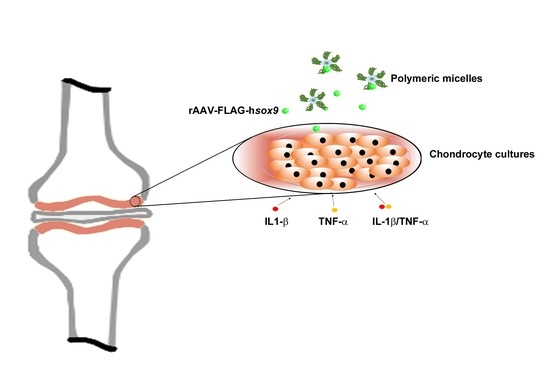
Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells
2.3. Plasmids and rAAV Vectors
2.4. Preparation of Micellar Copolymer Solutions Containing rAAV-FLAG-hsox9 Vectors
2.5. Gene Transfer in Inflammatory Conditions via rAAV-FLAG-hsox9/Polymeric Micelles
2.6. Histological and Immunocytochemical Analyses
2.7. Histomorphometry
2.8. Evaluation of Cell Proliferation and Viability
2.9. Statistical Analysis
3. Results
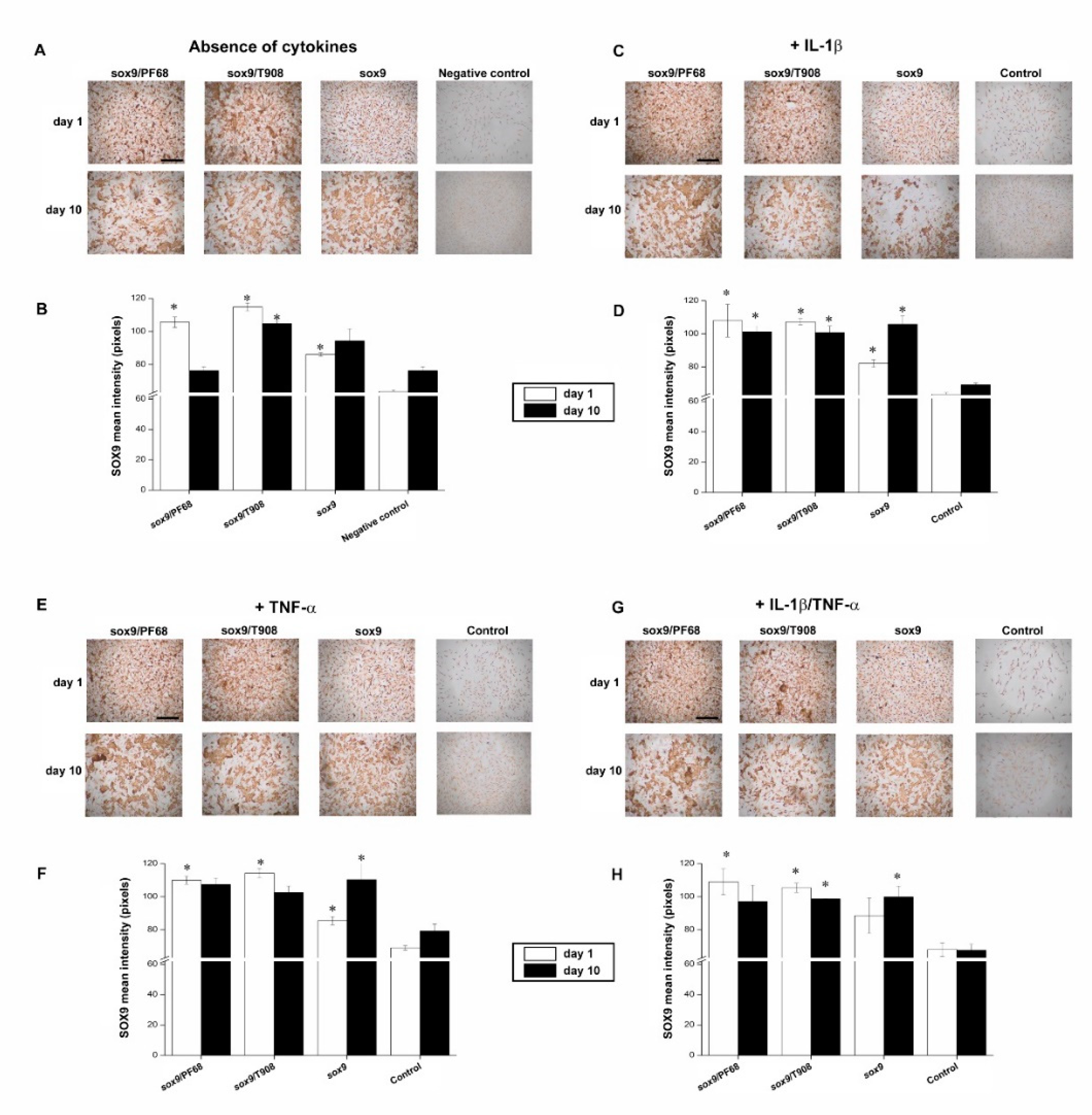
3.1. Efficacy of rAAV-Mediated sox9 Overexpression in Conditions of Inflammation upon Vector Delivery via Polymeric Micelles
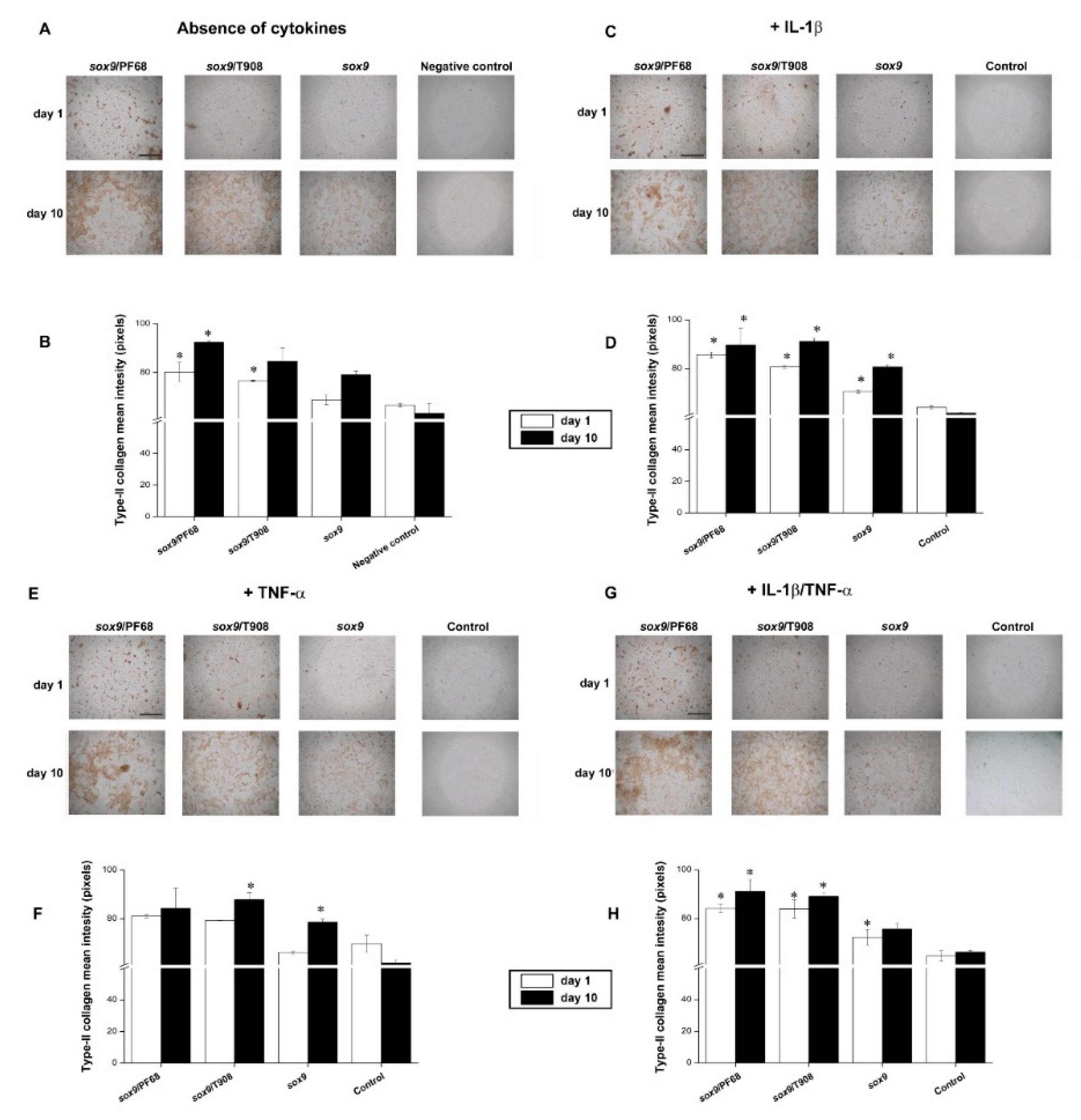
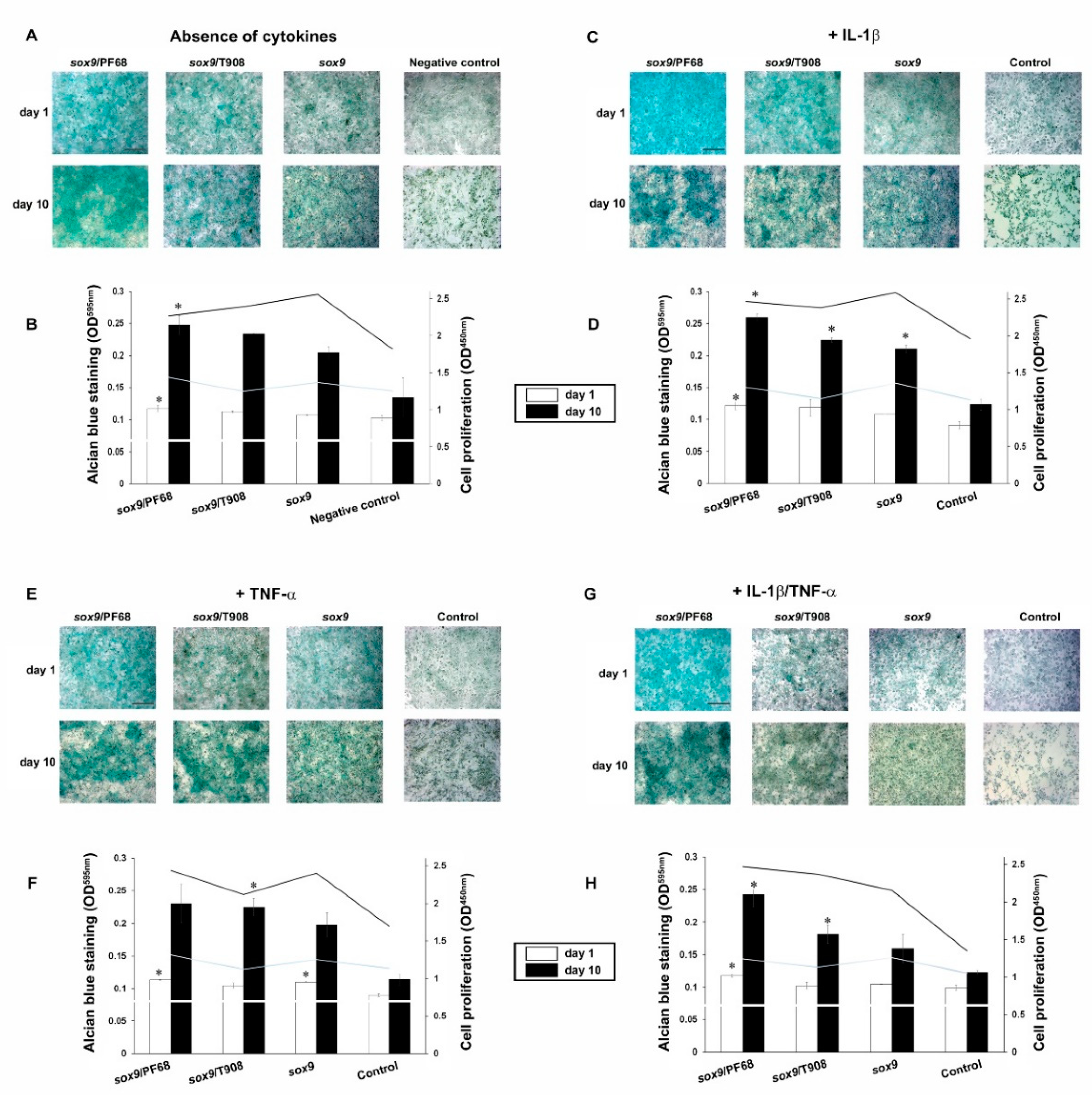
3.2. Effects of rAAV-FLAG-hsox9/Polymeric Micelle Delivery on the Anabolic Activities of Human OA Chondrocytes in Inflammatory Conditions
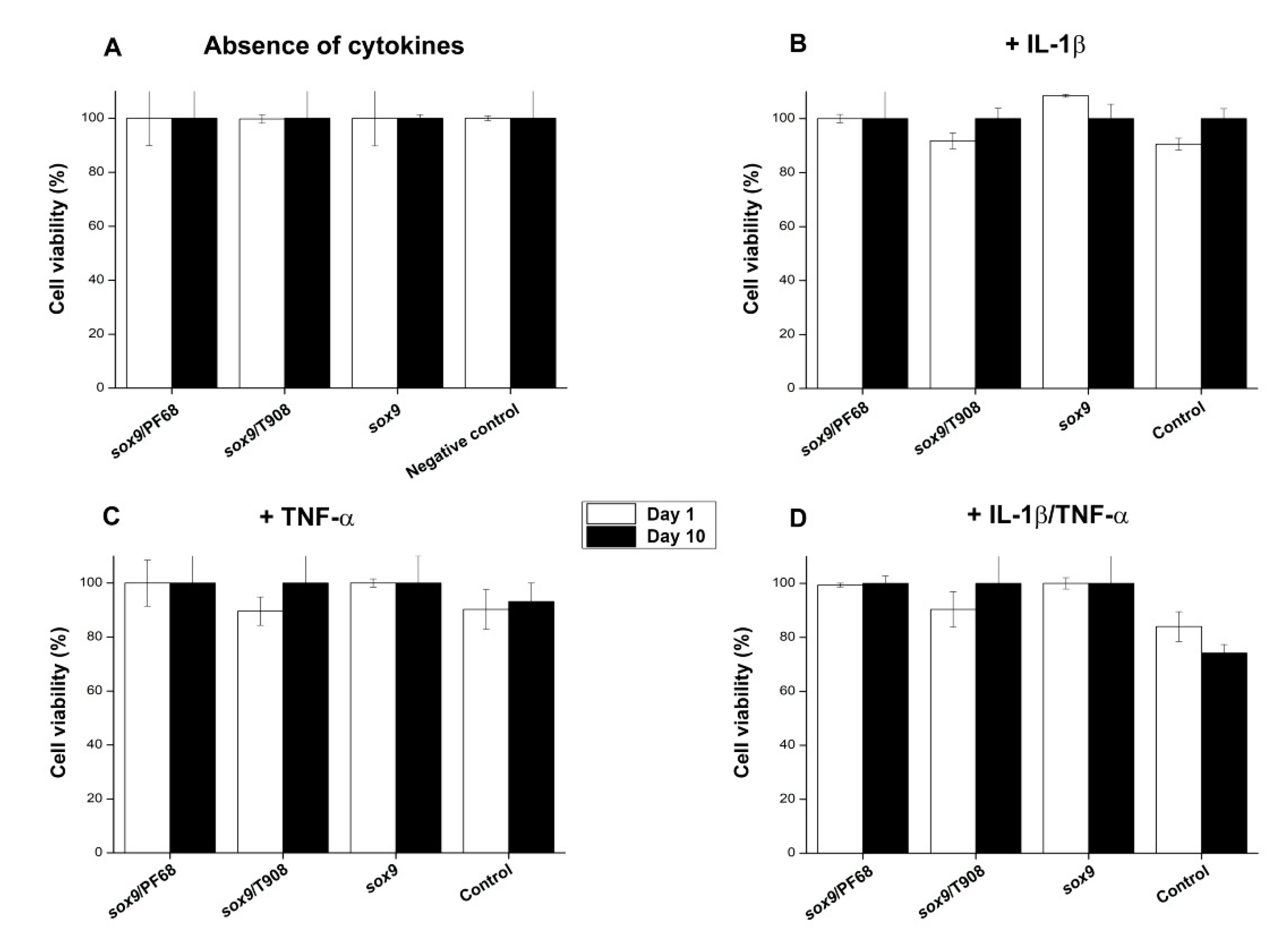
3.3. Effects of rAAV-FLAG-hsox9/Polymeric Micelle Delivery on the Viability Processes in Human OA Chondrocytes in Inflammatory Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current treatment options for osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R. Osteoarthritis as a whole joint disease. HSS J. 2012, 8, 4–6. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Hwang, S.G.; Yu, S.S.; Poo, H.; Chun, J.S. C-jun/activator protein-1 mediates interleukin-1beta-induced dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J. Biol. Chem. 2005, 280, 29780–29787. [Google Scholar] [CrossRef]
- Lu, H.; Zeng, C.; Chen, M.; Lian, L.; Dai, Y.; Zhao, H. Lentiviral vector-mediated over-expression of sox9 protected chondrocytes from il-1beta induced degeneration and apoptosis. Int. J. Clin. Exp. Pathol. 2015, 8, 10038–10049. [Google Scholar]
- Schlimgen, R.; Howard, J.; Wooley, D.; Thompson, M.; Baden, L.R.; Yang, O.O.; Christiani, D.C.; Mostoslavsky, G.; Diamond, D.V.; Duane, E.G.; et al. Risks associated with lentiviral vector exposures and prevention strategies. J. Occup. Environ. Med. 2016, 58, 1159–1166. [Google Scholar] [CrossRef]
- Smith, R.H. Adeno-associated virus integration: Virus versus vector. Gene Ther. 2008, 15, 817–822. [Google Scholar] [CrossRef]
- Madry, H.; Cucchiarini, M.; Terwilliger, E.F.; Trippel, S.B. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum. Gene Ther. 2003, 14, 393–402. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Madry, H.; Ma, C.; Thurn, T.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Trippel, S.B.; Terwilliger, E.F. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol. Ther. 2005, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, A.; Yokoo, N.; Xin, K.Q.; Okuda, K.; Mizukami, H.; Ozawa, K.; Saito, T. Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector. Hum. Gene Ther. 2005, 16, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Orth, P.; Madry, H. Direct rAAV sox9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J. Mol. Med. 2013, 91, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther. 2014, 21, 811–819. [Google Scholar] [CrossRef]
- Ortved, K.F.; Begum, L.; Mohammed, H.O.; Nixon, A.J. Implantation of rAAV5-IGF-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol. Ther. 2015, 23, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Vinther, M.; Stengaard, C.; Schwarz, E.M.; Goldring, M.B.; Soballe, K. Adeno-associated vector mediated gene transfer of transforming growth factor-beta1 to normal and osteoarthritic human chondrocytes stimulates cartilage anabolism. Eur. Cell Mater. 2005, 10, 40–50. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Wezel, A.; Madry, H.; Cucchiarini, M. rAAV-mediated overexpression of TGF-beta stably restructures human osteoarthritic articular cartilage in situ. J. Transl. Med. 2013, 11, 211. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Thurn, T.; Weimer, A.; Kohn, D.; Terwilliger, E.F.; Madry, H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor sox9. Arthritis Rheum. 2007, 56, 158–167. [Google Scholar] [CrossRef]
- Cottard, V.; Valvason, C.; Falgarone, G.; Lutomski, D.; Boissier, M.C.; Bessis, N. Immune response against gene therapy vectors: Influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 2004, 24, 162–169. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Rial-Hermida, I.; Taboada, P.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO-PPO-PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl. Mater. Interfaces 2016, 8, 20600–20613. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. rAAV-mediated overexpression of TGF-beta via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int. J. Nanomed. 2017, 12, 6985–6996. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Speicher-Mentges, S.; Madry, H.; Cucchiarini, M. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol. Pharm. 2018, 15, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Chang, L.S.; Shenk, T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989, 63, 3822–3828. [Google Scholar] [CrossRef]
- Samulski, R.J.; Chang, L.S.; Shenk, T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 1987, 61, 3096–3101. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Ekici, M.; Madry, H.; Schmitt, G.; Kohn, D.; Cucchiarini, M. Sox9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res. Ther. 2012, 3, 22. [Google Scholar] [CrossRef]
- Tao, K.; Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Lin, J.; Cucchiarini, M. rAAV-mediated combined gene transfer and overexpression of tgf-beta and sox9 remodels human osteoarthritic articular cartilage. J. Orthop. Res. 2016, 34, 2181–2190. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Frisch, J.; Rey-Rico, A.; Schmitt, G.; Madry, H.; Cucchiarini, M. Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors. J. Exp. Orthop. 2017, 4, 22. [Google Scholar] [CrossRef]
- Frisch, J.; Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Madry, H.; Cucchiarini, M. Determination of the chondrogenic differentiation processes in human bone marrow-derived mesenchymal stem cells genetically modified to overexpress transforming growth factor-beta via recombinant adeno-associated viral vectors. Hum. Gene Ther. 2014, 25, 1050–1060. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. Peo-ppo-peo micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1beta. Arthritis Res. 2001, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Schuerwegh, A.J.; Dombrecht, E.J.; Stevens, W.J.; Van Offel, J.F.; Bridts, C.H.; De Clerck, L.S. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (il-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef]
- Stanton, L.A.; Sabari, S.; Sampaio, A.V.; Underhill, T.M.; Beier, F. P38 map kinase signalling is required for hypertrophic chondrocyte differentiation. Biochem. J. 2004, 378, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Wang, G.; Beier, F. Rhoa/rock signaling regulates sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 2005, 280, 11626–11634. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Ikeda, T.; Kamekura, S.; Mabuchi, A.; Kou, I.; Seki, S.; Takato, T.; Nakamura, K.; Kawaguchi, H.; Ikegawa, S.; Chung, U.I. The combination of sox5, sox6, and sox9 (the sox trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004, 50, 3561–3573. [Google Scholar] [CrossRef]
- Salminen, H.; Vuorio, E.; Saamanen, A.M. Expression of sox9 and type IIa procollagen during attempted repair of articular cartilage damage in a transgenic mouse model of osteoarthritis. Arthritis Rheum. 2001, 44, 947–955. [Google Scholar] [CrossRef]
- Aigner, T.; Gebhard, P.M.; Schmid, E.; Bau, B.; Harley, V.; Poschl, E. Sox9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol. 2003, 22, 363–372. [Google Scholar] [CrossRef]
- Madry, H.; Cucchiarini, M. Advances and challenges in gene-based approaches for osteoarthritis. J. Gene Med. 2013, 15, 343–355. [Google Scholar] [CrossRef]
- Evans, C.H.; Huard, J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Calcedo, R.; Wilson, J.M. Humoral immune response to AAV. Front. Immunol. 2013, 4, 341. [Google Scholar] [CrossRef] [PubMed]
- Traister, R.S.; Fabre, S.; Wang, Z.; Xiao, X.; Hirsch, R. Inflammatory cytokine regulation of transgene expression in human fibroblast-like synoviocytes infected with adeno-associated virus. Arthritis Rheum. 2006, 54, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.F.; Zhang, X.; Sakano, S.; Lefebvre, V.; Sandell, L.J. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by sox9. J. Bone Miner. Res. 1999, 14, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Furumatsu, T.; Matsumoto-Ogawa, E.; Tanaka, T.; Lu, Z.; Ozaki, T. Rock inhibition enhances aggrecan deposition and suppresses matrix metalloproteinase-3 production in human articular chondrocytes. Connect. Tissue Res. 2014, 55, 89–95. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urich, J.; Cucchiarini, M.; Rey-Rico, A. Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes. Nanomaterials 2020, 10, 1238. https://doi.org/10.3390/nano10061238
Urich J, Cucchiarini M, Rey-Rico A. Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes. Nanomaterials. 2020; 10(6):1238. https://doi.org/10.3390/nano10061238
Chicago/Turabian StyleUrich, Jonas, Magali Cucchiarini, and Ana Rey-Rico. 2020. "Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes" Nanomaterials 10, no. 6: 1238. https://doi.org/10.3390/nano10061238
APA StyleUrich, J., Cucchiarini, M., & Rey-Rico, A. (2020). Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes. Nanomaterials, 10(6), 1238. https://doi.org/10.3390/nano10061238