Synthesis of ZnO Nanoparticles Doped with Cobalt Using Bimetallic ZIFs as Sacrificial Agents
Abstract
1. Introduction
2. Experimental
2.1. Methods
2.2. Synthesis
3. Results and Discussion
3.1. XRD
3.2. TEM
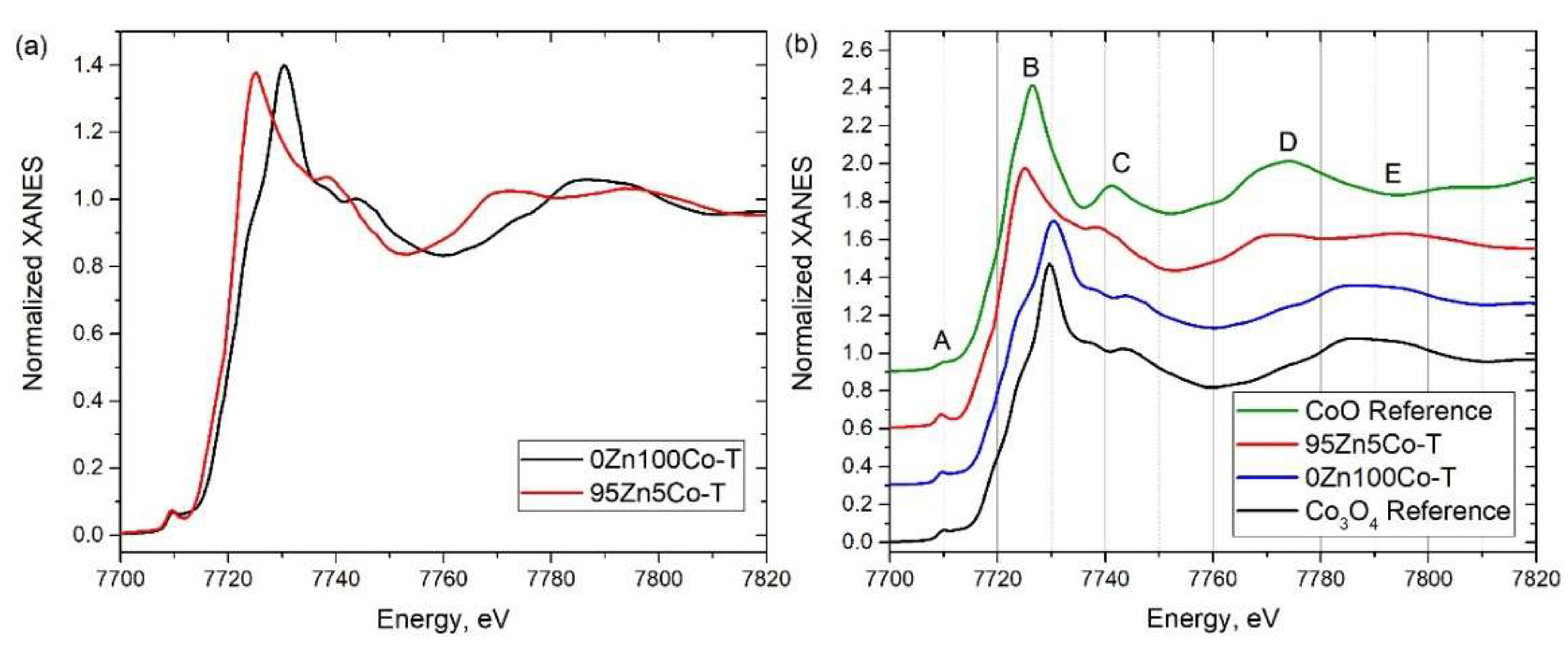
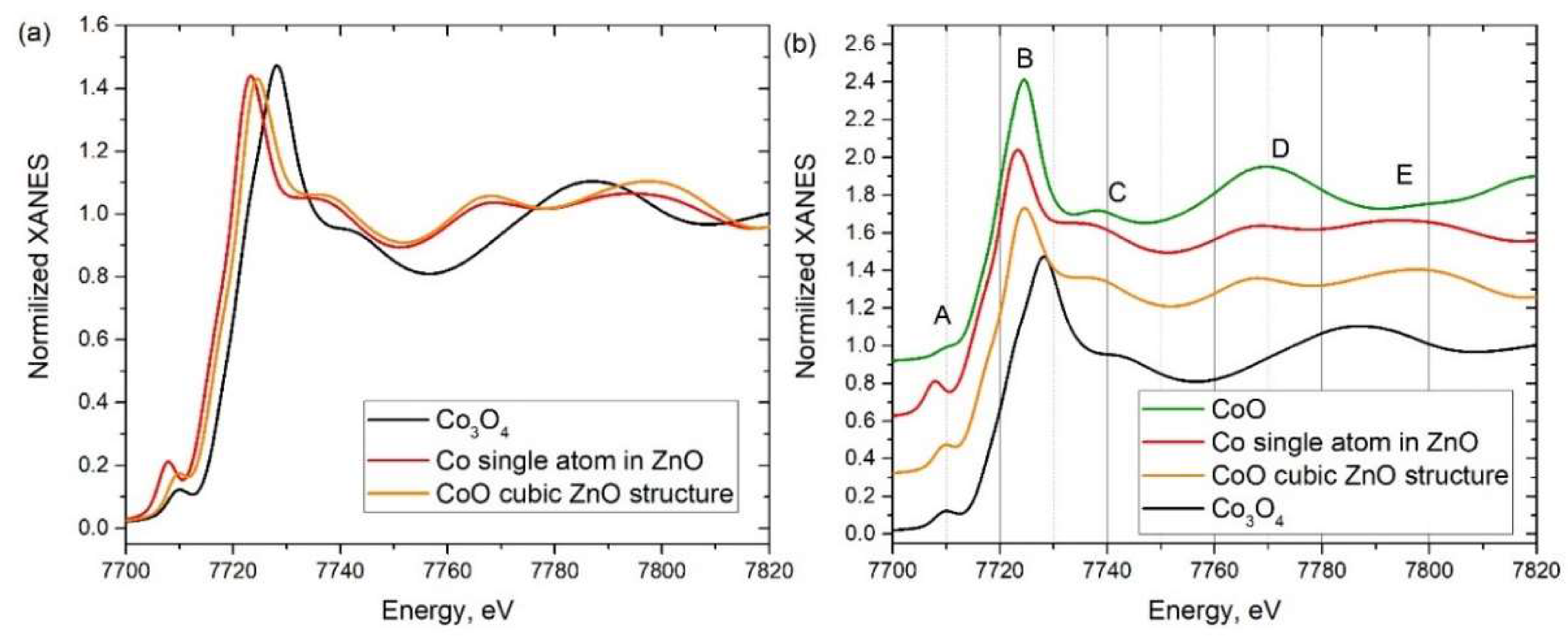
3.3. XANES
3.4. Magnetic Properties
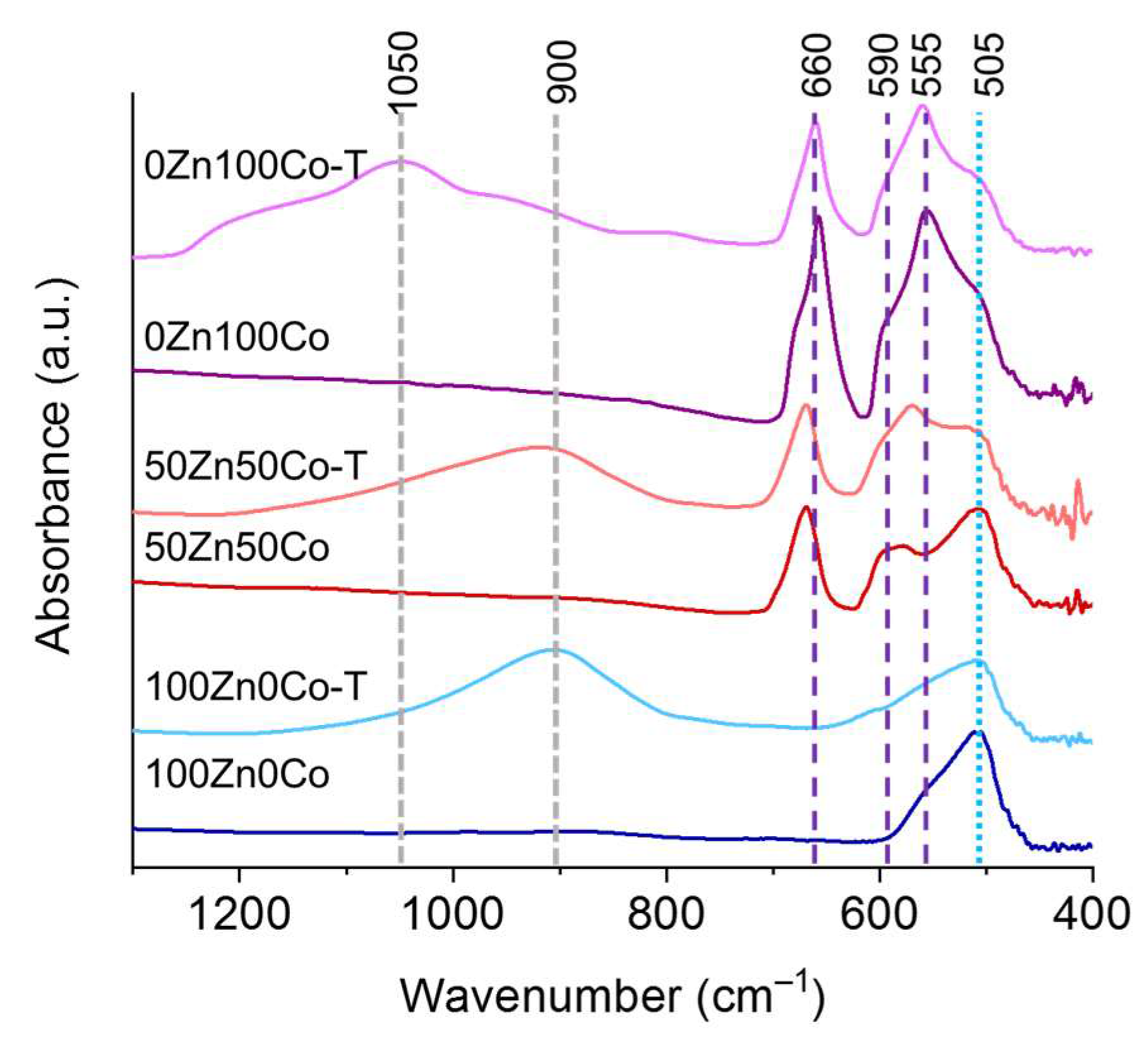
3.5. FTIR
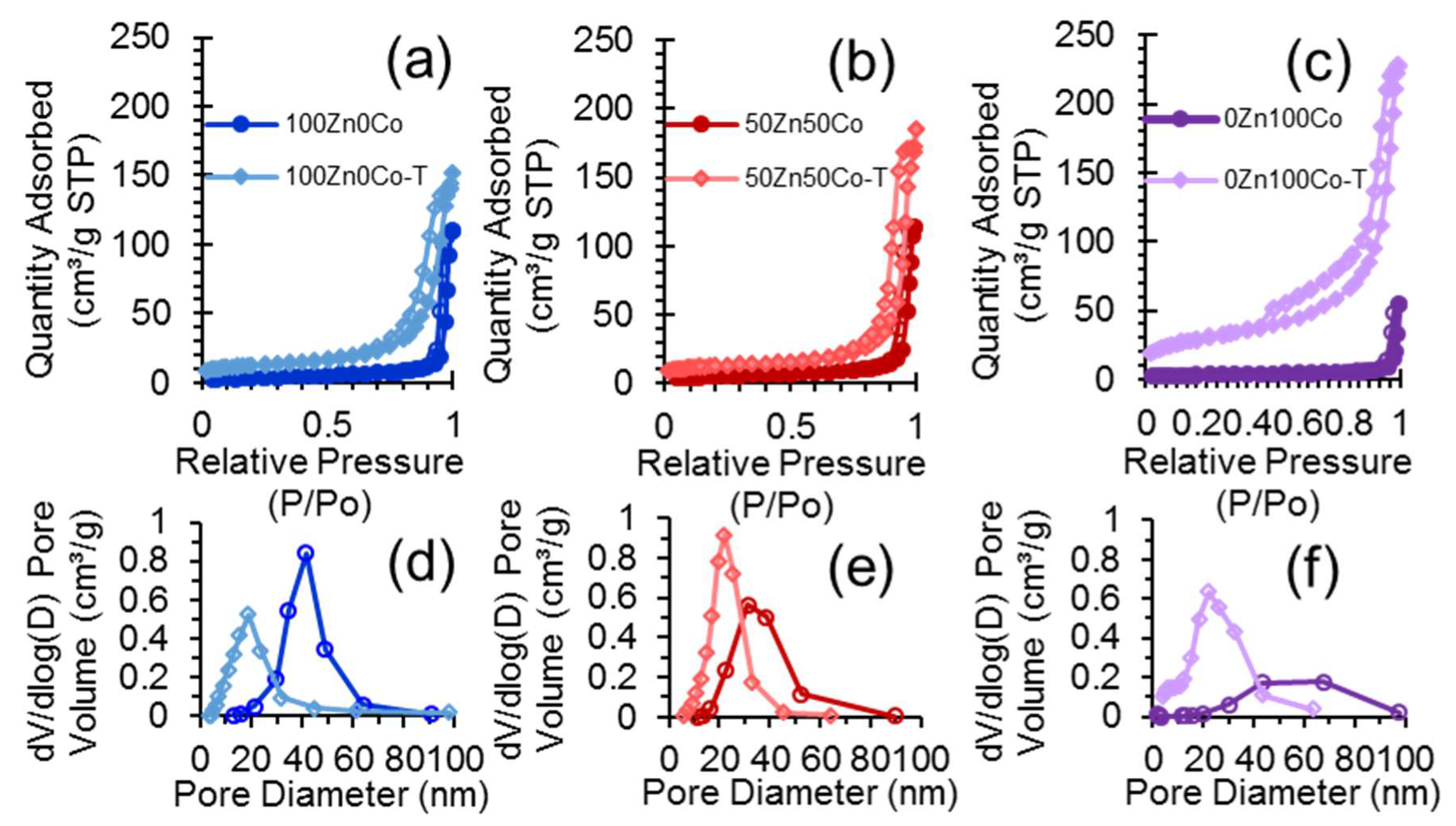
3.6. Nitrogen Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortes, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef]
- Butova, V.V.; Budnyk, A.P.; Guda, A.A.; Lomachenko, K.A.; Bugaev, A.L.; Soldatov, A.V.; Chavan, S.M.; Oien-Odegaard, S.; Olsbye, U.; Lillerud, K.P.; et al. Modulator Effect in UiO-66-NDC (1,4-Naphthalenedicarboxylic Acid) Synthesis and Comparison with UiO-67-NDC Isoreticular Metal-Organic Frameworks. Cryst. Growth Des. 2017, 17, 5422–5431. [Google Scholar] [CrossRef]
- Butova, V.V.; Budnyk, A.P.; Charykov, K.M.; Vetlitsyna-Novikova, K.S.; Lamberti, C.; Soldatov, A.V. Water as a structure-driving agent between the UiO-66 and MIL-140A metal-organic frameworks. Chem. Commun. 2019, 55, 901–904. [Google Scholar] [CrossRef]
- Chavan, S.; Vitillo, J.G.; Gianolio, D.; Zavorotynska, O.; Civalleri, B.; Jakobsen, S.; Nilsen, M.H.; Valenzano, L.; Lamberti, C.; Lillerud, K.P.; et al. H-2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Budnyk, A.P.; Charykov, K.M.; Vetlitsyna-Novikova, K.S.; Bugaev, A.L.; Guda, A.A.; Damin, A.; Chavan, S.M.; Øien-ØDegaard, S.; Lillerud, K.P.; et al. Partial and Complete Substitution of the 1,4-Benzenedicarboxylate Linker in UiO-66 with 1,4-Naphthalenedicarboxylate: Synthesis, Characterization, and H 2 -Adsorption Properties. Inorg. Chem. 2019, 58, 1607–1620. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Titanium-Based Zeolitic Imidazolate Framework for Chemical Fixation of Carbon Dioxide. Green Chem. 2016, 18, 4855–4858. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Guda, A.A.; Lomachenko, K.A.; Kamyshova, E.G.; Soldatov, M.A.; Kaur, G.; Oien-Odegaard, S.; Braglia, L.; Lazzarini, A.; Manzoli, M.; et al. Operando study of palladium nanoparticles inside UiO-67 MOF for catalytic hydrogenation of hydrocarbons. Faraday Discuss. 2018, 208, 287–306. [Google Scholar] [CrossRef]
- Butova, V.V.; Polyakov, V.A.; Budnyk, A.P.; Aboraia, A.M.; Bulanova, E.A.; Guda, A.A.; Reshetnikova, E.A.; Podkovyrina, Y.S.; Lamberti, C.; Soldatov, A.V. Zn/Co ZIF family: MW synthesis, characterization and stability upon halogen sorption. Polyhedron 2018, 154, 457–464. [Google Scholar] [CrossRef]
- Butova, V.V.; Bulanova, E.A.; Polyakov, V.A.; Guda, A.A.; Aboraia, A.M.; Shapovalov, V.V.; Zahran, H.Y.; Yahia, I.S.; Soldatov, A.V. The effect of cobalt content in Zn/Co-ZIF-8 on iodine capping properties. Inorg. Chim. Acta 2019, 492, 18–22. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nystrom, A.M.; Zou, X. One-pot Synthesis of Metal Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Wang, Y.; Li, H.; Lin, Y.; Jiang, Z.; Xie, Z.; Kuang, Q.; Zheng, L. MOF-Derived Porous Co/C Nanocomposites with Excellent Electromagnetic Wave Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 13604–13611. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Liu, X.; Niu, C.; Pang, Q.; Li, J.; Liu, F.; Liu, Z.; Mai, L. Advances in Metal–Organic Framework Coatings: Versatile Synthesis and Broad Applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.D.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Butova, V.V.; Polyakov, V.A.; Bulanova, E.A.; Soldatov, M.A.; Yahia, I.S.; Zahran, H.Y.; El-Rehim, A.F.A.; Algarni, H.; Aboraia, A.M.; Soldatov, A.V. MW synthesis of ZIF-65 with a hierarchical porous structure. Microporous Mesoporous Mat. 2020, 293. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.-L.; Xu, Q. Nanomaterials derived from metal–organic frameworks. Nat. Rev. Mater. 2018, 3, 17075. [Google Scholar] [CrossRef]
- Lv, B.; Zeng, S.; Yang, W.; Qiao, J.; Zhang, C.; Zhu, C.F.; Chen, M.H.; Di, J.T.; Li, Q.W. In-situ embedding zeolitic imidazolate framework derived Co-N-C bifunctional catalysts in carbon nanotube networks for flexible Zn-air batteries. J. Energy Chem. 2019, 38, 170–176. [Google Scholar] [CrossRef]
- Zhao, X.R.; He, X.B.; Chen, B.H.; Yin, F.X.; Li, G.R. MOFs derived metallic cobalt-zinc oxide@nitrogen-doped carbon/carbon nanotubes as a highly-efficient electrocatalyst for oxygen reduction. Appl. Surf. Sci. 2019, 487, 1049–1057. [Google Scholar] [CrossRef]
- Zhu, J.K.; Tu, W.M.; Bai, Z.Y.; Pan, H.F.; Ji, P.X.; Zhang, H.; Deng, Z.; Zhang, H.N. Zeolitic-imidazolate-framework-derived Co@Co3O4 embedded into iron, nitrogen, sulfur Co-doped reduced graphene oxide as efficient electrocatalysts for overall water splitting and zinc-air batteries. Electrochim. Acta 2019, 323, 134821. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Liu, X.Y.; Zhang, S.; Yuan, H.R.; Zhu, C.L.; Zhang, X.T.; Chen, Y.J. Metal organic framework-derived three-dimensional graphene-supported nitrogen-doped carbon nanotube spheres for electromagnetic wave absorption with ultralow filler mass loading. Carbon 2019, 155, 233–242. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, S.W.; Hu, C.G.; Li, Z.T.; Wang, J.; Feng, X.X. Porous ZnO/Co3O4/CoO/Co composite derived from Zn-Co-ZIF as improved performance anodes for lithium-ion batteries. Mater. Lett. 2019, 250, 75–78. [Google Scholar] [CrossRef]
- Shang, L.; Yu, H.; Huang, X.; Bian, T.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Well-Dispersed ZIF-Derived Co,N-Co-doped Carbon Nanoframes through Mesoporous-Silica-Protected Calcination as Efficient Oxygen Reduction Electrocatalysts. Adv. Mater. 2016, 28, 1668–1674. [Google Scholar] [CrossRef]
- Abbas, I.; Kim, H.; Shin, C.H.; Yoon, S.; Jung, K.D. Differences in bifunctionality of ZnO and ZrO2 in Cu/ZnO/ZrO2/Al2O3 catalysts in hydrogenation of carbon oxides for methanol synthesis. Appl. Catal. B-Environ. 2019, 258, 117971. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, K.L.; Bu, W.B.; Ni, D.L.; Liu, Y.Y.; Feng, J.W.; Shi, J.L. Marriage of Scintillator and Semiconductor for Synchronous Radiotherapy and Deep Photodynamic Therapy with Diminished Oxygen Dependence. Angew. Chem. Int. Edit. 2015, 54, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Kaur, G.; Kapil, S.; Sharma, V.; Sharma, S. Biogenic PLGA-Zinc oxide nanocomposite as versatile tool for enhanced photocatalytic and antibacterial activity. Appl. Nanosci. 2019, 9, 2001–2016. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State Mat. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Zhou, X.R.; Zou, Y.D.; Ma, J.H.; Cheng, X.W.; Li, Y.Y.; Deng, Y.H.; Zhao, D.Y. Cementing Mesoporous ZnO with Silica for Controllable and Switchable Gas Sensing Selectivity. Chem. Mat. 2019, 31, 8112–8120. [Google Scholar] [CrossRef]
- Venkatesan, M.; Fitzgerald, C.B.; Lunney, J.G.; Coey, J.M.D. Anisotropic ferromagnetism in substituted zinc oxide. Phys. Rev. Lett. 2004, 93, 177206. [Google Scholar] [CrossRef]
- Du, F.F.; Li, Y.C.; Li, X.L.; Yang, J.; Bai, Y.H.; Quan, Z.Y.; Liu, C.L.; Xu, X.H. Resistive switching and its modulating ferromagnetism and magnetoresistance of a ZnO-Co/SiO2-Co film. J. Magn. Magn. Mater. 2019, 489, 165445. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallog. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrot. Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Bunau, O.; Joly, Y. Self-consistent aspects of x-ray absorption calculations. J. Phys. Condes. Matter 2009, 21, 345501. [Google Scholar] [CrossRef] [PubMed]
- Guda, A.A.; Guda, S.A.; Soldatov, M.A.; Lomachenko, K.A.; Bugaev, A.L.; Lamberti, C.; Gawelda, W.; Bressler, C.; Smolentsev, G.; Soldatov, A.V.; et al. Finite difference method accelerated with sparse solvers for structural analysis of the metal-organic complexes. In Proceedings of the 16th International Conference on X-ray Absorption Fine Structure; Iop Publishing Ltd.: Bristol, UK, 2016. [Google Scholar]
- Guda, S.A.; Guda, A.A.; Soldatov, M.A.; Lomachenko, K.A.; Bugaev, A.L.; Lamberti, C.; Gawelda, W.; Bressler, C.; Smolentsev, G.; Soldatov, A.V.; et al. Optimized Finite Difference Method for the Full-Potential XANES Simulations: Application to Molecular Adsorption Geometries in MOFs and Metal-Ligand Intersystem Crossing Transients. J. Chem. Theory Comput. 2015, 11, 4512–4521. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Nanophase ZnCo2O4 as a high performance anode material for Li-ion batteries. Adv. Funct. Mater. 2007, 17, 2855–2861. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Single Crystalline Co3O4 Nanocrystals Exposed with Different Crystal Planes for Li-O-2 Batteries. Sci. Rep. 2014, 4, 5767. [Google Scholar] [CrossRef]
- Carbone, M.R.; Yoo, S.; Topsakal, M.; Lu, D. Classification of Local Chemical Environments from X-ray Absorption Spectra Using Supervised Machine Learning. Phys. Rev. Mater. 2019, 3, 033604. [Google Scholar] [CrossRef]
- Fernandez-Perez, A.; Rodriguez-Casado, V.; Valdes-Solis, T.; Marban, G. Room temperature sintering of polar ZnO nanosheets: II-mechanism. Phys. Chem. Chem. Phys. 2017, 19, 16413–16425. [Google Scholar] [CrossRef]
- Jiratova, K.; Perekrestov, R.; Dvorakova, M.; Balabanova, J.; Topka, P.; Kostejn, M.; Olejnicek, J.; Cada, M.; Hubicka, Z.; Kovanda, F. Cobalt Oxide Catalysts in the Form of Thin Films Prepared by Magnetron Sputtering on Stainless-Steel Meshes: Performance in Ethanol Oxidation. Catalysts 2019, 9, 806. [Google Scholar] [CrossRef]
- Sulciute, A.; Baltrusaitis, J.; Valatka, E. Structure, morphology and electrochemical properties of zinc-cobalt oxide films on AISI 304 type steel. J. Appl. Electrochem. 2015, 45, 405–417. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.L.; Qin, F.; Fang, H.; Hadjiev, V.G.; Litvinov, D.; Bao, J.M. Identification of Cobalt Oxides with Raman Scattering and Fourier Transform Infrared Spectroscopy. J. Phys. Chem. C 2016, 120, 4511–4516. [Google Scholar] [CrossRef]
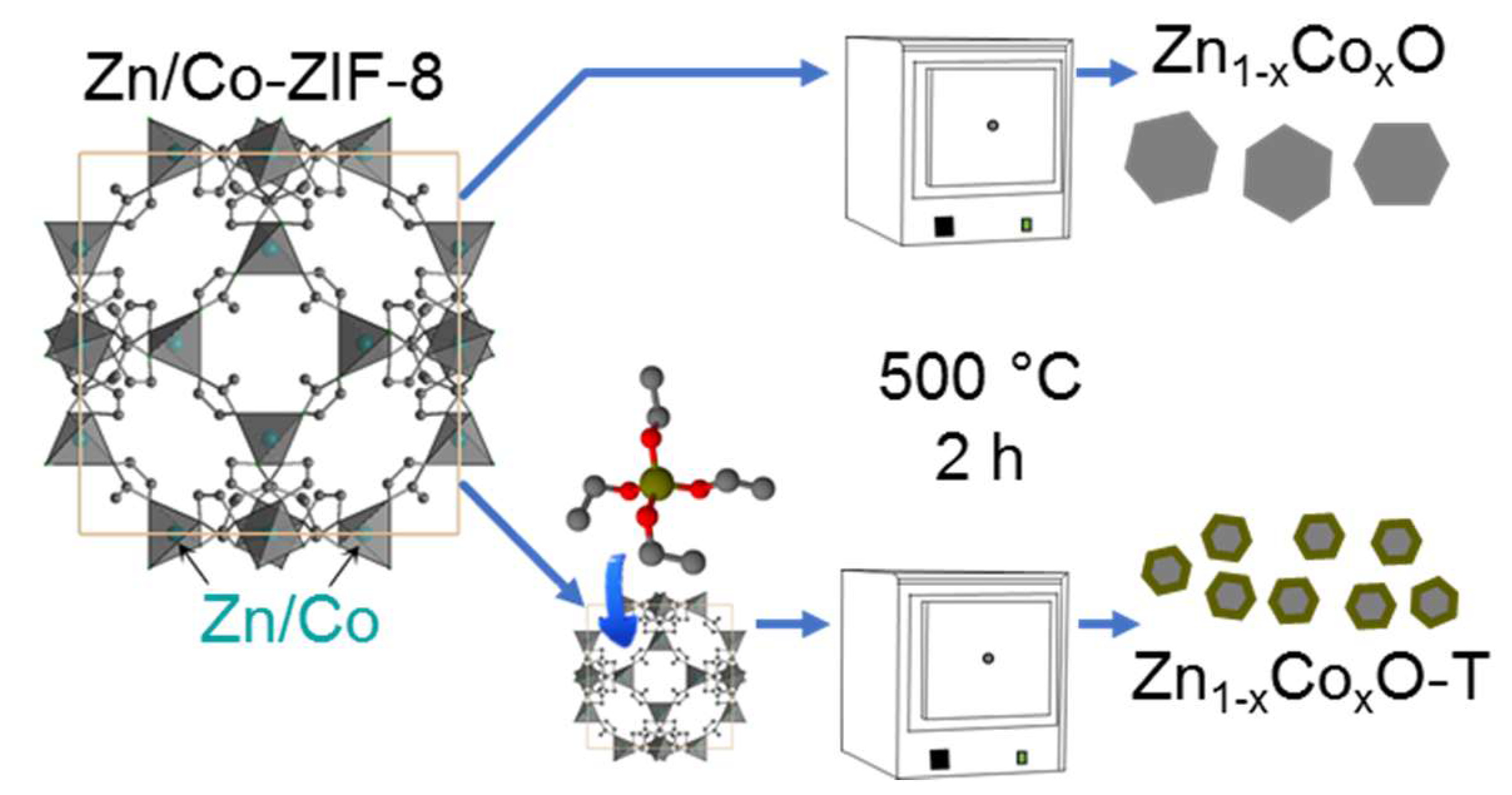
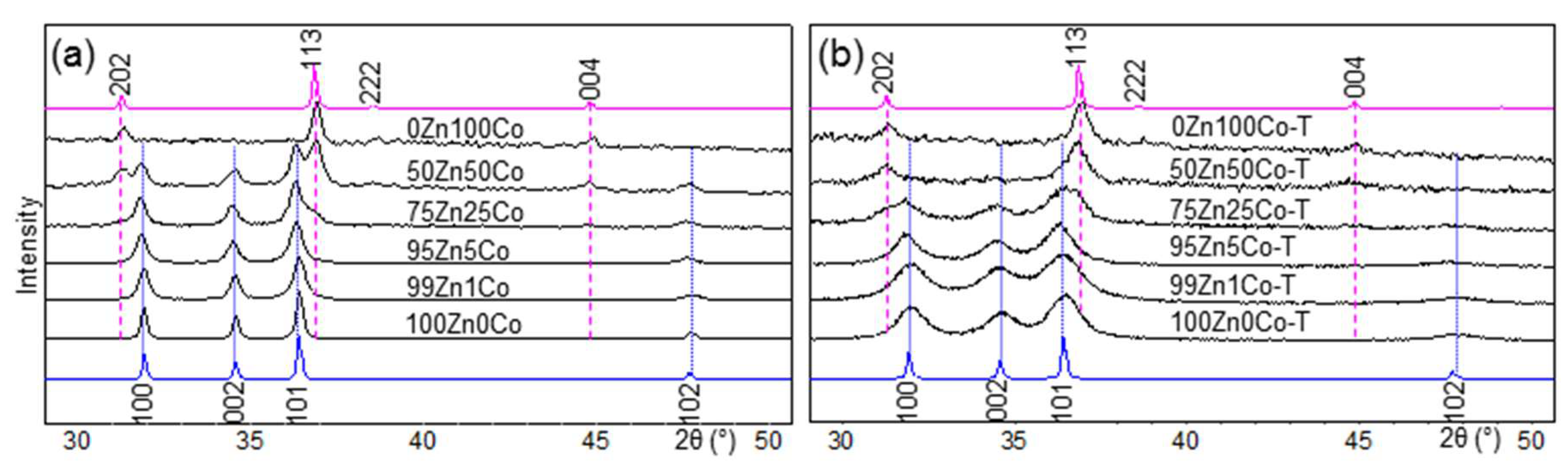
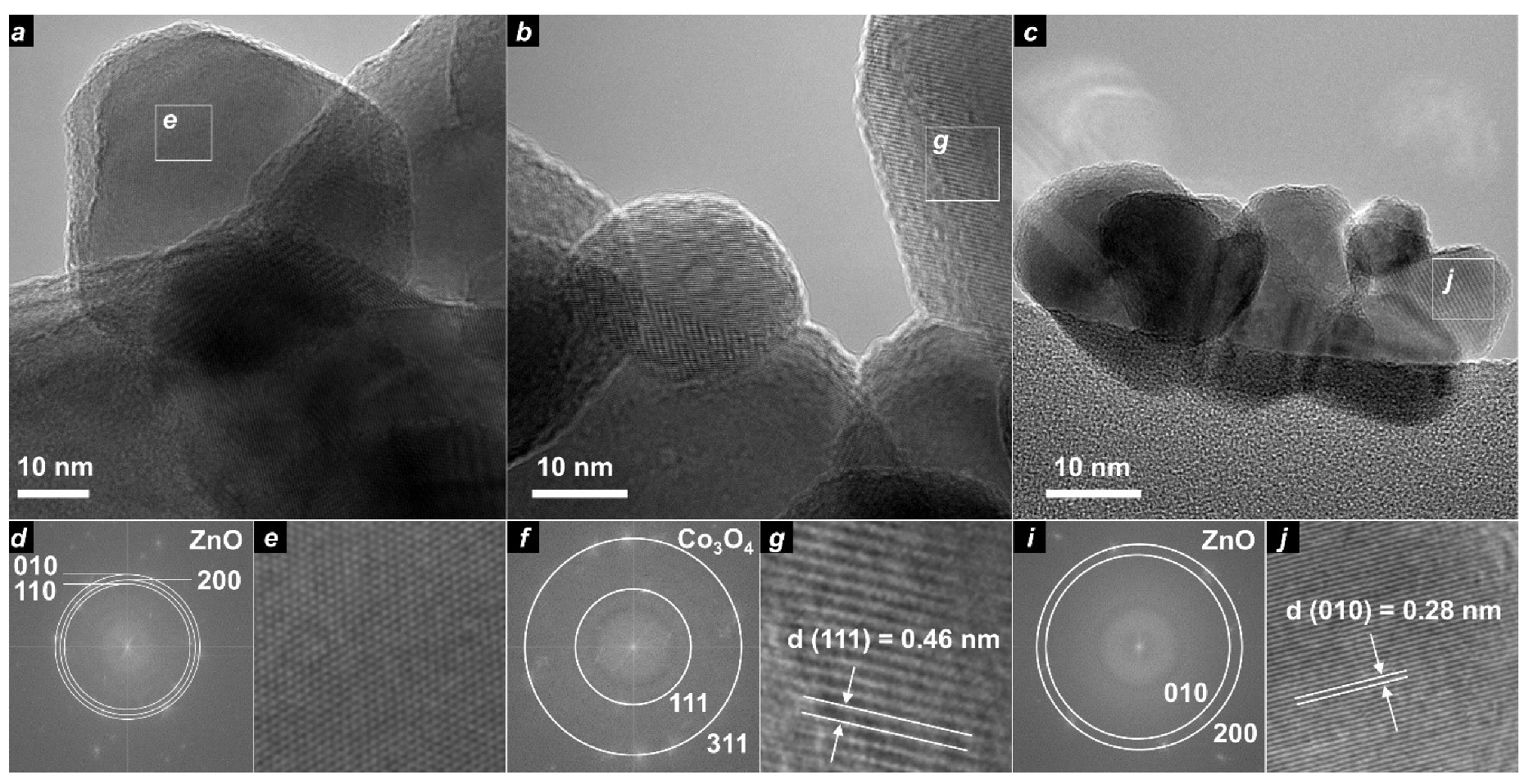
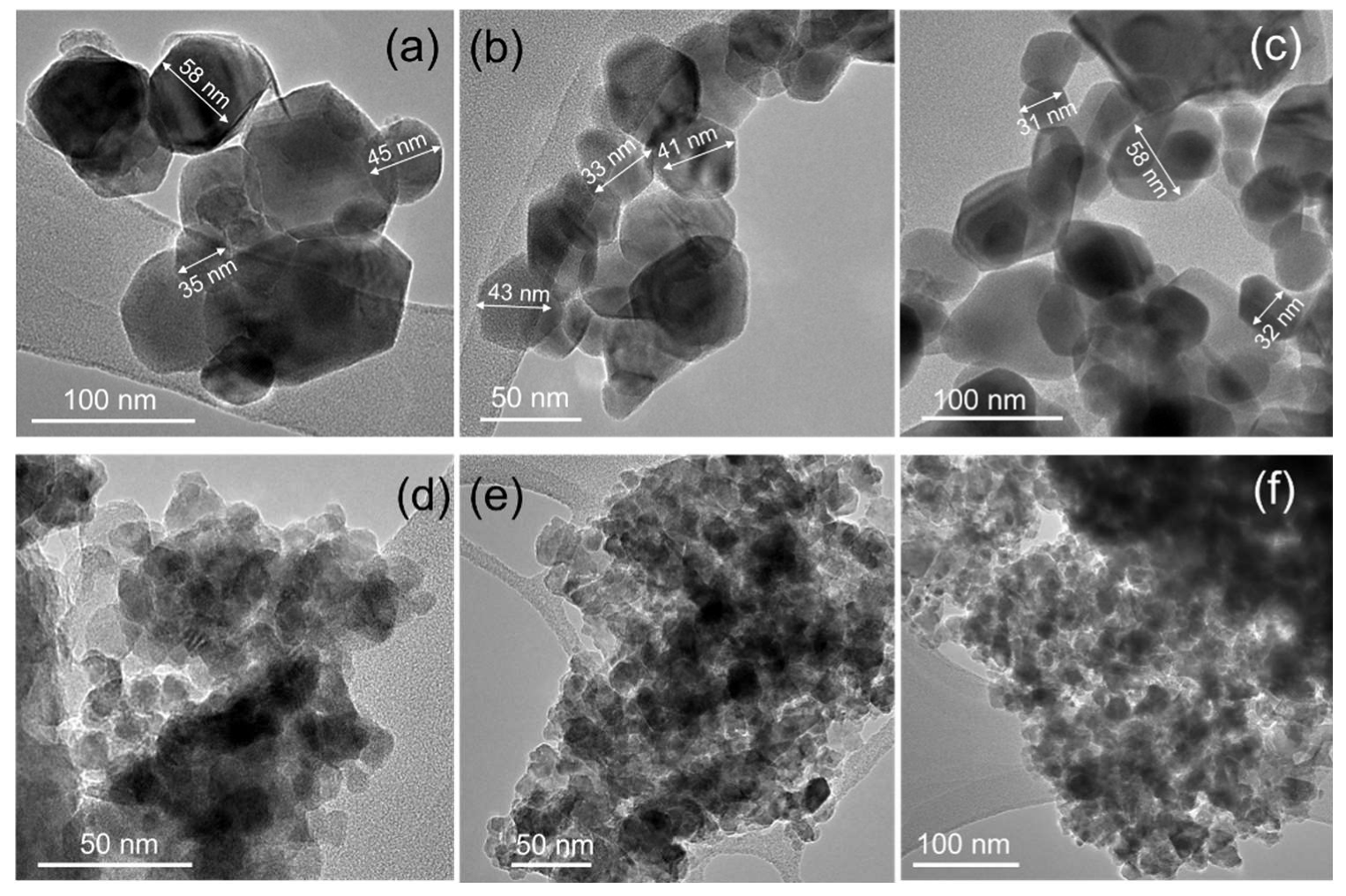
| Sample Designation | Molar Ratio Zn:Co | Precursor | Impregnation with TEOS |
|---|---|---|---|
| 100Zn0Co | 1:0 | Zn(C4N2H5)2 | no |
| 100Zn0Co-T | yes | ||
| 99Zn1Co | 0.99:0.01 | Zn0.99Co0.01(C4N2H5)2 | no |
| 99Zn1Co-T | yes | ||
| 95Zn5Co | 0.95:0.05 | Zn0.95Co0.05(C4N2H5)2 | no |
| 95Zn5Co-T | yes | ||
| 75Zn25Co | 0.75:0.25 | Zn0.75Co025(C4N2H5)2 | no |
| 75Zn25Co-T | yes | ||
| 50Zn50Co | 0.5:0.5 | Zn0.5Co0.5(C4N2H5)2 | no |
| 50Zn50Co-T | yes | ||
| 0Zn100Co | 0:1 | Co(C4N2H5)2 | no |
| 0Zn100Co-T | yes |
| Sample Designation | XRF (at.%) | Phase (%) | Unit Cell Parameters (Å) | Particle Size (nm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Zn | Co | Si | a | c | Sh | W-H | TEM | ||
| 100Zn0Co | 100 | - | - | W | 3.24872(8) | 5.20630(14) | 31.16 | 29.7 | 34 |
| 100Zn0Co-T | 90.5 | - | 7.4 | W | 3.2477(3) | 5.2084(6) | 10.24 | 6.7 | 5.6 |
| 99Zn1Co | 99.3 | 0.7 | - | W | 3.24738(10) | 5.20518(18) | 27.91 | 23.5 | 18.2 |
| 99Zn1Co-T | 93.3 | 0.6 | 6.0 | W | 3.2449(4) | 5.2100(7) | 7.93 | 5.3 | 6.7 |
| 95Zn5Co | 96.6 | 3.4 | - | W | 3.2487(2) | 5.2055(4) | 22.45 | 15.3 | |
| 95Zn5Co-T | 91.7 | 3.3 | 4.8 | W | 3.2472(3) | 5.2069(6) | 9.19 | 8.1 | 5.9 |
| 75Zn25Co | 78.2 | 21.8 | - | W(29) | 3.25016(16) | 5.2063(3) | 23.15 | 20.1 | - |
| S(71) | 8.0992(5) | 16.86 | 18.2 | ||||||
| 75Zn25Co-T | 74.0 | 17.9 | 8.1 | W(30) | 3.2478(6) | 5.2099(10) | 7.24 | 6.3 | 6.7 |
| S(70) | 8.0991(13) | 12.7 | 10.8 | ||||||
| 50Zn50Co | 50.9 | 49.1 | - | W(46) | 3.2530(3) | 5.2047(5) | 29.4 | 19.8 | |
| S(53) | 8.0993(6) | 26.65 | 18.6 | ||||||
| 50Zn50Co-T | 50.1 | 40.2 | 9.7 | S | 8.0998(11) | 15.76 | 12.9 | 5.5 | |
| 0Zn100Co | 0 | 100 | - | S | 8.0968(9) | 32.2 | 25.5 | ||
| 0Zn100Co-T | 0 | 87.4 | 12.6 | S | 8.0811(12) | 21.9 | 20.9 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butova, V.V.; Polyakov, V.A.; Erofeeva, E.A.; Efimova, S.A.; Soldatov, M.A.; Trigub, A.L.; Rusalev, Y.V.; Soldatov, A.V. Synthesis of ZnO Nanoparticles Doped with Cobalt Using Bimetallic ZIFs as Sacrificial Agents. Nanomaterials 2020, 10, 1275. https://doi.org/10.3390/nano10071275
Butova VV, Polyakov VA, Erofeeva EA, Efimova SA, Soldatov MA, Trigub AL, Rusalev YV, Soldatov AV. Synthesis of ZnO Nanoparticles Doped with Cobalt Using Bimetallic ZIFs as Sacrificial Agents. Nanomaterials. 2020; 10(7):1275. https://doi.org/10.3390/nano10071275
Chicago/Turabian StyleButova, Vera V., Vladimir A. Polyakov, Elena A. Erofeeva, Sofia A. Efimova, Mikhail A. Soldatov, Alexander L. Trigub, Yury V. Rusalev, and Alexander V. Soldatov. 2020. "Synthesis of ZnO Nanoparticles Doped with Cobalt Using Bimetallic ZIFs as Sacrificial Agents" Nanomaterials 10, no. 7: 1275. https://doi.org/10.3390/nano10071275
APA StyleButova, V. V., Polyakov, V. A., Erofeeva, E. A., Efimova, S. A., Soldatov, M. A., Trigub, A. L., Rusalev, Y. V., & Soldatov, A. V. (2020). Synthesis of ZnO Nanoparticles Doped with Cobalt Using Bimetallic ZIFs as Sacrificial Agents. Nanomaterials, 10(7), 1275. https://doi.org/10.3390/nano10071275







