Charge-Transporting-Layer-Free, Vacuum-Free, All-Inorganic CsPbIBr2 Perovskite Solar Cells Via Dipoles-Adjusted Interface
Abstract
1. Introduction
2. Experimental Section
2.1. Materials Information
2.2. Device Fabrication
2.3. Device Characterization
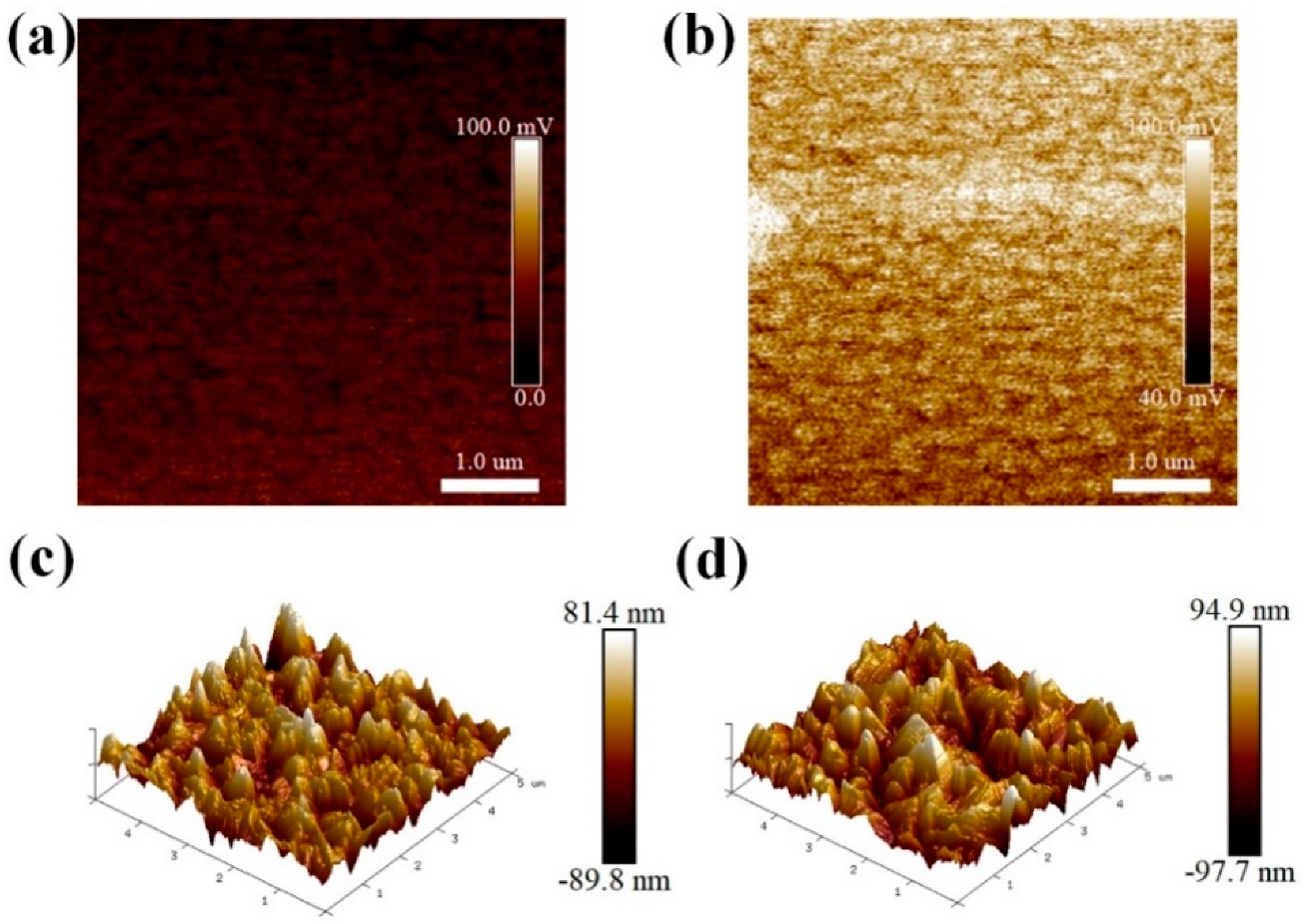
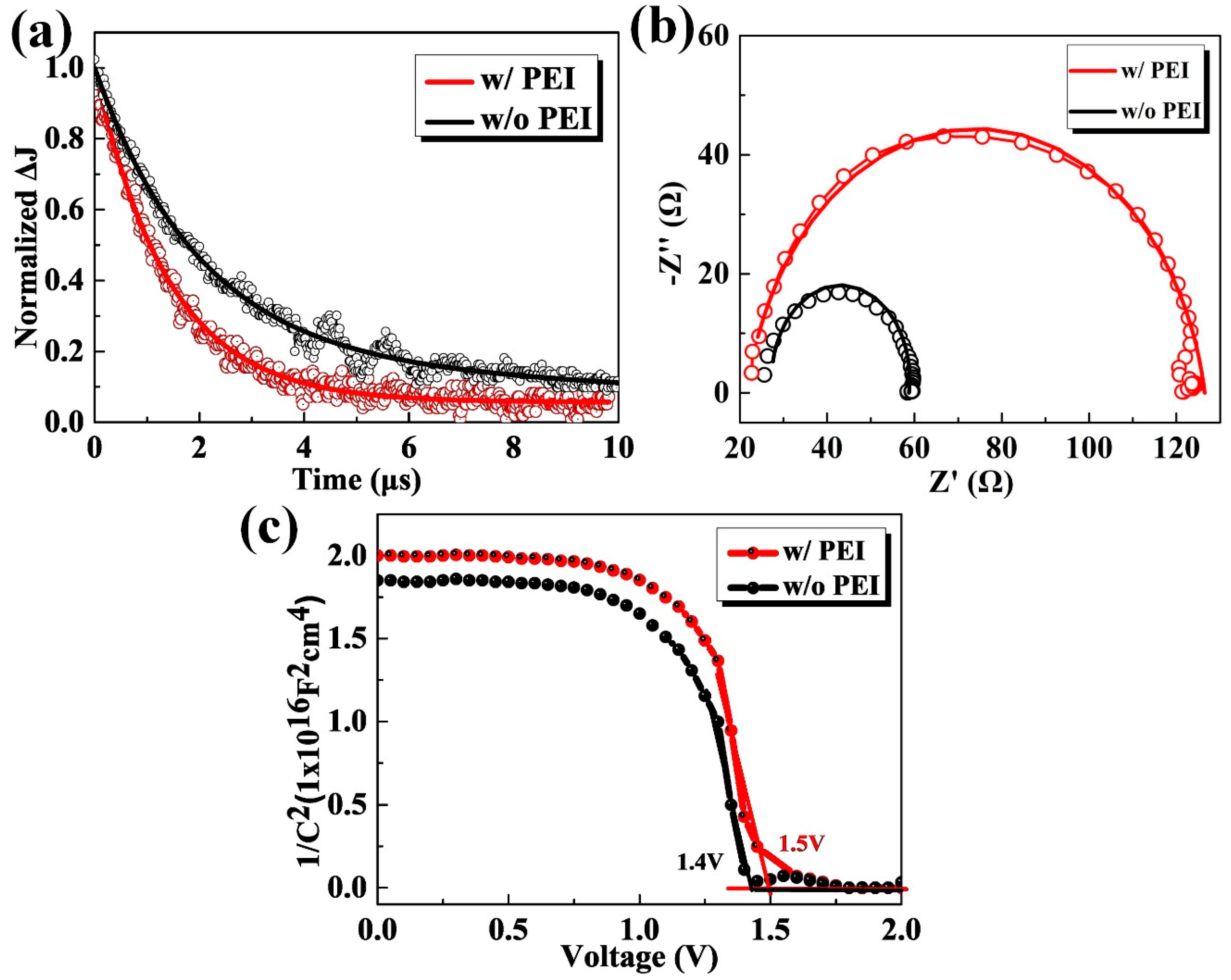
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ding, M.; Sun, L.; Chen, X.; Luo, T.; Ye, T.; Zhao, C.; Zhang, W.; Chang, H. Air-processed, large grain perovskite films with low trap density from perovskite crystal engineering for high-performance perovskite solar cells with improved ambient stability. J. Mater. Sci. 2019, 54, 12000–12011. [Google Scholar] [CrossRef]
- Pang, S.; Li, X.; Dong, H.; Chen, D.; Zhu, W.; Chang, J.; Lin, Z.; Xi, H.; Zhang, J.; Zhang, C.; et al. Efficient Bifacial Semitransparent Perovskite Solar Cells Using Ag/V2O5 as Transparent Anodes. ACS Appl. Mater. Interfaces 2018, 10, 12731–12739. [Google Scholar] [CrossRef]
- Liao, J.-F.; Wu, W.-Q.; Jiang, Y.; Kuang, D.-B.; Wang, L. Maze-Like Halide Perovskite Films for Efficient Electron Transport Layer-Free Perovskite Solar Cells. Sol. RRL 2019, 3. [Google Scholar] [CrossRef]
- Pang, S.; Chen, D.; Zhang, C.; Chang, J.; Lin, Z.; Yang, H.; Sun, X.; Mo, J.; Xi, H.; Han, G.; et al. Efficient bifacial semitransparent perovskite solar cells with silver thin film electrode. Sol. Energy Mater. Sol. Cells 2017, 170, 278–286. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic origins of high-performance in hybrid halide perovskite solar cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ge, Z. Simple, Robust, and Going More Efficient: Recent Advance on Electron Transport Layer-Free Perovskite Solar Cells. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Xiang, S.; Fu, Z.; Li, W.; Wei, Y.; Liu, J.; Liu, H.; Zhu, L.; Zhang, R.; Chen, H. Highly Air-Stable Carbon-Based α-CsPbI3 Perovskite Solar Cells with a Broadened Optical Spectrum. ACS Energy Lett. 2018, 3, 1824–1831. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. Simplified Perovskite Solar Cell with 4.1% Efficiency Employing Inorganic CsPbBr3 as Light Absorber. Small 2018, 14, e1704443. [Google Scholar] [CrossRef]
- Luo, D.Y.; Yang, W.Q.; Wang, Z.P.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.J.; et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Q.; Chen, D.; Zhang, Z.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Intermolecular Exchange Boosts Efficiency of Air-Stable, Carbon-Based All-Inorganic Planar CsPbIBr2 Perovskite Solar Cells to Over 9%. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Dar, M.I.; Ono, L.K.; Zhang, T.Y.; Kan, M.; Li, Y.W.; Zhang, L.J.; Wang, X.T.; Yang, Y.G.; Gao, X.Y.; et al. Thermodynamically stabilized β-CsPbI3-based perovskite solar cells with efficiencies > 18%. Science 2019, 365, 591. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Jiang, Q.; Wei, Z.; Deng, M.; Chen, D.; Zhu, W.; Zhang, J.; You, H. Toward High-Performance Electron/Hole-Transporting-Layer-Free, Self-Powered CsPbIBr2 Photodetectors via Interfacial Engineering. ACS Appl. Mater. Interfaces 2020, 12, 6607–6614. [Google Scholar] [CrossRef]
- Liu, P.; Yang, X.; Chen, Y.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Promoting the Efficiency and Stability of CsPbIBr2-Based All-Inorganic Perovskite Solar Cells through a Functional Cu2+ Doping Strategy. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.; Chai, W.; Zhang, Q.; Chen, D.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Band Alignment Engineering Towards High Efficiency Carbon-Based Inorganic Planar CsPbIBr2 Perovskite Solar Cells. ChemSusChem 2019, 12, 2318–2325. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of a-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Gao, L.; Cao, J.; Han, Q.; Yu, F.; Kamata, Y.; Zhang, C.; Fan, M.; Wei, G.; et al. Excellent Moisture Stability and Efficiency of Inverted All-Inorganic CsPbIBr2 Perovskite Solar Cells through Molecule Interface Engineering. ACS Appl. Mater. Interfaces 2020, 12, 13931–13940. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, C.X.; Wang, Y.R.; Xu, Z.R.; Lu, Z.P.; Ma, Y.; Zhu, H.F.; Hu, Y.; Xiao, C.C.; Yi, X.; et al. All-Inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Buonassisi, T.; Correa-Baena, J.-P. State-of-the-Art Electron-Selective Contacts in Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14. [Google Scholar] [CrossRef]
- Etgar, L.; Gao, P.; Xue, Z.; Peng, Q.; Chandiran, A.K.; Liu, B.; Nazeeruddin, M.K.; Gratzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 2012, 134, 17396–17399. [Google Scholar] [CrossRef]
- Batmunkh, M.; Shearer, C.J.; Biggs, M.J.; Shapter, J.G. Nanocarbons for mesoscopic perovskite solar cells. J. Mater. Chem. A 2015, 3, 9020–9031. [Google Scholar] [CrossRef]
- Ferguson, V.; Silva, S.R.P.; Zhang, W. Carbon Materials in Perovskite Solar Cells: Prospects and Future Challenges. Energy Environ. Mater. 2019, 2, 107–118. [Google Scholar] [CrossRef]
- Fagiolari, L.; Bella, F. Carbon-based materials for stable, cheaper and large-scale processable perovskite solar cells. Energy Environ. Sci. 2019, 12, 3437–3472. [Google Scholar] [CrossRef]
- Li, D.; Kong, W.; Zhang, H.; Wang, D.; Li, W.; Liu, C.; Chen, H.; Song, W.; Gao, F.; Amini, A.; et al. Bifunctional Ultrathin PCBM Enables Passivated Trap States and Cascaded Energy Level toward Efficient Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 20103–20109. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, M.; Zhou, X.; Wu, J.; Zhang, L.; Kong, W.; Li, X.; Zhao, X.; Dai, S.; Xu, B.; et al. Efficiency and stability enhancement of perovskite solar cells by introducing CsPbI3 quantum dots as an interface engineering layer. NPG Asia Mater. 2018, 10, 552–561. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Wang, K.; Wu, C.; Yang, R.; Hou, Y.; Jiang, Y.; Liu, S.; Priya, S. Stable Efficiency Exceeding 20.6% for Inverted Perovskite Solar Cells through Polymer-Optimized PCBM Electron-Transport Layers. Nano Lett. 2019, 19, 3313–3320. [Google Scholar] [CrossRef]
- O’Boyle, M.P.; Hwang, T.T.; Wickramasinghe, H.K. Atomic force microscopy of work functions on the nanometer scale. Appl. Phys. Lett. 1999, 74, 2641–2642. [Google Scholar] [CrossRef]
- Li, W.; Rothmann, M.U.; Liu, A.; Wang, Z.; Zhang, Y.; Pascoe, A.R.; Lu, J.; Jiang, L.; Chen, Y.; Huang, F.; et al. Phase Segregation Enhanced Ion Movement in Efficient Inorganic CsPbIBr2 Solar Cells. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Slotcavage, D.J.; Karunadasa, H.I.; McGehee, M.D. Light-Induced Phase Segregation in Halide-Perovskite Absorbers. ACS Energy Lett. 2016, 1, 1199–1205. [Google Scholar] [CrossRef]
- Subhani, W.S.; Wang, K.; Du, M.; Wang, X.; Liu, S. Interface-Modification-Induced Gradient Energy Band for Highly Efficient CsPbIBr2 Perovskite Solar Cells. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Subhani, W.S.; Wang, K.; Du, M.; Liu, S.F. Goldschmidt-rule-deviated perovskite CsPbIBr2 by barium substitution for efficient solar cells. Nano Energy 2019, 61, 165–172. [Google Scholar] [CrossRef]
- Yang, B.; Wang, M.; Hu, X.; Zhou, T.; Zang, Z. Highly efficient semitransparent CsPbIBr2 perovskite solar cells via low-temperature processed In2S3 as electron-transport-layer. Nano Energy 2019, 57, 718–727. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.; Ma, Q.; Zheng, J.; Yun, J.S.; Green, M.A.; Huang, S.; Ho-Baillie, A.W.Y. CsPblBr2 Perovskite Solar Cell by Spray-Assisted Deposition. ACS Energy Lett. 2016, 1, 573–577. [Google Scholar] [CrossRef]
- Adinolfi, V.; Peng, W.; Walters, G.; Bakr, O.M.; Sargent, E.H. The Electrical and Optical Properties of Organometal Halide Perovskites Relevant to Optoelectronic Performance. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Cao, F.; Meng, L.; Wang, M.; Tian, W.; Li, L. Gradient Energy Band Driven High-Performance Self-Powered Perovskite/CdS Photodetector. Adv. Mater. 2019, 31, e1806725. [Google Scholar] [CrossRef]

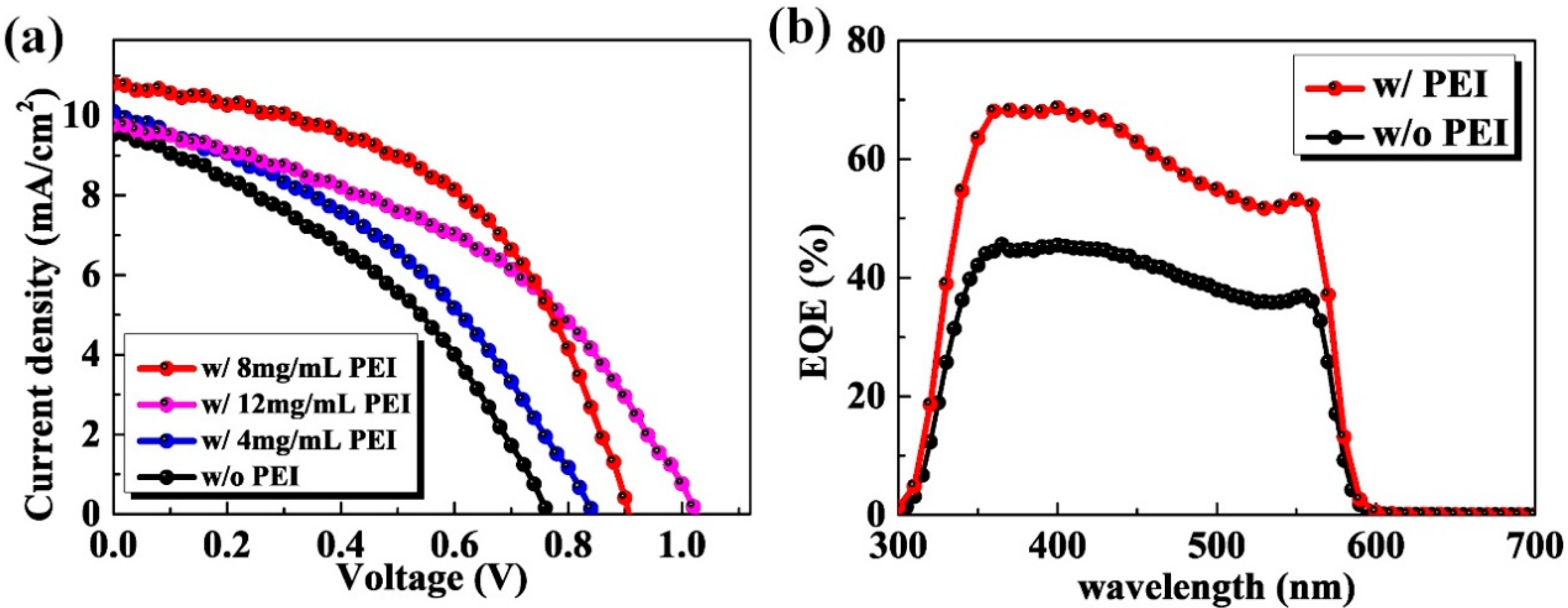
| Samples | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (Average PCE) (%) |
|---|---|---|---|---|
| Without PEI modification | 9.5 | 0.76 | 39 | 2.82 (2.42 ± 0.32) |
| With 4 mg/mL PEI modification | 10.1 | 0.84 | 39 | 3.31 (2.91 ± 0.30) |
| With 8 mg/mL PEI modification | 10.8 | 0.90 | 50 | 4.86 (4.51 ± 0.33) |
| With 12 mg/mL PEI modification | 9.8 | 1.02 | 44 | 4.39 (3.82 ± 0.34) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, Z.; Jiang, Q.; Wei, Z.; Zhang, Y.; You, H.; Chen, D.; Zhu, W.; He, F.; Zhang, C. Charge-Transporting-Layer-Free, Vacuum-Free, All-Inorganic CsPbIBr2 Perovskite Solar Cells Via Dipoles-Adjusted Interface. Nanomaterials 2020, 10, 1324. https://doi.org/10.3390/nano10071324
Zhang W, Zhang Z, Jiang Q, Wei Z, Zhang Y, You H, Chen D, Zhu W, He F, Zhang C. Charge-Transporting-Layer-Free, Vacuum-Free, All-Inorganic CsPbIBr2 Perovskite Solar Cells Via Dipoles-Adjusted Interface. Nanomaterials. 2020; 10(7):1324. https://doi.org/10.3390/nano10071324
Chicago/Turabian StyleZhang, Wentao, Zeyulin Zhang, Qubo Jiang, Ziming Wei, Yuting Zhang, Hailong You, Dazheng Chen, Weidong Zhu, Fengqin He, and Chunfu Zhang. 2020. "Charge-Transporting-Layer-Free, Vacuum-Free, All-Inorganic CsPbIBr2 Perovskite Solar Cells Via Dipoles-Adjusted Interface" Nanomaterials 10, no. 7: 1324. https://doi.org/10.3390/nano10071324
APA StyleZhang, W., Zhang, Z., Jiang, Q., Wei, Z., Zhang, Y., You, H., Chen, D., Zhu, W., He, F., & Zhang, C. (2020). Charge-Transporting-Layer-Free, Vacuum-Free, All-Inorganic CsPbIBr2 Perovskite Solar Cells Via Dipoles-Adjusted Interface. Nanomaterials, 10(7), 1324. https://doi.org/10.3390/nano10071324







