Electrochemical DNA Sensor Based on the Copolymer of Proflavine and Azure B for Doxorubicin Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Electropolymerization of Azure B and Proflavine and DNA Sensor Preparation
2.4. Doxorubicin Determination
3. Results
3.1. Copolymerization of Azure B and Electrochemical Properties of the Polymerization Product
3.2. The Comparison of the Redox Properties of Poly(Azure B), Poly(proflavine) and Their Copolymer
3.3. DNA Deposition on the Copolymer of Azure B and Proflavine
3.3.1. SEM Monitoring of the Surface Layer Assembling
3.3.2. EIS Measurements
3.4. Determination of Doxorubicin
3.4.1. Doxorubicin Oxidation on Glassy Carbon Prior to and After the Electropolymerization Stage
3.4.2. Determination of Doxorubicin with DNA Sensor Based on Copolymer of Azure B and Proflavine
3.4.3. Measurement Precision and DNA Sensor Lifetime
3.4.4. Real Sample Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palma-Cando, A.; Rendón-Enríquez, I.; Tausch, M.; Scherf, U. Thin functional polymer films by electropolymerization. Nanomaterials 2019, 9, 1125. [Google Scholar] [CrossRef]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Hianik, T. Electrochemical DNA sensors and aptasensors based on electropolymerized materials and polyelectrolyte complexes. TrAC Trends Anal. Chem. 2016, 79, 168–178. [Google Scholar] [CrossRef]
- Berkes, B.B.; Bandarenka, A.S.; Inzelt, G. Electropolymerization: Further insight into the formation of conducting polyindole thin films. J. Phys. Chem. C 2015, 119, 1996–2003. [Google Scholar] [CrossRef]
- Inzelt, G. Rise and rise of conducting polymers. J. Solid State Electrochem. 2011, 15, 1711–1718. [Google Scholar] [CrossRef]
- Omar, F.S.; Duraisamy, N.; Ramesh, K.R.S. Conducting polymer and its composite materials based electrochemical sensor for nicotinamide adenine dinucleotide (NADH). Biosens. Bioelectron. 2016, 79, 763–775. [Google Scholar] [CrossRef]
- Runsewe, D.; Betancourt, T.; Irvin, J.A. Biomedical application of electroactive polymers in electrochemical sensors: A review. Materials 2019, 12, 2629. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef]
- Moro, G.; De Vael, K.; Moretto, L.M. Challenges in the electrochemical (bio)sensing of nonelectroactive food and environmental contaminants. Curr. Opin. Electrochem. 2019, 16, 57–65. [Google Scholar] [CrossRef]
- Prajapati, D.G.; Kandasubramanian, B. Progress in the development of intrinsically conducting polymer composites as biosensors. Macromol. Chem. Phys. 2019, 220, 1800561. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, S.K. Advances in polyaniline-based nanocomposites. J. Mater. Sci. 2020, 55, 1331–1365. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: A review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jaina, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Hui, Y.; Bian, C.; Xia, S.; Tong, J.; Wang, J. Synthesis and electrochemical sensing application of poly(3,4-ethylenedioxythiophene)-based materials: A review. Anal. Chim. Acta 2018, 1022, 1–19. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Schmidt, H.-L. Electropolymerized azines: A new group of electroactive polymers. Electroanalysis 1999, 11, 149–155. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, E.M.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef]
- Topçu, E.; Alanyalıoğlu, M. Electrochemical formation of poly(thionine) thin films: The effect of amine group on the polymeric film formation of phenothiazine dyes. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Satpati, A.K.; D’Souza, O.J.; Dhason, A. Facile preparation of poly(methylene blue) modified carbon paste electrode for the detection and quantification of catechin. Mater. Sci. Eng. C 2017, 73, 552–561. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Ahmadi, E.; Haseli, M. A novel voltammetric sensor for nevirapine, based on modified graphite electrode by MWCNs/poly(methylene blue)/gold nanoparticle. Anal. Biochem. 2017, 552–561. [Google Scholar] [CrossRef]
- Devi, C.L.; Narayanan, S.S. Poly(amido amine) dendrimer/silver nanoparticles/multi-walled carbon nanotubes/poly (neutral red)-modified electrode for electrochemical determination of paracetamol. Ionics 2019, 25, 2323–2335. [Google Scholar] [CrossRef]
- Baluchová, S.; Barek, J.; Tomé, L.I.N.; Brett, C.M.A.; Schwarzová-Pecková, K. Vanillylmandelic and homovanillic acid: Electroanalysis at non-modified and polymer-modified carbon-based electrodes. J. Electroanal. Chem. 2018, 821, 22–32. [Google Scholar] [CrossRef]
- Kumar, T.S.S.; Swamy, B.E.K. Modification of carbon paste electrode by electrochemical polymerization of neutral red and its catalytic capability towards the simultaneous determination of catechol and hydroquinone: A voltammetric study. J. Electroanal. Chem. 2017, 804, 78–86. [Google Scholar] [CrossRef]
- Liu, L.; Zhai, J.; Zhu, C.; Han, L.; Ren, W.; Dong, S. One-step synthesis of functional pNR/rGO composite as a building block for enhanced ascorbic acid biosensing. Anal. Chim. Acta 2017, 981, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ihmels, H.; Otto, D. Intercalation of organic dye molecules into double-stranded DNA—General principles and recent developments. In Supermolecular Dye Chemistry. Topics in Current Chemistry; Würthner, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 258, pp. 161–204. [Google Scholar] [CrossRef]
- Han, X.; Yu, Z.; Li, F.; Shi, W.; Fu, C.; Yan, H.; Zhan, G. Two kanamycin electrochemical aptamer-based sensors using different signal transduction mechanisms: A comparison of electrochemical behavior and sensing performance. Bioelectrochemistry 2019, 129, 270–277. [Google Scholar] [CrossRef]
- Attar, A.; Ghica, M.E.; Amine, A.; Brett, C.M.A. Poly(neutral red) based hydrogen peroxide biosensor for chromium determination by inhibition measurements. J. Hazard. Mater. 2014, 279, 348–355. [Google Scholar] [CrossRef]
- Kuzin, Y.; Kappo, D.; Porfireva, A.; Shurpik, D.; Stoikov, I.; Evtugyn, G.; Hianik, T. Electrochemical DNA sensor based on carbon black—Poly(Neutral red) composite for detection of oxidative DNA damage. Sensors 2018, 18, 3489. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, R.; Chai, Y.; Xiang, Y.; Hong, C.; Ran, X. An amperometric hydrogen peroxide biosensor based on the immobilization of HRP on multi-walled carbon nanotubes/electro-copolymerized nano-Pt-poly(neutral red) composite membrane. Biochem. Eng. J. 2010, 51, 102–109. [Google Scholar] [CrossRef]
- Porfireva, A.; Vorobev, V.; Babkina, S.; Evtugyn, G. Electrochemical sensor based on poly(Azure B)-DNA composite for doxorubicin determination. Sensors 2019, 19, 2085. [Google Scholar] [CrossRef]
- Shen-Tu, C.; Liu, Z.; Kong, Y.; Yao, C.; Tao, Y. Electrochemical synthesis and properties of poly(azure B). J. Electrochem. Soc. 2013, 160, G83–G87. [Google Scholar] [CrossRef]
- Porfireva, A.V.; Goida, A.I.; Rogov, A.M.; Evtugyn, G.A. Impedimetric DNA sensor based on poly(proflavine) for determination of anthracycline drugs. Electroanalysis 2020, 32, 827–834. [Google Scholar] [CrossRef]
- Chen, C.; Gan, Z.; Xu, C.; Lu, L.; Liu, Y.; Gao, Y. Electrosynthesis of poly(aniline-co-azure B) for aqueous rechargeable zinc-conducting polymer batteries. Electrochim. Acta 2017, 252, 226–234. [Google Scholar] [CrossRef]
- Grützke, S.; Abdali, S.; Schuhmann, W.; Gebala, M. Detection of DNA hybridization using electrochemical impedance spectroscopy and surface enhanced Raman scattering. Electrochem. Commun. 2012, 19, 59–62. [Google Scholar] [CrossRef]
- Robinson, S.M.; Shen, Z.; Askim, J.R.; Montgomery, C.B.; Sintim, H.O.; Semancik, S. Ligand-based stability changes in duplex DNA measured with a microscale electrochemical platform. Biosensors 2019, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Bayındır, O.; Alanyalıoğlu, M. Formation mechanism of polymeric thin films of Azure B on gold electrodes. ChemistrySelect 2018, 3, 2167–2173. [Google Scholar] [CrossRef]
- Nishida, Y.; Domura, R.; Sakai, R.; Okamoto, M.; Arakawa, S.; Ishiki, R.; Salick, M.R.; Turng, L.-S. Fabrication of PLLA/HA composite scaffolds modified by DNA. Polymer 2015, 56, 73–81. [Google Scholar] [CrossRef]
- Hongpaisan, J.; Roomans, G.M. Retaining ionic concentrations during in vitro storage of tissue for microanalytical studies. J. Microsc. 1999, 193, 257–267. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications; J. Willey & Sons: New York, NY, USA, 1980. [Google Scholar]
- Evtugyn, G.A.; Kostyleva, V.B.; Porfireva, A.V.; Savelieva, V.A.; Evtugyn, V.G.; Sitdikov, R.R.; Stoikov, I.I.; Antipin, I.S.; Hianik, T. Label-free aptasensor for thrombin determination based on the nanostructured phenazine mediator. Talanta 2012, 102, 156–163. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Yang, Y.; Wu, F.; Cao, J.; Bai, K. A polyaniline-reduced graphene oxide nanocomposite as a redox nanoprobe in a voltammetric DNA biosensor for Mycobacterium tuberculosis. Microchim. Acta 2017, 184, 1801–1808. [Google Scholar] [CrossRef]
- Kuzin, Y.; Ivanov, A.; Evtugyn, G.; Hianik, T. Voltammetric detection of oxidative DNA damage based on interactions between polymeric dyes and DNA. Electroanalysis 2016, 28, 2956–2964. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Zhou, M.; Guo, L. A nanocomposite prepared from metal-free mesoporous carbon nanospheres and graphene oxide for voltammetric determination of doxorubicin. Microchim. Acta 2019, 186, 639. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Havran, L.; Fojta, M. Ex situ voltammetry and chronopotentiometry of doxorubicin at a pyrolytic graphite electrode: Redox and catalytic properties and analytical applications. Electroanalysis 2009, 21, 21399–22144. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.-M. Highly sensitive detection of daunorubicin based on carbon nanotubes–drug supramolecular interaction. Electrochem. Commun. 2009, 11, 126–129. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.R.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Ilkhani, H.; Hughes, T.; Li, J.; Zhong, C.J.; Hepel, H. Nanostructured SERS-electrochemical biosensors for testing of anticancer drug interactions with DNA. Biosens. Bioelectron. 2016, 80, 257–264. [Google Scholar] [CrossRef]
- Kulikova, T.; Porfireva, A.; Evtugyn, G.; Hianik, T. Electrochemical DNA sensors with layered polyaniline-DNA coating for detection of specific DNA interactions. Sensors 2019, 19, 469. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Xu, H.; Luo, C.; Ding, H. Application of a disposable doxorubicin sensor for direct determination of clinical drug concentration in patient blood. Int. J. Electrochem. Sci. 2016, 11, 6266–6278. [Google Scholar] [CrossRef]
- Evtugyn, A.; Porfireva, A.; Stepanova, V.; Budnikov, H. Electrochemical biosensors based on native DNA and nanosized mediator for the detection of anthracycline preparations. Electroanalysis 2015, 27, 629–637. [Google Scholar] [CrossRef]
- Hortobágyi, G.N. Anthracyclines in the treatment of cancer. An overview. Drugs 1997, 54, 1–7. [Google Scholar] [CrossRef]
- Li, M.; Russo, M.; Pirozzi, F.; Tocchetti, C.G.; Ghigo, A. Autophagy and cancer therapy cardiotoxicity: From molecular mechanisms to therapeutic opportunities. Biochim. Biophys. Acta 2020, 1867, 118493. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Brett, A.M.; Piedade, J.A.P.; Serrano, S.H.P. Electrochemical oxidation of 8-oxoguanine. Electroanalysis 2000, 12, 969–973. [Google Scholar] [CrossRef]


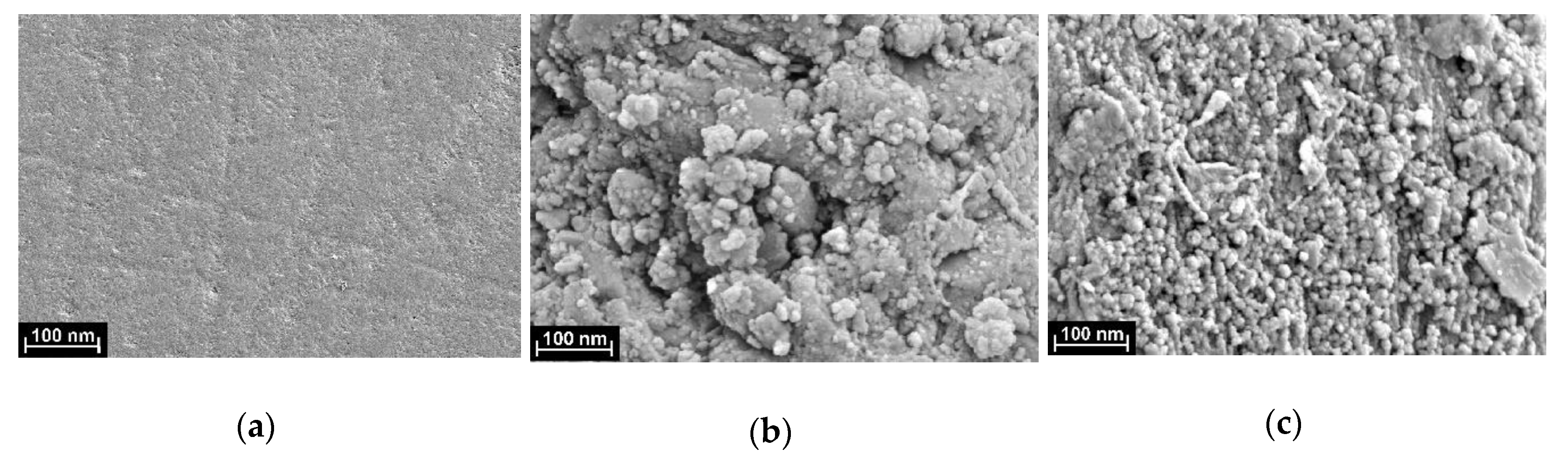
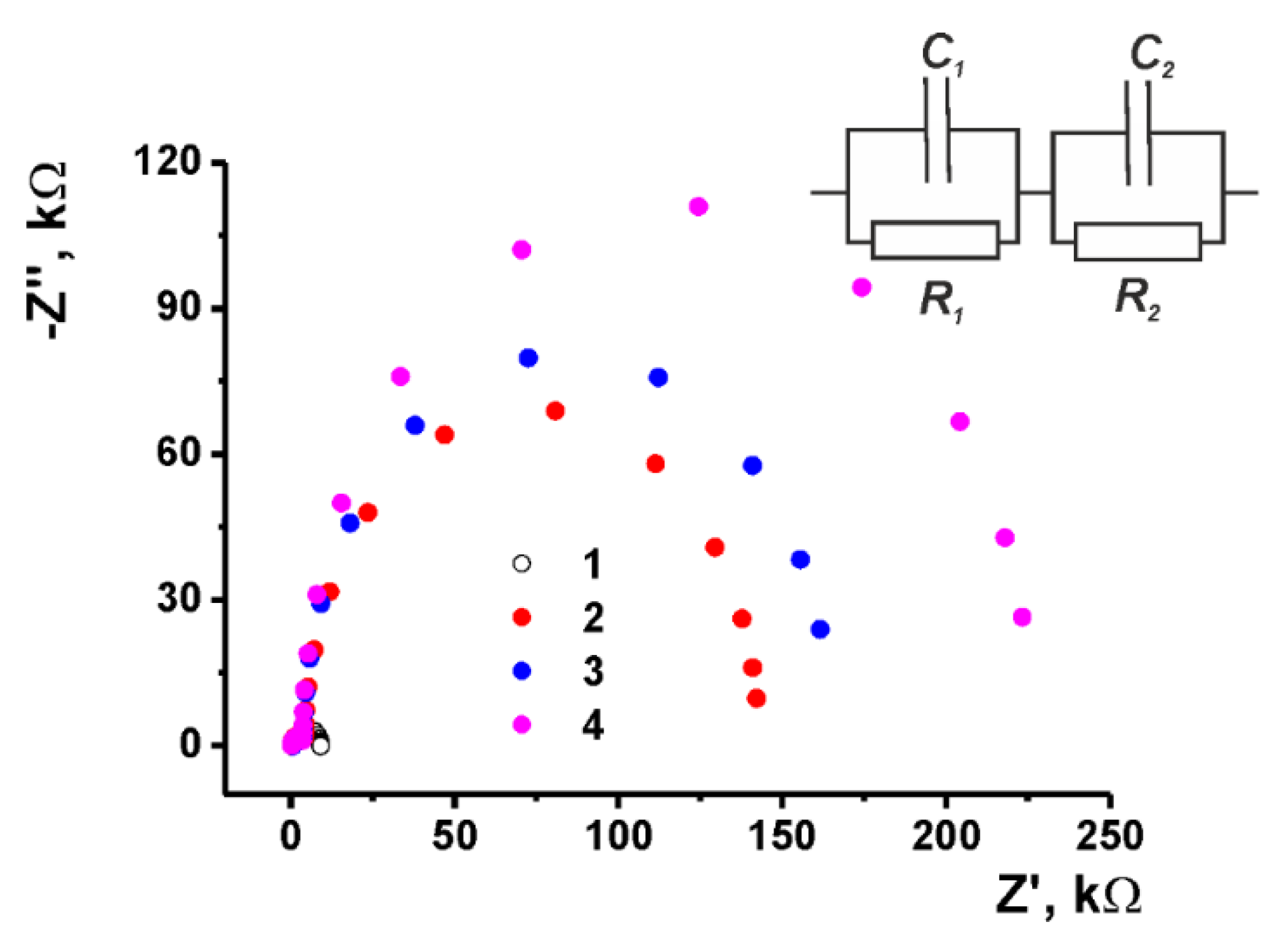
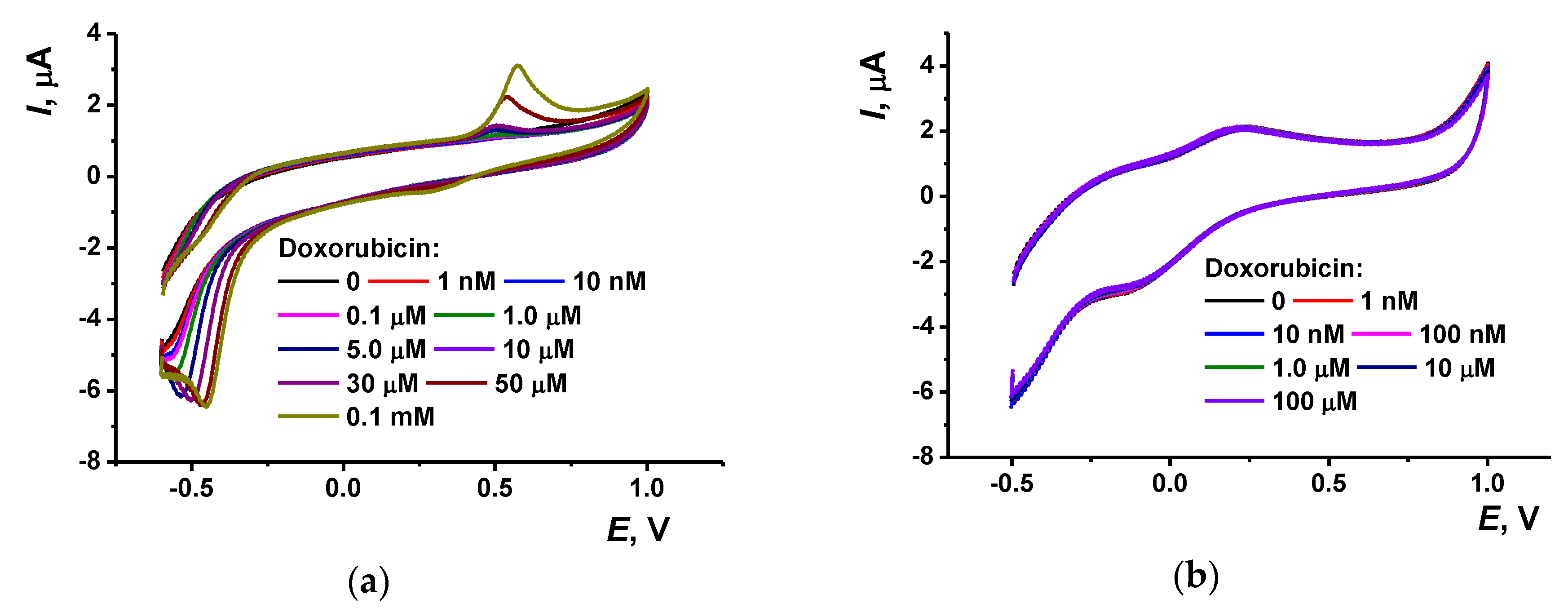
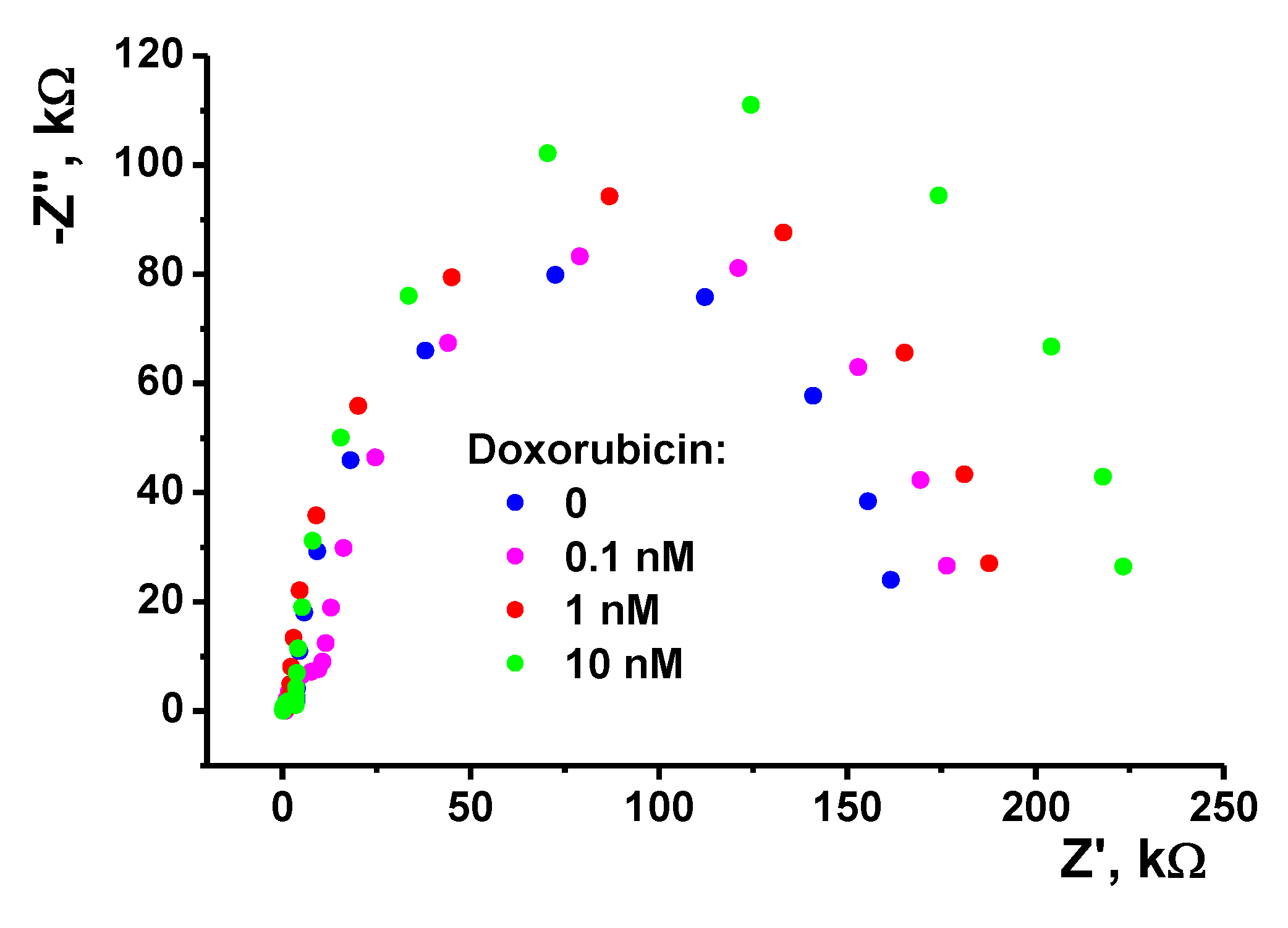
| Property | Poly(Azure B) [29] | Poly(proflavine) | Copolymer [31] |
|---|---|---|---|
| Stability of redox parameters | The currents regularly decrease in consecutive measurements | Stable | Stable to 2–3 cycle |
| logIp-logν slope | Oxidation: 1.1 (polymer), 0.83 (monomer) | Oxidation: 0.81 | Oxidation: 0.67 |
| Reduction: 0.96 (monomer) | Reduction: 0.64 | Reduction: 0.64 | |
| Transmission coefficient | 0.56 (polymer) | 0.57 | 0.83 |
| Surface Layer/Electrode | Concentration Range | LOD, nM | Ref |
|---|---|---|---|
| Electrochemical sensors | |||
| Carbon nanotubes | 20–500 nM | - | [46] |
| Ionic liquid/ZnO in carbon paste | 0.07–5000 μM | 9.0 | [47] |
| Mesoporous carbon nanospheres/reduced graphene oxide | 10 nM–10 μM | 1.5 | [44] |
| Basal plane pyrographite | 0.01–1 μM | 10 | [45] |
| Fe2Ni@Au/reduced graphene oxide | 5.5–9.2 μM | 1460 | [48] |
| Electrochemical DNA sensors | |||
| Poly(Azure B)/DNA | 0.1 μM–0.1 nM | 0.07 | [29] |
| Poly(proflavine)/DNA | 1 nM–0.1 μM | 0.3 | [31] |
| Polyaniline/DNA | 1.0 pM–1 mM | 0.0006 | [49] |
| Carbon nanotubes/polylysine/DNA | 2.5 nM–0.25 μM | 1.0 | [50] |
| Poly(Neutral red)/DNA | 0.01–100 μM | 0.1 | [51] |
| Poly(Azure B–proflavine) | 0.03–10 nM | 0.01 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porfireva, A.; Evtugyn, G. Electrochemical DNA Sensor Based on the Copolymer of Proflavine and Azure B for Doxorubicin Determination. Nanomaterials 2020, 10, 924. https://doi.org/10.3390/nano10050924
Porfireva A, Evtugyn G. Electrochemical DNA Sensor Based on the Copolymer of Proflavine and Azure B for Doxorubicin Determination. Nanomaterials. 2020; 10(5):924. https://doi.org/10.3390/nano10050924
Chicago/Turabian StylePorfireva, Anna, and Gennady Evtugyn. 2020. "Electrochemical DNA Sensor Based on the Copolymer of Proflavine and Azure B for Doxorubicin Determination" Nanomaterials 10, no. 5: 924. https://doi.org/10.3390/nano10050924
APA StylePorfireva, A., & Evtugyn, G. (2020). Electrochemical DNA Sensor Based on the Copolymer of Proflavine and Azure B for Doxorubicin Determination. Nanomaterials, 10(5), 924. https://doi.org/10.3390/nano10050924





