Graphene Oxide-Polypyrrole Coating for Functional Ceramics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PPy/rGO Ceramic-Supported Electrodes
2.2.1. Metallization of Ceramic Substrate by Electroless Process
2.2.2. Modification of CS Substrates with GO
2.2.3. Reduction of GO
2.2.4. Electrodeposition of PPy on rGO-Modified CS Substrates
2.3. Characterization
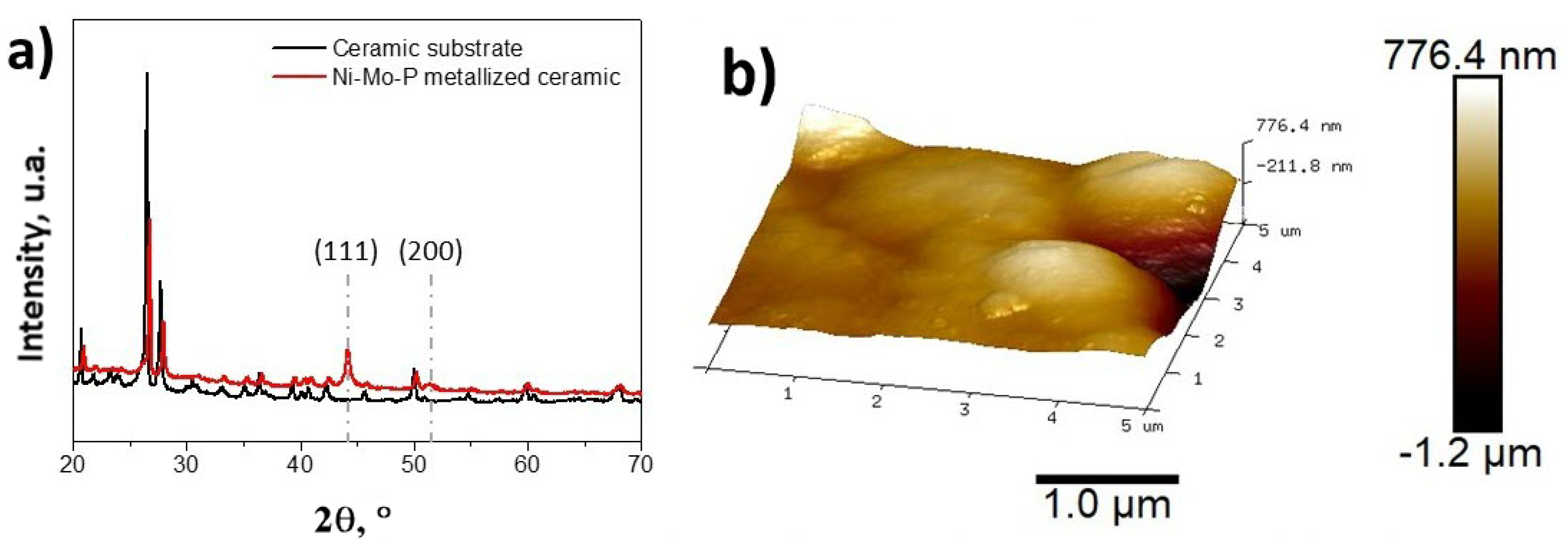
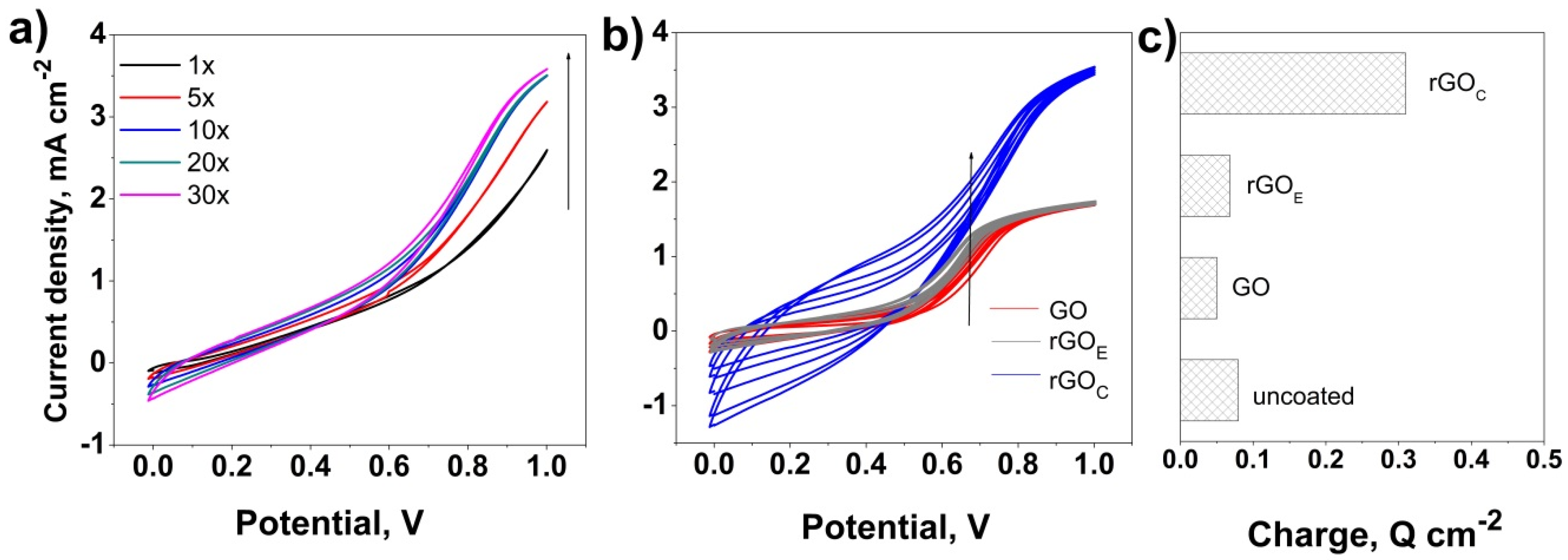
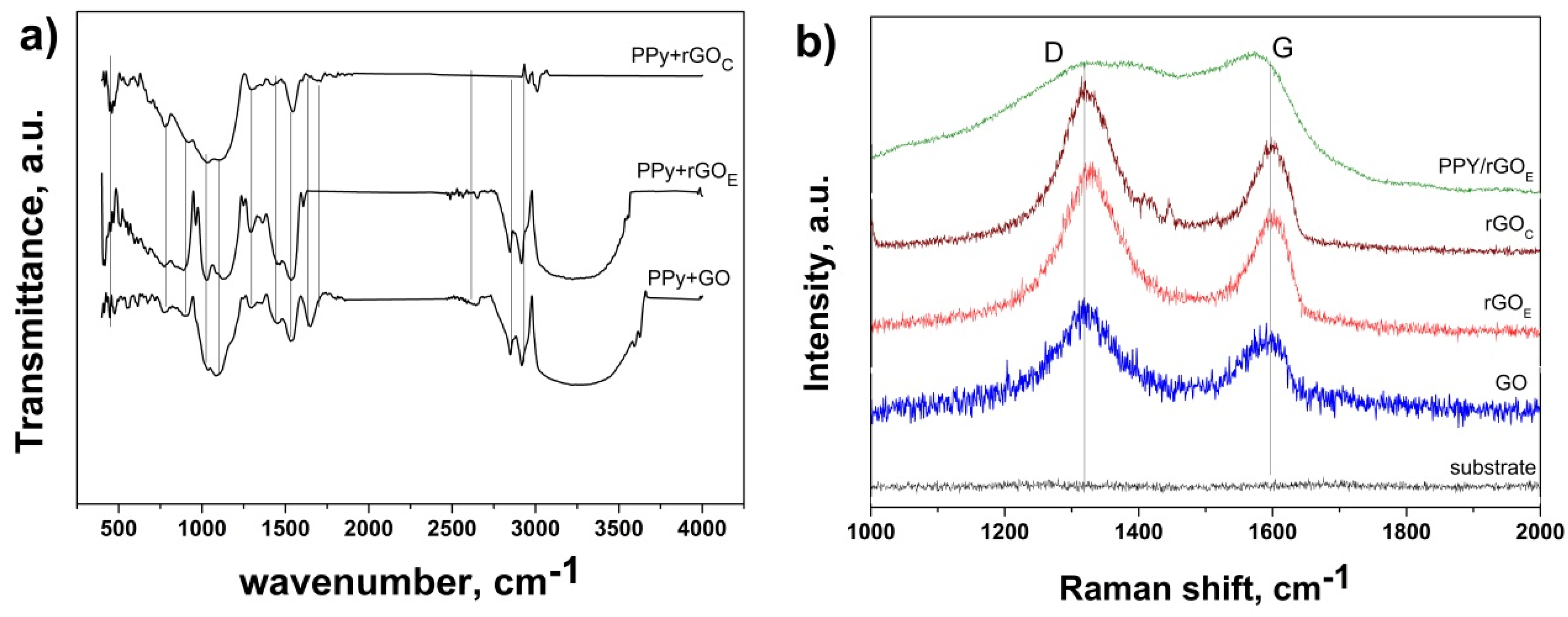
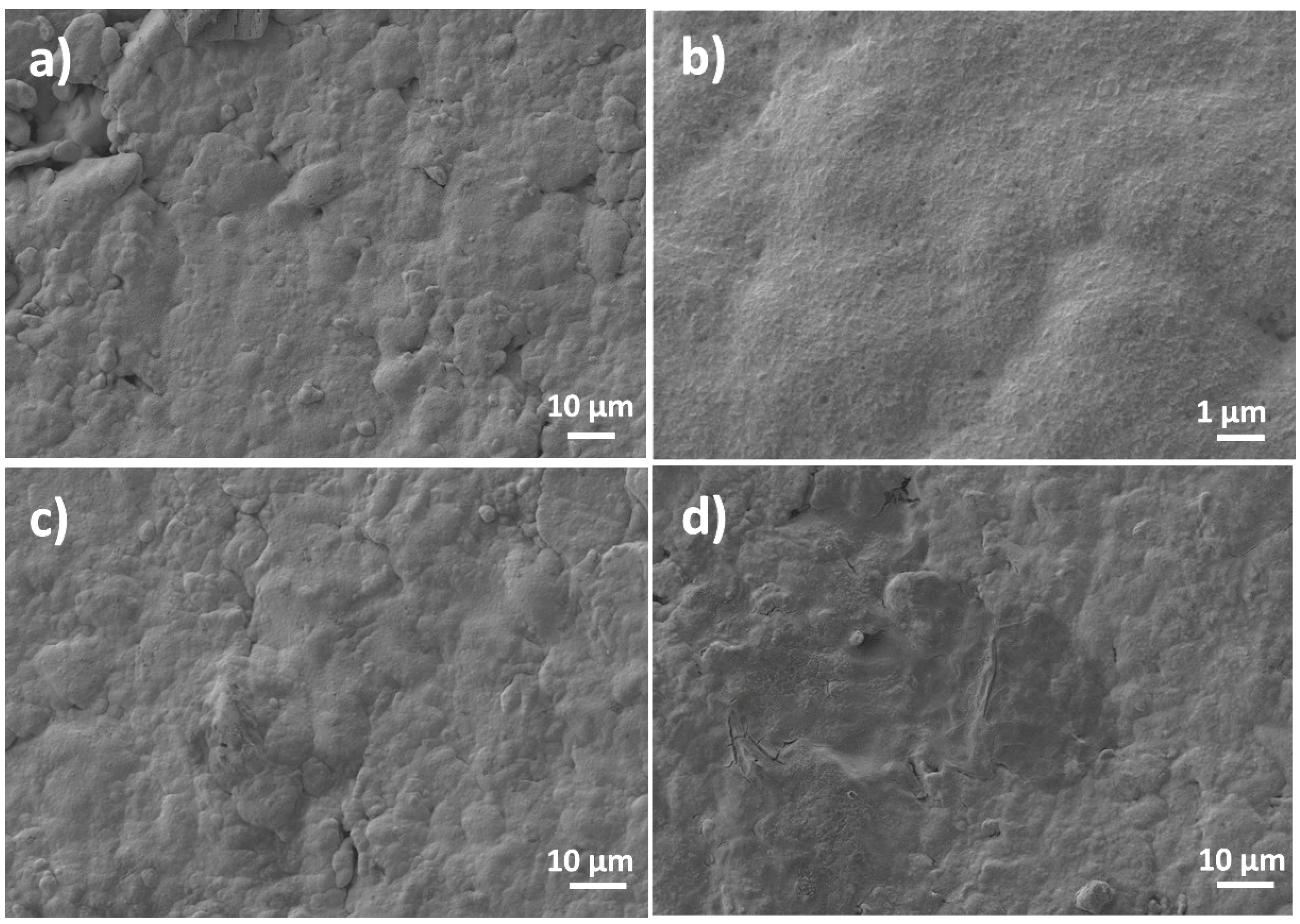
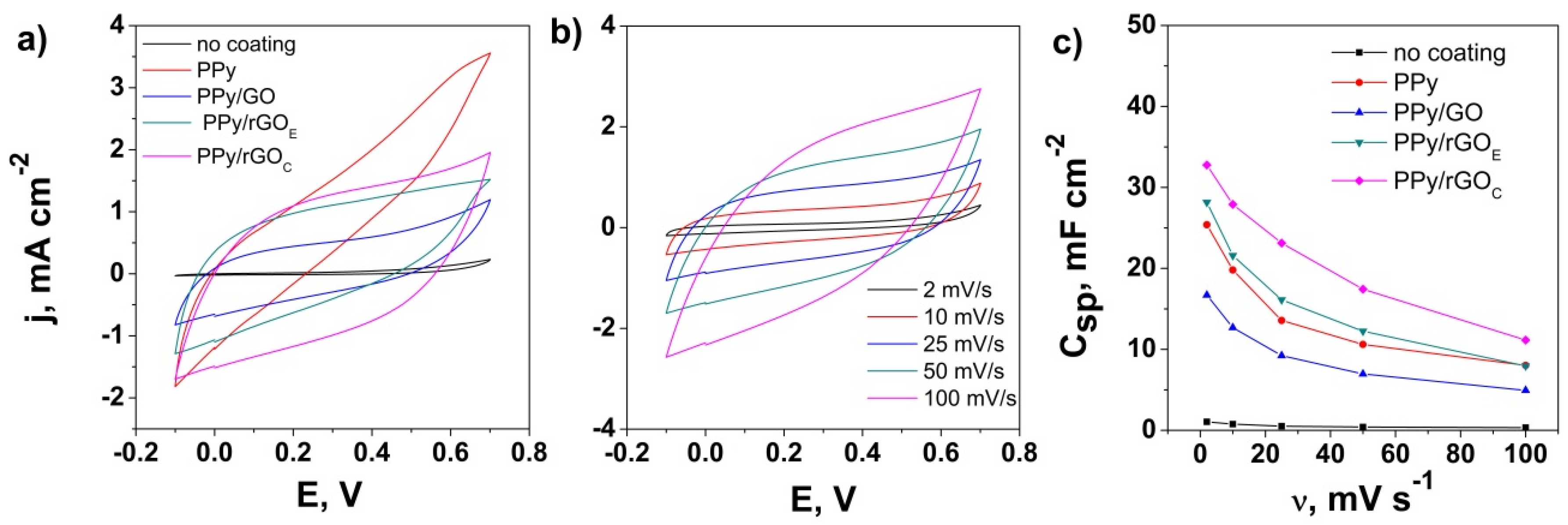
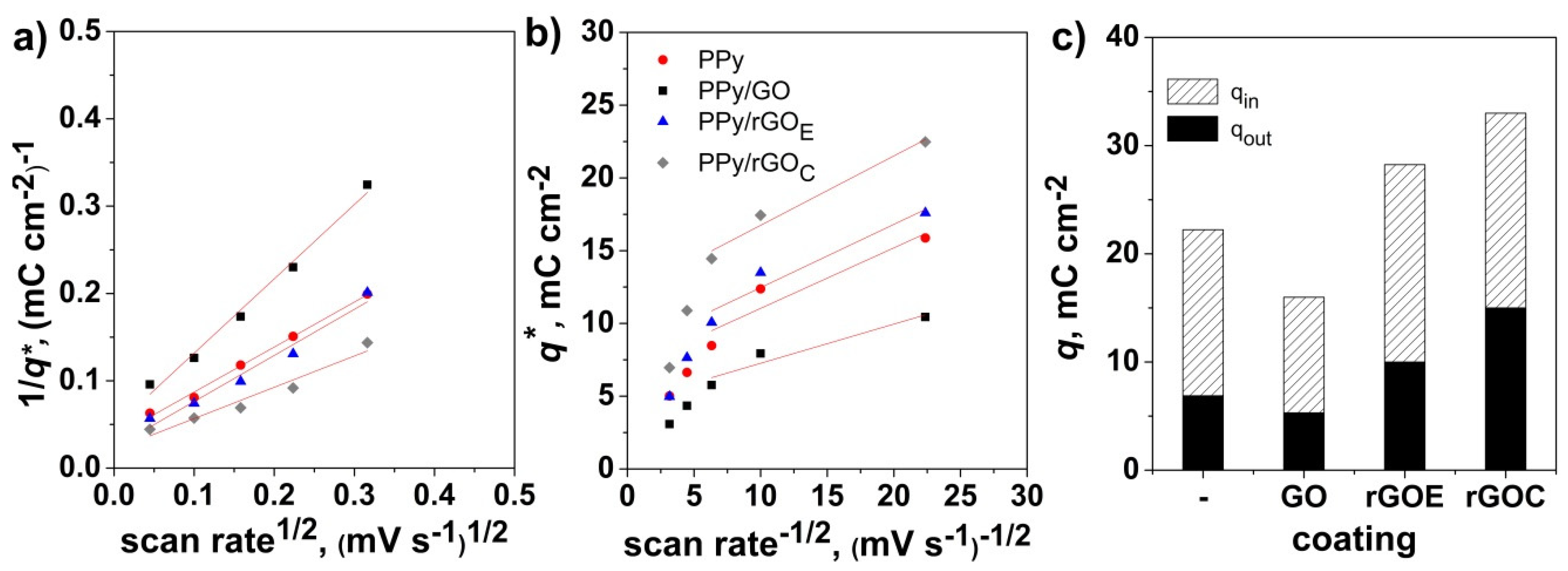
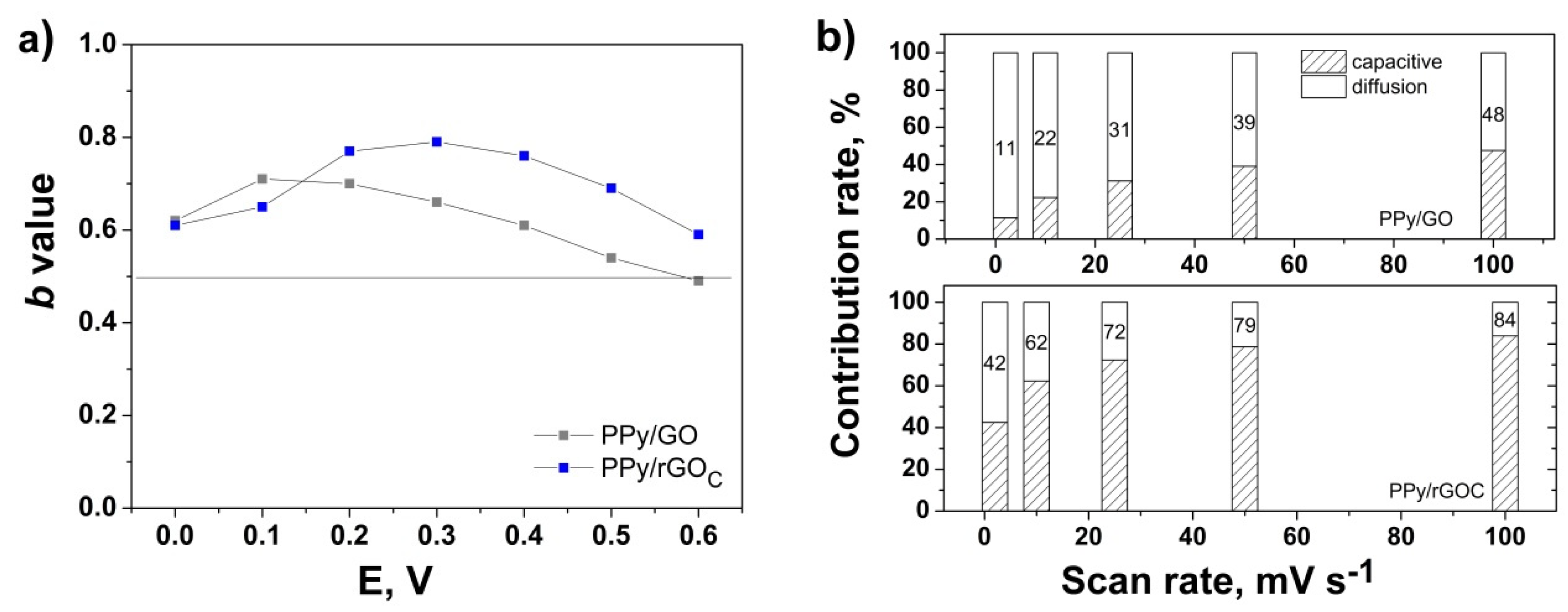
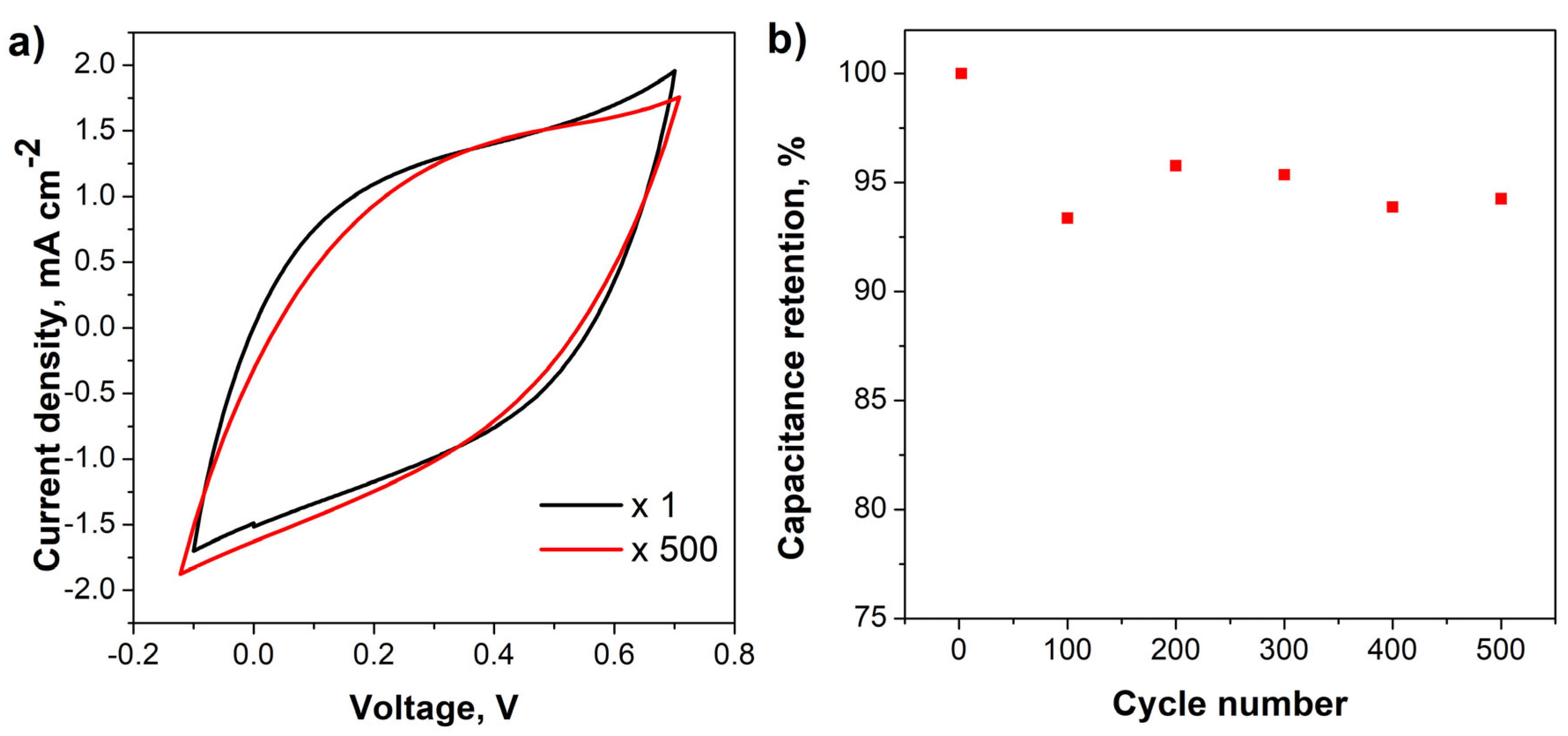
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosas-Laverde, N.M.; Pruna, A.; Cembrero, J.; Pascual, M.; Orozco-Messana, J. Optimizing Electroless Plating of Ni–Mo–P Coatings Toward Functional Ceramics. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 1–9. [Google Scholar] [CrossRef]
- Wu, H.; Susanto, A.; Lian, K. Thin and flexible Ni-P based current collectors developed by electroless deposition for energy storage devices. Appl. Surf. Sci. 2017, 394, 63–69. [Google Scholar] [CrossRef]
- Kobets, A.V.; Vorobyova, T.N. Palladium catalyst synthesis through sol-gel processing for electroless nickel deposition on glass. Thin Solid Film. 2016, 616, 793–799. [Google Scholar] [CrossRef]
- Heidarzadeh, A.; Mousavian, R.T.; Khosroshahi, R.A.; Afkham, Y.A.; Pouraliakbar, H. Empirical model to predict mass gain of cobalt electroless deposition on ceramic particles using response surface methodology. Rare Met. 2017, 36, 209–219. [Google Scholar] [CrossRef]
- Lin, J.D.; Kuo, C.L. Effects of hydrogen plasma treatment on microstructure evolution and electrical conductivity of electroless Ni-P coatings on polyimide and glass substrates. Surf. Coat. Technol. 2012, 209, 80–89. [Google Scholar] [CrossRef]
- Cao, J.; Yi, H.; Znang, Z. The Research on Heat Treatment Technology of Ni-Mo-P Electroless Plating. Adv. Intell. Syst. Res. 2016, 130, 1240–1244. [Google Scholar]
- Zuleta, A.A.; Ojewumi, M.; Yeboah, Y.D.; Kalu, E.E.; Elendu, O.; Ojewumi, M.; Yeboah, Y.D.; Kalu, E.E. Use of a mixed formaldehyde and sodium hypophosphite reducing agent bath in the electroless synthesis of Cu-Ni-Mo-P electro-catalyst active for glycerol oxidation. Int. J. Electrochem. Sci. 2016, 25, 778–787. [Google Scholar]
- Jiang, J.; Chen, H.; Wang, Y.; Zhu, L.; Sun, Y.; Lin, H.; Han, S.; Luo, Y.; Qian, W. Effect of ultrasonication and Na 2 MoO 4 content on properties of electroless Ni–Mo–P coatings. Surf. Eng. 2019, 35, 873–882. [Google Scholar] [CrossRef]
- Serin, I.G.; Göksenli, A.; Yüksel, B.; Yildiz, R.A. Effect of Annealing Temperature on the Corrosion Resistance of Electroless Ni-B-Mo Coatings. J. Mater. Eng. Perform. 2015, 24, 3032–3037. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Sources 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Wimalasiri, Y.; Fan, R.; Zhao, X.S.; Zou, L. Assembly of Ni-Al layered double hydroxide and graphene electrodes for supercapacitors. Electrochim. Acta 2014, 134, 127–135. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Cao, J.; Liu, Y.; Ouyang, J.-H.; Jia, D.; Zhou, Y. Flexible and solid-state asymmetric supercapacitor based on ternary graphene/MnO2/carbon black hybrid film with high power performance. Electrochim. Acta 2015, 182, 861–870. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Wang, S.; Wu, Q.; Xia, N.; Kong, F. Electroless decoration of cellulose paper with nickel nanoparticles: A hybrid carbon fiber for supercapacitors. Mater. Chem. Phys. 2018, 215, 157–162. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, B.; Gong, J.; Wen, J.; Hua, T.; Kan, C.-W.; Deng, J. Design of High-Performance Wearable Energy and Sensor Electronics from Fiber Materials. ACS Appl. Mater. Interfaces 2019, 11, 2120–2129. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.; Busquets-Mataix, D. Improving Electrochemical Properties of Polypyrrole Coatings by Graphene Oxide and Carbon Nanotubes. Nanomaterials 2020, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Luo, B.; Hao, L.; Zhou, M.; Zhang, X.; Fan, Z.; Zhi, L. Approaching the Downsizing Limit of Silicon for Surface-Controlled Lithium Storage. Adv. Mater. 2015, 27, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Fregonara, G.; Trasatti, S. “Inner” and “outer” active surface of RuO2 electrodes. Electrochim. Acta 1990, 35, 263–267. [Google Scholar] [CrossRef]
- Liu, T.-C.; Pell, W.G.; Conway, B.E.; Roberson, S.L. Behavior of Molybdenum Nitrides as Materials for Electrochemical Capacitors. J. Electrochem. Soc. 1998, 145, 1882. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO 2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Martín, N. Carbon Nanoforms for Photovoltaics: Myth or Reality? Adv. Energy Mater. 2017, 7, 1601102. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. Recent progress in carbon dot–metal based nanohybrids for photochemical and electrochemical applications. J. Mater. Chem. A 2017, 5, 1826–1859. [Google Scholar] [CrossRef]
- Huang, C.; Kang, L.; Zhang, J.; Li, J.; Wan, S.; Zhang, N.; Xu, H.; Wang, C.; Yu, Y.; Luo, C.; et al. RGO-Protected Electroless Plated Nickel Electrode with Enhanced Stability Performance for Flexible Micro-Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 7182–7190. [Google Scholar] [CrossRef]
- Nagaraju, G.; Chandra Sekhar, S.; Krishna Bharat, L.; Yu, J.S. Wearable Fabrics with Self-Branched Bimetallic Layered Double Hydroxide Coaxial Nanostructures for Hybrid Supercapacitors. ACS Nano 2017, 11, 10860–10874. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Lee, K.S.; Seo, H.W.; Jin, Y.K.; Kim, D.M. Reduced graphene oxide for Pt-free counter electrodes of dye-sensitized solar cells. Sol. Energy 2017, 158, 42–48. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X. Preparation and characterization of polypyrrole films for three-dimensional micro supercapacitor. J. Power Sources 2009, 193, 924–929. [Google Scholar] [CrossRef]
- Pruna, A.; Tamvakos, D.; Sgroi, M.; Pullini, D.; Nieto, E.A.; Busquets-Mataix, D. Electrocapacitance of hybrid film based on graphene oxide reduced by ascorbic acid. Int. J. Mater. Res. 2015, 106, 398–405. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. Structure, optical, and electrical properties of indium tin oxide thin films prepared by sputtering at room temperature and annealed in air or nitrogen. J. Appl. Phys. 2007, 101, 73514. [Google Scholar] [CrossRef]
- Saeed, K.; Park, S.-Y. Preparation and properties of multiwalled carbon nanotube/polycaprolactone nanocomposites. J. Appl. Polym. Sci. 2007, 104, 1957–1963. [Google Scholar] [CrossRef]
- Chien, H.H.; Liao, C.Y.; Hao, Y.C.; Hsu, C.C.; Cheng, I.C.; Yu, I.S.; Chen, J.Z. Improved performance of polyaniline/reduced-graphene-oxide supercapacitor using atmospheric-pressure-plasma-jet surface treatment of carbon cloth. Electrochim. Acta 2018, 260, 391–399. [Google Scholar] [CrossRef]
- Wang, N.; Han, G.; Song, H.; Xiao, Y.; Li, Y.; Zhang, Y.; Wang, H. Integrated flexible supercapacitor based on poly (3, 4-ethylene dioxythiophene) deposited on Au/porous polypropylene film/Au. J. Power Sources 2018, 395, 228–236. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K. Effect of heat treatment on tribological behavior of electroless Ni-B coating at elevated temperatures. Surf. Rev. Lett. 2018, 25, 1–22. [Google Scholar] [CrossRef]
- Wolfart, F.; Dubal, D.P.; Vidotti, M.; Holze, R.; Gómez-Romero, P. Electrochemical supercapacitive properties of polypyrrole thin films: Influence of the electropolymerization methods. J. Solid State Electrochem. 2016, 20, 901–910. [Google Scholar] [CrossRef]
- Wang, N.; Han, G.; Xiao, Y.; Li, Y.; Song, H.; Zhang, Y. Polypyrrole/graphene oxide deposited on two metalized surfaces of porous polypropylene films as all-in-one flexible supercapacitors. Electrochim. Acta 2018, 270, 490–500. [Google Scholar] [CrossRef]
- Pruna, A.; Shao, Q.; Kamruzzaman, M.; Zapien, J.A.A.; Ruotolo, A. Enhanced electrochemical performance of ZnO nanorod core/polypyrrole shell arrays by graphene oxide. Electrochim. Acta 2016, 187, 517–524. [Google Scholar] [CrossRef]
- Guo, H.-L.; Wang, X.-F.; Qian, Q.-Y.; Wang, F.-B.; Xia, X.-H. A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-Y.; Zhang, D. Aqueous colloids of graphene oxide nanosheets by exfoliation of graphite oxide without ultrasonication. Bull. Mater. Sci. 2011, 34, 25–28. [Google Scholar] [CrossRef]
- Yun, H.; Kim, J.D.; Choi, H.C.; Lee, C.W. Antibacterial Activity of CNT-Ag and GO-Ag Nanocomposites Against Gram-negative and Gram-positive Bacteria. Bull. Korean Chem. Soc. 2013, 34, 3261–3264. [Google Scholar] [CrossRef]
- Wang, S.; Pei, B.; Zhao, X.; Dryfe, R.A.W. Highly porous graphene on carbon cloth as advanced electrodes for flexible all-solid-state supercapacitors. Nano Energy 2013, 2, 530–536. [Google Scholar] [CrossRef]
- Nethravathi, C.; Nisha, T.; Ravishankar, N.; Shivakumara, C.; Rajamathi, M. Graphene–nanocrystalline metal sulphide composites produced by a one-pot reaction starting from graphite oxide. Carbon 2009, 47, 2054–2059. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A. Graphene Oxide Synthesized by using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Yourey, J.E.; Bartlett, B.M. Chemically Bonded TiO2—Bronze Nanosheet/Reduced Graphene Oxide Hybrid for High-Power Lithium Ion Batteries. ACS Nano 2014, 8, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, N.; Du, T.; Wang, X.; Chen, W. Transformation of graphene oxide by chlorination and chloramination: Implications for environmental transport and fate. Water Res. 2016, 103, 416–423. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, M.; Chen, Z.; Liu, X. Hierarchical Co3O4@PPy core-shell composite nanowires for supercapacitors with enhanced electrochemical performance. Mater. Res. Bull. 2017, 96, 463–470. [Google Scholar] [CrossRef]
- Pruna, A.; Pullini, D.; Busquets, D. Influence of synthesis conditions on properties of green-reduced graphene oxide. J. Nanopart. Res. 2013, 15, 1605. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; So, H.H.; Cho, J.W. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synth. Met. 2007, 157, 374–379. [Google Scholar] [CrossRef]
- Naidek, N.; Zarbin, A.J.G.; Orth, E.S. Covalently linked nanocomposites of polypyrrole with graphene: Strategic design toward optimized properties. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 579–588. [Google Scholar] [CrossRef]
- Ai, W.; Du, Z.-Z.; Liu, J.-Q.; Zhao, F.; Yi, M.-D.; Xie, L.-H.; Shi, N.-E.; Ma, Y.-W.; Qian, Y.; Fan, Q.-L.; et al. Formation of graphene oxide gel via the π-stacked supramolecular self-assembly. RSC Adv. 2012, 2, 12204. [Google Scholar] [CrossRef]
- Faraji, S.; Ani, F.N. Electroless nano zinc oxide–activate carbon composite supercapacitor electrode. J. Electroceram. 2016, 36, 122–128. [Google Scholar] [CrossRef]
- Pandit, B.; Devika, V.S.; Sankapal, B.R. Electroless-deposited Ag nanoparticles for highly stable energy-efficient electrochemical supercapacitor. J. Alloy. Compd. 2017, 726, 1295–1303. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, P.; Zhou, F.; Zeng, W.; Su, H.; Li, G.; Gao, J.; Sun, R.; Wong, C. Flexible Asymmetrical Solid-State Supercapacitors Based on Laboratory Filter Paper. ACS Nano 2016, 10, 1273–1282. [Google Scholar] [CrossRef]
- Chen, W.; He, Y.; Li, X.; Zhou, J.; Zhang, Z.; Zhao, C.; Gong, C.; Li, S.; Pan, X.; Xie, E. Facilitated charge transport in ternary interconnected electrodes for flexible supercapacitors with excellent power characteristics. Nanoscale 2013, 5, 11733–11741. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, Z.; Wang, J.; Ma, J.; Zhang, M.; Liu, L. Solvothermal one-step synthesis of Ni-Al layered double hydroxide/carbon nanotube/reduced graphene oxide sheet ternary nanocomposite with ultrahigh capacitance for supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 5443–5454. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Hao, Q.; Lei, W.; Wang, W.; Wang, H.; Wang, X. Reduced-graphene oxide/molybdenum oxide/polyaniline ternary composite for high energy density supercapacitors: Synthesis and properties. J. Mater. Chem. 2012, 22, 8314–8320. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Zhang, X.; Wang, G.; Ji, P.; Yin, F. WSe 2 /Reduced Graphene Oxide Nanocomposite with Superfast Sodium Ion Storage Ability as Anode for Sodium Ion Capacitors. J. Electrochem. Soc. 2018, 165, A3642–A3647. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Chen, C.; Guo, H.; Liu, X.; Xu, G.; Zhong, H.; Cheng, S.; Wu, P.; Meng, J.; et al. In Operando Mechanism Analysis on Nanocrystalline Silicon Anode Material for Reversible and Ultrafast Sodium Storage. Adv. Mater. 2017, 29, 1604708. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Sheng, J.; An, Q.; Tao, Z.; Kang, Y.M.; Chen, J.; Mai, L. Structural and chemical synergistic effect of CoS nanoparticles and porous carbon nanorods for high-performance sodium storage. Nano Energy 2017, 35, 281–289. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Zhao, Y.; Lushington, A.; Gao, J.; Li, Q.; Zuo, P.; Wang, B.; Gao, Y.; Ma, Y.; et al. Superior performance of ordered macroporous TiNb2O7 anodes for lithium ion batteries: Understanding from the structural and pseudocapacitive insights on achieving high rate capability. Nano Energy 2017, 34, 15–25. [Google Scholar] [CrossRef]
- Li, W.J.; Chou, S.L.; Wang, J.Z.; Wang, J.L.; Gu, Q.F.; Liu, H.K.; Dou, S.X. Multifunctional conducing polymer coated Na1+xMnFe(CN)6 cathode for sodium-ion batteries with superior performance via a facile and one-step chemistry approach. Nano Energy 2015, 13, 200–207. [Google Scholar] [CrossRef]
- Duay, J.; Sherrill, S.A.; Gui, Z.; Gillette, E.; Lee, S.B. Self-limiting electrodeposition of hierarchical MnO2 and M(OH)2/MnO2 nanofibril/nanowires: Mechanism and supercapacitor properties. ACS Nano 2013, 7, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Ryu, I.; Kim, G.; Park, D.; Yim, S. Ethanedithiol-treated manganese oxide nanoparticles for rapidly responsive and transparent supercapacitors. J. Power Sources 2015, 297, 98–104. [Google Scholar] [CrossRef]
- Yan, W.; Ayvazian, T.; Kim, J.; Liu, Y.; Donavan, K.C.; Xing, W.; Yang, Y.; Hemminger, J.C.; Penner, R.M. Mesoporous manganese oxide nanowires for high-capacity, high-rate, hybrid electrical energy storage. ACS Nano 2011, 5, 8275–8287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhai, H.-J.J. A highly flexible solid-state supercapacitor based on the carbon nanotube doped graphene oxide/polypyrrole composites with superior electrochemical performances. Org. Electron. 2016, 37, 197–206. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Kamboj, N.; Das, M.; Dey, R.S. All-porous heterostructure of reduced graphene oxide–polypyrrole–nanoporous gold for a planar flexible supercapacitor showing outstanding volumetric capacitance and energy density. J. Mater. Chem. A 2018, 6, 22858–22869. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Laverde, N.M.; Pruna, A.I.; Busquets-Mataix, D. Graphene Oxide-Polypyrrole Coating for Functional Ceramics. Nanomaterials 2020, 10, 1188. https://doi.org/10.3390/nano10061188
Rosas-Laverde NM, Pruna AI, Busquets-Mataix D. Graphene Oxide-Polypyrrole Coating for Functional Ceramics. Nanomaterials. 2020; 10(6):1188. https://doi.org/10.3390/nano10061188
Chicago/Turabian StyleRosas-Laverde, Nelly Ma., Alina Iuliana Pruna, and David Busquets-Mataix. 2020. "Graphene Oxide-Polypyrrole Coating for Functional Ceramics" Nanomaterials 10, no. 6: 1188. https://doi.org/10.3390/nano10061188
APA StyleRosas-Laverde, N. M., Pruna, A. I., & Busquets-Mataix, D. (2020). Graphene Oxide-Polypyrrole Coating for Functional Ceramics. Nanomaterials, 10(6), 1188. https://doi.org/10.3390/nano10061188




