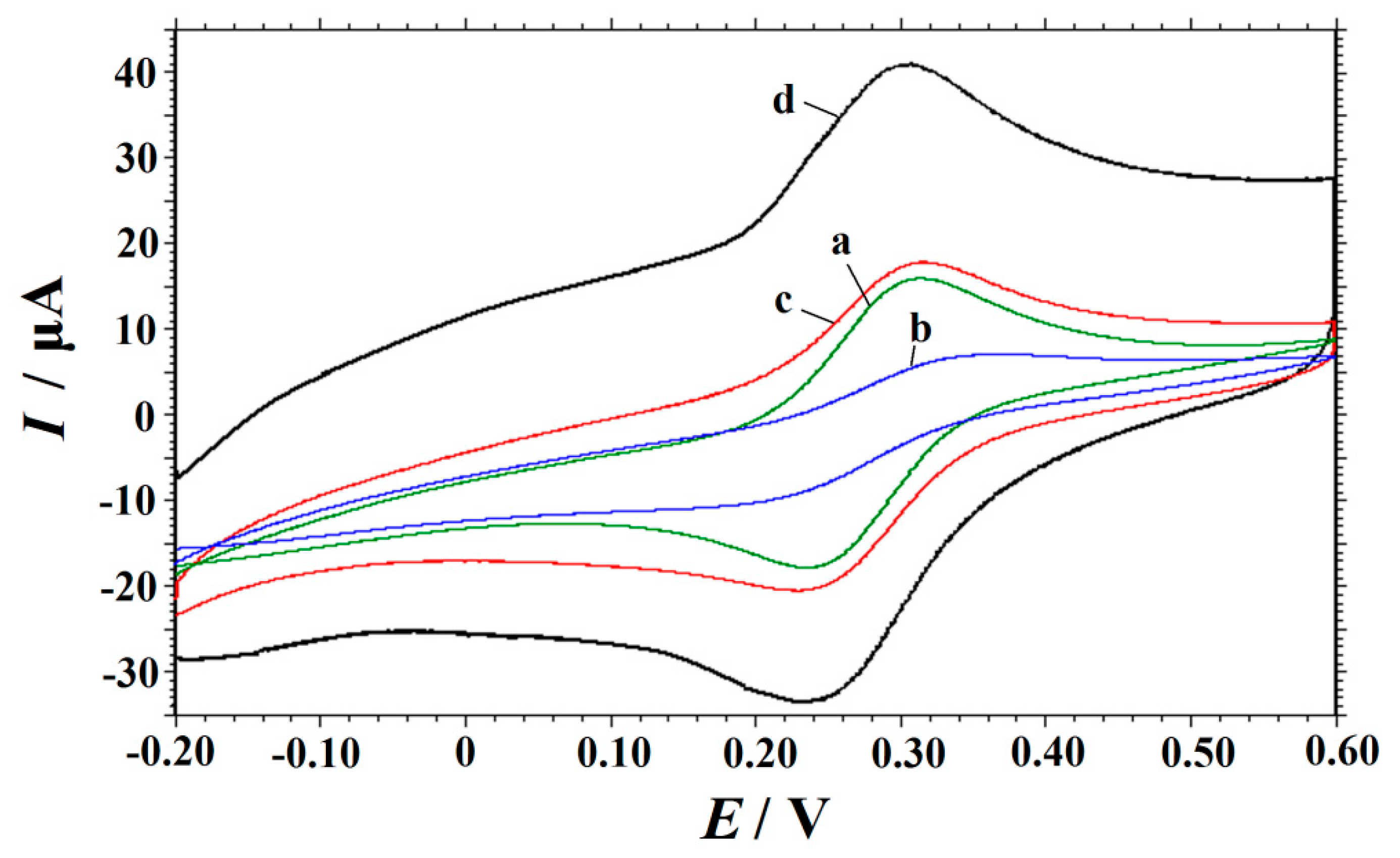
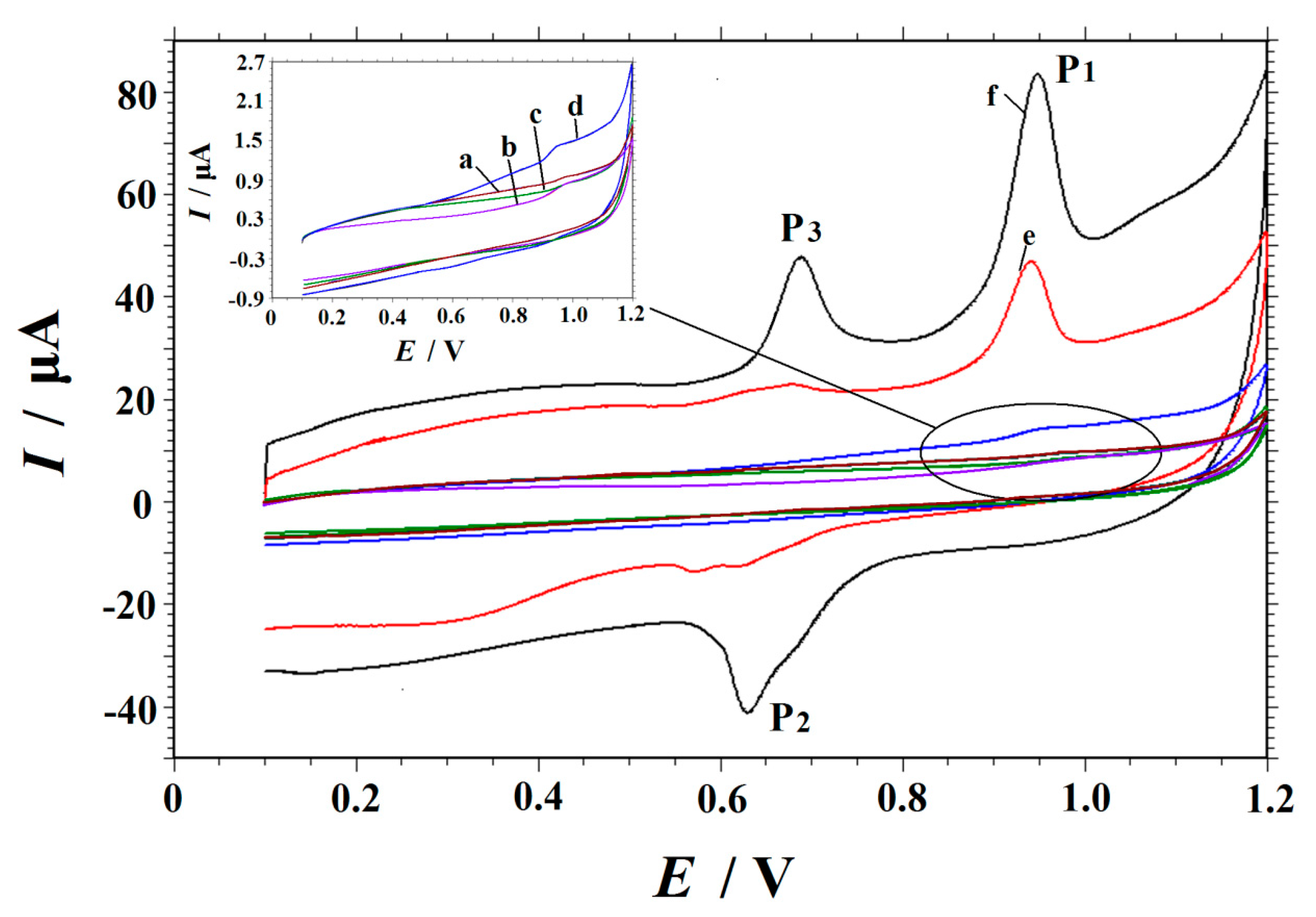
3.2. Electrochemical Characterization of the Modified Electrodes
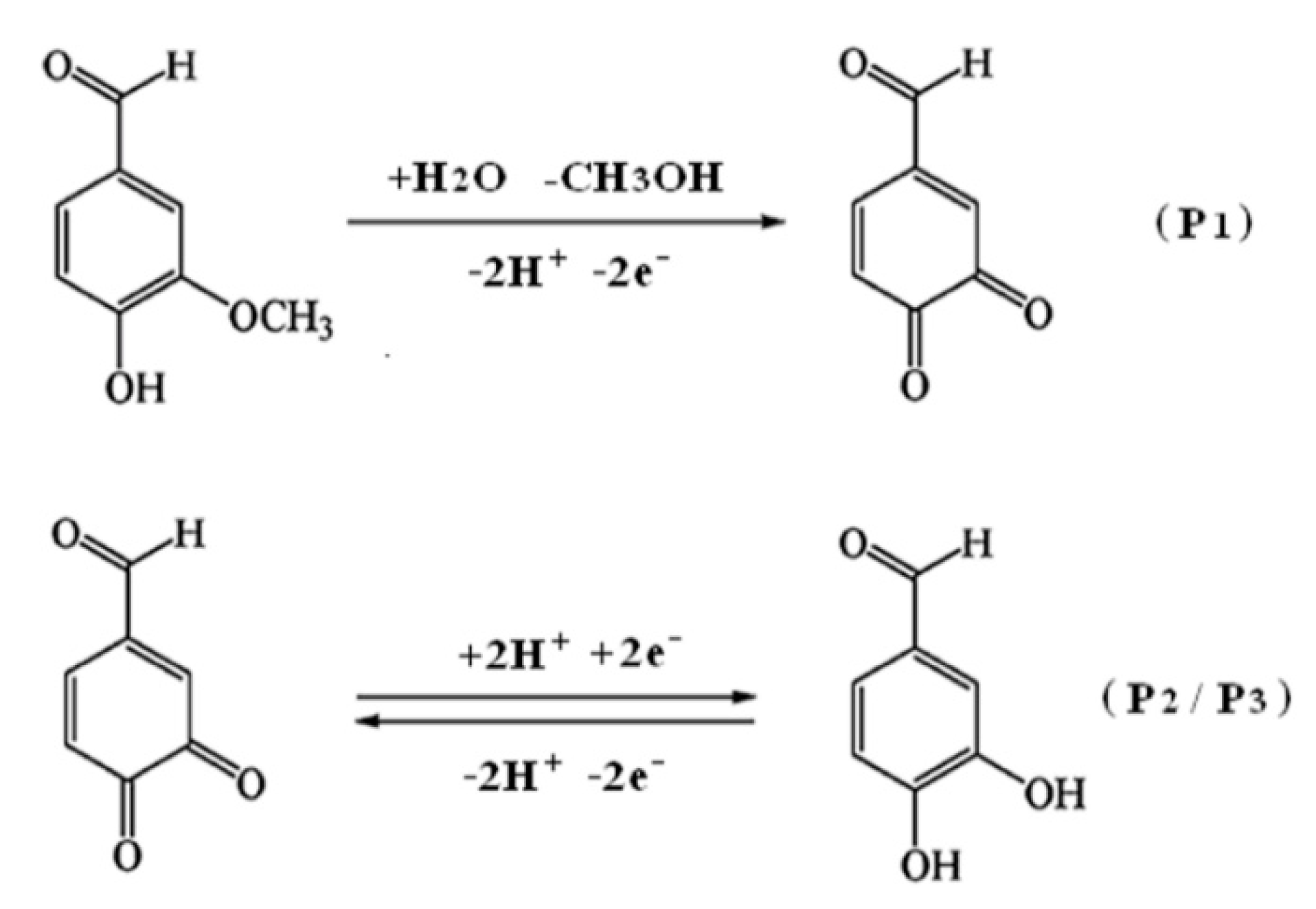
3.3. Electrochemical Oxidation of Vanillin at Different Electrodes
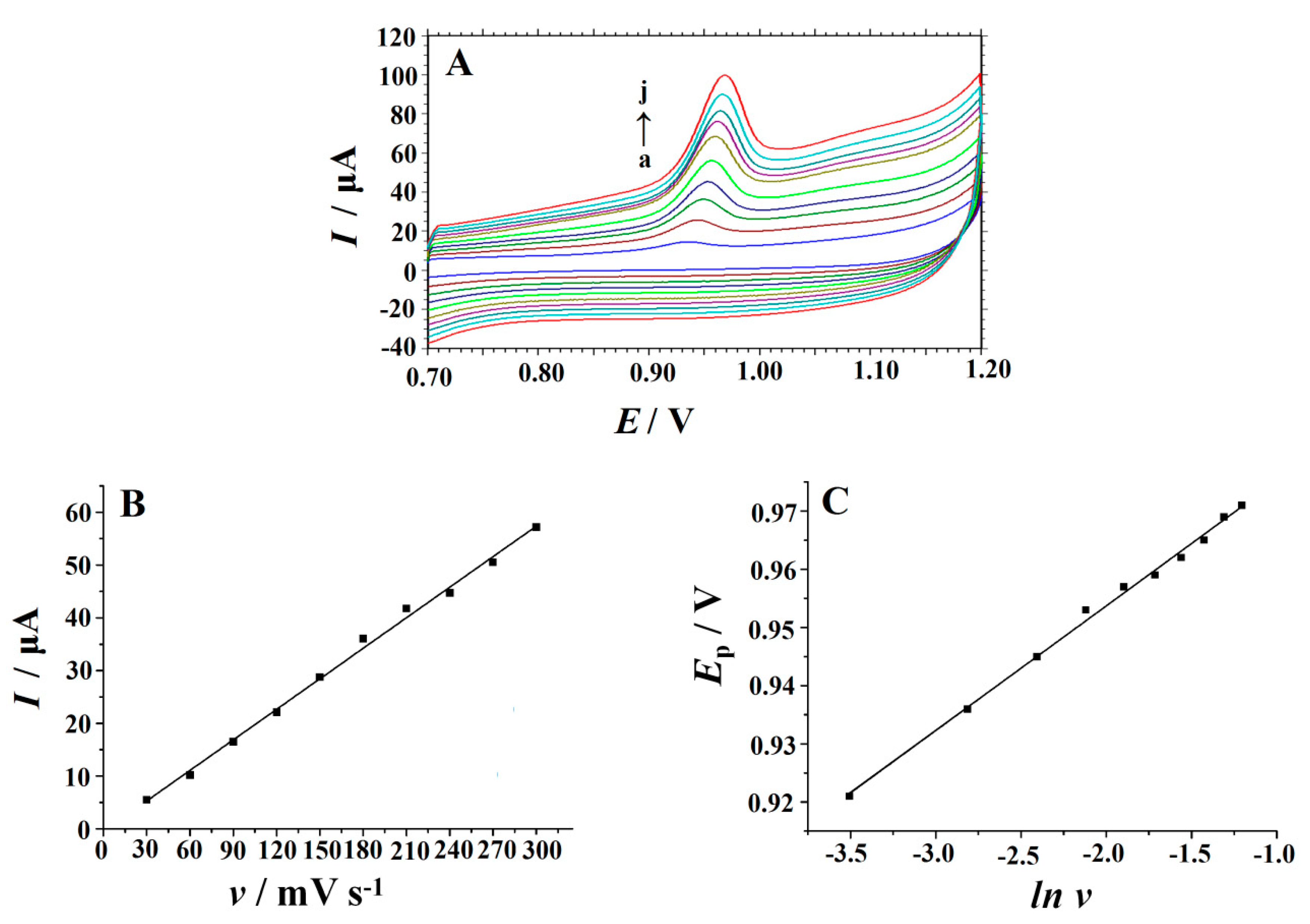
3.4. Effect of Scan Rate
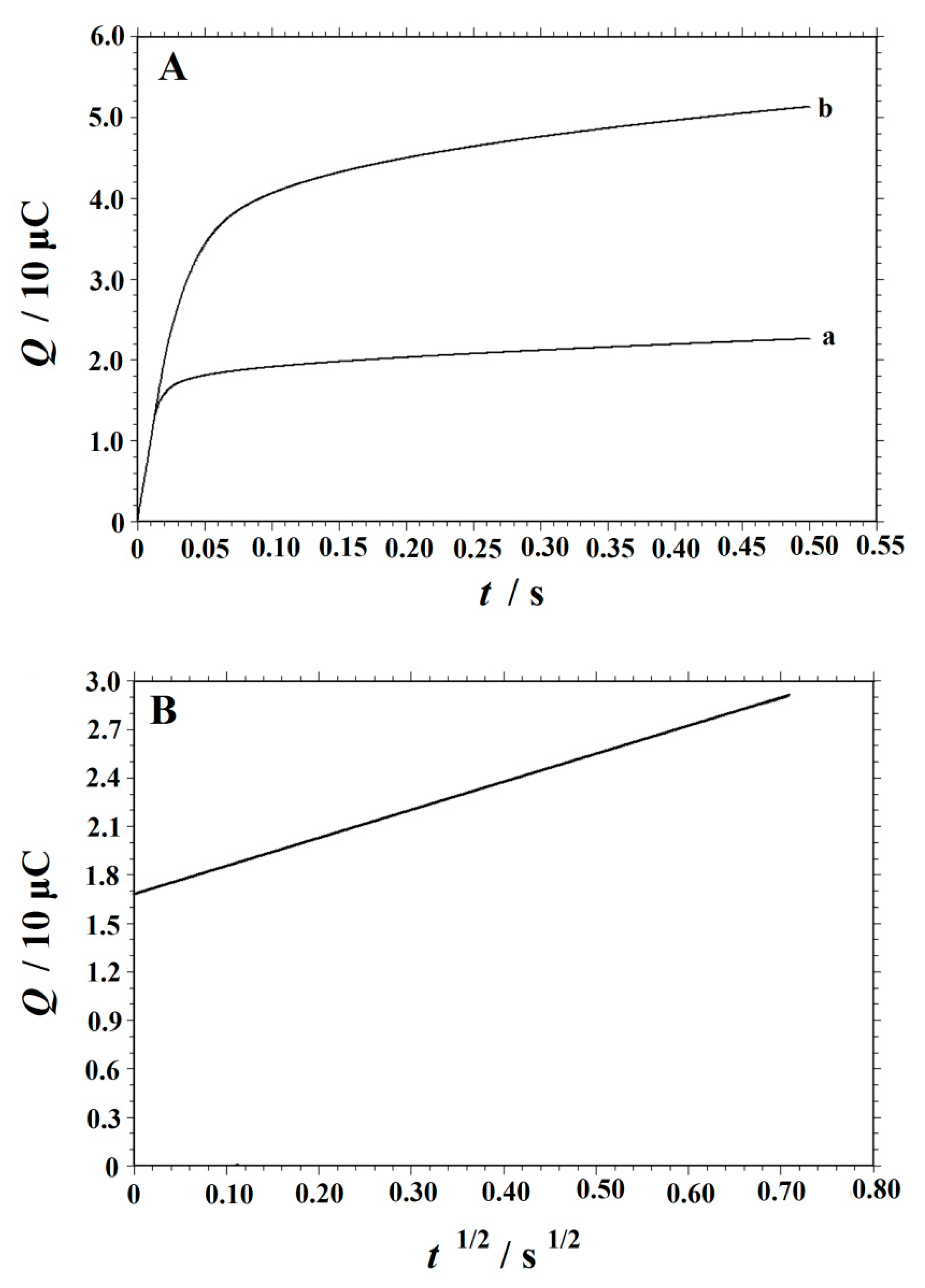
3.5. Chronocoulometric Studies
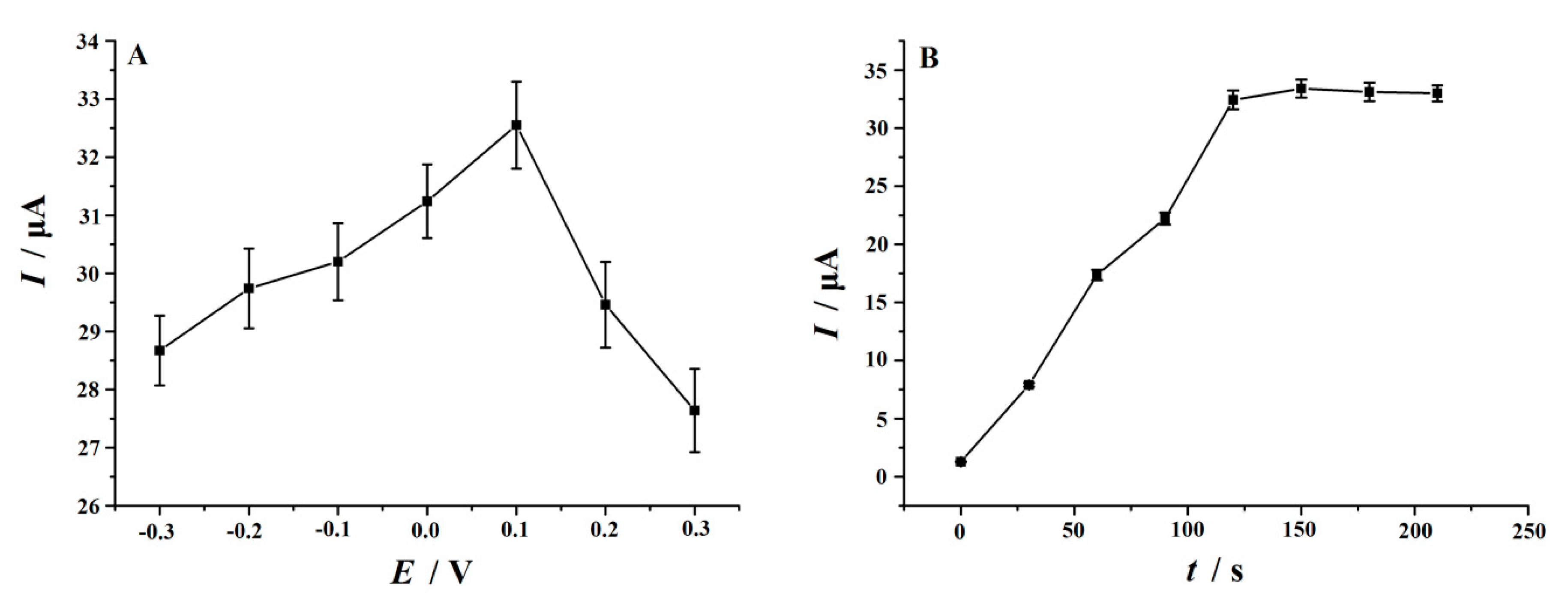
3.6. Optimal of Some Determination Conditions
3.7. Linear Range and Detection Limit
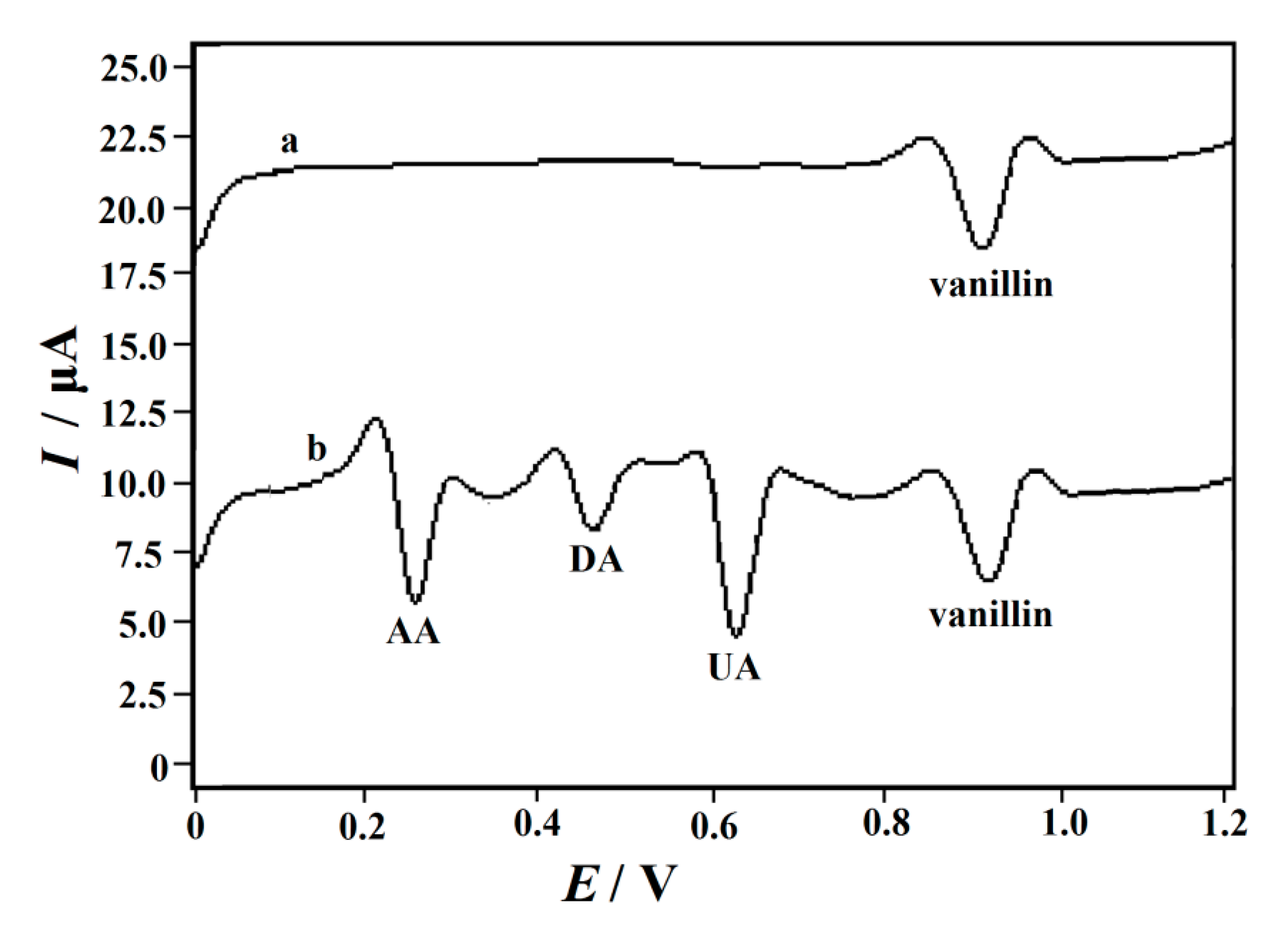
3.8. Reproducibility, Stability and Selectivity of CeO2/ERGO/GCE
3.9. Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Millour, S.; Noel, L.; Kadar, A.; Chekri, R.; Vastel, C.; Sirot, B.; Leblanc, J.; Guerin, T. Pb, Hg, Cd, As, Sb and Al levels in foodstuffs from the 2nd French total diet study. Food Chem. 2011, 126, 1787. [Google Scholar] [CrossRef] [PubMed]
- Yardım, Y.; Gülcan, M.; Şentürk, Z. Determination of vanillin in commercial food product by adsorptive stripping voltammetry using a boron-doped diamond electrode. Food Chem. 2013, 141, 1821–1827. [Google Scholar] [CrossRef]
- Pérez-Silva, A.; Odoux, E.; Brat, P.; Ribeyre, F.; Rodriguez-Jimenes, G.; Robles-Olvera, V.; García-Alvarado, M.A.; Günata, Z. GC–MS and GC–olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chem. 2006, 99, 728–735. [Google Scholar]
- Pérez-Esteve, É.; Lerma-García, M.J.; Fuentes, A.; Palomares, C.; Barat, J.M. Control of undeclared flavoring of cocoa powders by the determination of vanillin and ethyl vanillin by HPLC. Food Control 2016, 67, 171–176. [Google Scholar] [CrossRef]
- Gerasimov, A.V.; Gornova, N.V.; Rudometova, N.V. Determination of vanillin and ethylvanillin in vanilla flavorings by planar (thin-layer) chromatography. J. Anal. Chem. 2003, 58, 677–684. [Google Scholar] [CrossRef]
- Altunay, N. Development of vortex-assisted ionic liquid-dispersive microextraction methodology for vanillin monitoring in food products using ultraviolet-visible spectrophotometry. LWT 2018, 93, 9–15. [Google Scholar] [CrossRef]
- Timotheou-Potamia, M.; Calokerinos, A.C. Chemiluminometric determination of vanillin in commercial vanillin products. Talanta 2007, 71, 208–212. [Google Scholar] [CrossRef]
- Minematsu, S.; Xuan, G.S.; Wu, X.Z. Determination of vanillin in vanilla perfumes and air by capillary electrophoresis. J. Environ. Sci. 2013, 25, s8–s14. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, F.; Zeng, B. Sensitive voltammetric determination of vanillin with an AuPd nanoparticles graphene composite modified electrode. Food Chem. 2014, 151, 53–57. [Google Scholar] [CrossRef]
- Sivakumar, M.; Sakthivel, M.; Chen, S.M. Simple synthesis of cobalt sulfide nanorods for efficient electrocatalytic oxidation of vanillin in food samples. J. Colloid Interface Sci. 2017, 490, 719–726. [Google Scholar] [CrossRef]
- Mei, Q.; Ding, Y.; Li, L.; Wang, A.; Zhao, Y. Electrospun MoS2 composite carbon nanofibers for determination of vanillin. J. Electroanal. Chem. 2019, 833, 297–303. [Google Scholar]
- Huang, L.; Hou, K.; Jia, X.; Pan, H.; Du, M. Preparation of novel silver nanoplates/graphene composite and their application in vanillin electrochemical detection. Mater. Sci. Eng. C 2014, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Zeng, R.; Ding, C. Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 2015, 180, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Alpar, N.; Yardım, Y.; Şentürk, Z. Selective and simultaneous determination of total chlorogenic acids, vanillin and caffeine in foods and beverages by adsorptive stripping voltammetry using a cathodically pretreated boron-doped diamond electrode. Sens. Actuators B Chem. 2018, 257, 398–408. [Google Scholar] [CrossRef]
- Ali, H.S.; Abdullah, A.A.; Pınar, P.T.; Yardım, Y.; Şentürk, Z. Simultaneous voltammetric determination of vanillin and caffeine in food products using an anodically pretreated boron-doped diamond electrode: Its comparison with HPLC-DAD. Talanta 2017, 170, 384–391. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G. A simple approach for the electrochemical determination of vanillin at ionic surfactant modified graphene paste electrode. Microchem. J. 2020, 154, 104575. [Google Scholar] [CrossRef]
- Li, J.; Feng, H.; Li, J.; Jiang, J.; Feng, Y.; He, L.; Qian, D. Bimetallic Ag-Pd nanoparticles-decorated graphene oxide: A fascinating three-dimensional nanohybrid as an efficient electrochemical sensing platform for vanillin determination. Electrochim. Acta 2015, 176, 827–835. [Google Scholar] [CrossRef]
- Wu, W.; Yang, L.; Zhao, F.; Zeng, B. A vanillin electrochemical sensor based on molecularly imprinted poly(1-vinyl-3-octylimidazole hexafluoride phosphorus)–multi-walled carbon nanotubes@polydopamine–carboxyl single-walled carbon nanotubes composite. Sens. Actuators B Chem. 2017, 239, 481–487. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.L.X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301–021359. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Li, J. Simultaneous voltammetric determination of 2-nitrophenol and 4-nitrophenol based on an acetylene black paste electrode modified with a graphene-chitosan composite. Microchim Acta 2014, 181, 1077–1084. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Xu, Z.; Liu, M.; Tang, S.; Yang, C.; Qian, D. Facile synthesis of Ag@Cu2O heterogeneous nanocrystals decorated N-doped reduced graphene oxide with enhanced electrocatalytic activity for ultrasensitive detection of H2O2. Sens. Actuators B Chem. 2018, 260, 529–540. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Xu, Z.; Liu, M.; Tang, S.; Yang, C.; Qian, D. Facile synthesis of Pd−Cu@Cu2O/N-RGO hybrid and its application for electrochemical detection of tryptophan. Electrochim. Acta 2018, 260, 526–535. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Facile and Ultrasensitive Determination of 4-Nitrophenol Based on Acetylene Black Paste and Graphene Hybrid Electrode. Nanomaterials 2019, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, P.; Tian, Y.; Magesa, F.; Liu, J.; Li, G.; He, Q. Construction of effective electrochemical sensor for the determination of quinoline yellow based on different morphologies of manganese dioxide functionalized graphene. J. Food Compos. Anal. 2019, 84, 103280. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Li, J.; Liu, J.; Li, G.; He, Q. MnO2 Nanowires-Decorated Reduced Graphene Oxide Modified Glassy Carbon Electrode for Sensitive Determination of Bisphenol A. J. Electrochem. Soc. 2020, 167, 046514. [Google Scholar] [CrossRef]
- Haldorai, Y.; Yeon, S.H.; Huh, Y.S.; Han, Y.K. Electrochemical determination of tryptophan using a glassy carbon electrode modified with flower-like structured nanocomposite consisting of reduced graphene oxide and SnO2. Sens. Actuators B Chem. 2017, 239, 1221–1230. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; Deng, P.; Chen, D. Facile electrochemical sensor for nanomolar rutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials 2019, 9, 115. [Google Scholar] [CrossRef]
- Balla, P.; Sinha, A.; Wu, L.; Lu, X.; Tan, D.; Chen, J. Co3O4 nanoparticles supported mesoporous carbon framework interface for glucose biosensing. Talanta 2019, 203, 112–121. [Google Scholar]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles coated reduced graphene oxide nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Tian, Y.; Wu, Y.; Magesa, F.; Deng, P.; Li, G. Facile Preparation of Cu2O Nanoparticles and Reduced Graphene Oxide Nanocomposite for Electrochemical Sensing of Rhodamine B. Nanomaterials 2019, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef]
- Carbone, M.; Nesticò, A.; Bellucci, N.; Micheli, L.; Palleschi, G. Enhanced performances of sensors based on screen printed electrodes modified with nanosized NiO particles. Electrochim. Acta 2017, 246, 580–587. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of tartrazine based on TiO2-Electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- Sarpoushi, M.R.; Nasibi, M.; Golozar, M.A.; Shishesaz, M.R.; Borhani, M.R.; Noroozi, S. Electrochemical investigation of graphene/cerium oxide nanoparticles as an electrode material for supercapacitors. Mater. Sci. Semicond. Process. 2014, 26, 374–378. [Google Scholar] [CrossRef]
- Adarakatti, P.S.; Gangaiah, V.; Siddaramanna, A. Mesoporous CeO2 nanoparticles modified Glassy carbon electrode for individual and simultaneous determination of Cu(II) and Hg(II): Application to environmental samples. Mater. Sci. Semicond. Process. 2018, 84, 157–166. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, L.; Ding, Y.; Wang, J.; Li, J.; Du, X. Determination of sulfamethoxazole in foods based on CeO2/chitosan nanocomposite-modified electrodes. Mater. Sci. Eng. C 2012, 32, 2623–2627. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Qiao, F.; Liu, P.; Ai, S. Highly sensitive electrochemical stripping analysis of methyl parathion at MWCNTs–CeO2–Au nanocomposite modified electrode. Sens. Actuators B 2013, 186, 774–780. [Google Scholar] [CrossRef]
- Li, T.; Liu, H. A simple synthesis method of nanocrystals CeO2 modified rGO composites as electrode materials for supercapacitors with long time cycling stability. Powder Technol. 2018, 327, 275–281. [Google Scholar] [CrossRef]
- Ojha, G.P.; Pant, B.; Park, S.J.; Park, M.; Kim, H.Y. Synthesis and characterization of reduced graphene oxide decorated with CeO2-doped MnO2 nanorods for supercapacitor applications. J. Colloid Interface Sci. 2017, 494, 338–344. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Feng, Y. Acetylene black paste electrode modified with graphene as the voltammetric sensor for selective determination of tryptophan in the presence of high concentrations of tyrosine. Mater. Sci. Eng. C 2014, 35, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Anson, F.C. Application of potentiostatic current integration to the study of the adsorption of cobalt (III)-(ethylenedinitrilo(tetraacetate) on mercury electrodes. Anal. Chem. 1964, 36, 932–934. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef]
- Velasco, J.G. Determination of standard rate constants for electrochemical irreversible processes from linear sweep voltammograms. Electroanalysis 1997, 9, 880–882. [Google Scholar] [CrossRef]


| Electrode | Ip/μA | Ep/V |
|---|---|---|
| GCE | 0.3242 | 0.978 |
| GO/GCE | 0.6226 | 0.979 |
| CeO2/GCE | 0.3469 | 0.980 |
| CeO2/GO/GCE | 1.146 | 0.953 |
| ERGO/GCE | 25.24 | 0.949 |
| CeO2/ERGO/GCE | 52.22 | 0.942 |
| Electrochemical Sensors | Technique | Linear Range /μM | Detection Limit /μM | References |
|---|---|---|---|---|
| a AuPd-GR/GCE | k DPV | 0.1–7; 10–40 | 0.02 | [9] |
| b CoS NR@Nafion/GCE | DPV | 0.5–56 | 0.07 | [10] |
| c MoS2-CNF/GCE | amperometry | 0.3–135 | 0.15 | [11] |
| d Ag NPs/GN/GCE | l SWV | 2–100 | 0.332 | [12] |
| e GR-PVP/ABPE | SDLSV | 0.02–2.0; 2.0–40; 40–100 | 0.01 | [13] |
| f BDD electrode | m SWASV | 3.3–330 | 0.38 | [14] |
| g APT-BDD | SWASV | 6.6–660 | 1.47 | [15] |
| h CTABMGPE | DPV | 4–15; 20–70 | 1.29 | [16] |
| i Ag-Pd/GO/GCE | DPV | 0.02–45 | 0.005 | [17] |
| j MWNTs-PDA@ MIP/SWNTsCOOH/GCE | DPV | 0.2–10 | 0.1 | [18] |
| CeO2-ERGO/GCE | SDLSV | 0.04–20; 20–100 | 0.01 | This work |
| Samples | Added/μM | Found/μM | RSD/% | Recovery/% |
|---|---|---|---|---|
| Biscuit | 0 | 1.52 | 2.86 | - |
| 1.0 | 2.56 | 3.19 | 104.0 | |
| 2.0 | 3.48 | 2.14 | 98.0 | |
| Chocolate | 0 | 2.28 | 2.37 | - |
| 1.0 | 3.25 | 2.75 | 97.0 | |
| 2.0 | 4.32 | 2.84 | 102.0 | |
| Pudding powder | 0 | 4.37 | 2.16 | - |
| 2.0 | 6.44 | 2.51 | 103.5 | |
| 4.0 | 8.30 | 2.83 | 98.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Zhang, R.; Tang, Z.; Wang, H.; Deng, P.; Tang, Y. Facile Fabrication of CeO2/Electrochemically Reduced Graphene Oxide Nanocomposites for Vanillin Detection in Commercial Food Products. Nanomaterials 2020, 10, 1356. https://doi.org/10.3390/nano10071356
Nie X, Zhang R, Tang Z, Wang H, Deng P, Tang Y. Facile Fabrication of CeO2/Electrochemically Reduced Graphene Oxide Nanocomposites for Vanillin Detection in Commercial Food Products. Nanomaterials. 2020; 10(7):1356. https://doi.org/10.3390/nano10071356
Chicago/Turabian StyleNie, Xue, Rui Zhang, Zheng Tang, Haiyan Wang, Peihong Deng, and Yougen Tang. 2020. "Facile Fabrication of CeO2/Electrochemically Reduced Graphene Oxide Nanocomposites for Vanillin Detection in Commercial Food Products" Nanomaterials 10, no. 7: 1356. https://doi.org/10.3390/nano10071356
APA StyleNie, X., Zhang, R., Tang, Z., Wang, H., Deng, P., & Tang, Y. (2020). Facile Fabrication of CeO2/Electrochemically Reduced Graphene Oxide Nanocomposites for Vanillin Detection in Commercial Food Products. Nanomaterials, 10(7), 1356. https://doi.org/10.3390/nano10071356





