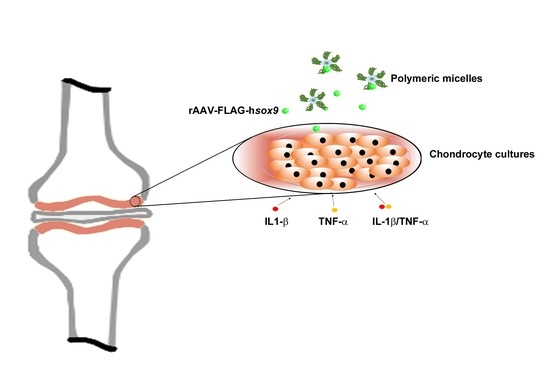
Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells
2.3. Plasmids and rAAV Vectors
2.4. Preparation of Micellar Copolymer Solutions Containing rAAV-FLAG-hsox9 Vectors
2.5. Gene Transfer in Inflammatory Conditions via rAAV-FLAG-hsox9/Polymeric Micelles
2.6. Histological and Immunocytochemical Analyses
2.7. Histomorphometry
2.8. Evaluation of Cell Proliferation and Viability
2.9. Statistical Analysis
3. Results
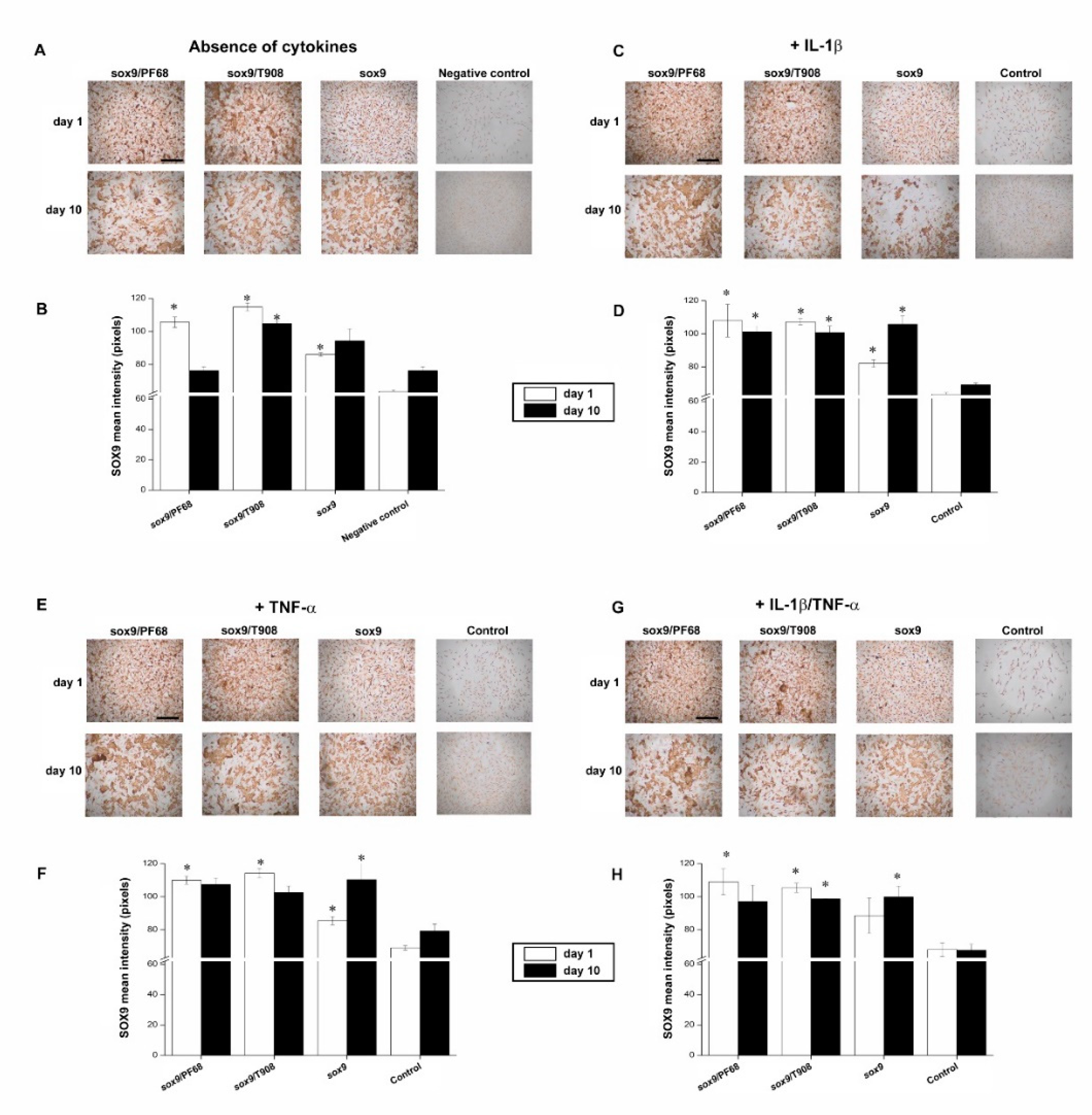
3.1. Efficacy of rAAV-Mediated sox9 Overexpression in Conditions of Inflammation upon Vector Delivery via Polymeric Micelles
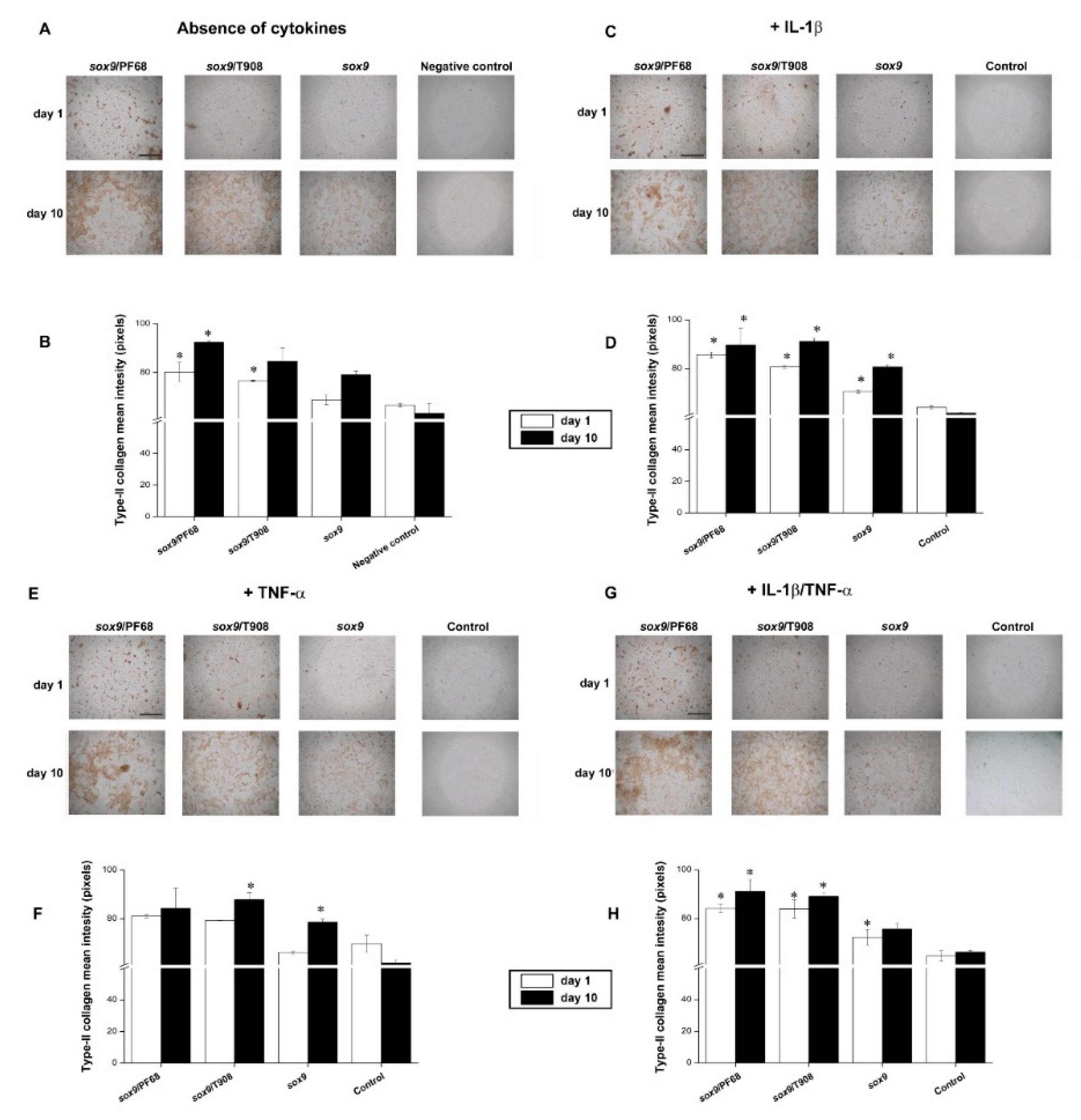
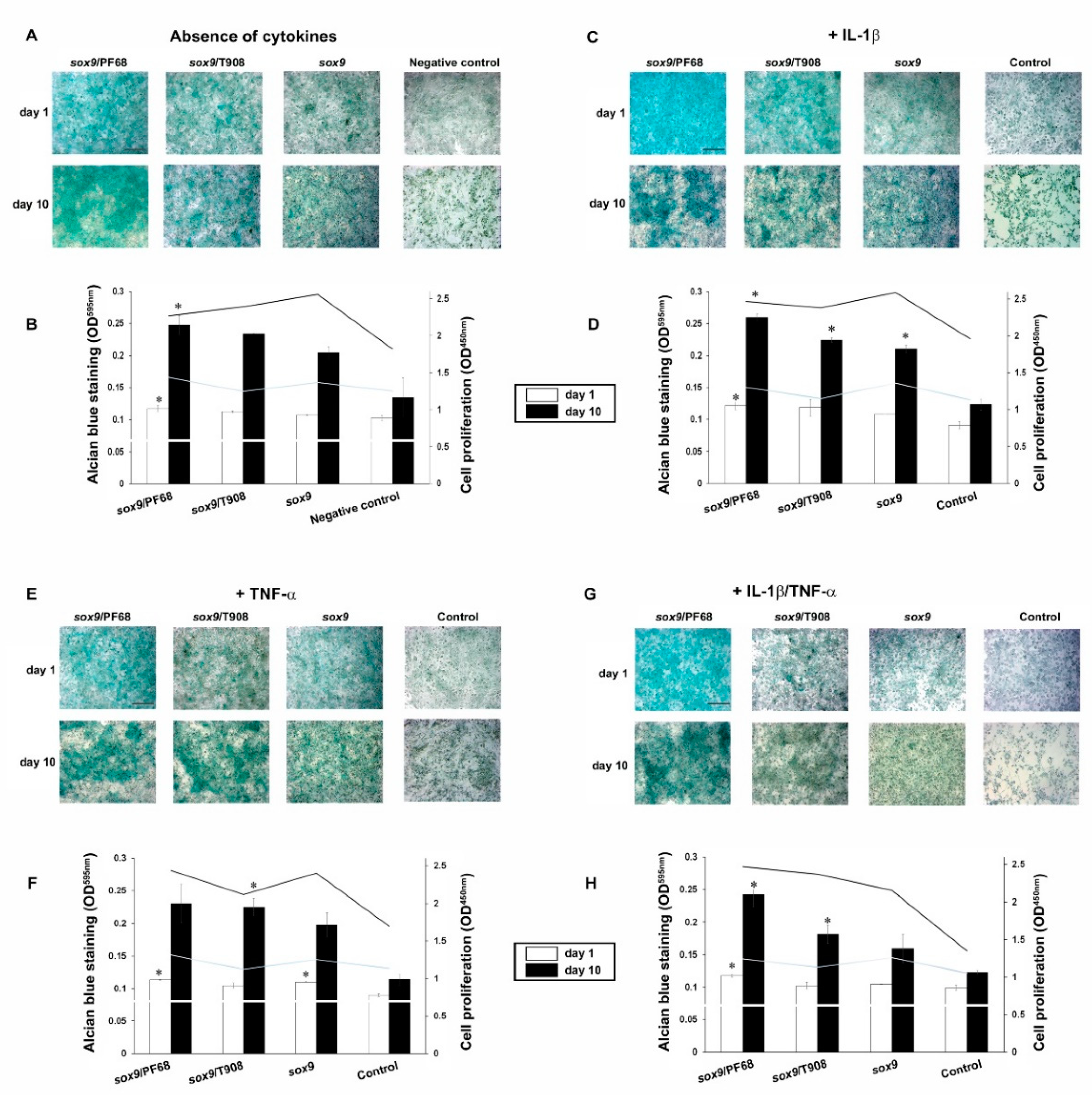
3.2. Effects of rAAV-FLAG-hsox9/Polymeric Micelle Delivery on the Anabolic Activities of Human OA Chondrocytes in Inflammatory Conditions
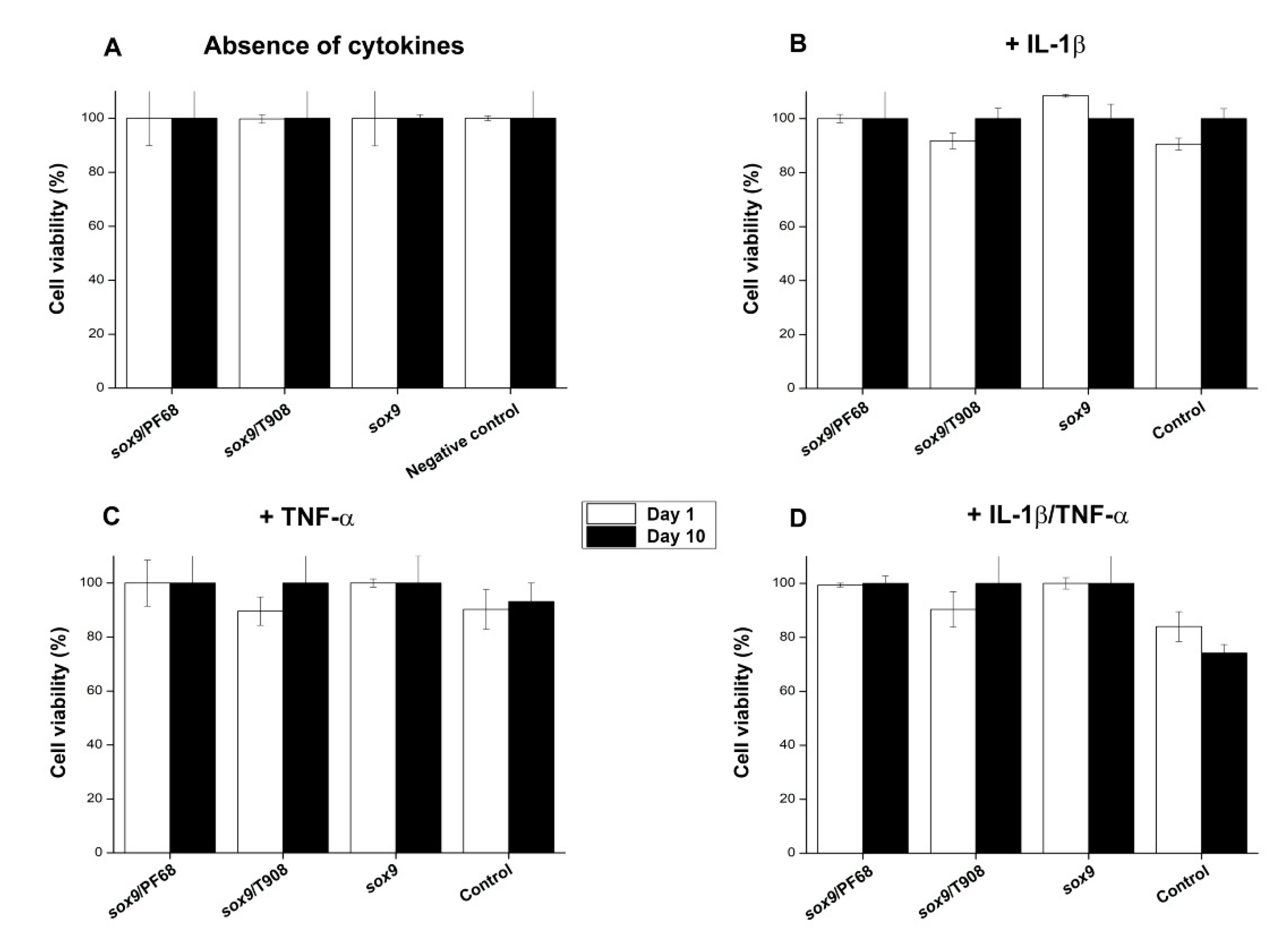
3.3. Effects of rAAV-FLAG-hsox9/Polymeric Micelle Delivery on the Viability Processes in Human OA Chondrocytes in Inflammatory Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current treatment options for osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, A.R. Osteoarthritis as a whole joint disease. HSS J. 2012, 8, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Hwang, S.G.; Yu, S.S.; Poo, H.; Chun, J.S. C-jun/activator protein-1 mediates interleukin-1beta-induced dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J. Biol. Chem. 2005, 280, 29780–29787. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zeng, C.; Chen, M.; Lian, L.; Dai, Y.; Zhao, H. Lentiviral vector-mediated over-expression of sox9 protected chondrocytes from il-1beta induced degeneration and apoptosis. Int. J. Clin. Exp. Pathol. 2015, 8, 10038–10049. [Google Scholar]
- Schlimgen, R.; Howard, J.; Wooley, D.; Thompson, M.; Baden, L.R.; Yang, O.O.; Christiani, D.C.; Mostoslavsky, G.; Diamond, D.V.; Duane, E.G.; et al. Risks associated with lentiviral vector exposures and prevention strategies. J. Occup. Environ. Med. 2016, 58, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.H. Adeno-associated virus integration: Virus versus vector. Gene Ther. 2008, 15, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Madry, H.; Cucchiarini, M.; Terwilliger, E.F.; Trippel, S.B. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum. Gene Ther. 2003, 14, 393–402. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Madry, H.; Ma, C.; Thurn, T.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Trippel, S.B.; Terwilliger, E.F. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol. Ther. 2005, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, A.; Yokoo, N.; Xin, K.Q.; Okuda, K.; Mizukami, H.; Ozawa, K.; Saito, T. Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector. Hum. Gene Ther. 2005, 16, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Orth, P.; Madry, H. Direct rAAV sox9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J. Mol. Med. 2013, 91, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther. 2014, 21, 811–819. [Google Scholar] [CrossRef]
- Ortved, K.F.; Begum, L.; Mohammed, H.O.; Nixon, A.J. Implantation of rAAV5-IGF-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol. Ther. 2015, 23, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich-Vinther, M.; Stengaard, C.; Schwarz, E.M.; Goldring, M.B.; Soballe, K. Adeno-associated vector mediated gene transfer of transforming growth factor-beta1 to normal and osteoarthritic human chondrocytes stimulates cartilage anabolism. Eur. Cell Mater. 2005, 10, 40–50. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Wezel, A.; Madry, H.; Cucchiarini, M. rAAV-mediated overexpression of TGF-beta stably restructures human osteoarthritic articular cartilage in situ. J. Transl. Med. 2013, 11, 211. [Google Scholar] [CrossRef] [Green Version]
- Cucchiarini, M.; Thurn, T.; Weimer, A.; Kohn, D.; Terwilliger, E.F.; Madry, H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor sox9. Arthritis Rheum. 2007, 56, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Cottard, V.; Valvason, C.; Falgarone, G.; Lutomski, D.; Boissier, M.C.; Bessis, N. Immune response against gene therapy vectors: Influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 2004, 24, 162–169. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Rial-Hermida, I.; Taboada, P.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO-PPO-PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl. Mater. Interfaces 2016, 8, 20600–20613. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. rAAV-mediated overexpression of TGF-beta via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int. J. Nanomed. 2017, 12, 6985–6996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Speicher-Mentges, S.; Madry, H.; Cucchiarini, M. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol. Pharm. 2018, 15, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samulski, R.J.; Chang, L.S.; Shenk, T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989, 63, 3822–3828. [Google Scholar] [CrossRef] [Green Version]
- Samulski, R.J.; Chang, L.S.; Shenk, T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 1987, 61, 3096–3101. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.K.; Ekici, M.; Madry, H.; Schmitt, G.; Kohn, D.; Cucchiarini, M. Sox9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res. Ther. 2012, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Tao, K.; Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Lin, J.; Cucchiarini, M. rAAV-mediated combined gene transfer and overexpression of tgf-beta and sox9 remodels human osteoarthritic articular cartilage. J. Orthop. Res. 2016, 34, 2181–2190. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Frisch, J.; Rey-Rico, A.; Schmitt, G.; Madry, H.; Cucchiarini, M. Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors. J. Exp. Orthop. 2017, 4, 22. [Google Scholar] [CrossRef]
- Frisch, J.; Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Madry, H.; Cucchiarini, M. Determination of the chondrogenic differentiation processes in human bone marrow-derived mesenchymal stem cells genetically modified to overexpress transforming growth factor-beta via recombinant adeno-associated viral vectors. Hum. Gene Ther. 2014, 25, 1050–1060. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. Peo-ppo-peo micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1beta. Arthritis Res. 2001, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Schuerwegh, A.J.; Dombrecht, E.J.; Stevens, W.J.; Van Offel, J.F.; Bridts, C.H.; De Clerck, L.S. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (il-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Stanton, L.A.; Sabari, S.; Sampaio, A.V.; Underhill, T.M.; Beier, F. P38 map kinase signalling is required for hypertrophic chondrocyte differentiation. Biochem. J. 2004, 378, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, A.; Wang, G.; Beier, F. Rhoa/rock signaling regulates sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 2005, 280, 11626–11634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Ikeda, T.; Kamekura, S.; Mabuchi, A.; Kou, I.; Seki, S.; Takato, T.; Nakamura, K.; Kawaguchi, H.; Ikegawa, S.; Chung, U.I. The combination of sox5, sox6, and sox9 (the sox trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004, 50, 3561–3573. [Google Scholar] [CrossRef]
- Salminen, H.; Vuorio, E.; Saamanen, A.M. Expression of sox9 and type IIa procollagen during attempted repair of articular cartilage damage in a transgenic mouse model of osteoarthritis. Arthritis Rheum. 2001, 44, 947–955. [Google Scholar] [CrossRef]
- Aigner, T.; Gebhard, P.M.; Schmid, E.; Bau, B.; Harley, V.; Poschl, E. Sox9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol. 2003, 22, 363–372. [Google Scholar] [CrossRef]
- Madry, H.; Cucchiarini, M. Advances and challenges in gene-based approaches for osteoarthritis. J. Gene Med. 2013, 15, 343–355. [Google Scholar] [CrossRef]
- Evans, C.H.; Huard, J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcedo, R.; Wilson, J.M. Humoral immune response to AAV. Front. Immunol. 2013, 4, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traister, R.S.; Fabre, S.; Wang, Z.; Xiao, X.; Hirsch, R. Inflammatory cytokine regulation of transgene expression in human fibroblast-like synoviocytes infected with adeno-associated virus. Arthritis Rheum. 2006, 54, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.F.; Zhang, X.; Sakano, S.; Lefebvre, V.; Sandell, L.J. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by sox9. J. Bone Miner. Res. 1999, 14, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Furumatsu, T.; Matsumoto-Ogawa, E.; Tanaka, T.; Lu, Z.; Ozaki, T. Rock inhibition enhances aggrecan deposition and suppresses matrix metalloproteinase-3 production in human articular chondrocytes. Connect. Tissue Res. 2014, 55, 89–95. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urich, J.; Cucchiarini, M.; Rey-Rico, A. Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes. Nanomaterials 2020, 10, 1238. https://doi.org/10.3390/nano10061238
Urich J, Cucchiarini M, Rey-Rico A. Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes. Nanomaterials. 2020; 10(6):1238. https://doi.org/10.3390/nano10061238
Chicago/Turabian StyleUrich, Jonas, Magali Cucchiarini, and Ana Rey-Rico. 2020. "Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes" Nanomaterials 10, no. 6: 1238. https://doi.org/10.3390/nano10061238
APA StyleUrich, J., Cucchiarini, M., & Rey-Rico, A. (2020). Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes. Nanomaterials, 10(6), 1238. https://doi.org/10.3390/nano10061238