Nanoporous Carbon Derived from Green Material by an Ordered Activation Method and Its High Capacitance for Energy Storage
Abstract
1. Introduction
2. Preparations and Measurements
2.1. Preparation of Activated Water Hyacinth-Derived Carbon (AAWHC)
2.2. Characterization
2.3. Electrochemical Measurements
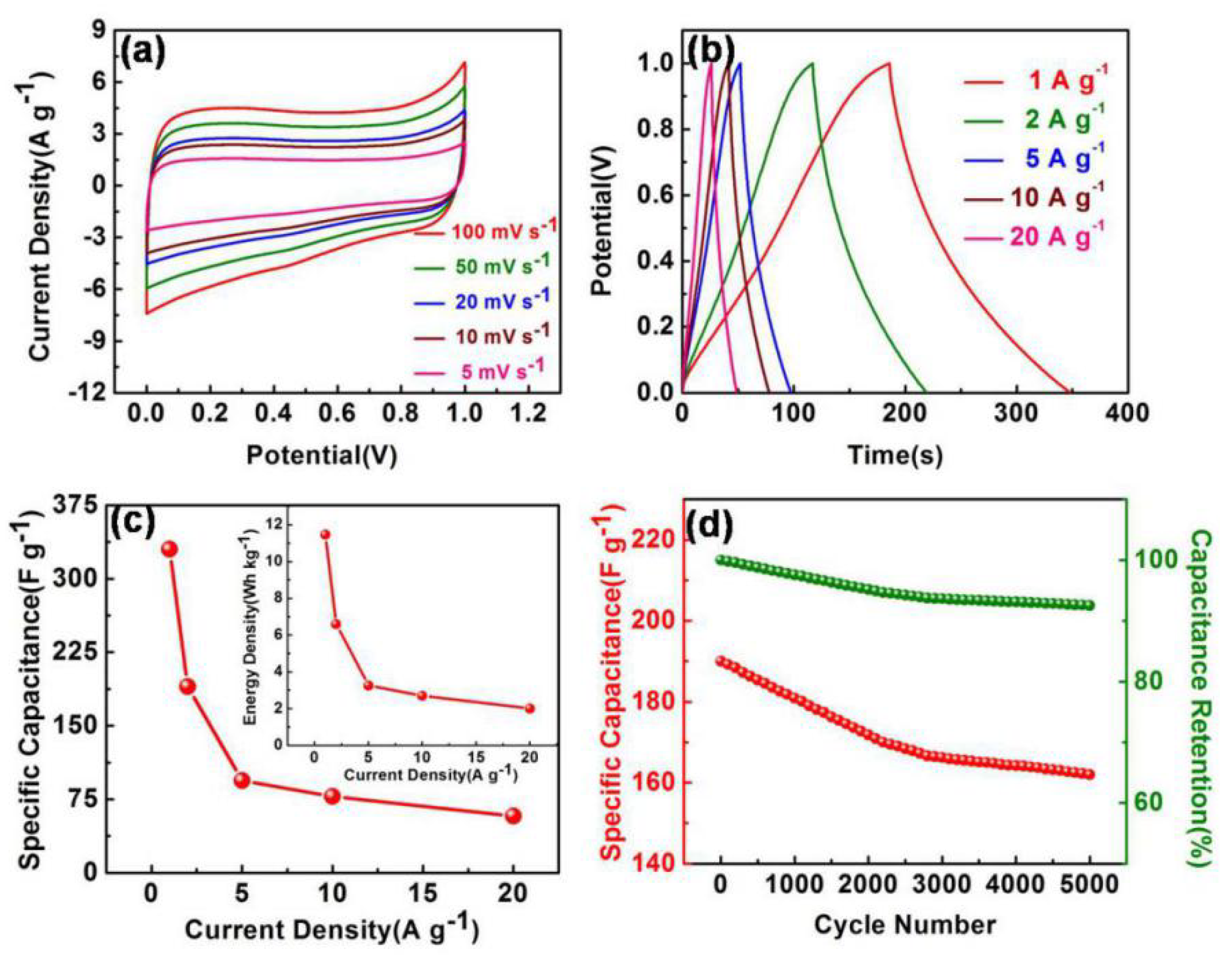
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.-W.; Yin, B.-S.; Liu, X.-X.; Gu, D.-M.; Gong, H.; Wang, Z.-B. A high energy density aqueous hybrid supercapacitor with widened potential window through multi approaches. Nano Energy 2019, 59, 41–49. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Z.; Kim, J.; Yamauchi, Y. Hollow functional materials derived from metal-organic frameworks: Synthetic strategies, conversion mechanisms, and electrochemical applications. Adv. Mater. 2019, 31, e1804903. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- He, W.; Zhao, G.; Sun, P.; Hou, P.; Zhu, L.; Wang, T.; Li, L.; Xu, X.; Zhai, T. Construction of Longan–like hybrid structures by anchoring nickel hydroxide on yolk–shell polypyrrole for asymmetric supercapacitors. Nano Energy. [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.; Maleski, K.; Hatter, C.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/Graphene Films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent progress in ruthenium oxide-based composites for supercapacitor applications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, K.; Zhou, J.; Hu, H.; Chen, W.; Yu, Y. Design of a unique 3D-nanostructure to make MnO2 work as supercapacitor material in acid environment. Chem. Eng. J. 2017, 321, 554–563. [Google Scholar] [CrossRef]
- Yu, N.; Yin, H.; Zhang, W.; Liu, Y.; Tang, Z.; Zhu, M. High-performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics. Adv. Energy Mater. 2016, 6, 1501458. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Wang, Z.; Yu, J.; Huang, H.; Qiu, J. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives. Nano Energy 2019, 57, 459–472. [Google Scholar] [CrossRef]
- Ali, A.A.; Eltabey, M.M.; Abdelbary, B.M.; Zoalfakar, S.H. MWCNTs/carbon nano fibril composite papers for fuel cell and super capacitor applications. J. Electrost. 2015, 73, 12–18. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lai, C.C.; Ho, C.L.; Lo, C.T. Preparation of interconnected carbon nanofibers as electrodes for supercapacitors. Electrochim. Acta 2014, 127, 369–376. [Google Scholar] [CrossRef]
- Yang, W.; Deng, B.; Hou, L.; Wang, T.; Tian, J.; Wang, S.; Lia, R.; Yang, F.; Li, Y. Sulfur-fixation strategy toward controllable synthesis of molybdenum-based/carbon nanosheets derived from petroleum asphalt. Chem. Eng. J. 2020, 380, 122552. [Google Scholar] [CrossRef]
- Qiu, H.; An, S.; Sun, X.; Yang, H.; Zhang, Y.; He, W. MWCNTs-GONRs/Co3O4@Ni(OH)2 core-shell array structure with a high performance electrode for supercapacitor. Chem. Eng. J. 2020, 380, 122490. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, Y.; Xia, Y.; Hung, C.T.; Liu, L.; Bi, W.; Li, W. Synthesis of sandwich-like graphene@mesoporous nitrogen-doped carbon nanosheets for application in high-performance supercapacitors. Nanotechnology 2020, 31, 024001. [Google Scholar] [CrossRef]
- Kostoglou, C.; Koczwara, C.; Prehal, V.; Terziyska, B.; Babic, B.; Matovic, G.; Constantinides, C.; Tampaxis, G.; Charalambopoulou, T.; Steriotis, S.; et al. Nanoporous activated carbon cloth as a versatile material for hydrogen adsorption, selective gas separation and electrochemical energy storage. Nano Energy 2017, 40, 49–64. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, X.; Zhan, H.; Long, S.; Xi, H.; Hu, Z. High-performance all-solid state flexible supercapacitors based on two-step activated carbon cloth. J. Power Sources 2014, 272, 16–23. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Lu, Y.; Ling, M.; Yu, T.; Zhai, Y. Solid-state supercapacitor based on activated carbon cloths exhibits excellent rate capability. Adv. Mater. 2014, 26, 2676–2682. [Google Scholar] [CrossRef]
- Ma, C.; Liu, X.; Min, J.; Li, J.; Mijoska, E. Sustainable recycling of waste polystyrene into hierarchical porous carbon nanosheets with potential applications in supercapacitors. Nanotechnology 2020, 31, 035402. [Google Scholar] [CrossRef]
- Shu, Y.; Bai, Q.; Fu, G.; Xiong, Q.; Uyama, H. Hierarchical porous carbons from polysaccharides carboxymethyl cellulose, bacterial cellulose, and citric acid for supercapacitor. Carbohydr. Polym. 2020, 227, 115346. [Google Scholar] [CrossRef]
- Sun, H.; Pan, J.; Yan, X.; Shen, W.; Zhong, W.; Cheng, X. MnO2 nanoneedles loaded on silicon oxycarbide-derived hierarchically porous carbon for supercapacitor electrodes with enhanced electrochemical performance. Ceram. Int. 2019, 45, 24802. [Google Scholar] [CrossRef]
- Kim, C.H.; Yang, C.M.; Kim, Y.A.; Yang, K.S. Pore engineering of nanoporous carbon nanofibers toward enhanced supercapacitor performance. Appl. Surface Sci. 2019, 497, 143693. [Google Scholar] [CrossRef]
- Chen, C.J.; Zhang, Y.; Li, Y.J.; Dai, J.Q.; Song, J.W.; Yao, Y.G.; Gong, Y.H.; Kierzewski, I.; Xie, J.; Hu, L.B. All-wood, low tortuosity, aqueous, biodegradable supercapacitors with ultra-high capacitance. Energy Environ. Sci. 2017, 10, 538–545. [Google Scholar] [CrossRef]
- Xu, D.; Su, Y.; Zhang, S.; Xiong, Y. Highly porous N-doped carbons production from biomass for high-performance supercapacitors without chemical nitrogen-containing dopants. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 1797–1807. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Y.G.; Wan, J.Y.; Henderson, D.; Zhang, X.G.; Hu, L.B. High temperature carbonized grass as a high performance sodium ion battery anode. ACS Appl. Mater. Interfaces 2017, 9, 391–397. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Akiyama, T. Cotton derived porous carbon via an MgO template method for high performance lithium ion battery anodes. Green Chem. 2016, 18, 2106–2114. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, S.; Li, B.; Wei, H.; Zhang, D.; Hu, J.; Zhang, L.; Zhang, J.; Liu, Q. Mesopore-rich carbon flakes derived from lotus leaves and it’s ultrahigh performance for supercapacitors. Electrochim. Acta 2020, 333, 135481. [Google Scholar] [CrossRef]
- Sajjad, M.; Jiang, Y.; Guan, L.; Chen, X.; Iqbal, A.; Zhang, S.Y.; Ren, Y.; Zhou, X.W.; Liu, Z. NiCo2S4 nanosheet grafted SiO2@C core-shelled spheres as a novel electrode for high performance supercapacitors. Nanotechnology 2020, 31, 045403. [Google Scholar] [CrossRef]
- Duan, B.; Gao, X.; Yao, X.; Fang, Y.; Huang, L.; Zhou, J.; Zhang, L.N. Unique elastic N-doped carbon nanofibrous microspheres with hierarchical porosity derived from renewable chitin for high rate supercapacitors. Nano Energy 2016, 27, 482–491. [Google Scholar] [CrossRef]
- Jiao, Z.; Wu, Q.; Cardon, L.; Qiu, J. Preparation and electrochemical performance of hollow activated carbon fiber self-supported electrode for supercapacitor. J. Nanosci. Nanotechnol. 2020, 20, 2316–2323. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.J.; Gao, Y.F.; An, W.D.; Cao, Z.Z.; Liu, J.R. Biomass-swelling assisted synthesis of hierarchical porous carbon fibers for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28283–28290. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Jiang, K.; Tahir, M.; Wu, X.; Idrees, F.; Shen, M.; Cao, C. Tunable porous structure of carbon nanosheets derived from puffed rice for high energy density supercapacitors. J. Power Sources 2017, 371, 148–155. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.F.; Liu, S.H.; Wang, Y.W.; Qiu, J.S. Sustainable synthesis and assembly of biomass-derived B/N co-doped carbon nanosheets with ultrahigh aspect ratio for high-performance supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Wu, H.; Chen, A. N-Doped yolk-shell carbon nanotube composite for enhanced electrochemical performance in a supercapacitor. Nanoscale 2019, 11, 22796–22803. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, W.; Quan, H.; Huang, Z.; Chen, D.; Guo, L. Nitrogen-doped foam-like carbon plate consisting of carbon tubes as high-performance electrode materials for supercapacitors. ChemElectroChem 2016, 3, 814–821. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Fan, Z. Biomass-derived carbon materials with structural diversities and their applications in energy storage. Sci. China Mater. 2017, 61, 133–158. [Google Scholar] [CrossRef]
- Tian, X.; Ma, H.G.; Li, Z.; Yan, S.; Ma, L.; Yu, F.; Wang, G.; Guo, X.; Ma, Y.; Wong, C. Flute type micropores activated carbon from cotton stalk for high performance supercapacitors. J. Power Sources 2017, 359, 88–96. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, P.; Zhu, B.; Chen, S.; Yao, K.; Han, R. Microporous carbon derived from acacia gum with tuned porosity for high-performance electrochemical capacitors. Int. J. Hydrog. Energy 2015, 40, 6188–6196. [Google Scholar] [CrossRef]
- Zhu, G.; Ma, L.; Lv, H.; Hu, Y.; Chen, T.; Chen, R.; Liang, J.; Wang, X.; Wang, Y.; Yan, C. Pine needle-derived microporous nitrogen-doped carbon frameworks exhibit high performances in electrocatalytic hydrogen evolution reaction and supercapacitors. Nanoscale 2017, 9, 1237–1243. [Google Scholar] [CrossRef]
- Liu, C.; Han, G.; Chang, Y.; Xiao, Y.; Li, M.; Zhou, W.; Fu, D.; Hou, W. Properties of porous carbon derived from cornstalk core in high-performance electrochemical capacitors. ChemElectroChem 2016, 3, 323–331. [Google Scholar] [CrossRef]
- Enock, T.K.; King’ondu, C.K.; Pogrebnoi, A.; Jande, Y.A.C. Status of biomass derived carbon materials for supercapacitor application. Int. J. Electrochem. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Zapata-Benabithe, Z.; Carrasco-Marin, F.; Moreno-Castilla, C. Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes. Bioresour. Technol. 2012, 111, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Tian, P.; Yang, G.; Jia, S.; Zhou, S.; Xu, H.; Wang, Y. A facile preparation of pomegranate-like porous carbon by carbonization and activation of phenolic resin prepared via hydrothermal synthesis in KOH solution for high performance supercapacitor electrodes. Adv. Powder Technol. 2019, 30, 2900–2907. [Google Scholar] [CrossRef]
- Chen, C.; Yu, D.; Zhao, G.; Du, B.; Tang, W.; Sun, L.; Sun, Y.; Besenbacher, F.; Yu, M. Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors. Nano Energy 2016, 27, 377–389. [Google Scholar] [CrossRef]
- Long, C.; Jiang, L.; Wu, X.; Jiang, Y.; Yang, D.; Wang, C.; Wei, T.; Fan, Z. Facile synthesis of functionalized porous carbon with three-dimensional interconnected pore structure for high volumetric performance supercapacitors. Carbon 2015, 93, 412–420. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, W.; Gong, Y.; Dong, L.; Deng, Y. High-performance pseudocapacitors from kraft lignin modified active carbon. Electrochim. Acta 2019, 320, 134640. [Google Scholar] [CrossRef]
- Fang, J.; Guo, D.; Kang, C.; Wan, S.; Li, S.; Fu, L.; Liu, G.; Liu, Q. Enhanced hetero-elements doping content in biomass waste-derived carbon for high performance supercapacitor. Int. J. Energy Res. 2017, 14, 8811–8821. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liu, L.; Zhang, Y.; Yang, J.; Zeng, Z.; Deng, S. Controllable synthesis of bifunctional porous carbon for efficient gas-mixture separation and high-performance supercapacitor. Chem. Eng. J. 2018, 348, 57–66. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, C.; Yu, D.; Sun, L.; Yang, C.H.; Zhang, H.; Sun, Y.; Besenbacher, F.; Yu, M. One-step production of O-N-S co-doped three-dimensional hierarchical porous carbons for high-performance supercapacitors. Nano Energy 2018, 47, 547–555. [Google Scholar] [CrossRef]
- Dong, S.; He, X.; Zhang, H.; Xie, X.Y.; Yu, M.X.; Yu, C.; Xiao, N.; Qiu, J.S. Surface modification of biomass-derived hard carbon by grafting porous carbon nanosheets for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15954–15960. [Google Scholar] [CrossRef]
- Zhang, D.; Xue, Y.; Chen, J.; Zhang, D.; Xue, Y.C.; Chen, J.L.; Guo, X.M.; Yang, D.D.; Wang, J.C.; Zhang, J.; et al. N, S, O self-doped porous carbon nanoarchitectonics derived from pinecone with outstanding supercapacitance performances. J. Nanosci. Nanotechnol. 2020, 20, 2728–2735. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, H.; Sun, H.; Wang, J.G.; Liu, H.Z.; Sun, H.H.; Hua, W.; Wang, H.W.; Liu, X.R.; Wei, B.Q. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Su, H.; Zhang, H.; Liu, F.; Chun, F.J.; Zhang, B.; Chu, X.; Huang, H.C.; Deng, W.L.; Gu, B.; Zhang, H.P.; et al. High power supercapacitors based on hierarchically porous sheet-like nanocarbons with ionic liquid electrolytes. Chem. Eng. J. 2017, 322, 73–81. [Google Scholar] [CrossRef]
- Hao, E.; Liu, W.; Liu, S.; Zhang, Y.; Wang, H.; Chen, S.; Cheng, F.; Zhao, S.; Yang, H. Rich sulfur doped porous carbon materials derived from ginkgo leaves for multiple electrochemical energy storage devices. J. Mater. Chem. A 2017, 5, 2204–2214. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Z. Recent breakthroughs in supercapacitors boosted by nitrogen-rich porous carbon materials. Adv. Sci. (Weinh) 2017, 4, 1600408. [Google Scholar] [CrossRef]
- Yuan, G.; Huang, W.; Guan, K.; Li, H.; Xie, Y.; Liang, Y.R.; Liu, Y.L.; Zheng, M. A universal K OH-free strategy towards nitrogen-doped carbon nanosheets for high-rate and high-energy storage devices. J. Mater. Chem. A 2019, 7, 26469–26478. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect graphene as a trifunctional catalyst for electrochemical reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Naz, R.; Imtiaz, M.; Liu, Q.; Yao, L.; Abbas, W.; Li, T.F.; Zada, I.; Yuan, Y.; Chen, W.S.; Gu, J.J. Highly defective 1T-MoS2 nanosheets on 3D reduced graphene oxide networks for supercapacitors. Carbon 2019, 152, 697–703. [Google Scholar] [CrossRef]
- Deb Nath, N.C.; Shah, S.S.; Qasem, M.A.A.; Zahir, M.H.; Aziz, M.A. Defective carbon nanosheets derived from syzygium cumini leaves for electrochemical energy-storage. ChemistrySelect 2019, 4, 9079–9083. [Google Scholar] [CrossRef]
- Zhu, Y.E.; Yang, L.; Sheng, J.; Chen, Y.N.; Gu, H.; Wei, J.P.; Zhou, Z. Fast sodium storage in TiO2 @CNT@C nanorods for high-performance Na-ion capacitors. Adv. Energy Mater. 2017, 7, 1701222. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Li, H.; Deng, X.; Xu, X.; Zhai, T. Ultrathin and porous Ni3S2/CoNi2S4 3D-network structure for superhigh energy density asymmetric supercapacitors. Adv. Energy Mater. 2017, 7, 1700983. [Google Scholar] [CrossRef]
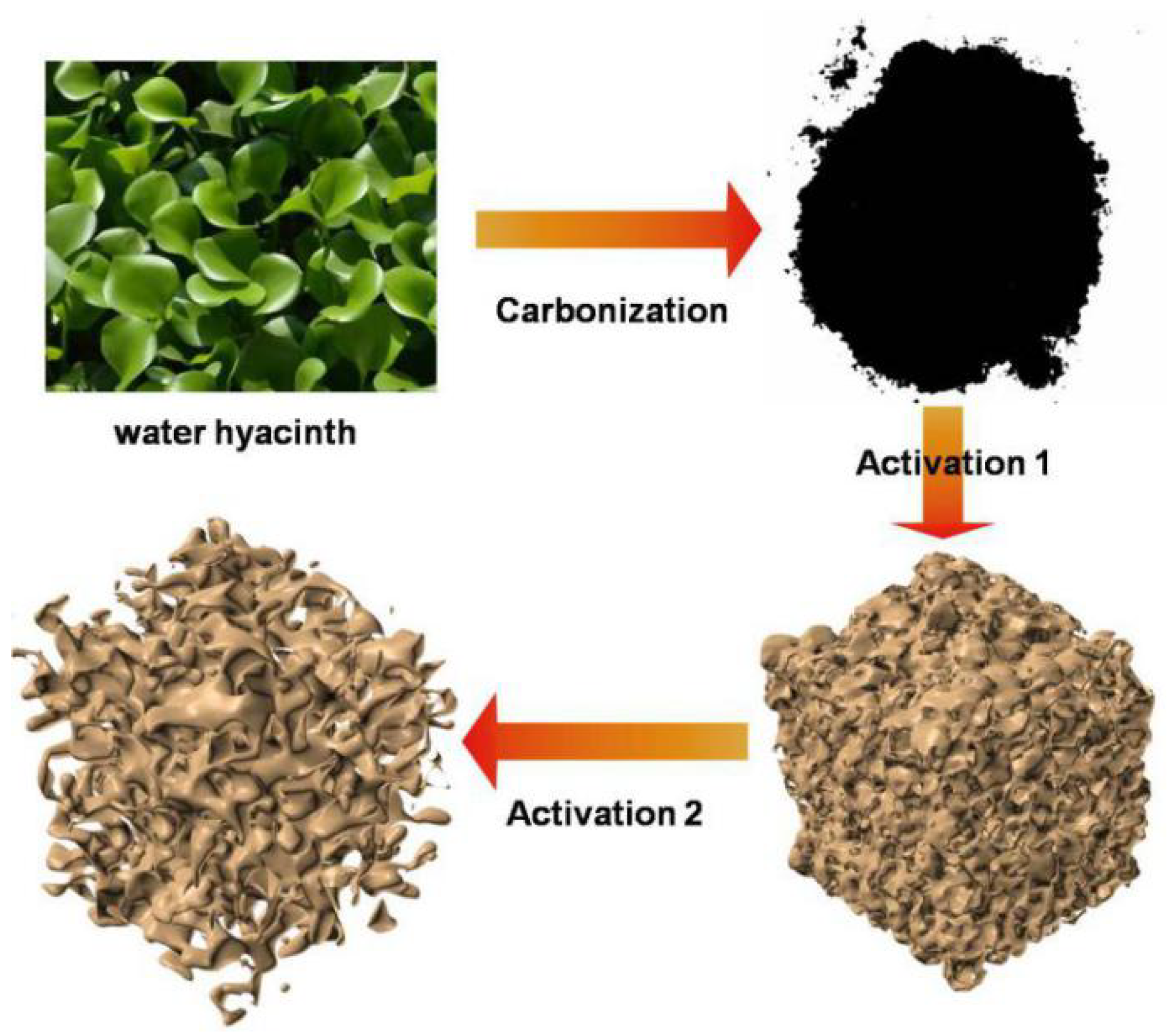
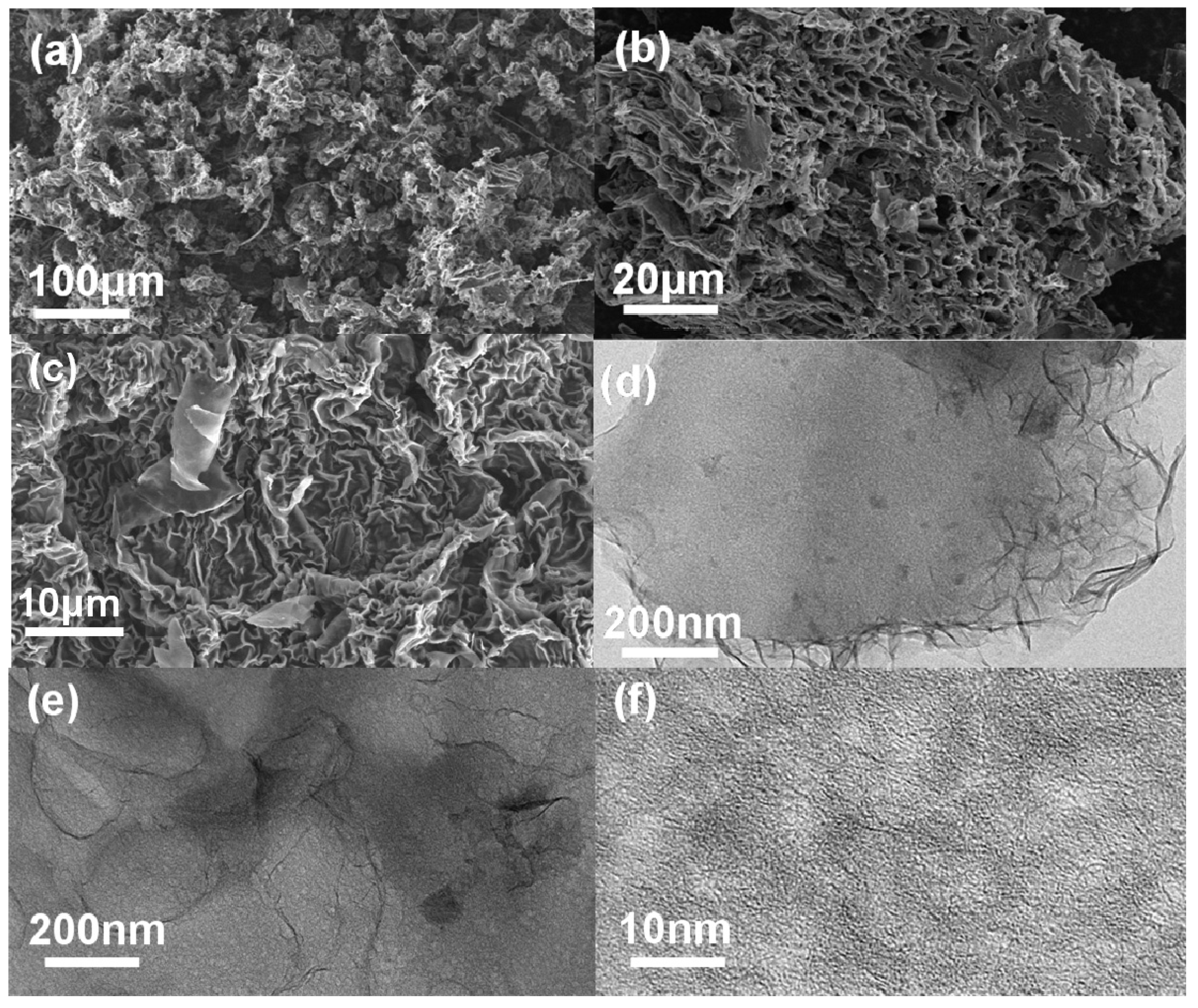
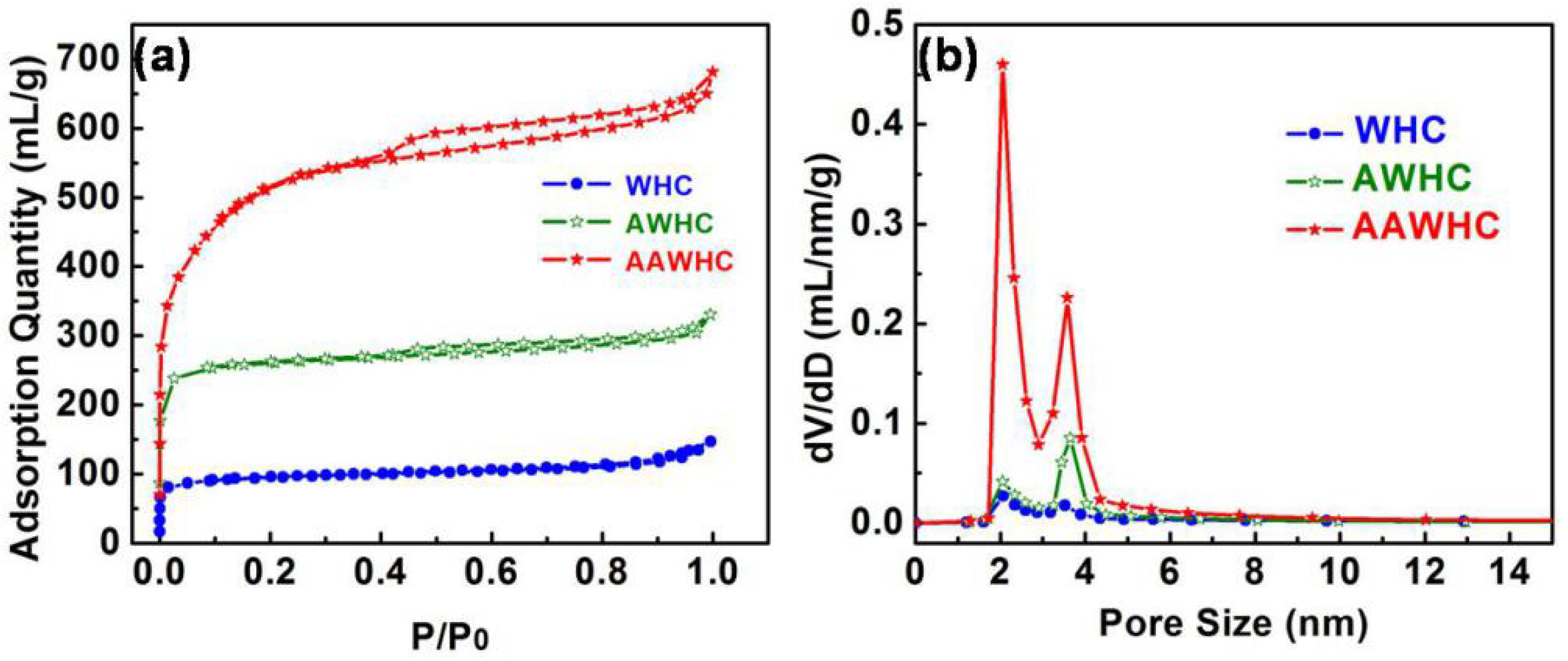
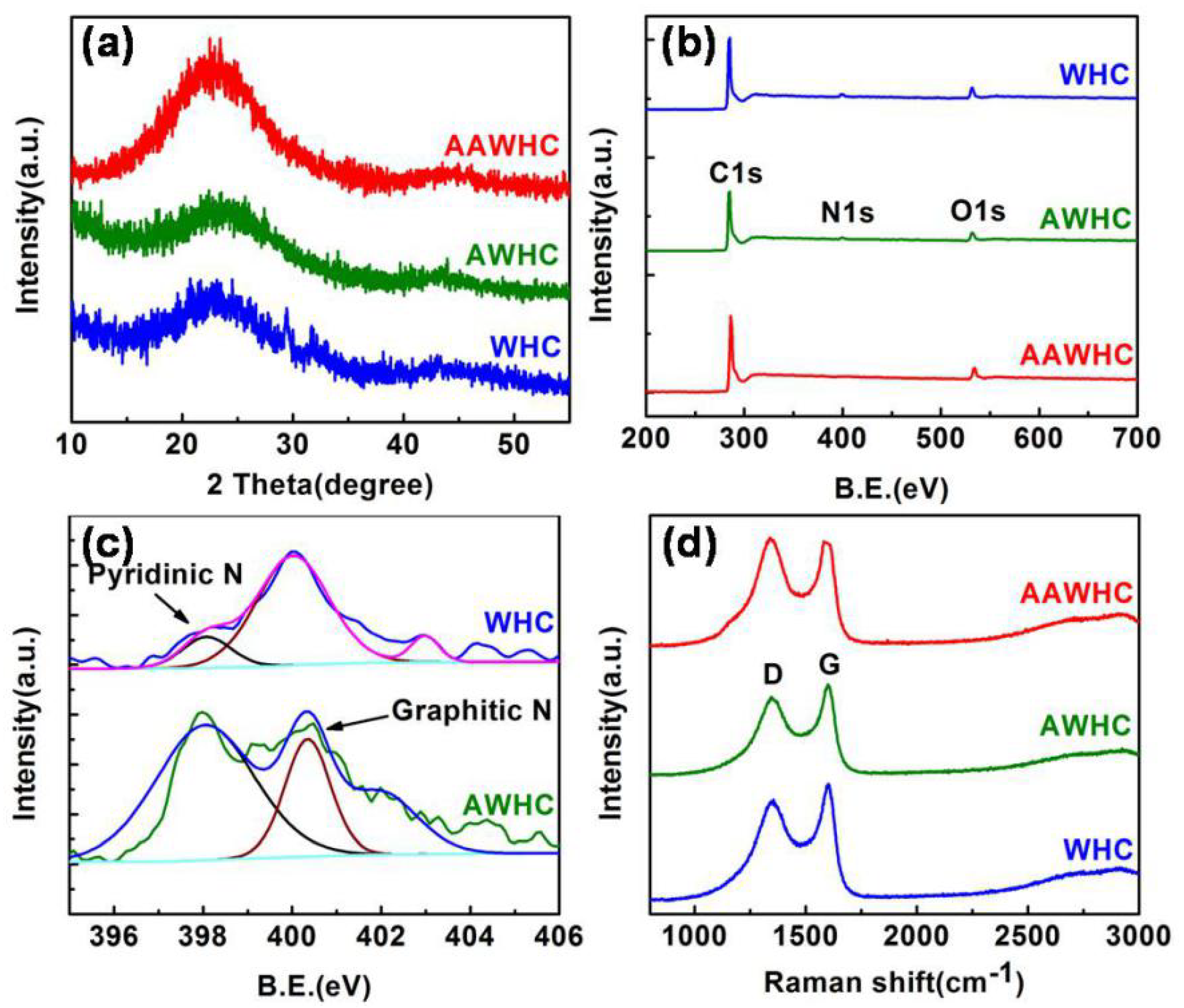

| Samples | SSA/m2 g−1 | Pore Volume/mL g−1 | Mesopore Volume/mL g−1 |
|---|---|---|---|
| WHC | 352 | 0.2288 | 0.0856 |
| AWHC | 741 | 0.5116 | 0.1127 |
| AAWHC | 1623 | 1.0547 | 0.3962 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Zhou, S.; Zhang, Y.; Chen, M.; Li, B.; Wei, H.; Zhang, D.; Zhang, J.; Liu, Q. Nanoporous Carbon Derived from Green Material by an Ordered Activation Method and Its High Capacitance for Energy Storage. Nanomaterials 2020, 10, 1058. https://doi.org/10.3390/nano10061058
Lu Q, Zhou S, Zhang Y, Chen M, Li B, Wei H, Zhang D, Zhang J, Liu Q. Nanoporous Carbon Derived from Green Material by an Ordered Activation Method and Its High Capacitance for Energy Storage. Nanomaterials. 2020; 10(6):1058. https://doi.org/10.3390/nano10061058
Chicago/Turabian StyleLu, Qingjie, Shiqiang Zhou, Yumin Zhang, Mingpeng Chen, Bo Li, Haitang Wei, Dongming Zhang, Jin Zhang, and Qingju Liu. 2020. "Nanoporous Carbon Derived from Green Material by an Ordered Activation Method and Its High Capacitance for Energy Storage" Nanomaterials 10, no. 6: 1058. https://doi.org/10.3390/nano10061058
APA StyleLu, Q., Zhou, S., Zhang, Y., Chen, M., Li, B., Wei, H., Zhang, D., Zhang, J., & Liu, Q. (2020). Nanoporous Carbon Derived from Green Material by an Ordered Activation Method and Its High Capacitance for Energy Storage. Nanomaterials, 10(6), 1058. https://doi.org/10.3390/nano10061058






