In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Preparation and Characterization of TiO2NPs
2.3. Sample Collection, Evaluation and Exposure Procedure
2.4. Comet Assay
2.5. TUNEL Test
2.6. Genomic DNA Extraction, RAPD-PCR Technique and Genomic Template Stability
2.7. DCF Assay
2.8. Statistical Analysis
3. Results
3.1. Sperm Motility Is Reduced after 90 min TiO2NPs and CdCl2 Co-Exposure
3.2. TiO2NPs and CdCl2 Co-Exposure Causes Time-Dependent Loss of Sperm DNA Integrity
3.3. TiO2NPs and CdCl2 Combined Exposure Induces Sperm DNA Fragmentation
3.4. TiO2NPs in Combination with CdCl2 Produce Intracellular Oxidative Stress in Sperm Cells
3.5. TiO2NPs and CdCl2 Co-Exposure Generates Sperm DNA Polymorphic Profiles Alterations
3.6. TiO2NPs in Combination with CdCl2 Reduce Sperm Genome Stability
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. Identification of the mechanisms that drive the toxicity of TiO 2particulates: The contribution of physicochemical characteristics. Part. Fibre Toxicol. 2009, 6, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wang, X.; Marsch, S.; Hunziker, P. Intelligent nanomaterials for medicine: Carrier platforms and targeting strategies in the context of clinical application. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 742–757. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef]
- Demir, E.; Akça, H.; Turna, F.; Aksakal, S.; Burgucu, D.; Kaya, B.; Tokgün, O.; Vales, G.; Creus, A.; Marcos, R. Genotoxic and cell-transforming effects of titanium dioxide nanoparticles. Environ. Res. 2015, 136, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V.; et al. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef]
- Rocco, L.; Santonastaso, M.; Mottola, F.; Costagliola, D.; Suero, T.; Pacifico, S.; Stingo, V. Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol. Environ. Saf. 2015, 113, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Mørck, T.A.; Andersen, D.N.; Hougaard, K.S. A Critical Review of Studies on the Reproductive and Developmental Toxicity of Nanomaterials Month 20xx. Available online: https://euon.echa.europa.eu/documents/23168237/24095696/critical_review_of_studies_on_reproductive_and_developmental_toxicity_of_nanomaterials_en.pdf/c83f78ef-7136-ef4b-268c-c5d9b7bf1fea (accessed on 4 June 2020).
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO2) in human sperm cells. Mol. Reprod. Dev. 2019, 86, 1369–1377. [Google Scholar] [CrossRef]
- D’Agata, A.; Fasulo, S.; Dallas, L.J.; Fisher, A.S.; Maisano, M.; Readman, J.W.; Jha, A.N. Enhanced toxicity of “bulk” titanium dioxide compared to “fresh” and “aged” nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology 2014, 8, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Frenzilli, G.; Bernardeschi, M.; Guidi, P.; Scarcelli, V.; Lucchesi, P.; Marsili, L.; Fossi, M.C.; Brunelli, A.; Pojana, G.; Marcomini, A.; et al. Effects of in vitro exposure to titanium dioxide on DNA integrity of bottlenose dolphin (Tursiops truncatus) fibroblasts and leukocytes. Mar. Environ. Res. 2014, 100, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Buonocore, F.; Frenzilli, G.; Corsolini, S.; Brunelli, A.; Guidi, P.; Kocan, A.; Mariottini, M.; Mottola, F.; Nigro, M.; et al. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax). Environ. Pollut. 2015, 196, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Frenzilli, G.; Balbi, T.; Bernardeschi, M.; Ciacci, C.; Corsolini, S.; Della Torre, C.; Fabbri, R.; Faleri, C.; Focardi, S.; et al. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2014, 153, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Pintado-Herrera, M.G.; Aguirre-Martínez, G.V.; Moreno-Garrido, I.; Martin-Díaz, L.M.; Lara-Martín, P.A.; Blasco, J. Are the TiO2 NPs a “Trojan horse” for personal care products (PCPs) in the clam Ruditapes philippinarum? Chemosphere 2017, 185, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Mottola, F.; Iovine, C.; Santonastaso, M.; Romeo, M.L.; Pacifico, S.; Cobellis, L.; Rocco, L. NPs-TiO2 and lincomycin coexposure induces DNA damage in cultured human amniotic cells. Nanomaterials 2019, 9, 1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.H.; Cui, M.; Shi, Z.W.; Cheng Tan, X.P.Y. Enhanced Oxidative Stress and Physiological Damage in Daphnia magna by Copper in the Presence of Nano-TiO2. J. Nanomater. 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Miao, W.; Zhu, B.; Xiao, X.; Li, Y.; Dirbaba, N.B.; Zhou, B.; Wu, H. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 2015, 161, 117–126. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, X.; Zhang, Z.; Chen, Y.; Crittenden, J.C. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ. Pollut. 2009, 157, 1165–1170. [Google Scholar] [CrossRef]
- Tan, C.; Fan, W.H.; Wang, W.X. Role of titanium dioxide nanoparticles in the elevated uptake and retention of cadmium and zinc in Daphnia magna. Environ. Sci. Technol. 2012, 46, 469–476. [Google Scholar] [CrossRef]
- Tan, C.; Wang, W.X. Modification of metal bioaccumulation and toxicity in Daphnia magna by titanium dioxide nanoparticles. Environ. Pollut. 2014, 186, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qian, Y.; Li, H.; Cheng, Y.; Zhao, M. The reduced bioavailability of copper by nano-TiO2 attenuates the toxicity to Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2015, 22, 12407–12414. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, R.R.; Seitz, F.; Zubrod, J.P.; Feckler, A.; Merkel, T.; Lüderwald, S.; Bundschuh, R.; Schulz, R.; Bundschuh, M. Does the presence of titanium dioxide nanoparticles reduce copper toxicity? A factorial approach with the benthic amphipod Gammarus fossarum. Aquat. Toxicol. 2015, 165, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Li, Y.; Miao, A.J.; Yang, L.Y. Cd 2+ toxicity as affected by bare TiO 2 nanoparticles and their bulk counterpart. Ecotoxicol. Environ. Saf. 2012, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, H.; Nie, Y.; Wang, M.; Yang, Z.; Cheng, L.; Liu, Y.; Chen, S.; Zhao, G.; Wu, L.; et al. TiO2 nanoparticles enhance bioaccumulation and toxicity of heavy metals in Caenorhabditis elegans via modification of local concentrations during the sedimentation process. Ecotoxicol. Environ. Saf. 2018, 162, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P.; Coogan, T.P.; Barter, R.A. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit. Rev. Toxicol. 1992, 22, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, A.; Gay, F.; Scudiero, R.; Trinchella, F.; Caputo, I.; Lepretti, M.; Marabotti, A.; Esposito, C.; Laforgia, V. Histological changes, apoptosis and metallothionein levels in Triturus carnifex (Amphibia, Urodela) exposed to environmental cadmium concentrations. Aquat. Toxicol. 2016, 173, 63–73. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Summaries & evaluations: Cadmium and cadmium compounds (Group 1). Available online: http://www.inchem.org/documents/iarc/vol58/mono58-2.html (accessed on 4 June 2020).
- Nordberg, G.F. Cadmium and health in the 21st Century—Historical remarks and trends for the future. Proc. Biometals 2004, 17, 485–489. [Google Scholar] [CrossRef]
- Moitra, S.; Blanc, P.D.; Sahu, S. Adverse respiratory effects associated with Cadmium exposure in small-scale jewellery workshops in India. Thorax 2013, 68, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Pourahmad, J.; O’Brien, P.J. A comparison of hepatocyte cytotoxic mechanisms for Cu2+ and Cd2+. Toxicology 2000, 143, 263–273. [Google Scholar] [CrossRef]
- Benoff, S.; Jacob, A.; Hurley, I.R. Male Infertility and Environmental Exposure to Lead and Cadmium. Hum. Reprod. Update 2000, 6, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Alabi, N.S.; Whanger, P.D.; Wu, A.S.H. Interactive Effects of Organic and Inorganic Selenium with Cadmium and Mercury on Spermatozoal Oxygen Consumption and Motility in Vitro1. Biol. Reprod. 1985, 33, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchiani, S.; Tamburrino, L.; Farnetani, G.; Muratori, M.; Vignozzi, L.; Baldi, E. Acute effects on human sperm exposed in vitro to cadmium chloride and diisobutyl phthalate. Reproduction 2019, 158, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Ru, Y.F.; Liu, M.; Tang, J.N.; Zheng, J.F.; Wu, B.; Gu, Y.H.; Shi, H.J. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardneck, F.; Israel, G.; Pool, E.; Maree, L. Quantitative assessment of heavy metal effects on sperm function using computer-aided sperm analysis and cytotoxicity assays. Andrologia 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskey, J.W.; Berman, E.; Ferrell, J.M. The use of cultured ovarian fragments to assess toxicant alterations in steroidogenesis in the sprague-dawley rat. Reprod. Toxicol. 1995, 9, 131–141. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Q.; Jiang, L.; Yu, Z.; Jiang, D.; Yin, D. Combined effects of titanium dioxide and humic acid on the bioaccumulation of cadmium in Zebrafish. Environ. Pollut. 2011, 159, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhou, Y.; Ma, C.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Le, V.N.; Han, Y.; et al. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017, 110, 82–93. [Google Scholar] [CrossRef]
- Manesh, R.R.; Grassi, G.; Bergami, E.; Marques-Santos, L.F.; Faleri, C.; Liberatori, G.; Corsi, I. Co-exposure to titanium dioxide nanoparticles does not affect cadmium toxicity in radish seeds (Raphanus sativus). Ecotoxicol. Environ. Saf. 2018, 148, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Legros, S.; Von der Kammer, F.; Hofmann, T.; Baun, A. The potential of TiO2 nanoparticles as carriers for cadmium uptake in Lumbriculus variegatus and Daphnia magna. Aquat. Toxicol. 2012, 118–119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Engelbrekt, C.; Zhang, J.; Ulstrup, J.; Kusk, K.O.; Baun, A. The challenges of testing metal and metal oxide nanoparticles in algal bioassays: Titanium dioxide and gold nanoparticles as case studies. Nanotoxicology 2013, 7, 1082–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.W.; Miao, A.J.; Yang, L.Y. Cd 2+ toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO 2 engineered nanoparticles. PLoS ONE 2012, 7, e32300. [Google Scholar]
- Rocco, L.; Santonastaso, M.; Nigro, M.; Mottola, F.; Costagliola, D.; Bernardeschi, M.; Guidi, P.; Lucchesi, P.; Scarcelli, V.; Corsi, I.; et al. Genomic and chromosomal damage in the marine mussel Mytilus galloprovincialis: Effects of the combined exposure to titanium dioxide nanoparticles and cadmium chloride. Mar. Environ. Res. 2015, 111, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Alaizeri, Z.A.M.; Alhadlaq, H.A. TiO2 nanoparticles potentiated the cytotoxicity, oxidative stress and apoptosis response of cadmium in two different human cells. Environ. Sci. Pollut. Res. 2020, 27, 10425–10435. [Google Scholar] [CrossRef] [PubMed]
- WHO laboratory manual for the Examination and processing of human semen. World Health Organ. 2010, 1, 10–17.
- Rocco, L.; Peluso, C.; Cesaroni, F.; Morra, N. Genomic Damage in Human Sperm Cells Exposed In Vitro to Environmental Pollutants. J. Environ. Anal. Toxicol. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.V. OpenComet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Noel, S.; Rath, S.K. Randomly amplified polymorphic DNA as a tool for genotoxicity: An assessment. Toxicol. Ind. Health 2006, 22, 267–275. [Google Scholar] [CrossRef]
- Rocco, L.; Valentino, I.V.; Scapigliati, G.; Stingo, V. RAPD-PCR analysis for molecular characterization and genotoxic studies of a new marine fish cell line derived from Dicentrarchus labrax. Cytotechnology 2014, 66, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Chenlo, P.H.; Curi, S.M.; Pugliese, M.N.; Ariagno, J.I.; Sardi-Segovia, M.; Furlan, M.J.; Repetto, H.E.; Zeitler, E.; Cohen, M.; Mendeluk, G.R. Fragmentation of sperm DNA using the TUNEL method. Actas Urológicas Españolas 2014, 38, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-W.; Ge Song, Q.W.; Liu, S.; Zhu, X.; Deng, S.; Zhong, A.; Tan, Y. Ying Tan Sperm DNA damage has a negative effect on early embryonic development following in vitro fertilization. Asian J. Androl. 2018, 20, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, B.; Maleki, A.; Johari, S.A.; Shahmoradi, B.; Mohammadi, E.; Shahsavari, S.; Davari, B. Copper Bioaccumulation and Depuration in Common Carp (Cyprinus carpio) Following Co-exposure to TiO2 and CuO Nanoparticles. Arch. Environ. Contam. Toxicol. 2016, 71, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Shahin, N.N.; Mohamed, M.M. Nano-sized titanium dioxide toxicity in rat prostate and testis: Possible ameliorative effect of morin. Toxicol. Appl. Pharmacol. 2017, 334, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lin, L.; Liu, L.; Wang, K.; Ding, Y.; Niu, Q.; Mu, L.; Wang, H.; Shen, H.; Guo, S. Toxic Effects of Anatase Titanium DioxideNanoparticles on Spermatogenesis and Testiclesin Male Mice. Polish J. Environ. Stud. 2017, 26, 2739–2745. [Google Scholar] [CrossRef]
- Lauvås, A.J.; Skovmand, A.; Poulsen, M.S.; Kyjovska, Z.O.; Roursgaard, M.; Goericke-Pesch, S.; Vogel, U.; Hougaard, K.S. Airway exposure to TiO2 nanoparticles and quartz and effects on sperm counts and testosterone levels in male mice. Reprod. Toxicol. 2019, 90, 134–140. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Erboga, M.; Kanter, M. Effect of Cadmium on Trophoblast Cell Proliferation and Apoptosis in Different Gestation Periods of Rat Placenta. Biol. Trace Elem. Res. 2016, 169, 285–293. [Google Scholar] [CrossRef]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Tascioglu, S. Protective effects of grape seed extract on cadmium-induced testicular damage, apoptosis, and endothelial nitric oxide synthases expression in rats. Toxicol. Ind. Health 2016, 32, 1486–1494. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, C.; Shi, C.; Cao, H.; Han, Z.; Jia, X. Cadmium-induced oxidative stress and apoptosis in the testes of frog Rana limnocharis. Aquat. Toxicol. 2012, 122–123, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Su, L.; Sun, Y.; Han, A.; Chang, X.; Zhu, A.; Liu, F.; Li, J.; Sun, Y. Nickel sulfate induced apoptosis via activating ROS-dependent mitochondria and endoplasmic reticulum stress pathways in rat Leydig cells. Environ. Toxicol. 2017, 32, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F. Cadmium and cellular signaling cascades: To be or not to be? Toxicol. Appl. Pharmacol. 2009, 238, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Bosco, L.; Notari, T.; Ruvolo, G.; Roccheri, M.C.; Martino, C.; Chiappetta, R.; Carone, D.; Lo Bosco, G.; Carrillo, L.; Raimondo, S.; et al. Sperm DNA fragmentation: An early and reliable marker of air pollution. Environ. Toxicol. Pharmacol. 2018, 58, 243–249. [Google Scholar] [CrossRef]
- Payne, J.F.; Raburn, D.J.; Couchman, G.M.; Price, T.M.; Jamison, M.G.; Walmer, D.K. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil. Steril. 2005, 84, 356–364. [Google Scholar] [CrossRef]
- Liu, W.; Li, P.J.; Qi, X.M.; Zhou, Q.X.; Zheng, L.; Sun, T.H.; Yang, Y.S. DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere 2005, 61, 158–167. [Google Scholar] [CrossRef]
- Atienzar, F.A.; Jha, A.N. The random amplified polymorphic DNA (RAPD) assay and related techniques applied to genotoxicity and carcinogenesis studies: A critical review. Mutat. Res. Rev. Mutat. Res. 2006, 613, 76–102. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.S.; Zhou, Q.; Xie, L.; Li, P.; Sun, T. Impact assessment of cadmium contamination on rice (Oryza sativa L.) seedlings at molecular and population levels using multiple biomarkers. Chemosphere 2007, 67, 1155–1163. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Alahmar, A. Role of oxidative stress in male infertility: An updated review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Xia, B.; Chen, J.; Zhou, Y. Cellular oxidative damage of HEK293T cells induced by combination of CdCl2 and nano-TiO2. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 290–294. [Google Scholar] [CrossRef]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Sager, T.M.; Porter, D.W.; Robinson, V.A.; Lindsley, W.G.; Schwegler-Berry, D.E.; Castranova, V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology 2007, 1, 118–129. [Google Scholar] [CrossRef]
- Leisegang, K.; Henkel, R.; Agarwal, A. Redox Regulation of Fertility in Aging Male and the Role of Antioxidants: A Savior or Stressor. Curr. Pharm. Des. 2016, 23, 4438–4450. [Google Scholar] [CrossRef] [PubMed]
- Muster, W.; Breidenbach, A.; Fischer, H.; Kirchner, S.; Müller, L.; Pähler, A. Computational toxicology in drug development. Drug Discov. Today 2008, 13, 303–310. [Google Scholar] [CrossRef] [PubMed]
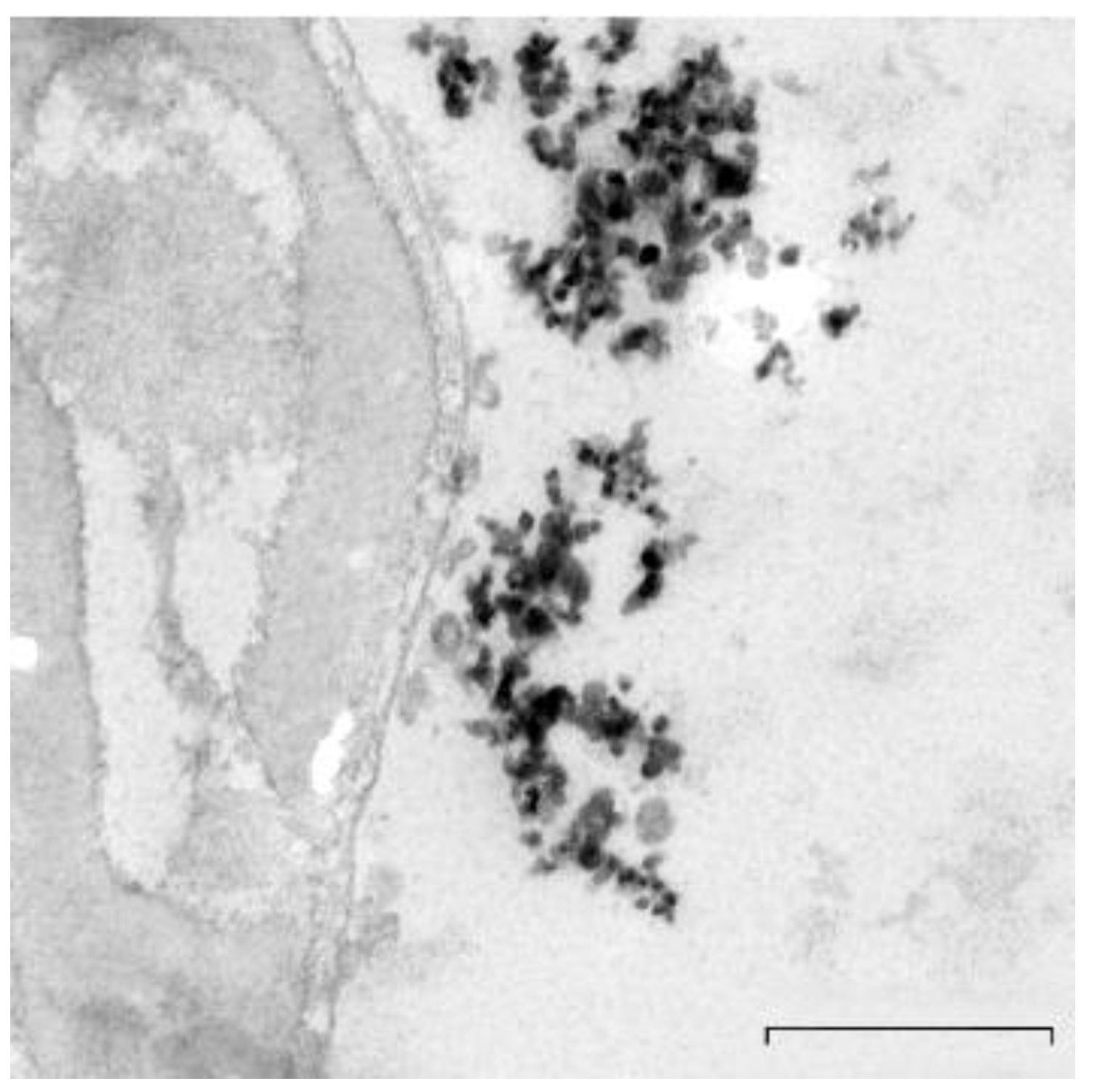
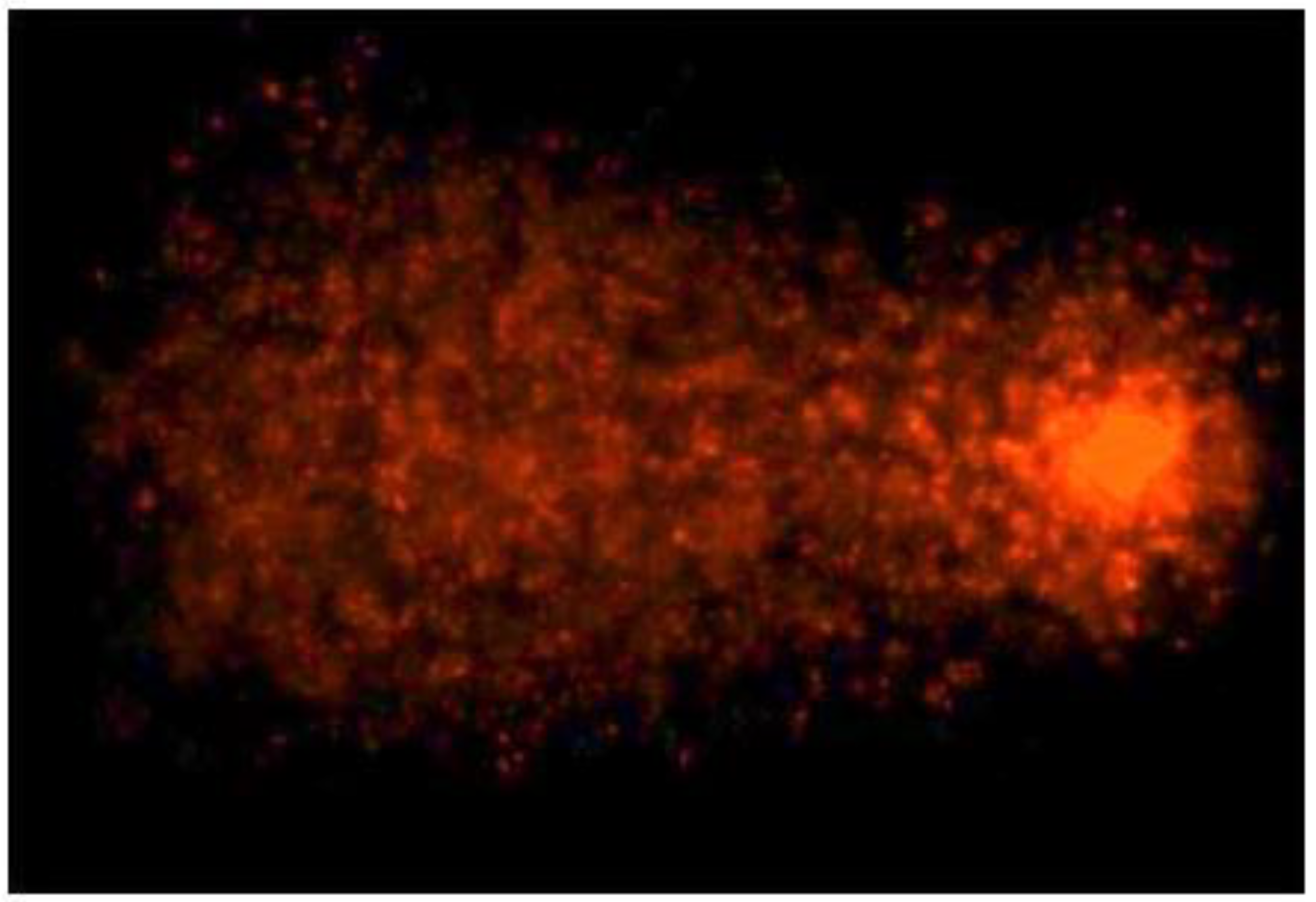

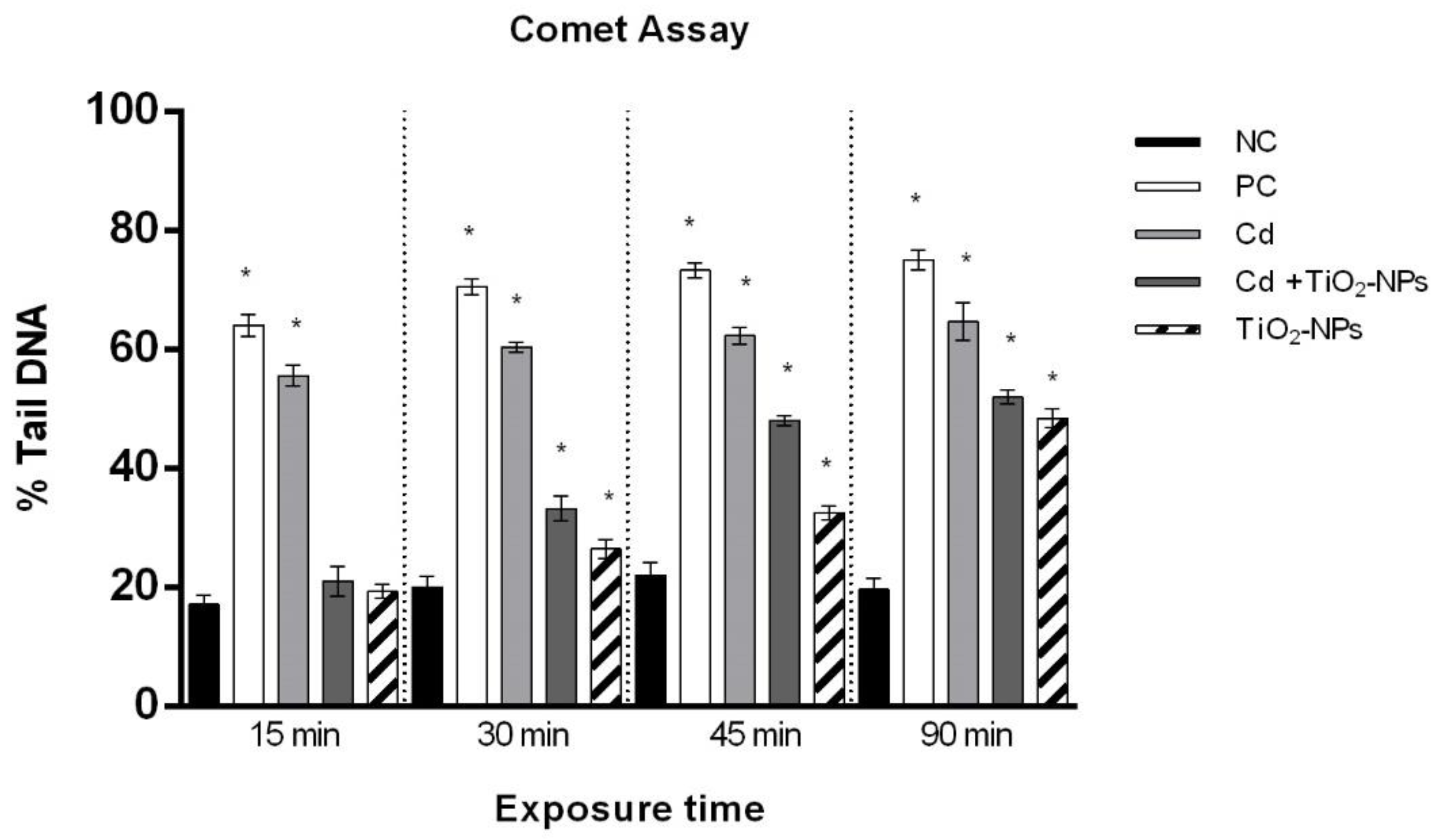

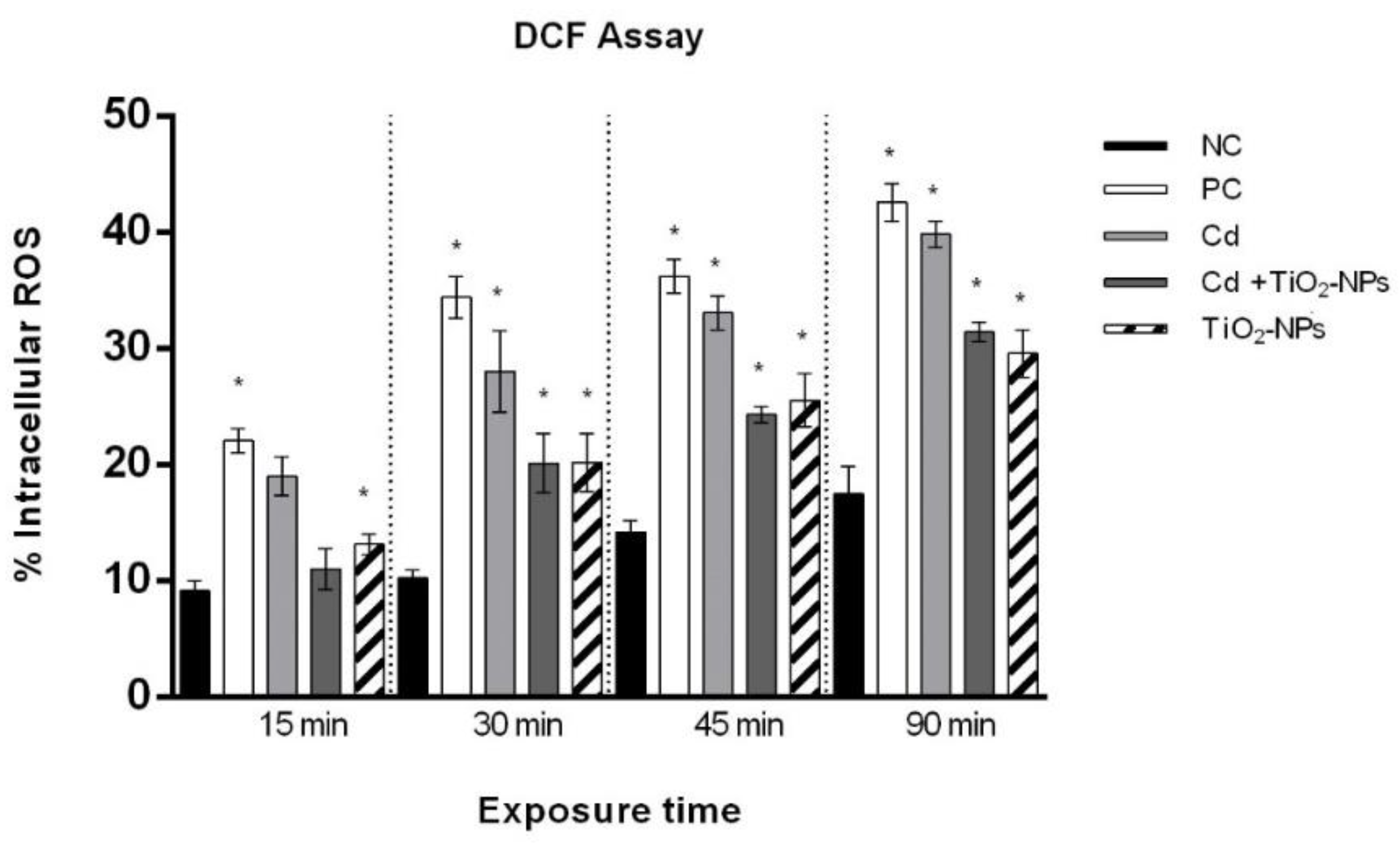

| Time (min) | nTiO2NPs [1 μg/L] |
|---|---|
| 0 | 1.01 ± 0.01 |
| 15 | 0.91 ± 0.05 |
| 30 | 0.87 ± 0.02 |
| 45 | 0.69 ± 0.01 |
| 90 | 0.27 ± 0.03 |
| Sperm Parameters | Mean± SD |
|---|---|
| Semen volume (mL) | 3.5 ± 0.5 |
| Ph | 6.9 ± 0.3 |
| Sperm concentration (*106 sperm/mL) | 60.4 ± 15.6 |
| Vitality (%) | 70.8 ± 5.8 |
| Motility (%) | |
| Progressive | 69.0 ± 15.7 |
| Non-progressive | 20.0 ± 5.5 |
| Immobile | 11 ± 9 |
| Normal morphology (%) | 15 ± 6.5 |
| Substances Concentration | Exposure Minutes | Vitality (%) | Motility (P + NP) (%) |
|---|---|---|---|
| CdCl2 10 µM µg/L | 15 30 45 90 | 71 ± 4.5 65 ± 2.5 65 ± 5.6 61 ± 4.5 | 81 ± 5.5 69 ± 8.0 * 57 ± 5.8 * 55 ± 7.5 * |
| CdCl2 10 µM + TiO2NPs 1 µg/L | 15 30 45 90 | 72 ± 3.2 70 ± 2.7 69 ± 4.1 65 ± 4.5 | 78 ± 6.9 74 ± 8.5 70 ± 8.7 58 ± 5.5 * |
| Substances Concentration | Exposure Minutes | Gained Bands | Lost Bands * |
|---|---|---|---|
| CdCl2 10 µM µg/L | 15 30 45 90 | 210 300, 350, 900 210, 700, 900 600 | 560, 850 560 270,850 270, 450, 500, 560, 850 |
| CdCl2 10 µM + TiO2NPs 1 µg/L | 15 30 45 90 | 700 210, 350, 900 210, 530, 900 300, 400, 900 | - - 850 850 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells. Nanomaterials 2020, 10, 1118. https://doi.org/10.3390/nano10061118
Santonastaso M, Mottola F, Iovine C, Cesaroni F, Colacurci N, Rocco L. In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells. Nanomaterials. 2020; 10(6):1118. https://doi.org/10.3390/nano10061118
Chicago/Turabian StyleSantonastaso, Marianna, Filomena Mottola, Concetta Iovine, Fulvio Cesaroni, Nicola Colacurci, and Lucia Rocco. 2020. "In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells" Nanomaterials 10, no. 6: 1118. https://doi.org/10.3390/nano10061118
APA StyleSantonastaso, M., Mottola, F., Iovine, C., Cesaroni, F., Colacurci, N., & Rocco, L. (2020). In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells. Nanomaterials, 10(6), 1118. https://doi.org/10.3390/nano10061118







