Grey Rutile TiO2 with Long-Term Photocatalytic Activity Synthesized Via Two-Step Calcination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2-GR and TiO2-WR
2.3. Photocurrent Measurements
2.4. Photoactivity Measurements
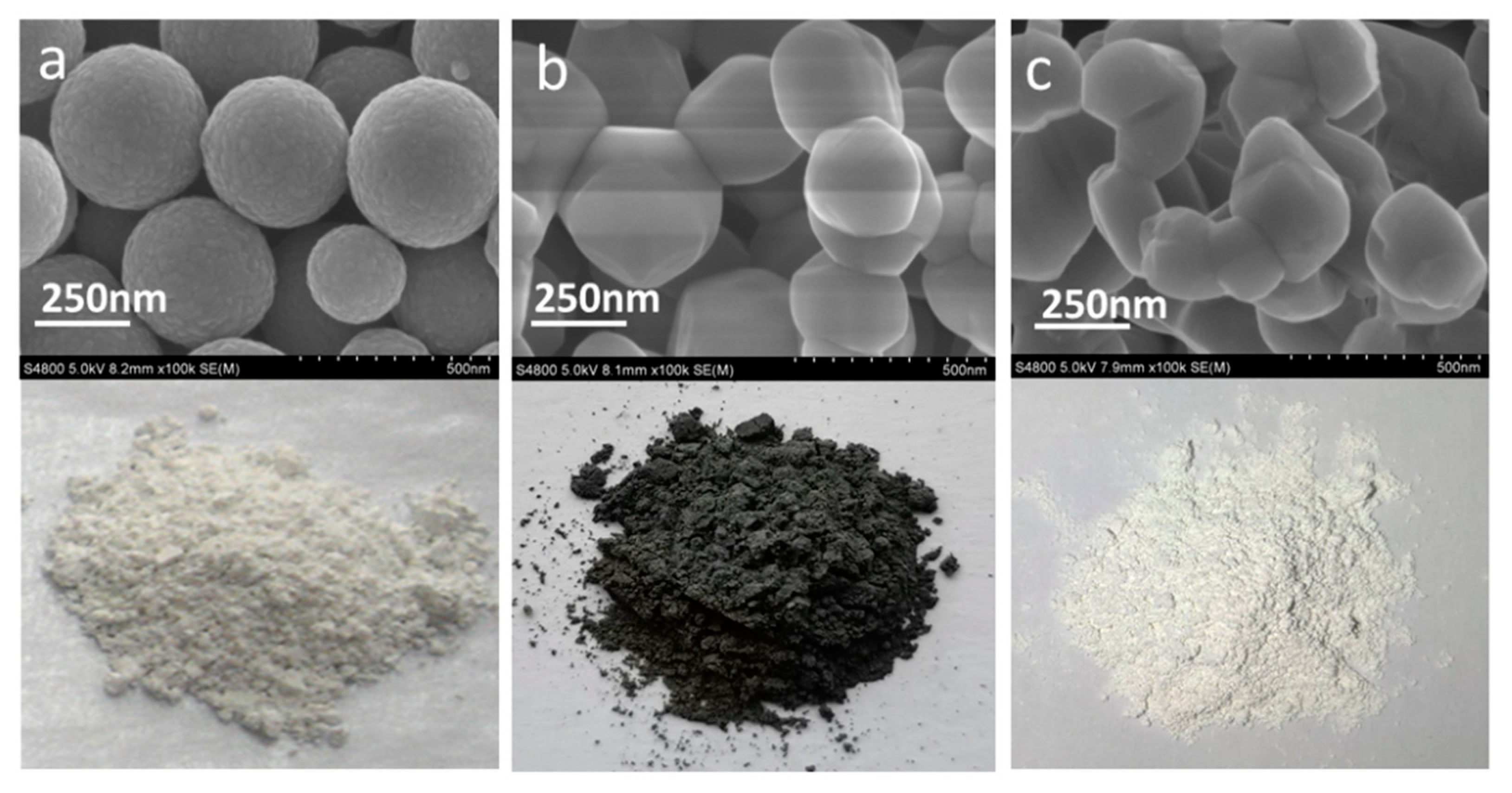
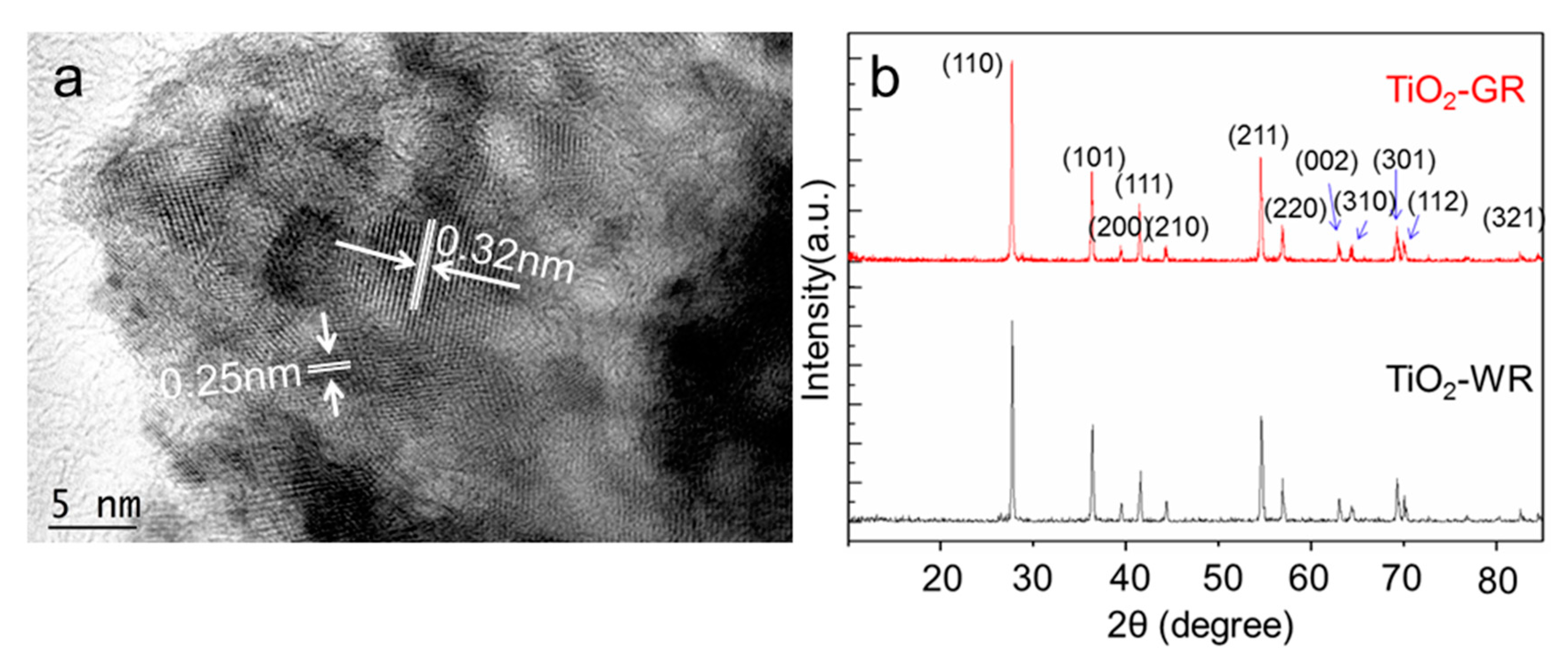
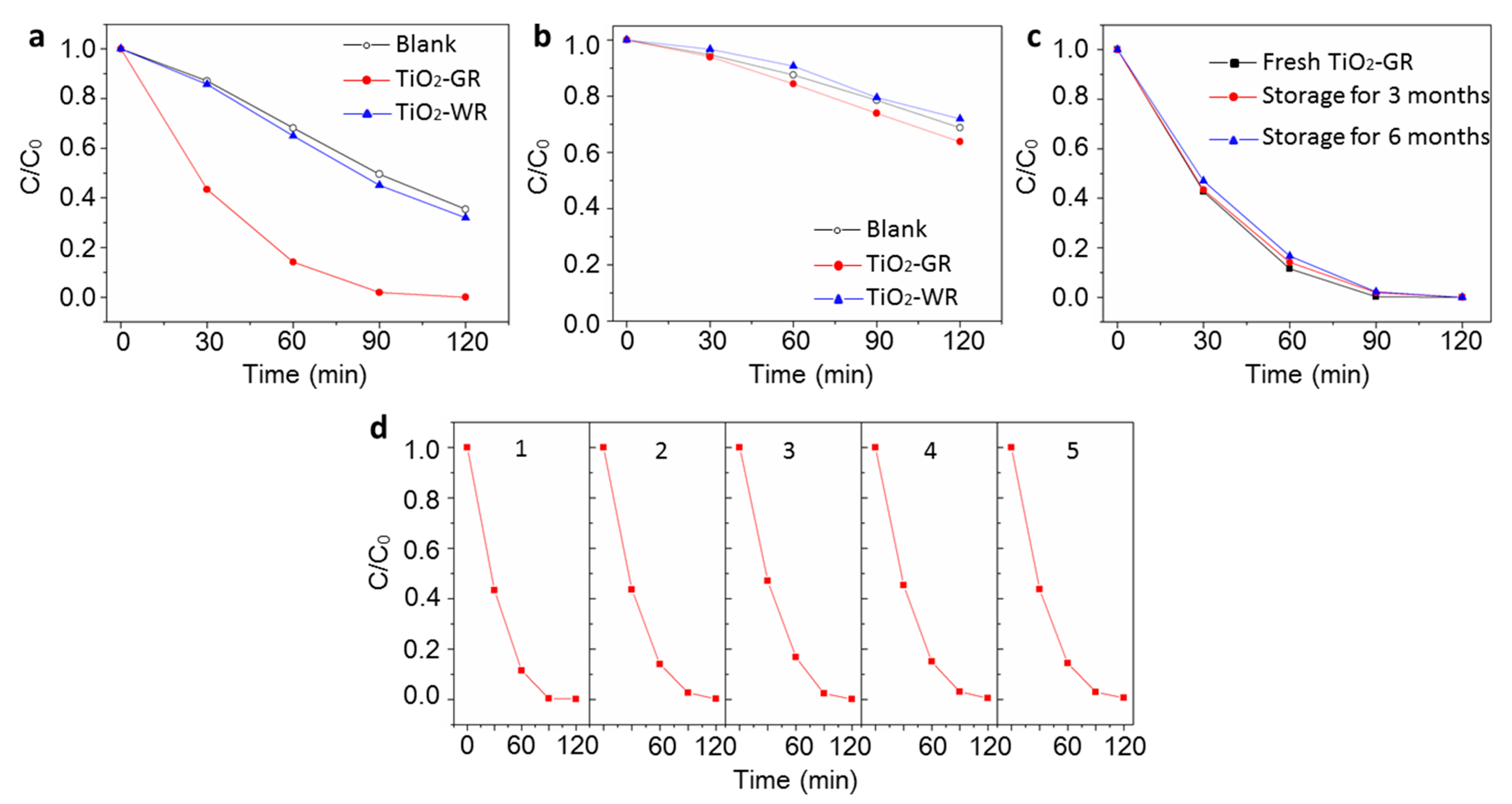
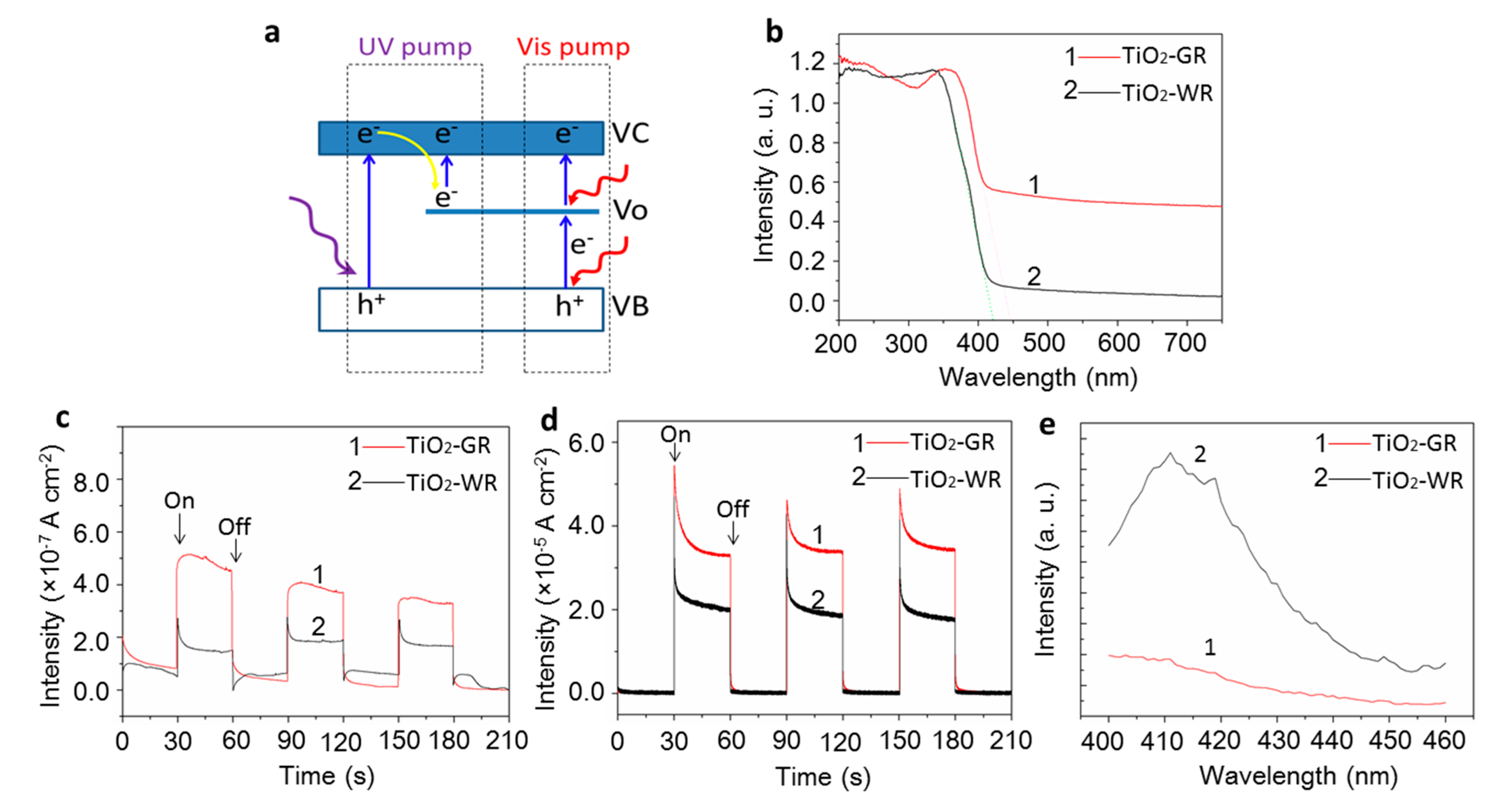
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Fu, X.; Jing, L.; Luan, P.; Feng, Y.; Fu, H. Long-Lived, Visible-Light-Excited Charge Carriers of TiO2/BiVO4 Nanocomposites and their Unexpected Photoactivity for Water Splitting. Adv. Energy Mater. 2014, 4, 1300995. [Google Scholar] [CrossRef]
- Cui, G.; Wang, W.; Ma, M.; Zhang, M.; Xia, X.; Han, F.; Shi, X.; Zhao, Y.; Dong, Y.; Tang, B. Rational design of carbon and TiO2 assembly materials: Covered or strewn, which is better for photocatalysis? Chem. Commun. 2013, 49, 6415. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Xiao, X.; Jung, H.S.; Giannini, V.; Maier, S.A.; Kim, D.-H.; Lee, Y.-I.; Park, S.-G. Compact Integration of TiO2 Nanoparticles into the Cross-Points of 3D Vertically Stacked Ag Nanowires for Plasmon-Enhanced Photocatalysis. Nanomaterials 2019, 9, 468. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Wang, X.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y. One-Step Low Temperature Hydrothermal Synthesis of Flexible TiO2/PVDF@MoS2 Core-Shell Heterostructured Fibers for Visible-Light-Driven Photocatalysis and Self-Cleaning. Nanomaterials 2019, 9, 431. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, W.; Yu, L. Photocatalytic Decomposition of Gaseous HCHO over Ag Modified TiO2 Nanosheets at Ambient Temperature. Nanomaterials 2019, 9, 338. [Google Scholar] [CrossRef]
- Sahrin, N.T.; Nawaz, R.; Kait, C.F.; Lee, S.L.; Wirzal, M.D.H. Visible Light Photodegradation of Formaldehyde over TiO2 Nanotubes Synthesized via Electrochemical Anodization of Titanium Foil. Nanomaterials 2020, 10, 128. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-Doped Ti3+ Enhanced Photocatalyst for Hydrogen Production under Visible Light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef]
- Livraghi, S.; Maurelli, S.; Paganini, M.C.; Chiesa, M.; Giamello, E. Probing the Local Environment of Ti3+ Ions in TiO2 (Rutile) by 17O HYSCORE. Angew. Chem. Int. Ed. 2011, 50, 8038–8040. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.-M.; Gao, G.-Q.; Wang, R.-X.; Xu, A.-W. Stable blue TiO2−x nanoparticles for efficient visible light photocatalysts. J. Mater. Chem. A 2014, 2, 4429–4437. [Google Scholar] [CrossRef]
- Bellardita, M.; Garlisi, C.; Ozer, L.Y.; Venezia, A.M.; Sá, J.; Mamedov, F.; Palmisano, L.; Palmisano, G. Highly stable defective TiO2−x with tuned exposed facets induced by fluorine: Impact of surface and bulk properties on selective UV/visible alcohol photo-oxidation. Appl. Surf. Sci. 2020, 510, 145419. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Santo, V.D. The effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, M.S.; Amrollahi, R.; Mul, G. Surface Ti3+-Containing (blue) Titania: A Unique Photocatalyst with High Activity and Selectivity in Visible Light-Stimulated Selective Oxidation. ACS Catal. 2012, 2, 2641–2647. [Google Scholar] [CrossRef]
- Hu, Y.H. A Highly Efficient Photocatalyst-Hydrogenated Black TiO2 for the Photocatalytic Splitting of Water. Angew. Chem. Int. Ed. 2012, 51, 12410–12412. [Google Scholar] [CrossRef]
- Grabstanowicz, L.R.; Gao, S.; Li, T.; Rickard, R.M.; Rajh, T.; Liu, D.-J.; Xu, T. Facile Oxidative Conversion of TiH2 to High-Concentration Ti3+-Self-Doped Rutile TiO2 with Visible-Light Photoactivity. Inorg. Chem. 2013, 52, 3884–3890. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Hartmann, M.; Venkatesan, U.; Müller, J.; Spiecker, E.; Schmuki, P. Black TiO2 Nanotubes: Cocatalyst-Free Open-Circuit Hydrogen Generation. Nano Lett. 2014, 14, 3309–3313. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Zhang, G.; Liu, Z.; Qu, D.; Miao, X.; Feng, P.; Sun, Z. Effect of defects on photocatalytic activity of rutile TiO2 nanorods. Nano Res. 2015, 8, 4061–4071. [Google Scholar] [CrossRef]
- Liu, N.; Haublein, V.; Zhou, X.; Venkatesan, U.; Hartmann, M.; Mackovic, M.; Nakajima, T.; Spiecker, E.; Osvet, A.; Frey, L.; et al. “Black” TiO2 Nanotubes Formed by High-Energy Proton Implantation Show Noble-Metal-co-Catalyst Free Photocatalytic H2-Evolution. Nano Lett. 2015, 15, 6815–6820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, W.; Hou, H.; Zhang, Y.; Jing, M.; Jia, X.; Ji, X. Ti3+ Self-Doped Dark Rutile TiO2 Ultrafine Nanorods with Durable High-Rate Capability for Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 6793–6801. [Google Scholar] [CrossRef]
- Tian, M.; Mahjouri-Samani, M.; Eres, G.; Sachan, R.; Yoon, M.; Chisholm, M.F.; Wang, K.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; et al. Structure and Formation Mechanism of Black TiO2 Nanoparticles. ACS Nano 2015, 9, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, J.; Tao, H.; Wang, C.; Shen, Y.; Jiang, J.; Zhu, L.; Zeng, X.; Wang, T. One-Step Solvothermal Synthesis of Black TiO2 Films for Enhanced Visible Absorption. J. Nanosci. Nanotechnol. 2016, 16, 3146–3149. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, D.; Liu, K.; Wang, C.; Liu, L.; Li, B.; Zhang, Z.; Shen, D. Laser-Modified Black Titanium Oxide Nanospheres and Their Photocatalytic Activities under Visible Light. ACS Appl. Mater. Interfaces 2015, 7, 16070–16077. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, A.; Luo, Y.; Lu, P.; Dai, Y.; Enriquez, E.; Dowden, P.; Xu, H.; Kotula, P.G.; Azad, A.K.; et al. Conducting Interface in Oxide Homojunction: Understanding of Superior Properties in Black TiO2. Nano Lett. 2016, 16, 5751–5755. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Wang, N.; Li, Y.; Ding, H. Stable Ti3+ Self-Doped Anatase-Rutile Mixed TiO2 with Enhanced Visible Light Utilization and Durability. J. Phys. Chem. C 2016, 120, 6116–6124. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Wang, Y.; Hu, Z.; Zhao, H.; Xie, W. A Facile Approach to Prepare Black TiO2 with Oxygen Vacancy for Enhancing Photocatalytic Activity. Nanomaterials 2018, 8, 245. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.-A.; Yu, P.Y.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, J. Some interesting properties of black hydrogen-treated TiO2 nanowires and their potential application in solar energy conversion. Sci. China Chem. 2015, 58, 1810–1815. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y.; Li, Z.; Xie, Y. Large-Scale Fabrication of TiO2 Hierarchical Hollow Spheres. Inorg. Chem. 2006, 45, 3493–3495. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Osterlund, L.; Ljungstrom, S.; Palmqvist, A. Preparation of Nanosize Anatase and Rutile TiO2 by Hydrothermal Treatment of Microemulsions and Their Activity for Photocatalytic Wet Oxidation of Phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Liu, S.; Han, G.; Shu, M.; Han, L.; Che, S. Monodispersed inorganic/organic hybrid spherical colloids: Versatile synthesis and their gas-triggered reversibly switchable wettability. J. Mater. Chem. 2010, 20, 10001–10009. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liu, Q.; Gu, J.; Guo, Z.; Chen, Z.; Feng, C.; Zhang, D.; Moon, W.-J. Carbon-coated SnO2@C with hierarchically porous structures and graphite layers inside for a high-performance lithium-ion battery. J. Mater. Chem. 2012, 22, 2766–2773. [Google Scholar] [CrossRef]
- Xie, Y.; Sherwood, P.M.A. Ultrahigh Purity Graphite Electrode by Core Level and Valence Band XPS. Surf. Sci. Spectra 1992, 1, 367. [Google Scholar] [CrossRef]
- Ocal, C.; Ferrer, S. The strong metal–support interaction (SMSI) in Pt–TiO2 model catalysts. A new CO adsorption state on Pt–Ti atoms. J. Chem. Phys. 1986, 84, 6474. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Pelaez, M.; Zhu, D.; Liao, S.; Likodimos, V.; Ioannidis, N.; Kontos, A.G.; Falaras, P.; Dunlop, P.S.M.; et al. Synthesis, characterization and photocatalytic evaluation of visible light activated C-doped TiO2 nanoparticles. Nanotechnology 2012, 23, 294003. [Google Scholar] [CrossRef]
- Galuska, A.A.; Uht, J.C.; Marquez, N. Reactive and nonreactive ion mixing of Ti films on carbon substrates. J. Vac. Sci. Technol. A 1988, 6, 110. [Google Scholar] [CrossRef]
- Dementjev, A.P.; Ivanova, O.P.; Vasilyev, L.A.; Naumkin, A.V.; Nemirovsky, D.M.; Shalaev, D.Y. Altered layer as sensitive initial chemical state indicator. J. Vac. Sci. Technol. A 1994, 12, 423. [Google Scholar] [CrossRef]
- Gonbeau, D.; Guimon, C.; Pfister-Guillouzo, G.; Levasseur, A.; Meunier, G.; Dormoy, R. XPS study of thin films of titanium oxysulfides. Surf. Sci. 1991, 254, 81–89. [Google Scholar] [CrossRef]
- Franzen, H.F.; Umana, M.X.; McCreary, J.R.; Thorn, R.J. XPS spectra of some transition metal and alkaline earth monochalcogenides. J. Solid State Chem. 1976, 18, 363–368. [Google Scholar] [CrossRef]
- Haukka, S.; Lakomaa, E.-L.; Jylha, O.; Vilhunen, J.; Hornytzkyj, S. Dispersion and distribution of titanium species bound to silica from titanium tetrachloride. Langmuir 1993, 9, 3497–3506. [Google Scholar] [CrossRef]
- Kuznetsov, M.V.; Zhuravlev, J.F.; Gubanov, V.A. XPS analysis of adsorption of oxygen molecules on the surface of Ti and TiNx films in vacuum. J. Electron. Spectrosc. Relat. Phenom. 1992, 58, 169–176. [Google Scholar] [CrossRef]
- Kuznetsov, M.V.; Zhuravlev, J.F.; Zhilyaev, V.A.; Gubanov, V.A. XPS study of the nitrides, oxides and oxynitrides of titanium. J. Electron. Spectrosc. Relat. Phenom. 1992, 58, 1–9. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Bukhtiyarov, V.I.; Boronin, A.I.; Prosvirin, I.P.; Savchenko, V.I.J. Stages in the Modification of a Silver Surface for Catalysis of the Partial Oxidation of Ethylene: II. Action of the Reaction Medium. J. Catal. 1994, 150, 268–273. [Google Scholar] [CrossRef]
- Deskins, N.A.; Rousseau, R.; Dupuis, M. Distribution of Ti3+ Surface Sites in Reduced TiO2. J. Phys. Chem. C 2011, 115, 7562–7572. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Ling, Y.C.; Dillon, R.J.; Fitzmorris, R.C.; Dudzik, C.G.; Zavodivker, L.; Rajh, T.; Dimitrijevic, N.M.; Millhauser, G.; Bardeen, C.; et al. Probing the nature of bandgap states in hydrogen-treated TiO2 nanowires. J. Phys. Chem. 2013, 117, 26821–26830. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Wang, G.; Fitzmorris, R.C.; Adams, S.A.; Li, Y.; Zhang, J.Z. Ultrafast charge carrier dynamics and photoelectrochemical properties of hydrogen-treated TiO2 nanowire arrays. MRS Proc. 2012, 1387, e04–e07. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Chen, S.; Liu, Q. Novel Fe doped mesoporous TiO2 microspheres: Ultrasonic–hydrothermal synthesis, characterization, and photocatalytic properties. Physica E 2010, 42, 1844–1849. [Google Scholar] [CrossRef]
- Acharya, K.P.; Khnayzer, R.S.; Connor, T.; Diederich, G.; Kirsanova, M.; Klinkova, A.; Roth, D.; Kinder, E.; Imboden, M.; Zamkov, M. The Role of Hole Localization in Sacrificial Hydrogen Production by Semiconductor–Metal Heterostructured Nanocrystals. Nano Lett. 2011, 11, 2919. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, P.; Fan, Y.; Fan, Y.; Shi, X.; Cui, G.; Tang, B. Grey Rutile TiO2 with Long-Term Photocatalytic Activity Synthesized Via Two-Step Calcination. Nanomaterials 2020, 10, 920. https://doi.org/10.3390/nano10050920
Liu Y, Chen P, Fan Y, Fan Y, Shi X, Cui G, Tang B. Grey Rutile TiO2 with Long-Term Photocatalytic Activity Synthesized Via Two-Step Calcination. Nanomaterials. 2020; 10(5):920. https://doi.org/10.3390/nano10050920
Chicago/Turabian StyleLiu, Yan, Ping Chen, Yaqi Fan, Yanfei Fan, Xifeng Shi, Guanwei Cui, and Bo Tang. 2020. "Grey Rutile TiO2 with Long-Term Photocatalytic Activity Synthesized Via Two-Step Calcination" Nanomaterials 10, no. 5: 920. https://doi.org/10.3390/nano10050920
APA StyleLiu, Y., Chen, P., Fan, Y., Fan, Y., Shi, X., Cui, G., & Tang, B. (2020). Grey Rutile TiO2 with Long-Term Photocatalytic Activity Synthesized Via Two-Step Calcination. Nanomaterials, 10(5), 920. https://doi.org/10.3390/nano10050920



