Exploration of the Interaction Strength at the Interface of Anionic Chalcogen Anchors and Gold (111)-Based Nanomaterials
Abstract
1. Introduction
2. Models, Computation, and Methodology
3. Results and Discussion
3.1. Binding Mode Conformation
3.2. Interaction Strength Analysis
3.3. Energy Decomposition Analysis
3.4. Charge Transfer Analysis
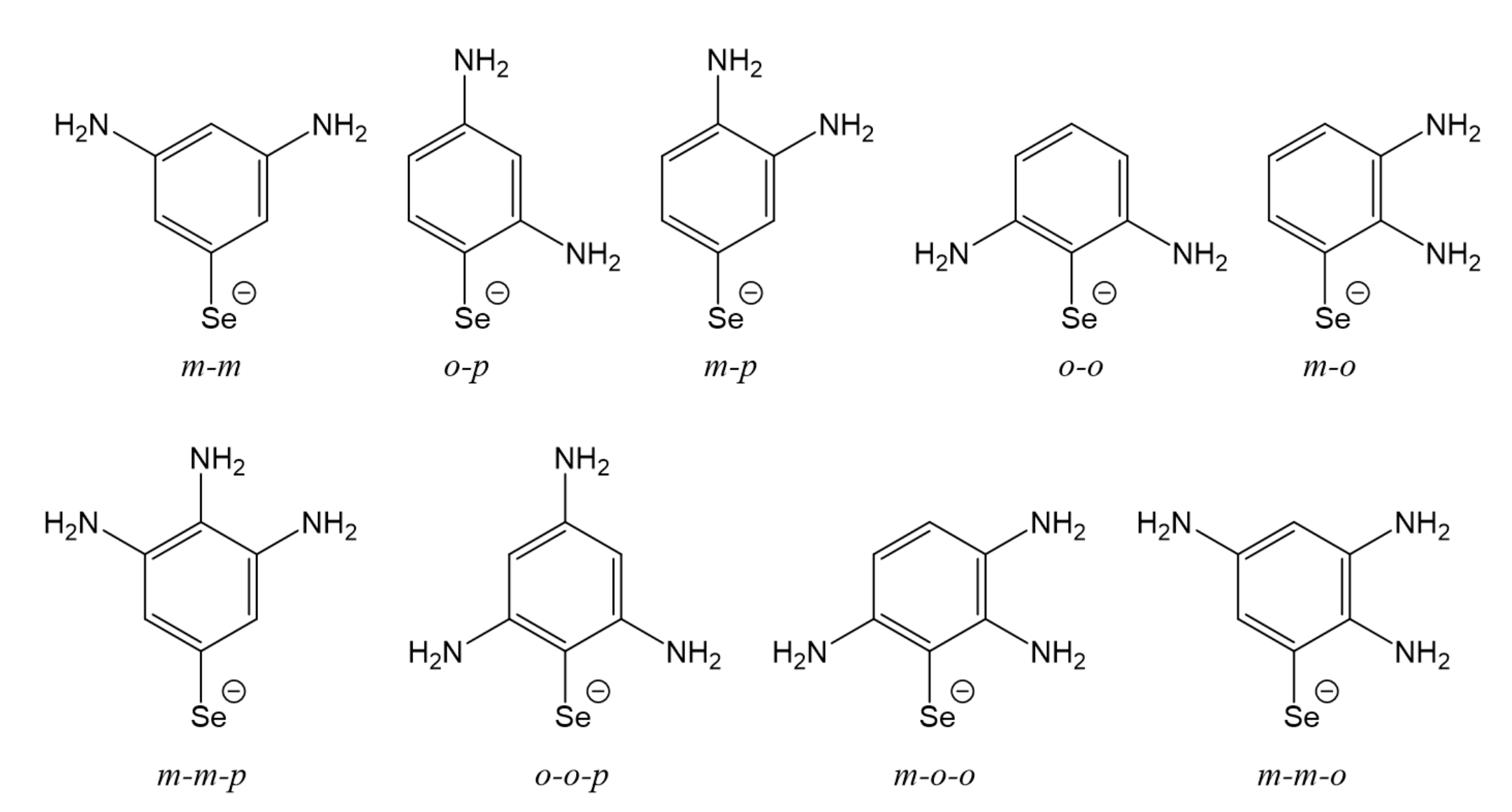
3.5. Multi-Substituted Selenophenolate Ligands
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Puck, A.; Graupe, M.; Colorado, R.; Shon, Y.-S.; Lee, T.R.; Perry, S.S. Structure, Wettability, and Frictional Properties of Phenyl-Terminated Self-Assembled Monolayers on Gold. Langmuir 2001, 17, 7364–7370. [Google Scholar] [CrossRef]
- Houston, J.E.; Kim, H.I. Adhesion, Friction, and Mechanical Properties of Functionalized Alkanethiol Self-Assembled Monolayers. Acc. Chem. Res. 2002, 35, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Kara, A.; Pasquali, L.; Bendounan, A.; Sirotti, F.; Esaulov, V.A. On sulfur core level binding energies in thiol self-assembly and alternative adsorption sites: An experimental and theoretical study. J. Chem. Phys. 2015, 143, 104702. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Thompson, D.T. Using gold nanoparticles for catalysis. Nano Today 2007, 2, 40–43. [Google Scholar] [CrossRef]
- Kang, B.; Mackey, M.A.; El-Sayed, M.A. Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis. J. Am. Chem. Soc. 2010, 132, 1517–1519. [Google Scholar] [CrossRef]
- Pasquato, L.; Pengo, P.; Scrimin, P. Functional gold nanoparticles for recognition and catalysis. J. Mater. Chem. 2004, 14, 3481–3487. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Newton, L.; Slater, T.; Clark, N.; Vijayaraghavan, A. Self assembled monolayers (SAMs) on metallic surfaces (gold and graphene) for electronic applications. J. Mater. Chem. C 2013, 1, 376–393. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Liu, Z.; Li, H.; Campos, L.M.; Neaton, J.B.; Venkataraman, L. Non-chemisorbed gold–sulfur binding prevails in self-assembled monolayers. Nat. Chem. 2019, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- McGuiness, C.L.; Diehl, G.A.; Blasini, D.; Smilgies, D.-M.; Zhu, M.; Samarth, N.; Weidner, T.; Ballav, N.; Zharnikov, M.; Allara, D.L. Molecular Self-Assembly at Bare Semiconductor Surfaces: Cooperative Substrate−Molecule Effects in Octadecanethiolate Monolayer Assemblies on GaAs(111), (110), and (100). ACS Nano 2010, 4, 3447–3465. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jamison, A.C.; Lee, T.R. Surface Dipoles: A Growing Body of Evidence Supports Their Impact and Importance. Acc. Chem. Res. 2015, 48, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold Nanoparticles: Past, Present, and Future. Langmuir 2009, 25, 13840–13851. [Google Scholar] [CrossRef]
- Mameka, N.; Lührs, L.; Heissler, S.; Gliemann, H.; Wöll, C. Tailoring the Strength of Nanoporous Gold by Self-Assembled Monolayers of Alkanethiols. ACS Appl. Nano Mater. 2018, 1, 6613–6621. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Pensa, E.; Cortés, E.; Corthey, G.; Carro, P.; Vericat, C.; Fonticelli, M.H.; Benítez, G.; Rubert, A.A.; Salvarezza, R.C. The Chemistry of the Sulfur–Gold Interface: In Search of a Unified Model. Acc. Chem. Res. 2012, 45, 1183–1192. [Google Scholar] [CrossRef]
- Häkkinen, H. The gold–sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef]
- Zenasni, O.; Marquez, M.D.; Jamison, A.C.; Lee, H.J.; Czader, A.; Lee, T.R. Inverted Surface Dipoles in Fluorinated Self-Assembled Monolayers. Chem. Mater. 2015, 27, 7433–7446. [Google Scholar] [CrossRef]
- Chen, H.; Heng, C.K.; Puiu, P.D.; Zhou, X.D.; Lee, A.C.; Lim, T.M.; Tan, S.N. Detection of Saccharomyces cerevisiae immobilized on self-assembled monolayer (SAM) of alkanethiolate using electrochemical impedance spectroscopy. Anal. Chim. Acta 2005, 554, 52–59. [Google Scholar] [CrossRef]
- Li, S.; Luo, Q.; Zhang, Z.; Shen, G.; Wu, H.; Chen, A.; Liu, X.; Li, M.; Zhang, A. Electrochemistry Study of Permselectivity and Interfacial Electron Transfers of a Branch-Tailed Fluorosurfactant Self-Assembled Monolayer on Gold. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Weidner, T.; Shaporenko, A.; Müller, J.; Schmid, M.; Cyganik, P.; Terfort, A.; Zharnikov, M. Effect of the Bending Potential on Molecular Arrangement in Alkaneselenolate Self-Assembled Monolayers. J. Phys. Chem. C 2008, 112, 12495–12506. [Google Scholar] [CrossRef]
- Wang, M.; Ren, K.; Wang, L. Iron-Catalyzed Ligand-Free Carbon-Selenium (or Tellurium) Coupling of Arylboronic Acids with Diselenides and Ditellurides. Adv. Synth. Catal. 2009, 351, 1586–1594. [Google Scholar] [CrossRef]
- Liotta, D.; Sunay, U.; Santiesteban, H.; Markiewicz, W. Phenyl selenide anion, a superior reagent for the SN2 cleavage of esters and lactones. J. Org. Chem. 1981, 46, 2605–2610. [Google Scholar] [CrossRef]
- Trenner, J.; Depken, C.; Weber, T.; Breder, A. Direct Oxidative Allylic and Vinylic Amination of Alkenes through Selenium Catalysis. Angew. Chem. Int. Ed. 2013, 52, 8952–8956. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Selenium-Catalyzed Regioselective Cyclization of Unsaturated Carboxylic Acids Using Hypervalent Iodine Oxidants. Org. Lett. 2011, 13, 6504–6507. [Google Scholar] [CrossRef]
- Yu, L.; Li, H.; Zhang, X.; Ye, J.; Liu, J.; Xu, Q.; Lautens, M. Organoselenium-Catalyzed Mild Dehydration of Aldoximes: An Unexpected Practical Method for Organonitrile Synthesis. Org. Lett. 2014, 16, 1346–1349. [Google Scholar] [CrossRef]
- Prasad, C.D.; Balkrishna, S.J.; Kumar, A.; Bhakuni, B.S.; Shrimali, K.; Biswas, S.; Kumar, S. Transition-Metal-Free Synthesis of Unsymmetrical Diaryl Chalcogenides from Arenes and Diaryl Dichalcogenides. J. Org. Chem. 2013, 78, 1434–1443. [Google Scholar] [CrossRef]
- Shaporenko, A.; Cyganik, P.; Buck, M.; Terfort, A.; Zharnikov, M. Self-Assembled Monolayers of Aromatic Selenolates on Noble Metal Substrates. J. Phys. Chem. B 2005, 109, 13630–13638. [Google Scholar] [CrossRef]
- Nakayama, Y.; Watanabe, K.; Ueyama, N.; Nakamura, A.; Harada, A.; Okuda, J. Titanium Complexes Having Chelating Diaryloxo Ligands Bridged by Tellurium and Their Catalytic Behavior in the Polymerization of Ethylene. Organometallics 2000, 19, 2498–2503. [Google Scholar] [CrossRef]
- Takashima, Y.; Nakayama, Y.; Watanabe, K.; Itono, T.; Ueyama, N.; Nakamura, A.; Yasuda, H.; Harada, A.; Okuda, J. Polymerizations of Cyclic Esters Catalyzed by Titanium Complexes Having Chalcogen-Bridged Chelating Diaryloxo Ligands. Macromolecules 2002, 35, 7538–7544. [Google Scholar] [CrossRef]
- Weidner, T.; Shaporenko, A.; Müller, J.; Höltig, M.; Terfort, A.; Zharnikov, M. Self-Assembled Monolayers of Aromatic Tellurides on (111)-Oriented Gold and Silver Substrates. J. Phys. Chem. C 2007, 111, 11627–11635. [Google Scholar] [CrossRef]
- Ossowski, J.; Wächter, T.; Silies, L.; Kind, M.; Noworolska, A.; Blobner, F.; Gnatek, D.; Rysz, J.; Bolte, M.; Feulner, P.; et al. Thiolate versus Selenolate: Structure, Stability, and Charge Transfer Properties. ACS Nano 2015, 9, 4508–4526. [Google Scholar] [CrossRef]
- Miranda-Rojas, S.; Muñoz-Castro, A.; Arratia-Pérez, R.; Mendizábal, F. Theoretical insights into the adsorption of neutral{,} radical and anionic thiophenols on gold(111). Phys. Chem. Chem. Phys. 2013, 15, 20363–20370. [Google Scholar] [CrossRef]
- Miranda-Rojas, S.; Salazar-Molina, R.; Kästner, J.; Arratia-Pérez, R.; Mendizábal, F. Theoretical exploration of seleno and tellurophenols as promising alternatives to sulfur ligands for anchoring to gold (111) materials. RSC Adv. 2016, 6, 4458–4468. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta–generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Johansson, M.P.; Lechtken, A.; Schooss, D.; Kappes, M.M.; Furche, F. 2D-3D transition of gold cluster anions resolved. Phys. Rev. A 2008, 77, 53202. [Google Scholar] [CrossRef]
- Goel, S.; Velizhanin, K.A.; Piryatinski, A.; Tretiak, S.; Ivanov, S.A. DFT Study of Ligand Binding to Small Gold Clusters. J. Phys. Chem. Lett. 2010, 1, 927–931. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Igel-Mann, G.; Stoll, H.; Preuss, H. Pseudopotentials for main group elements (IIIa through VIIa). Mol. Phys. 1988, 65, 1321–1328. [Google Scholar] [CrossRef]
- Bergner, A.; Dolg, M.; Küchle, W.; Stoll, H.; Preuß, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Hujo, W.; Grimme, S. Performance of the van der Waals Density Functional VV10 and (hybrid)GGA Variants for Thermochemistry and Noncovalent Interactions. J. Chem. Theory Comput. 2011, 7, 3866–3871. [Google Scholar] [CrossRef]
- Grimme, S. Comment on: “On the Accuracy of DFT Methods in Reproducing Ligand Substitution Energies for Transition Metal Complexes in Solution: The Role of Dispersive Interactions” by H. Jacobsen and L. Cavallo. ChemPhysChem 2012, 13, 1407–1409. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the calculation of bonding energies by the Hartree Fock Slater method. Theor. Chim. Acta 1977, 46, 1–10. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic total energy using regular approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Sánchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Understanding Regium Bonds and their Competition with Hydrogen Bonds in Au2:HX Complexes. ChemPhysChem. 2019, 20, 1572–1580. [Google Scholar] [CrossRef]
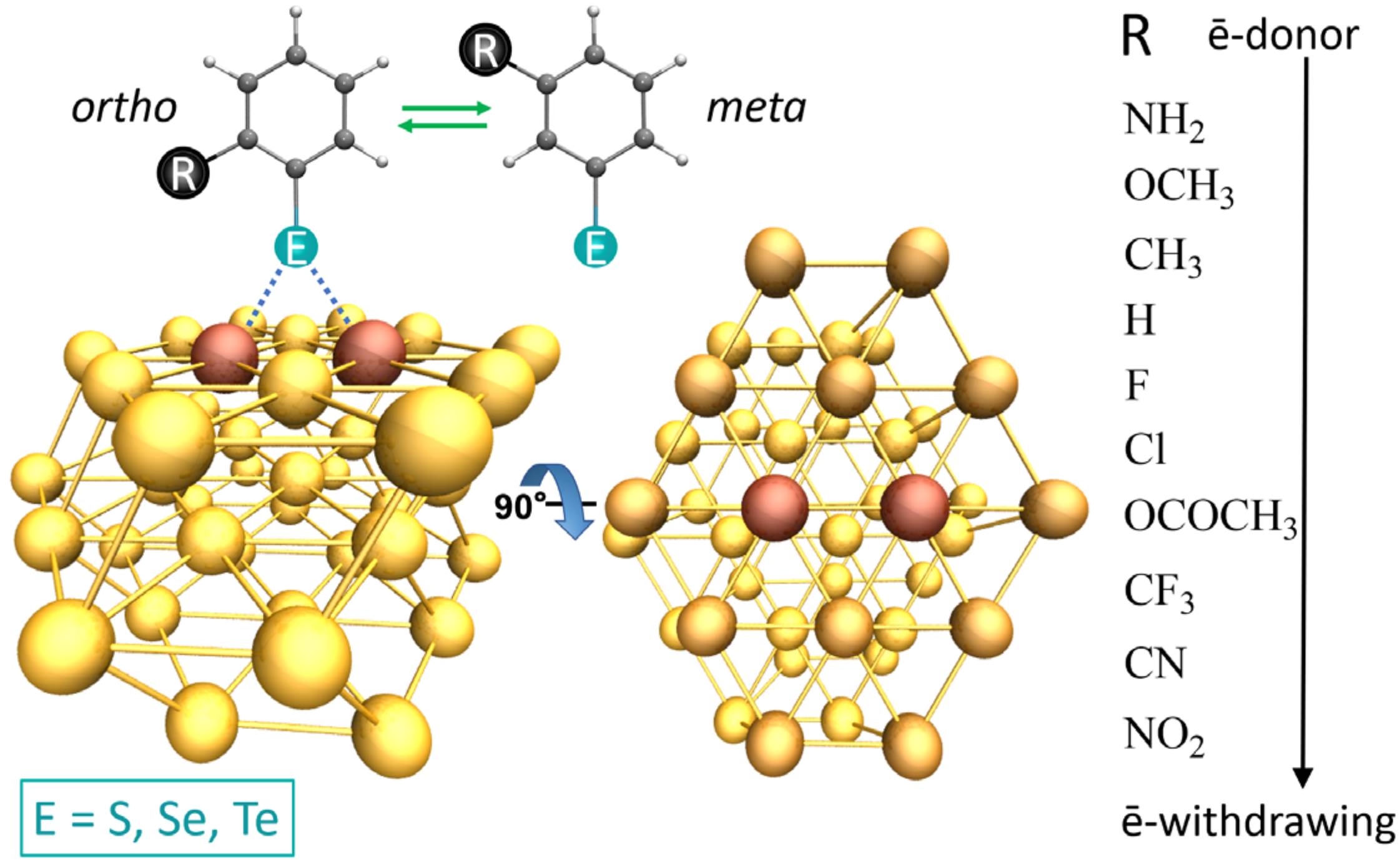
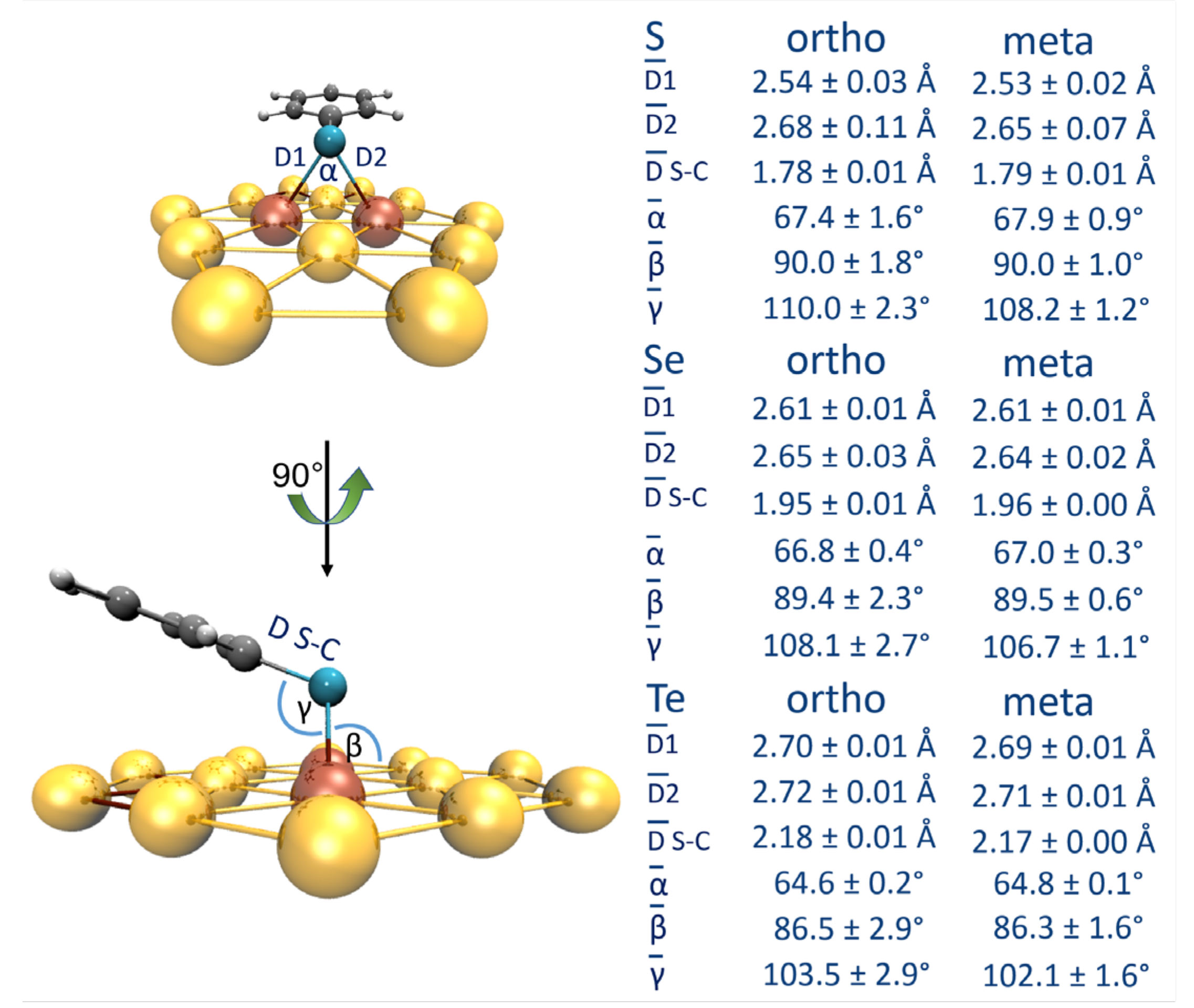
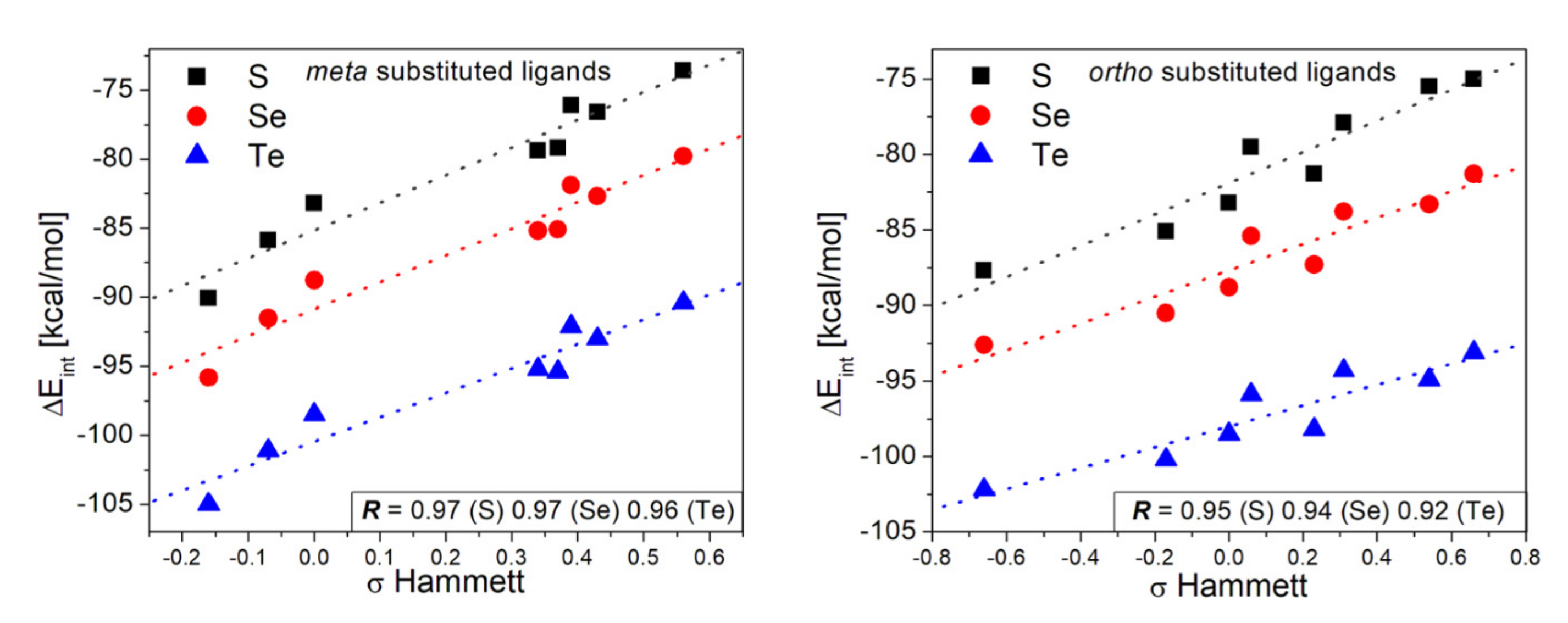
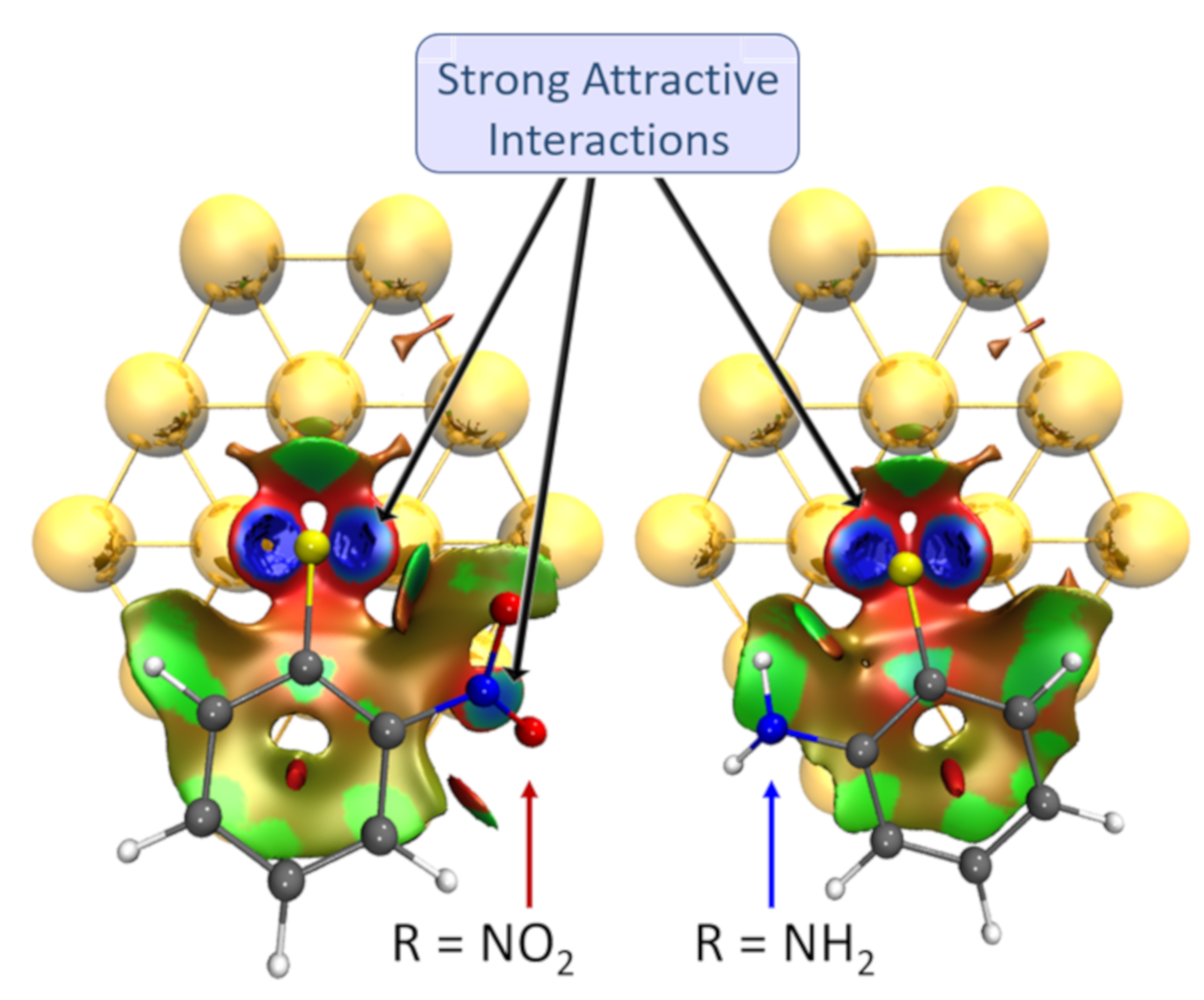
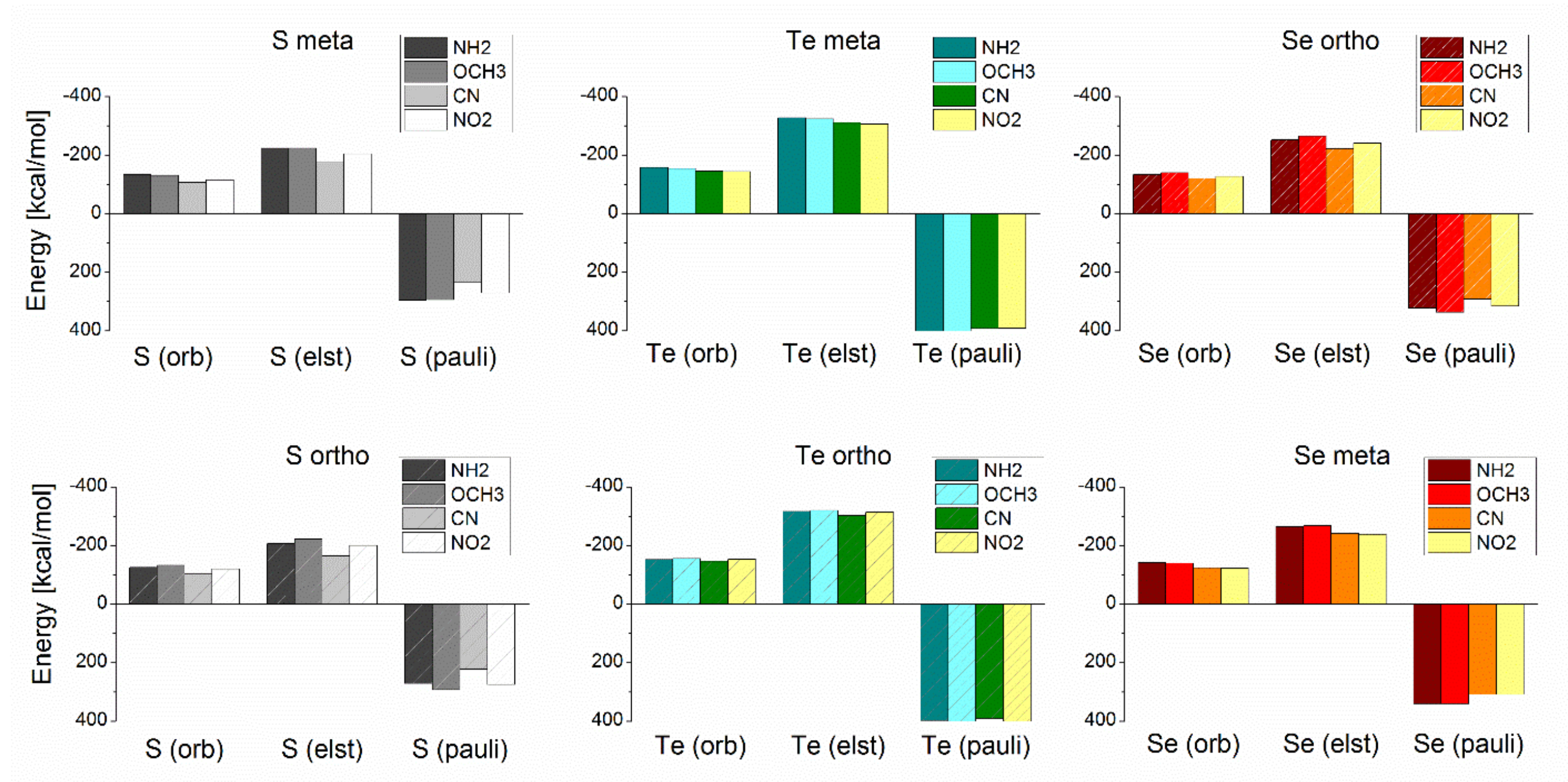

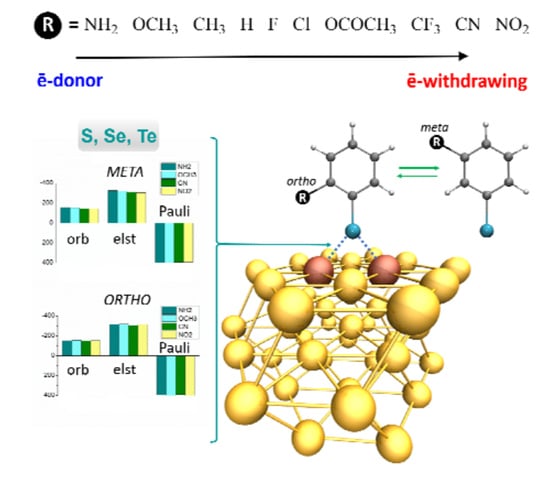
| [Au42-EPhRo]− | ΔEint(TPSS-D3) | ΔEint(TPSS) | % Dispersion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S | Se | Te | S | Se | Te | S | Se | Te | |
| Au42-EC6H4(NH2)o | −87.7 | −92.6 | −102.2 | −55.4 | −59.2 | −66.6 | 36.8 | 36.0 | 34.8 |
| Au42-EC6H4(OCH3)o | −89.3 | −95.1 | −105.3 | −57.1 | −61.2 | −69.3 | 36.1 | 35.7 | 34.1 |
| Au42-EC6H4(CH3)o | −85.1 | −90.5 | −100.2 | −54.4 | −58.0 | −65.3 | 36.1 | 35.9 | 34.8 |
| Au42-EC6H5 | −83.2 | −88.8 | −98.5 | −55.4 | −59.1 | −66.7 | 33.4 | 33.5 | 32.3 |
| Au42-EC6H4(F)o | −79.5 | −85.4 | −95.9 | −51.4 | −55.5 | −64.1 | 35.4 | 35.0 | 33.2 |
| Au42-EC6H4(Cl)o | −81.3 | −87.3 | −98.2 | −50.0 | −54.0 | −62.7 | 38.5 | 38.1 | 36.1 |
| Au42-EC6H4(OCOCH3)o | −77.9 | −83.8 | −94.3 | −43.8 | −48.1 | −57.2 | 43.7 | 42.6 | 39.4 |
| Au42-EC6H4(CF3)o | −75.5 | −83.3 | −94.9 | −45.3 | −51.7 | −61.3 | 40.0 | 37.9 | 35.4 |
| Au42-EC6H4(CN)o | −75.0 | −81.3 | −93.1 | −43.6 | −48.1 | −57.3 | 41.8 | 40.9 | 38.5 |
| Au42-EC6H4(NO2)o | −76.7 | −84.3 | −97.8 | −42.9 | −48.5 | −59.5 | 44.0 | 42.5 | 39.1 |
| [Au42–EPhRo]− | ΔEint(TPSS-D3) | ΔEint(TPSS) | % Dispersion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S | Se | Te | S | Se | Te | S | Se | Te | |
| Au42-EC6H4(NH2)m | −90.1 | −95.8 | −105.0 | −58.7 | −62.5 | −69.4 | 34.9 | 34.7 | 33.9 |
| Au42-EC6H4(OCH3)m | −86.5 | −92.1 | −101.7 | −55.7 | −59.5 | −67.0 | 35.6 | 35.4 | 34.1 |
| Au42-EC6H4(CH3)m | −85.9 | −91.5 | −101.1 | −55.6 | −59.6 | −67.3 | 35.3 | 34.9 | 33.5 |
| Au42-EC6H5 | −83.2 | −88.8 | −98.5 | −55.4 | −59.0 | −66.7 | 33.4 | 33.6 | 32.3 |
| Au42-EC6H4(F)m | −79.4 | −85.2 | −95.2 | −51.4 | −55.4 | −63.1 | 35.3 | 35.0 | 33.7 |
| Au42-EC6H4(Cl)m | −79.2 | −85.1 | −95.4 | −48.9 | −53.2 | −61.5 | 38.3 | 37.5 | 35.5 |
| Au42-EC6H4(OCOCH3)m | −76.1 | −81.9 | −92.1 | −47.0 | −51.3 | −59.2 | 38.2 | 37.4 | 35.7 |
| Au42-EC6H4(CF3)m | −76.6 | −82.7 | −93.0 | −46.9 | −51.2 | −59.3 | 38.8 | 38.1 | 36.2 |
| Au42-EC6H4(CN)m | −73.6 | −79.8 | −90.4 | −44.4 | −49.0 | −57.4 | 39.7 | 38.6 | 36.5 |
| Au42-EC6H4(NO2)m | −74.7 | −80.6 | −91.0 | −43.3 | −47.7 | −56.0 | 42.1 | 40.8 | 38.4 |
| Model System | % Orb (meta) | % Orb (ortho) | % Elect (meta) | % Elect (ortho) |
|---|---|---|---|---|
| Au42-SC6H4(NH2) | 37.4 | 37.6 | 62.6 | 62.4 |
| Au42-SeC6H4(NH2) | 34.8 | 34.7 | 65.2 | 65.3 |
| Au42-TeC6H4(NH2) | 32.6 | 32.4 | 67.4 | 67.6 |
| Au42-SC6H4(OCH3) | 37.0 | 37.2 | 63.0 | 62.8 |
| Au42-SeC6H4(OCH3) | 34.3 | 34.5 | 65.7 | 65.5 |
| Au42-TeC6H4(OCH3) | 32.3 | 32.7 | 67.7 | 67.3 |
| Au42-SC6H4(CN) | 37.6 | 38.7 | 62.4 | 61.3 |
| Au42-SeC6H4(CN) | 33.8 | 34.7 | 66.2 | 65.3 |
| Au42-TeC6H4(CN) | 31.6 | 32.5 | 68.4 | 67.5 |
| Au42-SC6H4(NO2) | 36.0 | 37.4 | 64.0 | 62.6 |
| Au42-SeC6H4(NO2) | 33.9 | 35.1 | 66.1 | 64.9 |
| Au42-TeC6H4(NO2) | 31.9 | 32.7 | 68.1 | 67.3 |
| S | Se | Te | ||||
|---|---|---|---|---|---|---|
| Substituent | Au42 | ∆S a | Au42 | ∆Se a | Au42 | ∆Te a |
| -NH2 | −0.52 | 0.28 | −0.61 | 0.41 | −0.80 | 0.65 |
| -OCH3 | −0.49 | 0.26 | −0.61 | 0.41 | −0.81 | 0.66 |
| -CH3 | −0.45 | 0.25 | −0.56 | 0.39 | −0.76 | 0.63 |
| -H | −0.48 | 0.27 | −0.60 | 0.42 | −0.79 | 0.66 |
| -F | −0.45 | 0.24 | −0.57 | 0.39 | −0.77 | 0.63 |
| -Cl | −0.49 | 0.23 | −0.56 | 0.36 | −0.75 | 0.61 |
| -OCOCH3 | −0.39 | 0.18 | −0.50 | 0.32 | −0.68 | 0.54 |
| -CF3 | −0.40 | 0.20 | −0.53 | 0.34 | −0.73 | 0.58 |
| -CN | −0.37 | 0.16 | −0.50 | 0.30 | −0.70 | 0.55 |
| -NO2 | −0.35 | 0.14 | −0.46 | 0.27 | −0.66 | 0.50 |
| S | Se | Te | ||||
|---|---|---|---|---|---|---|
| Substituent | Au42 | ∆Sa | Au42 | ∆Se a | Au42 | ∆Te a |
| -NH2 | −0.51 | 0.27 | −0.62 | 0.42 | −0.82 | 0.67 |
| -OCH3 | −0.48 | 0.27 | −0.60 | 0.42 | −0.80 | 0.66 |
| -CH3 | −0.47 | 0.27 | −0.59 | 0.42 | −0.79 | 0.66 |
| -H | −0.48 | 0.27 | −0.59 | 0.42 | −0.79 | 0.66 |
| -F | −0.45 | 0.25 | −0.57 | 0.40 | −0.77 | 0.64 |
| -Cl | −0.44 | 0.23 | −0.56 | 0.38 | −0.76 | 0.63 |
| -OCOCH3 | −0.42 | 0.22 | −0.54 | 0.36 | −0.74 | 0.62 |
| -CF3 | −0.42 | 0.22 | −0.55 | 0.38 | −0.75 | 0.63 |
| -CN | −0.39 | 0.21 | −0.53 | 0.36 | −0.74 | 0.61 |
| -NO2 | −0.36 | 0.20 | −0.49 | 0.34 | −0.71 | 0.58 |
| ΔEint(TPSS-D3) | ΔEint(TPSS) | % Dispersion | |
|---|---|---|---|
| (NH2)m-m-p | −97.3 | −71.0 | 37.2 |
| (NH2)o-o-p | −96.3 | −68.7 | 40.2 |
| (NH2)m-o-o | −95.3 | −67.0 | 42.4 |
| (NH2)m-m-o | −94.5 | −67.3 | 40.5 |
| (NH2)m-m | −93.0 | −68.7 | 35.3 |
| (NH2)o-p | −92.9 | −68.6 | 35.4 |
| (NH2)m-p | −91.8 | −68.1 | 34.8 |
| (NH2)o-o | −90.7 | −64.7 | 40.2 |
| (NH2)m-o | −87.9 | −62.2 | 41.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Rojas, S.; Mendizabal, F. Exploration of the Interaction Strength at the Interface of Anionic Chalcogen Anchors and Gold (111)-Based Nanomaterials. Nanomaterials 2020, 10, 1237. https://doi.org/10.3390/nano10061237
Miranda-Rojas S, Mendizabal F. Exploration of the Interaction Strength at the Interface of Anionic Chalcogen Anchors and Gold (111)-Based Nanomaterials. Nanomaterials. 2020; 10(6):1237. https://doi.org/10.3390/nano10061237
Chicago/Turabian StyleMiranda-Rojas, Sebastián, and Fernando Mendizabal. 2020. "Exploration of the Interaction Strength at the Interface of Anionic Chalcogen Anchors and Gold (111)-Based Nanomaterials" Nanomaterials 10, no. 6: 1237. https://doi.org/10.3390/nano10061237
APA StyleMiranda-Rojas, S., & Mendizabal, F. (2020). Exploration of the Interaction Strength at the Interface of Anionic Chalcogen Anchors and Gold (111)-Based Nanomaterials. Nanomaterials, 10(6), 1237. https://doi.org/10.3390/nano10061237