Abstract
As one of the most frequently-used electron-transporting materials, the mesoporous titanium dioxide (m-TiO2) film used in mesoporous structured perovskite solar cells (PSCs) can be employed for the scaffold of the perovskite film and as a pathway for electron transport, and the contact area between the perovskite and m-TiO2 directly determines the comprehensive performance of the PSCs. Because of the substandard interface combining quality between the all-inorganic perovskite CsPbIBr2 and m-TiO2, the development of the mesoporous structured CsPbIBr2 PSCs synthesized by the one-step method is severely limited. Here, we used a solution containing PbI2, monoethanolamine (EA) and dimethyl sulfoxide (DMSO) (PED) as the interfacial modifier to enhance the contact area and modify the m-TiO2/CsPbIBr2 contact characteristics. Comparatively, the performance of the solar device based on the PED-modified m-TiO2 layer has improved considerably, and its power conversion efficiency is up to 6.39%.
1. Introduction
Despite the advantages of simple fabrication processes, high conversion efficiency and successful techniques, the organic-inorganic perovskite solar cells (PSCs) under high humidity, high temperature and light conditions show poor stability [1,2,3,4,5,6], and since this poor stability seems unlikely to improve in the short term, scholars began researching ways to enhance the stability and efficiency of PSCs by investigating the all-inorganic hybrid PSCs [7,8,9]. As Cs+ ions have replaced organic molecules in organic-inorganic halide perovskites, the all-inorganic CsPbX3 (X = I or Br) materials present much better ultra-violet, thermal and humid stabilities in air [10,11,12,13]. Although CsPbI3 [14,15,16,17] and CsPbI2Br [18,19,20] materials exhibit acceptable light absorption capacity and performance with a relatively narrow band gap of 1.73 eV and 1.92 eV, respectively, both are difficult to be applied in practice due to their unstable structures, which easily lead to structure or performance degradation accompanied by color changes from dark red to yellow under laboratory conditions [10,13,15,21,22]. Many important physical properties of CsPbBr3 are known, such as high tolerance to humidity, light illumination [23] and a wide band gap of 2.30 eV [24,25]. However, extensive application in the field of photovoltaic is limited because of the low solubility of perovskite precursors (CsBr and PbBr2) in most organic solvents [26]. In contrast, CsPbIBr2 displays the best balance features for the all-inorganic PSCs as an absorption material in terms of stability and optical properties [27,28,29,30,31,32].
In mesoporous structured perovskite solar cells, the mesoporous TiO2 is an important electron transport material that has unique physical and chemical properties; it not only supplies the pathway for electron transport but also contacts the perovskite material to enhance the separation rate of photoinduced charges. Moreover, the interface relationships of the m-TiO2 exert a significant influence on the performance of PSCs. Many researchers have been focused on interfacial engineering to improve the comprehensive performance of the m-TiO2 films and have achieved many results in theory and application in the PSCs field. Briefly, the use of the interfacial modifier can effectively boost the performance and photostability of PSCs. In addition, all-inorganic CsPbIBr2 PSCs could be fabricated either on planar or mesoporous structures, but the former have received more research concentration on stable power output and the latter less. To our knowledge, TiO2 is the only mesoporous material used, and CsPbIBr2 films can only be synthesized by the only two-step solution method to date [26,33,34].
Herein, we successfully fabricated the CsPbIBr2 PSCs on a mesoporous structure, and the perovskite films were synthesized by a typical one-step method. The PED solution was used as the interfacial modifier to modify the m-TiO2/CsPbIBr2 interface and promote the precursor solution of CsPbIBr2 further diffusing into the grain boundaries of m-TiO2 film. Therefore, using PED on the interface can not only restrain the interface recombination to enhance the electronic transmission capability but can also promote CsPbIBr2 to fill the space in m-TiO2 film to enhance the interface combination. Compared with the standard mesoporous CsPbIBr2 PSCs we synthesized, the devices with PED modification presented a better performance with a power conversion efficiency (PCE) of 6.39%.
2. Experiment
2.1. Device Fabrication
Fluorine-doped tin oxide glass substrates (FTO, 6Ω/□) were patterned with a laser etcher (OPV Tech New Energy Co., Ltd., Yingkou, China.) and cleaned sequentially by a neutral detergent, deionized water, acetone, isopropanol and ethanol by ultrasound treatment. After being dried in the air, the FTO substrate was further cleaned in an ultraviolet treatment for 15 min, and then the TiO2 compact layer (c-TiO2) was spin-coated on the pre-conditioned FTO substrate according to the previous report [35]. Afterwards, the mesoporous TiO2 layer (m-TiO2) (Dysol, 30NR, diluted with ethanol at a ratio of 1:8, w/w) was coated on the FTO/c-TiO2 substrate by spin-coating at 5000 rpm for 30 s. After the film was dried on a hotplate at 125 °C for 5 min followed by the muffle furnace at 500 °C for 30 min, the pre-coated substrates were attained.
The following synthetic processes were carried out in a glove box under the highly purified argon environment. PED solution, which contained 2 mL DMSO (99.8%, Aladdin, Shanghai, China), 0.5 mL EA (double distillation, Aladdin, Shanghai, China) and 0.25 M PbI2 (99.99%, Xi’an p-OLED, Xi’an, China) was heated on a hot plate at 150 °C for 30 min. While the PbI2 dissolved completely, the PED was spin-coated on the surface of the cooled pre-coated substrates at 5000 rpm for 60 s and then heated and naturally cooled once again. For CsPbIBr2, the precursor solution prepared by full dissolving 260 mg CsI and 370 mg PbBr2 in 1 mL DMSO at 150 °C was coated on the PED film by spin-coating at the 5000 rpm for 60 s and annealed at 280 °C for 10 min subsequently. After Spiro-OMeTAD (>99%, OPV Tech New Energy Co., Ltd., Yingkou, LN, China) was spin-coated onto the CsPbIBr2 films at 3000 rpm for 30 s, the samples were removed from the glove box, and a 100 nm thick Ag was t deposited by thermal evaporation on the top of the Spiro-OMeTAD layer as metal electrode to complete the solar energy devices.
2.2. Device Characterizations
The crystal structures and composition of the synthesized samples were identified by X-ray diffraction (XRD, Cu Kα radiation, λ = 1.5418 Å, Rigaku D/max2500). The morphologies and structures of the films and solar devices were observed by a scanning electron microscope (SEM, FEI MAGELLAN 400, FEI, Hillsboro, OR, USA), and an energy dispersive spectroscope (EDS) attached to the SEM column was used to analysis the element composition of the corresponding samples. X-ray photoelectron spectroscopy (XPS) spectra were measured using ESCALAB 250Xi (Thermo Scientific, Waltham, MA, UK). A UV-Vis spectrometer (UV-3600, Shimadzu, TKY, Japan) was utilized to measure the absorption spectrum at a range of 300 nm to 800 nm. The steady-state photoluminescence (PL) spectrum was collected on a Ramascope System 1000 (λex = 633 nm). The current-voltage (J-V) characteristics and external quantum efficiency (EQE) of the fabricated solar devices were measured by a solar cells test system (XP3000, Sanyou, Beijing, China) and an external quantum efficiency (EQE) measured system (Solar Cell Scan100, Zolix, Beijing, China), respectively.
3. Results and Discussion
3.1. X-ray Diffraction Studies and Compositional Analysis
Figure 1 shows the XRD patterns of CsPbIBr2 films synthesized on c-TiO2/m-TiO2 substrates with and without PED modification. Both patterns show the main diffraction peaks at 2θ of 15.00°, 21.40°, 30.19° and 37.20° corresponding to (111), (110), (200) and (211) planes of α-phase perovskite CsPbIBr2 [29,36,37], which confirms that both CsPbIBr2 films synthesized by the one-step method are pure, that the PED modification has no influence on the perovskite phase or crystallinity and that no PbI2 diffraction peak can be observed. However, as the PED solution is spin-coated on c-TiO2/m-TiO2 substrates without CsPbIBr2 film, a PbI2 characteristic peak at 12.60° can be found in the XRD pattern, and the I/Pb atomic ratio is approximate to 2:1, as confirmed from EDS spectra (Figure S1a,b respectively, Supporting Information), which means that PbI2 presents in the m-TiO2 film as crystals. Because of being easily soluble in DMSO, PbI2 in the PED-modified m-TiO2 film is redissolved in the perovskite precursor solution during the successively synthetic process of CsPbIBr2, which explains why no PbI2 diffraction peak is observed in Figure 1.
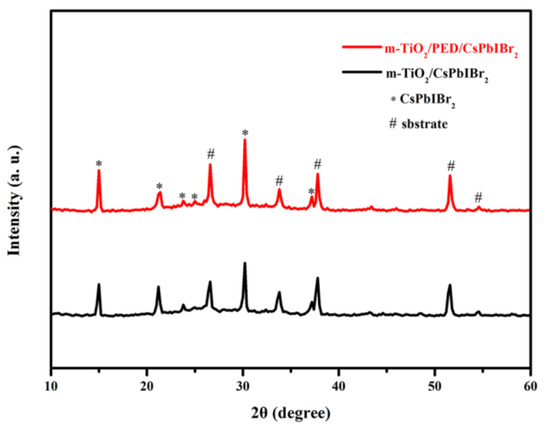
Figure 1.
XRD patterns of m-TiO2/CsPbIBr2 films with and without PED modification.
3.2. Morphological Characterization
The influences of PED on morphologies of m-TiO2 and CsPbIBr2 were investigated by using SEM as shown in Figure 2. As demonstrated in Figure 2a, CsPbIBr2 film deposited on m-TiO2 shows a relatively porous attribute with non-uniform distribution but a smooth surface (Figure 2a). Apparently, compared with Figure 2a, the rough CsPbIBr2 film deposited on PED-modified m-TiO2 shown in Figure 2b is composed of almost homogeneous nanoparticles and pores that are uniformly distributed in the film. Because of the poor solubility of PbBr2 in the perovskite precursor solution, such abundant pores in the CsPbIBr2 film synthesized by the one-step method have seemed inevitable [26]. As illustrated in Figure 2c, pores can be clearly seen in the m-TiO2 film, and the thickness of the CsPbIBr2 film is about 210 nm. The cross-sectional SEM image of the PED-modified sample in Figure 2d shows that the perovskite film with a thickness of 160 nm is thinner than the pristine one and that the m-TiO2 film is almost filled up and only a very small number of pores remain. No interface between CsPbIBr2 and m-TiO2 layers can be clearly distinguished. Combined with the conclusions of XRD, we can confirm that the abundant perovskite precursor solution diffuses into the m-TiO2 film during the spin-coating process and generates CsPbIBr2 to fill up the pores, which is conductive to the transportation and collection process for the carriers [38,39].
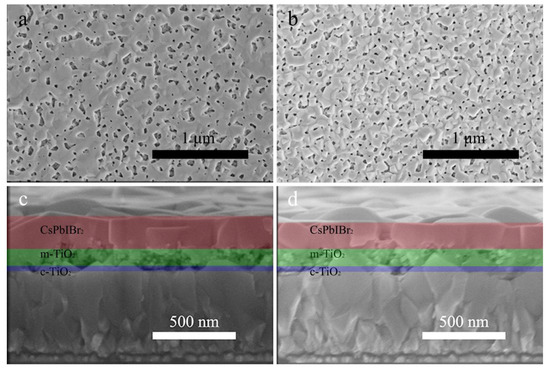
Figure 2.
Top-view SEM images and cross-sectional SEM images of m-TiO2/CsPbIBr2 films (a,c) without and (b,d) with PED.
3.3. XPS Analysis
XPS was utilized to probe the surficial elemental composition of CsPbIBr2 films with and without PED modification taking C 1s (284.8 eV) as the calibration. As seen from Figure 3a, obviously, the characteristic peaks of Cs, Pb, I, Br, C and O are detected, and no other element can be identified in each film. Figure 3b–e displays the XPS core spectra of corresponding Pb 4f, I 3d, Br 3d and Cs 3d, respectively. All of the binding energy peaks belonging to CsPbIBr2 constituent elements are in accordance with previous reports [36], and no notable peak shift can be observed for each of elements. The XPS results indicate that the existence or non-existence of PED has no essential influence on the stoichiometric of the CsPbIBr2 perovskite.
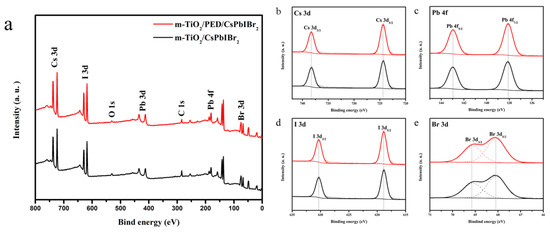
Figure 3.
(a) XPS spectra and (b) Cs 3d, (c) Pb 4f, (d) I 3d and (e) Br 3d XPS core spectra of the m-TiO2/CsPbIBr2 films with and without PED modification.
3.4. Optical Properties and Photovoltaic Performances
The photophysical properties of m-TiO2/CsPbIBr2 films with and without PED modification were then studied. Figure 4a presents their absorption spectra. Both the films exhibit similar absorption profiles in the visible region. The absorbance onset for the modified sample exhibits a slight red shift from about 606 to 610 nm, and the absorption intensity increases slightly in the whole measuring range compared with the pristine sample. Correspondingly, the calculated bandgap decreases from 2.09 to 2.08 eV (Figure S2, Supporting Information), which coincides with the relevant literature [40,41]. The PL spectra are demonstrated in Figure 4b, which signifies that when the peak position of the m-TiO2/CsPbIBr2 films with PED is blue-shifted (594.9 vs 597.6 nm), the luminous intensity also distinctly decreased. Such observations suggest that the trap states of the porous TiO2 layers that are full-filled by CsPbIBr2 and the spontaneous radiative recombination of CsPbIBr2 are passivated due to the interfacial modifier, PED, hence leading to the improvement of the charge separation and transfer process at the m-TiO2/CsPbIBr2 interface.
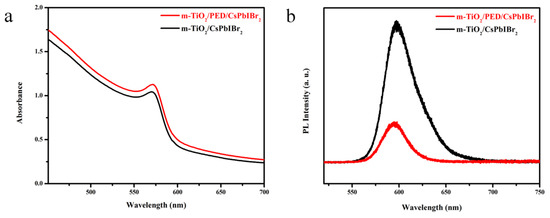
Figure 4.
(a) UV-vis absorbance spectra and (b) photoluminescence (PL) spectra of the m-TiO2/CsPbIBr2 films with and without PED modification.
The PSCs we successfully synthesized were based on the simple architecture of c-TiO2/m-TiO2/PED (or not)/CsPbIBr2/Spiro-OMeTAD/Ag, and the cross-section of the complete modified device is given in Figure 5a. Figure 5b provides the reverse- and forward-scanned J-V curves of two representative devices measured under simulated AM 1.5G illumination. The hysteresis index (HI) was applied to assess the hysteresis effect of the devices, which can be well defined according to the following equation [42]:
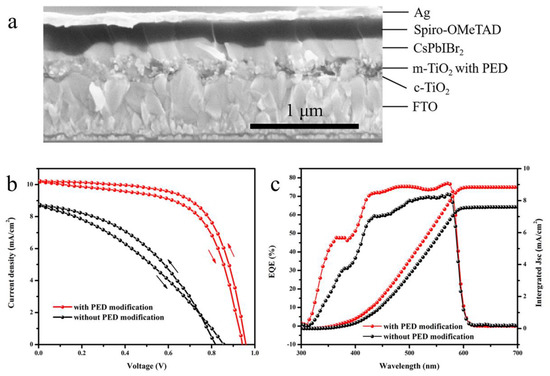
Figure 5.
(a) Cross-sectional SEM image of the modified CsPbIBr2 devices. (b) J-V curves and (c) EQE spectra and the integrated product of the EQE curve of the corresponding devices.
Compared with the pristine device, the m-TiO2/PED device shows an expected slighter hysteresis behavior (a decrease from 13.8% to 7.2%), and all photovoltaic parameters including short circuit current density (JSC), open circuit voltage (VOC), fill factor (FF) and PCE are enhanced evidently. As listed in Table 1, the PED modification of m-TiO2 has significantly increased the JSC from 8.69–8.74 mA∙cm−2 to 10.22–10.28 mA∙cm−2, VOC from 0.82–0.86 V to 0.94–0.96 V and FF from 0.35–0.43 to 0.62–0.65, leading to an efficiency improvement of more than 110% from 2.62–3.04% to 5.93–6.39%. The EQE spectra and the corresponding integral current densities are provided in Figure 5c. Both EQE spectra present the same onsets at the wavelength of about 600 nm, which are roughly consistent with the results of absorption spectrum. A significant enhancement of EQE values is observed for the m-TiO2/PED device in the light absorption wavelength range of 300–575 nm, which is consistent with the increment in JSC. Meanwhile, the integrated current densities from EQE are close to the results of JSC, while the discrepancy mainly originates from the spectral mismatch between the solar simulator and EQE measurement system.

Table 1.
Photovoltaic parameters of the CsPbIBr2 devices with and without PED modification.
3.5. Possible Mechanism
The enhancement of power conversion efficiency of the CsPbIBr2 device was mainly ascribed to the PED solution, which contained proper EA and PbI2. The –OH group of EA within the PED solution would interact with PbI2 colloid (such as PbI3− and PbI42−) to form stable chemical bonding –OH–Pb [43]. As shown in Figure 6, while PED was spin-coated on m-TiO2, the unreacted PbI2 was forced to penetrate into the TiO2 mesoporous film by –OH group [44]. While the CsPbIBr2 precursor solution was coated on the m-TiO2/PED substrate, PbI2 in the mesoporous film provided the paths for the precursor solution diffusing into the pores while –OH–Pb coordinated to the TiO2 surface to improve the interface contact with CsPbIBr2 [43]. Therefore, the holes and chink were packed and closed by CsPbIBr2 in the annealing process, leading to the decreasing thickness of the CsPbIBr2 film on the m-TiO2/PED substrate. It should be noted that the surface color of m-TiO2/PED gradually converted from light yellow into colorless during the spin-coated process of the CsPbIBr2 precursor solution. This phenomenon was caused by the fact that PbI2 deposited in porous TiO2 was redissolved in the perovskite precursor solution and participated in the CsPbIBr2 growth and film-forming processes, leaving native defects due to nonstoichiometry [45,46]. That explains why the diffraction peak of PbI2 could not be found in the XRD pattern and why the morphology of the CsPbIBr2 surface also changed for the m-TiO2/PED/ CsPbIBr2 film. Furthermore, the improvement of the optical properties of the modified sample based on UV-Vis spectra and EQE spectra was mainly attributable to the CsPbIBr2 filling behavior and the stoichiometric change in the mesoporous film. The pores filled by perovskite in m-TiO2 could effectively increase the contact area between TiO2 nanoparticles and CsPbIBr2. The –OH–Pb group was advantageous for enhancing the interface contact, reducing trap states of the perovskite film, improving the electron extraction and charging transport rates [47,48], as well as restraining the recombination of charge carriers at the interface to help decrease the hysteresis of CsPbIBr2 device, as proved by the PL and J-V measurements.
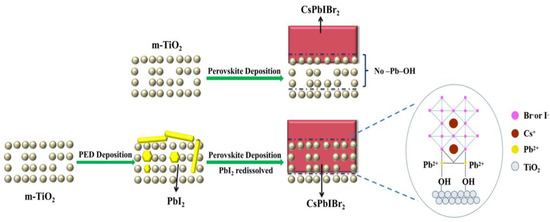
Figure 6.
Schematic diagram illustrating the deposition of CsPbIBr2 perovskite on m-TiO2. The upper row shows the normal procedure to synthesize CsPbIBr2 film on m-TiO2. The lower row is the CsPbIBr2 deposition on the PED/m-TiO2 substrate.
4. Conclusions
In this work, we have successfully utilized PED solution as the interfacial modifier to modify the c-TiO2/m-TiO2 substrates, and the influence of PED on the CsPbIBr2 PSCs, which were synthesized by the one-step method on the mesoporous structure, was studied. By using a PED solution, CsPbIBr2 efficiently fills the pores of the m-TiO2 to enhance the m-TiO2/CsPbIB2 interfacial contact area and to optimize the contact characteristics due to the combination of PbI2 with EA. The PCE of the modified solar device has been significantly promoted from about 3.0% to over 6.3%, with an enhancement of 110% compared with the pristine one. The –OH–Pb group in PED is the main reason for the improvement of optical properties and the optimization of comprehensive performance for the modified device.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-4991/10/5/962/s1, Figure S1: (a) XRD pattern and (b) EDS spectra of the m-TiO2/PED film; Figure S2: (αhν)2 vs. hν plots of the m-TiO2/CsPbIBr2 with and without PED modification.
Author Contributions
W.F. and H.Y. conceived the idea; X.M., K.C. and Q.L. performed research, analyzed data and wrote the paper; Y.C., G.S. and B.L. contributed to refining the ideas and finalizing the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (NO.51272086).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.B. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A. 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Park, Y.H.; Jeong, I.; Bae, S.; Son, H.J.; Lee, P.; Lee, J.; Lee, C.H.; Ko, M.J. Inorganic rubidium cation as an enhancer for photovoltaic performance and moisture stability of HC(NH2)2PbI3 perovskite solar cells. Adv. Funct. Mater. 2017, 27, 1605988. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Luchkin, S.Y.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the intrinsic thermal and photochemical stability of hybrid and inorganic lead halide perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.C.; Zhou, X.; Fu, R.; Li, Q.; Zhao, Y.; Liu, K.H.; Yu, D.P.; Zhao, Q. Light-independent ionic transport in inorganic perovskite and ultrastable Cs-based perovskite solar cells. J. Phys. Chem. Lett. 2017, 8, 4124–4128. [Google Scholar] [CrossRef]
- Sutter-Fella, C.M.; Ngo, Q.P.; Cefarin, N.; Gardner, K.L.; Tamura, N.; Stan, C.V.; Drisdell, W.S.; Javey, A.; Toma, F.M.; Sharp, I.D. Cation-dependent light-induced halide demixing in hybrid organic-inorganic perovskites. Nano Lett. 2018, 18, 3473–3480. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.-Y.; Lee, Y.G.; Kim, G.; Shin, H.-W.; Seok, S.I.; Lee, J.; Seo, J. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 2018, 3, 682–688. [Google Scholar] [CrossRef]
- Christians, J.A.; Schulz, P.; Tinkham, J.S.; Schloemer, T.H.; Harvey, S.P.; Tremolet de Villers, B.J.; Sellinger, A.; Berry, J.J.; Luther, J.M. Tailored interfaces of unencapsulated perovskite solar cells for >1,000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, S.; Li, W.; Liu, H.; Zhu, L.; Yang, S. Inorganic perovskite solar cells: A rapidly growing field. Solar RRL 2018, 2, 1700188. [Google Scholar] [CrossRef]
- Bian, H.; Bai, D.L.; Jin, Z.W.; Wang, K.; Liang, L.; Wang, H.R.; Zhang, J.R.; Wang, Q.; (Frank)Liu, S.Z. Graded bandgap CsPbI2+xBr1-x perovskite solar cells with a stabilized efficiency of 14.4%. Joule 2018, 2, 1500–1510. [Google Scholar] [CrossRef]
- Dastidar, S.; Li, S.; Smolin, S.Y.; Baxter, J.B.; Fafarman, A.T. Slow electron–hole recombination in lead iodide perovskites does not require a molecular dipole. ACS Energy Lett. 2017, 2, 2239–2244. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Jin, Z. All-inorganic halide perovskites for optoelectronics: Progress and prospects. Solar RRL 2017, 1, 1700086. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, X.; Deng, Y.; Zhao, J.; Chen, Z.; Huang, J. Stabilizing the α-Phase of CsPbI3 perovskite by sulfobetaine zwitterions in one-step spin-coating films. Joule 2017, 1, 371–382. [Google Scholar] [CrossRef]
- Eperon, G.E.; Paterno, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A. 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, F.; Liu, X.; Ji, Q.; Miao, X.; Qiu, T.; Zhang, S. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett. 2017, 2, 2219–2227. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, H.Y.; Chiang, K.M.; Tsai, W.L.; Huang, Y.C.; Tsao, C.S.; Lin, H.-W. All-vacuum-deposited stoichiometrically balanced inorganic cesium lead halide perovskite solar cells with stabilized efficiency exceeding 11%. Adv. Mater. 2017, 29, 1605290. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, Z.; Kubicki, D.J.; Tress, W.; Luo, J.; Prochowicz, D.; Akin, S.; Emsley, L.; Zhou, J.; Dietler, G.; et al. Hagfeldt, Europium-doped CsPbI2Br for stable and highly efficient inorganic perovskite solar cells. Joule 2019, 3, 205–214. [Google Scholar] [CrossRef]
- Shen, E.; Chen, J.; Tian, Y.; Luo, Y.; Shen, Y.; Sun, Q.; Jin, T.; Shi, G.; Li, Y.; Tang, J. Interfacial energy level tuning for efficient and thermostable CsPbI2Br perovskite solar cells. Adv. Sci. 2020, 7, 1901952. [Google Scholar] [CrossRef]
- Rao, H.; Ye, S.; Gu, F.; Zhao, Z.; Liu, Z.; Bian, Z.; Huang, C. Morphology controlling of all-inorganic perovskite at low temperature for efficient rigid and flexible solar cells. Adv. Energy Mater. 2018, 8, 1800758. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Kan, M.; Zhao, Y. Bifunctional stabilization of all-inorganic α-CsPbI3 perovskite for 17% efficiency photovoltaics. J. Am. Chem. Soc. 2018, 140, 12345–12348. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, S.; Liu, A.; Kamata, Y.; Teo, S.; Yang, S.; Xu, Z.; Hayase, S.; Ma, T. Niobium, Incorporation into CsPbI2Br for stable and efficient all-inorganic perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 19994–20003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Jin, Z.W.; Zhang, J.R.; Bai, D.L.; Bian, H.; Wang, K.; Sun, J.; Wang, Q.; Frank Liu, S.Z. All-ambient processed binary CsPbBr3-CsPb2Br5 perovskites with synergistic enhancement for high-efficiency Cs-Pb-Br-based solar cells. ACS Appl. Mater. Interfaces. 2018, 10, 7145–7154. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, C.; Wang, Y.; Xu, Z.; Lu, Z.; Ma, Y.; Zhu, H.; Hu, Y.; Xiao, C.; Yi, X.; et al. All-inorganic perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-purity inorganic perovskite films for solar cells with 9.72% efficiency. Angew. Chem. 2018, 57, 3787–3791. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.F.; Ma, Q.S.; Zheng, J.H.; Yun, J.S.; Green, M.A.; Huang, S.J.; Ho-Baillie, A.W.Y. CsPbIBr2 perovskite solar cell by spray-assisted deposition. ACS Energy Lett. 2016, 1, 573–577. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, S.; Wen, X.; Green, M.A.; Ho-Baillie, A.W.Y. Hole transport layer Free inorganic CsPbIBr2 perovskite solar cell by dual source thermal evaporation. Adv. Energy Mater. 2016, 6, 1502202. [Google Scholar] [CrossRef]
- Lin, J.; Lai, M.; Dou, L.; Kley, C.S.; Chen, H.; Peng, F.; Sun, J.; Lu, D.; Hawks, S.A.; Xie, C.; et al. Thermochromic halide perovskite solar cells. Nat. Mater. 2018, 17, 261–267. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, W.; Chen, D.; Zhang, Z.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Light processing enables efficient carbon-based, all-inorganic planar CsPbIBr2 solar cells with high photovoltages. ACS Appl. Mater. Interfaces 2019, 11, 2997–3005. [Google Scholar] [CrossRef]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Horantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T. Bandgap-tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, X.; Liu, J.; Wen, S.; Wu, Y.; Que, W. Inorganic CsPbIBr2-based perovskite solar cells: Fabrication technique modification and efficiency improvement. Sol. RRL 2019, 3, 1900135. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Chen, J.; Fan, J.; Mai, Y.; Schropp, R.E. Ultra-thin MoOx as cathode buffer layer for the improvement of all-inorganic CsPbIBr2 perovskite solar cells. Nano Energy 2017, 41, 75–83. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Z.H.; Qiu, L.B.; Hawash, Z.; Meng, L.Q.; Wu, Z.F.; Jiang, Y.; Ono, L.K.; Qi, Y.B. Enhancing optical, electronic, crystalline, and morphological properties of cesium lead halide by Mn substitution for high-stability all-inorganic perovskite solar cells with carbon electrodes. Adv. Energy Mater. 2018, 8, 1800504. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, P.Y.; Wang, C.X.; Wang, Y.R.; Hu, Y.; Zhu, G.Y.; Ma, L.B.; Liu, J.; Jin, Z. CsPb0.9Sn0.1IBr2 based all-Inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 2017, 139, 14009–14012. [Google Scholar] [CrossRef]
- Kim, S.; Chung, T.; Bae, S.; Lee, S.-W.; Lee, K.D.; Kim, H.; Lee, S.; Kang, Y.; Lee, H.S.; Kim, D. Improved performance and thermal stability of perovskite solar cells prepared via a modified sequential deposition process. Org. Electron. 2017, 41, 266–273. [Google Scholar] [CrossRef]
- Zhu, W.D.; Zhang, Q.N.; Chen, D.Z.; Zhang, Z.Y.; Lin, Z.H.; Chang, J.J.; Zhang, J.C.; Zhang, C.F.; Hao, Y. Intermolecular exchange boosts efficiency of air-stable, carbon-based all-Inorganic planar CsPbIBr2 perovskite solar cells to Over 9%. Adv. Energy Mater. 2018, 8, 1802080. [Google Scholar] [CrossRef]
- Lu, J.J.; Chen, S.C.; Zheng, Q.D. Defect Passivation of CsPbIBr2 perovskites for high-performance solar cells with large open-circuit voltage of 1.28 V. ACS Appl. Energy Mater. 2018, 1, 5872–5878. [Google Scholar] [CrossRef]
- Hwang, T.; Lee, S.; Kim, J.; Kim, J.; Kim, C.; Shin, B.; Park, B. Tailoring the mesoscopic TiO2 layer: Concomitant parameters for enabling high-performance perovskite solar cells. Nanoscale Res. Lett. 2017, 12, 57. [Google Scholar] [CrossRef]
- Divitini, G.; Cacovich, S.; Matteocci, F.; Cinà, L.; Carlo, A.D.; Ducati, C. In situ observation of heat-induced degradation of perovskite solar cells. Nat. Energy 2016, 1, 15012. [Google Scholar] [CrossRef]
- Yang, B.; Wang, M.; Hu, X.F.; Zhou, T.W.; Zang, Z.G. Highly efficient semitransparent CsPbIBr2 perovskite solar cells via low temperature processed In2S3 as electron-transport-layer. Nano Energy 2019, 57, 718–727. [Google Scholar] [CrossRef]
- Guo, Y.X.; Yin, X.T.; Liu, J.; Que, W.X. Highly efficient CsPbIBr2 perovskite solar cells with efficiency over 9.8% fabricated using a preheating-assisted spin-coating method. J. Mater. Chem. A 2019, 7, 19008–19016. [Google Scholar] [CrossRef]
- Ahn, N.; Jeon, I.; Yoon, J.; Kauppinen, E.; Matsuo, Y.; Maruyama, S.; Choi, M. Carbon-sandwiched perovskite solar cell. J. Mater. Chem. A 2018, 6, 1382–1389. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, H.; Xiao, S.; Xue, Q.; Zhang, T.; Zhu, Z.; Li, Q.; Hu, C.; Yang, Y.; Hu, Z.C.; et al. Effects of a molecular monolayer modification of NiO nanocrystal layer surfaces on perovskite crystallization and interface contact toward faster hole extraction and higher photovoltaic performance. Adv. Funct. Mater. 2016, 26, 2950–2958. [Google Scholar] [CrossRef]
- Peng, Q.M.; Guo, J.X.; Zhang, Q.R.; Xiang, J.Y.; Liu, B.Z.; Zhou, A.G.; Liu, R.P.; Tian, Y.J. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Shi, B.; Yao, X.; Hou, F.H.; Guo, S.; Li, Y.C.; Wei, C.C.; Ding, Y.; Li, Y.L.; Zhao, Y.; Zhang, X.D. Unraveling the passivation process of PbI2 to enhance the efficiency of planar perovskite solar cells. J. Phys. Chem. C. 2018, 122, 21269–21276. [Google Scholar] [CrossRef]
- Yu, H.; Lu, H.P.; Xie, F.Y.; Zhou, S.; Zhao, N. Native defect-induced hysteresis behavior in organolead iodide perovskite solar cells. Adv. Funct. Mater. 2016, 26, 1411–1419. [Google Scholar] [CrossRef]
- Ahn, N.; Kwak, K.; Jang, M.S.; Yoon, H.; Lee, B.Y.; Lee, J.K.; Pikhitsa, P.V.; Byun, J.; Choi, M. Trapped charge-driven degradation of perovskite solar cells. Nat. Commun. 2016, 7, 13422. [Google Scholar] [CrossRef]
- Lin, H.S.; Jeno, I.I.; Chen, Y.Q.; Yang, X.Y.; Nakagawa, T.; Maruyama, S.; Manzhos, S.; Matsuo, Y. Highly selective and scalable fullerene-cation-mediated synthesis accessing cyclo [60] fullerenes with five-membered carbon ring and their application to perovskite solar cells. Chem. Mater. 2019, 31, 8432–8439. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).