Synthesis, Characterization and Application of Iron(II) Doped Copper Ferrites (CuII(x)FeII(1-x)FeIII2O4) as Novel Heterogeneous Photo-Fenton Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NPs
2.3. Characterization of NPs
2.4. Assessment of Photocatalytic Activity
2.5. Assessment of Reusability
3. Results
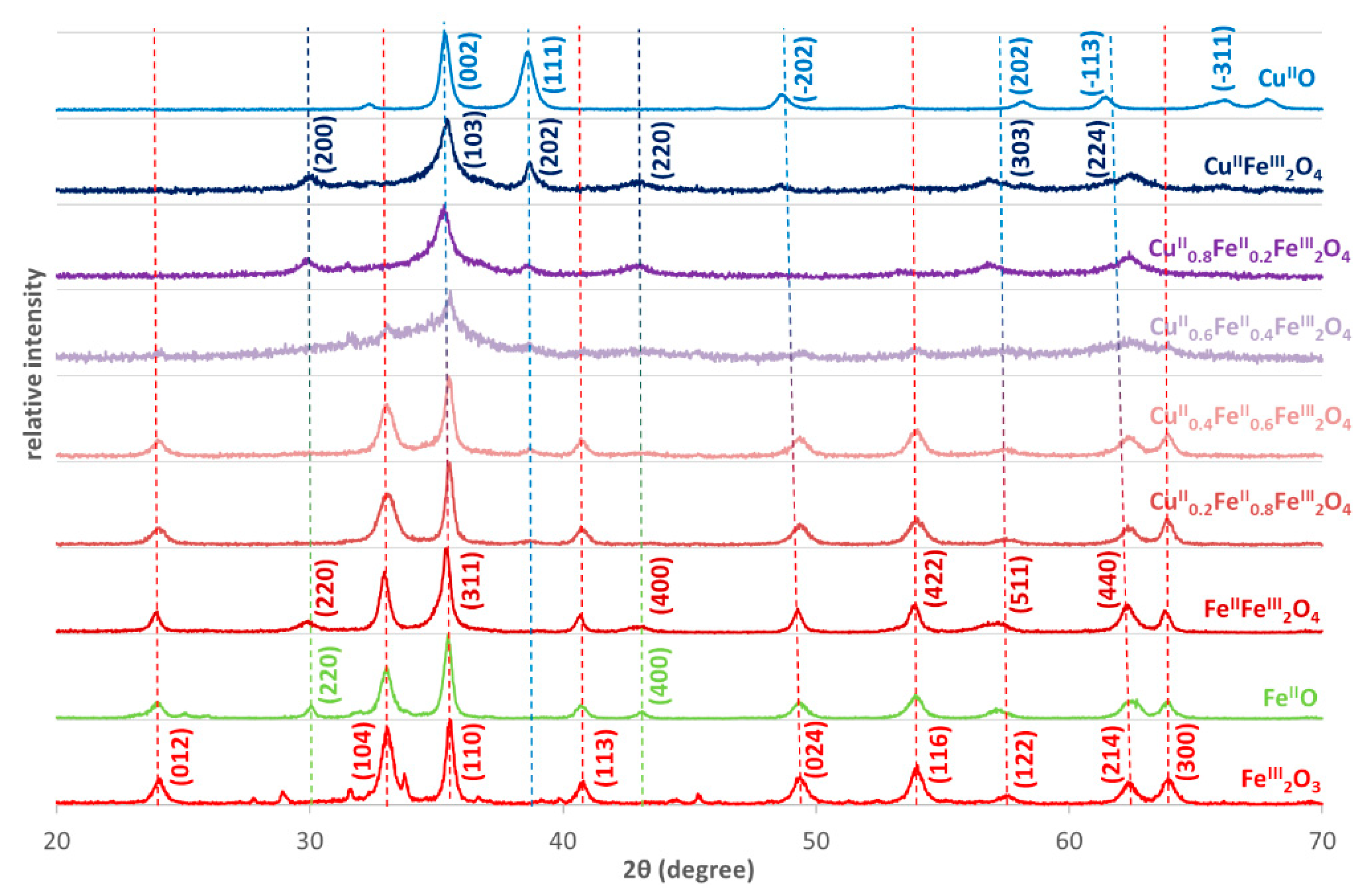
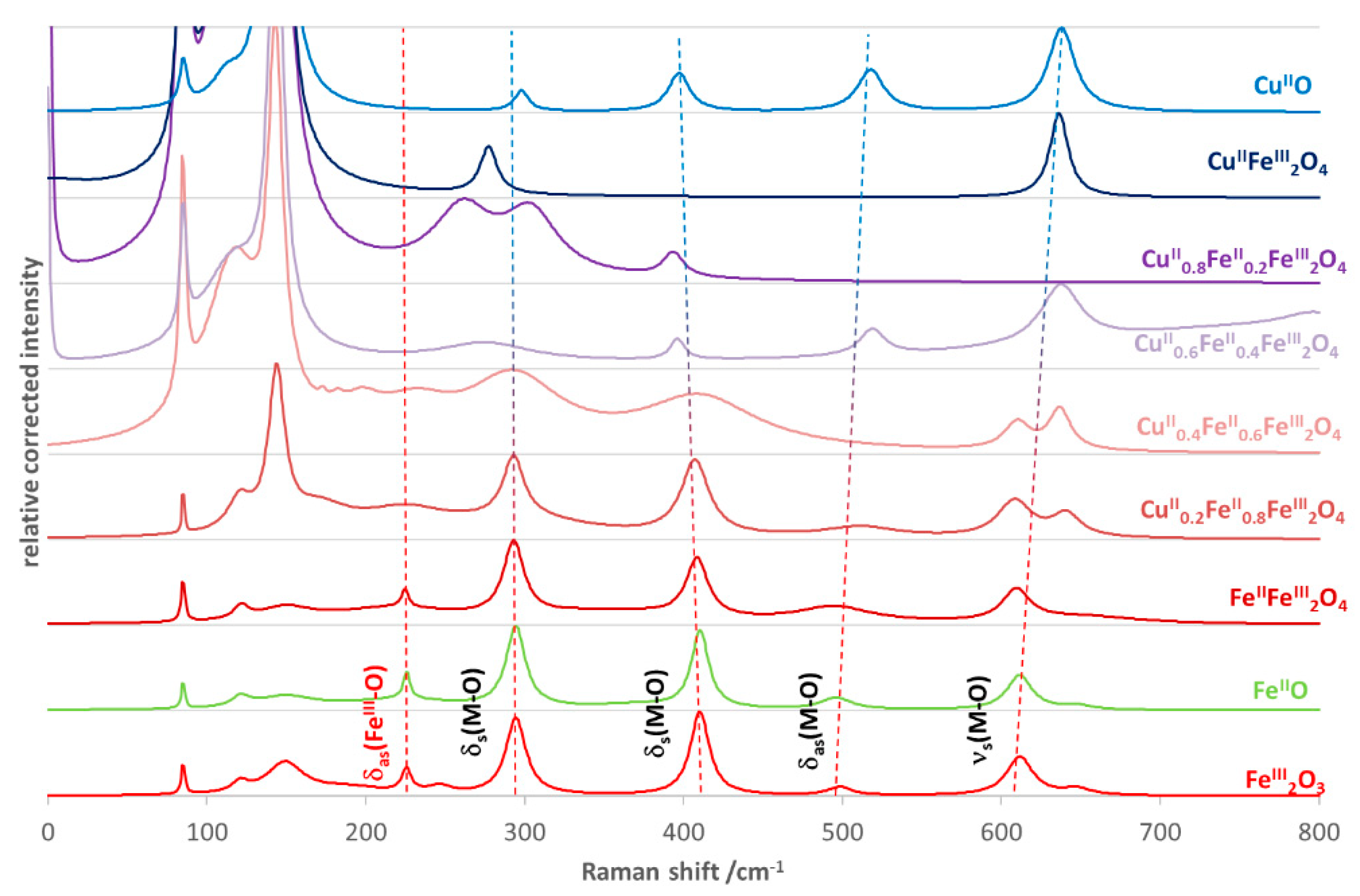
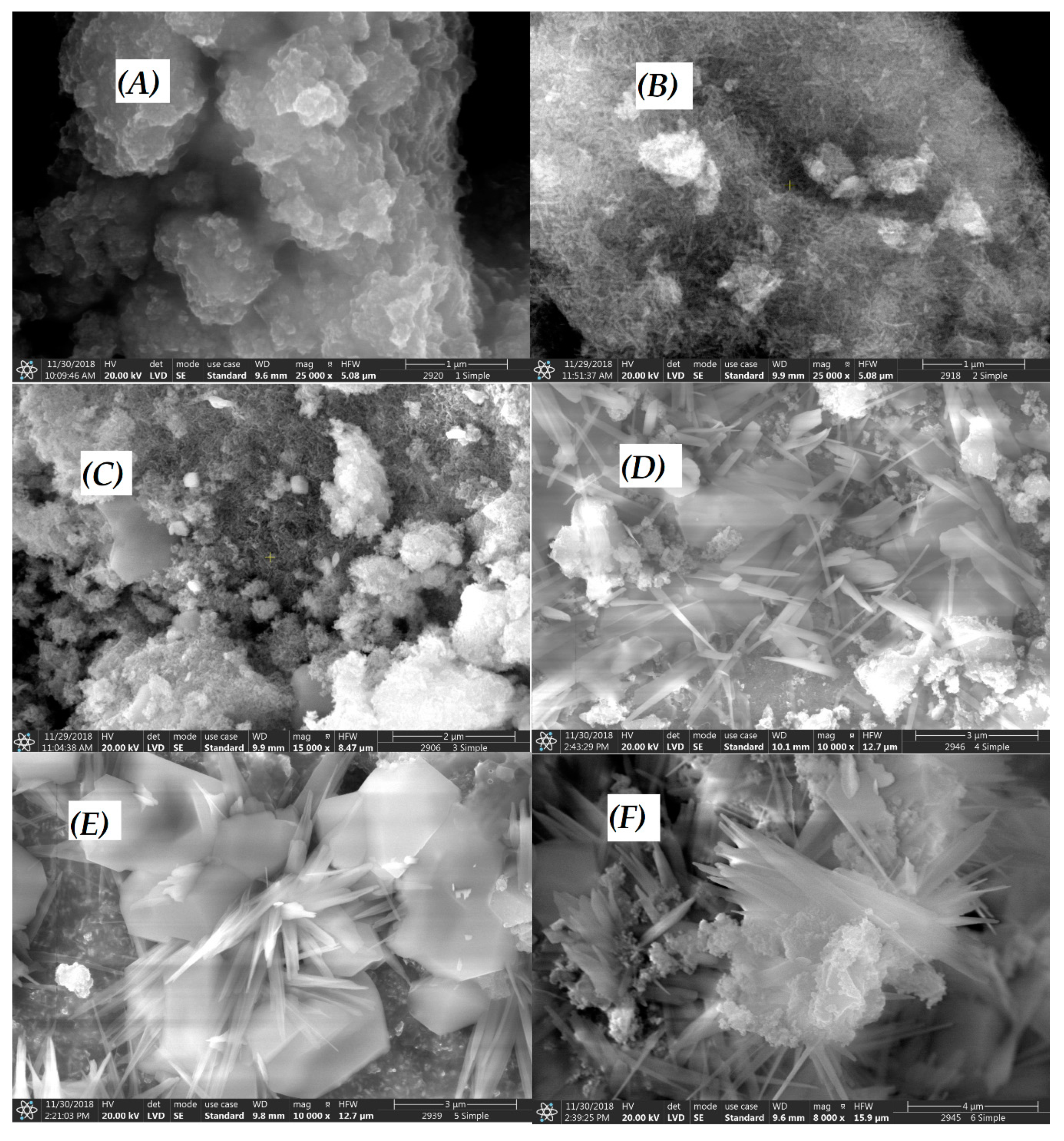
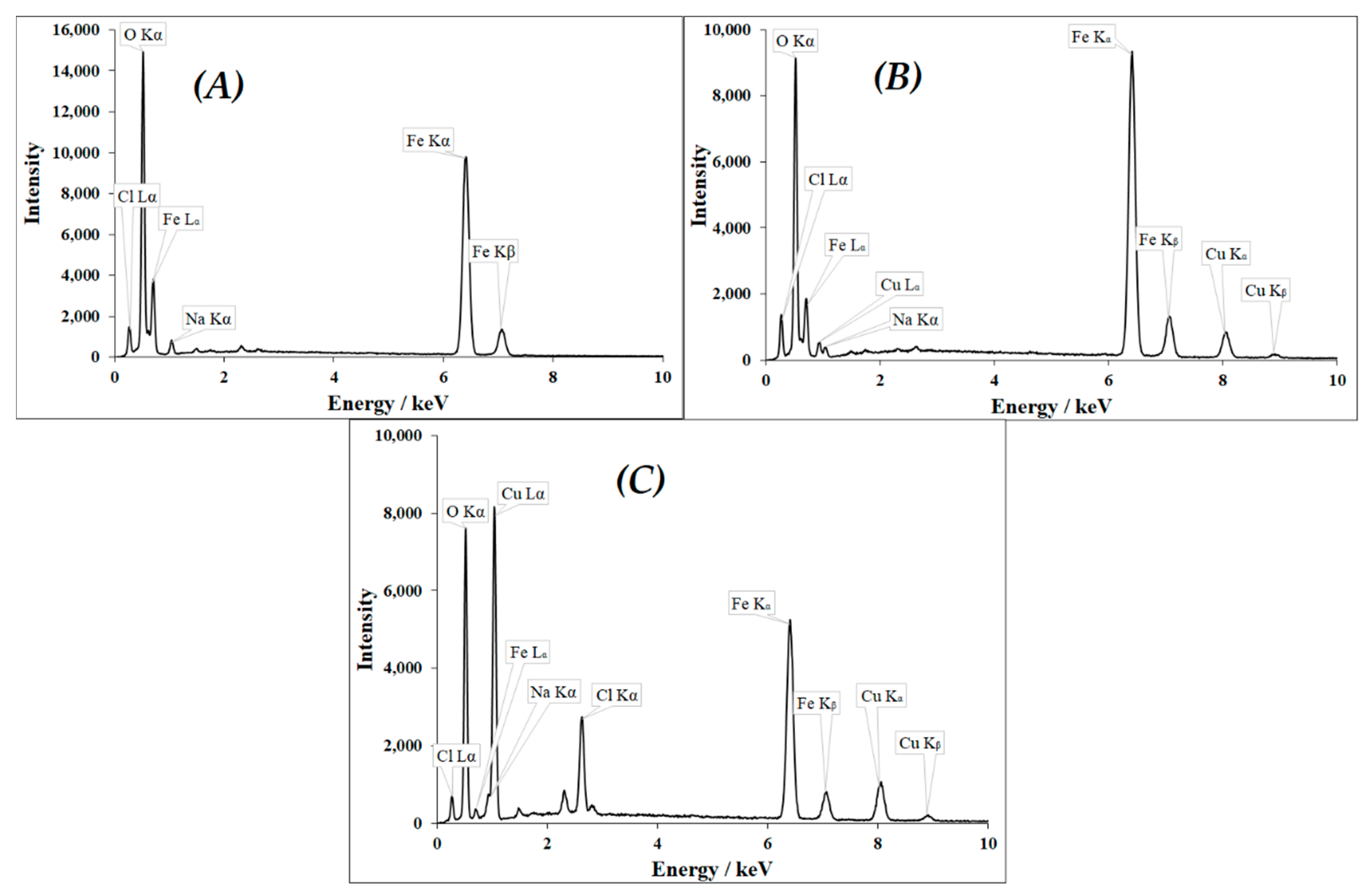
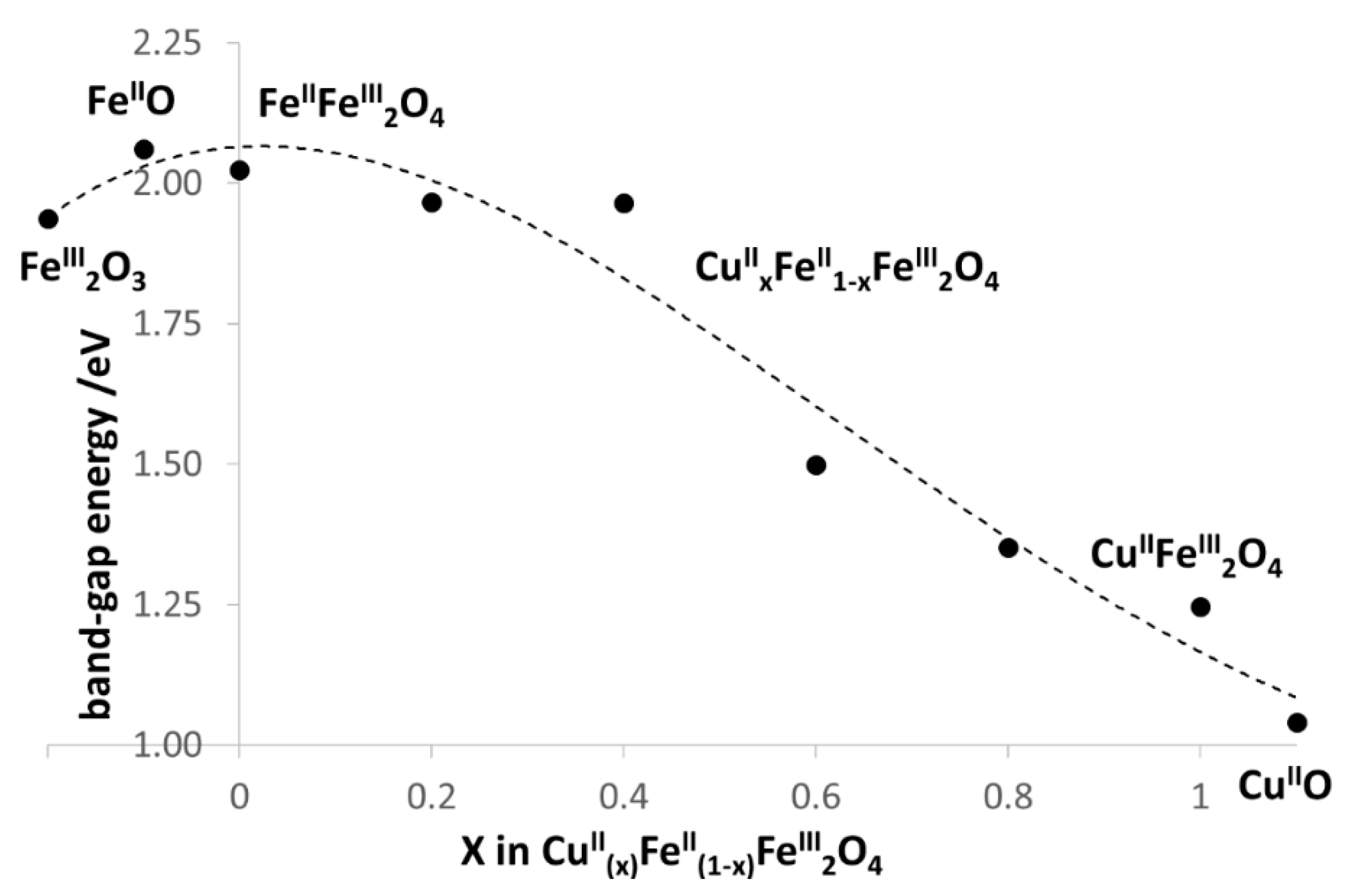
3.1. Characterization of CuII(x)FeII(1-x)FeIII2O4 NPs
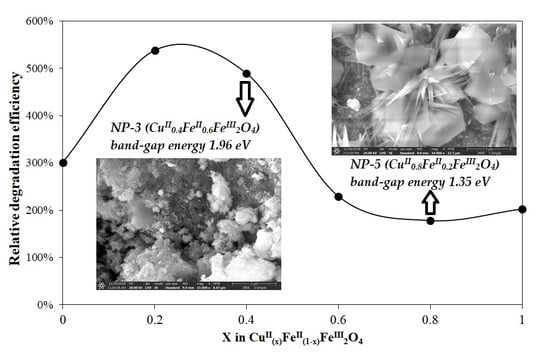
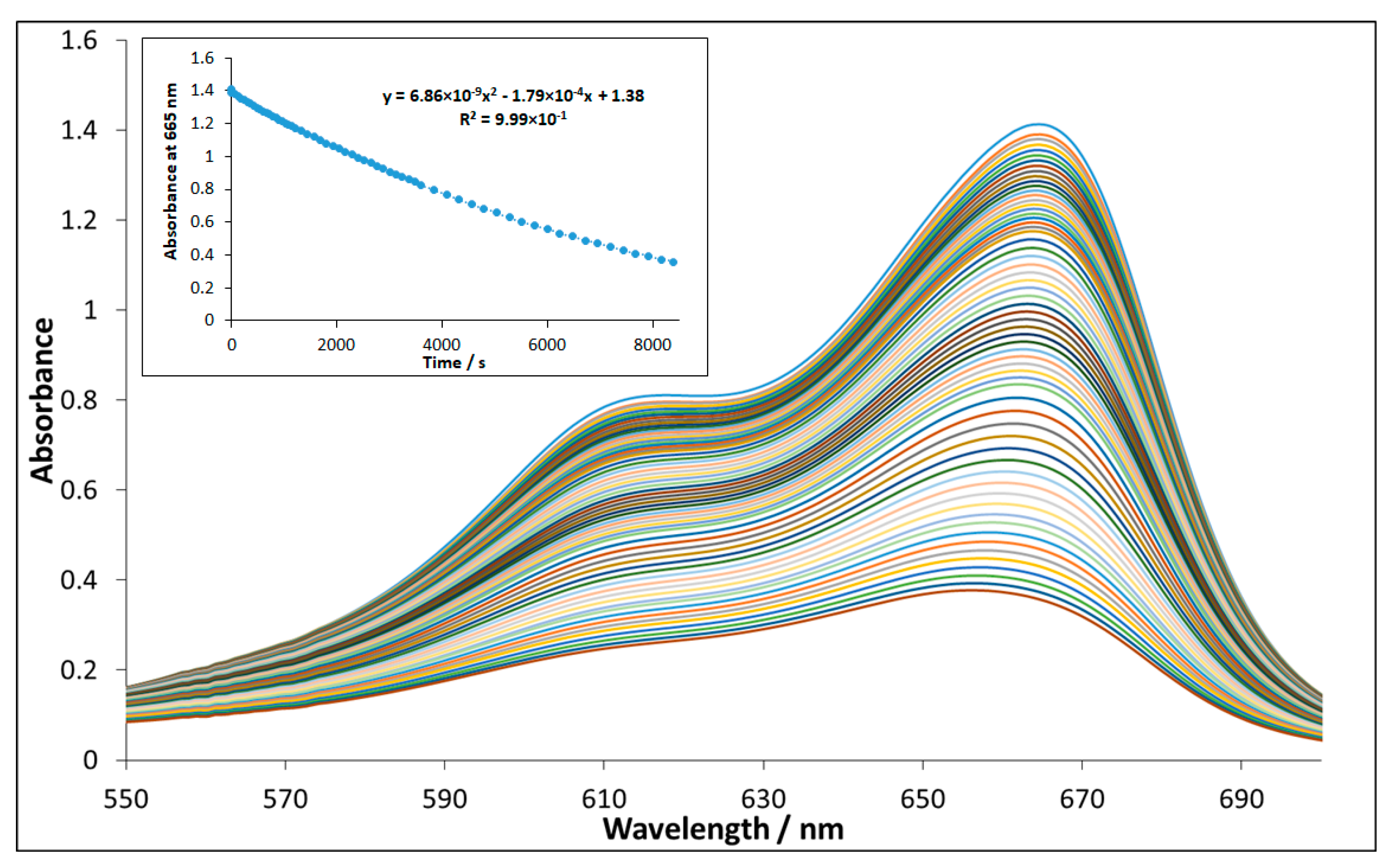
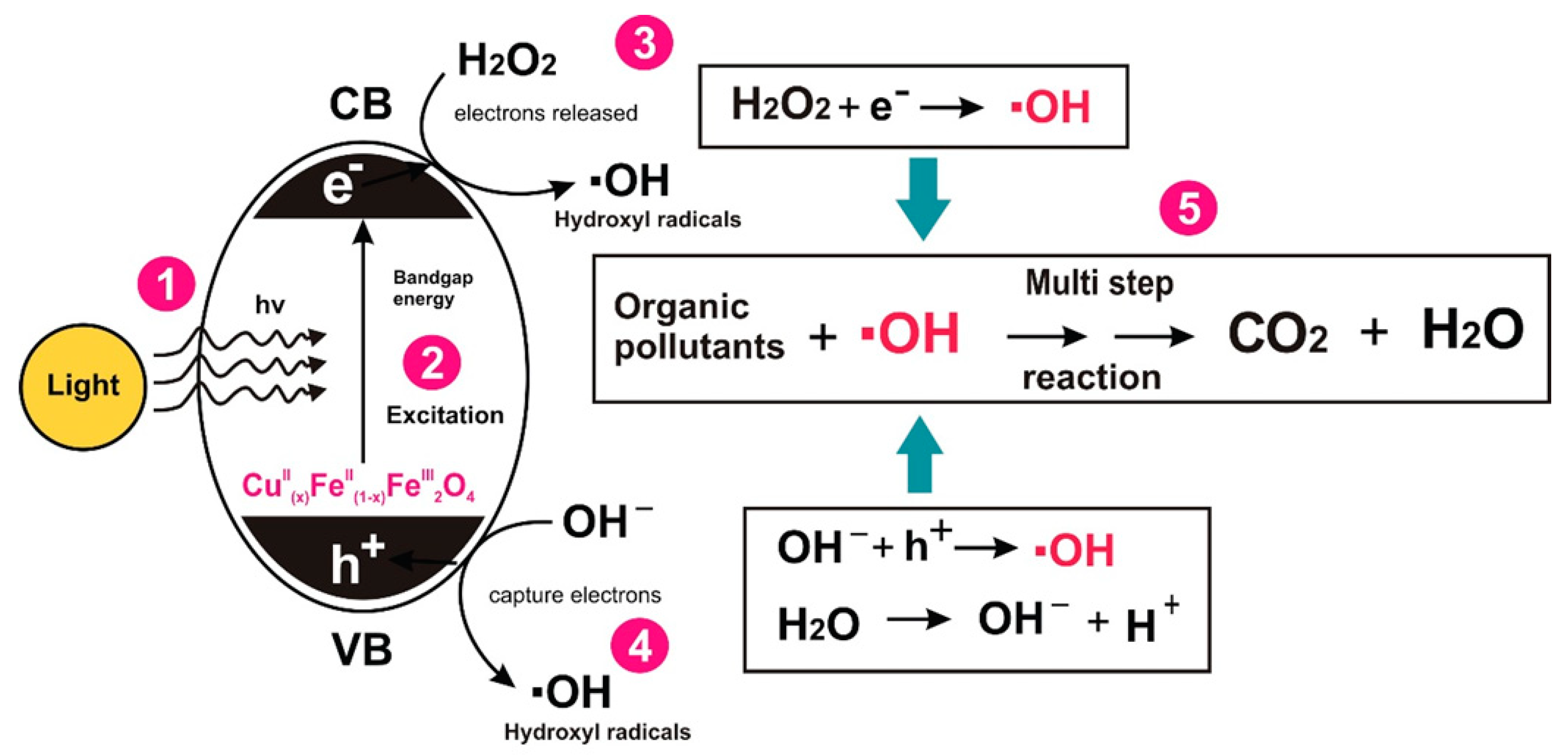
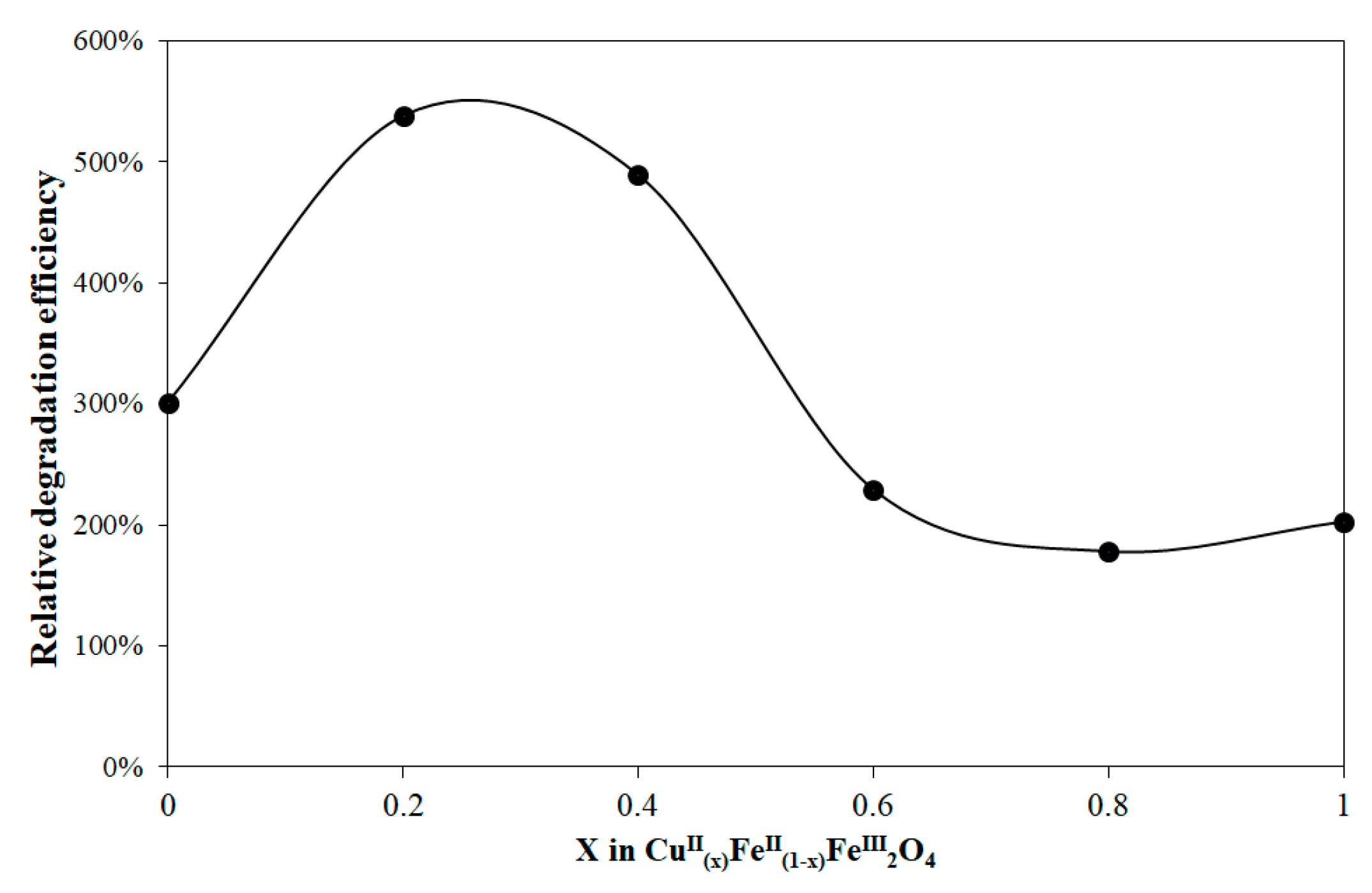
3.2. Evaluation of Photocatalytic Activity of CuII(x)FeII(1-x)FeIII2O4 NPs
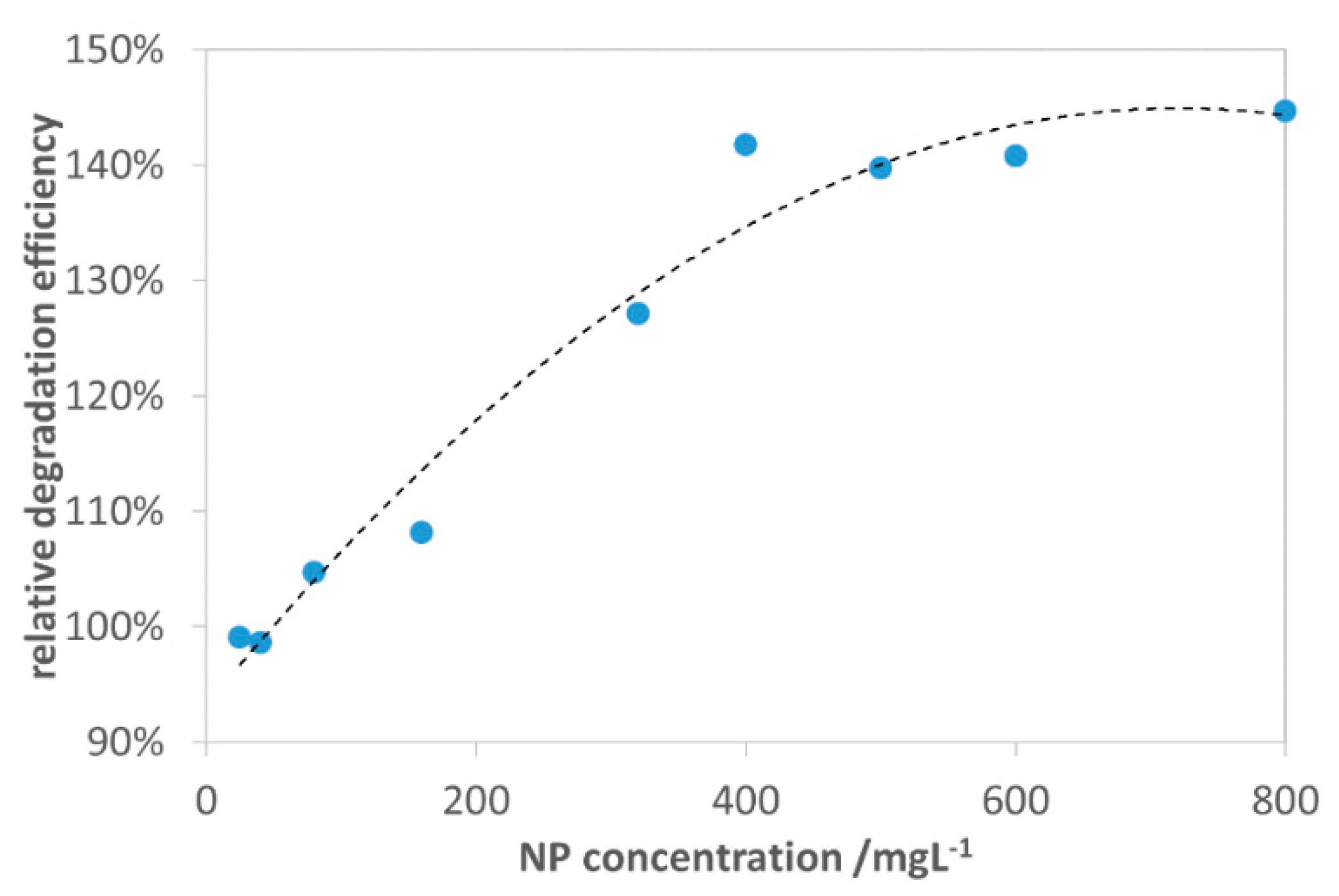
3.2.1. Effect of CuII0.4FeII0.6FeIII2O4 Dosage
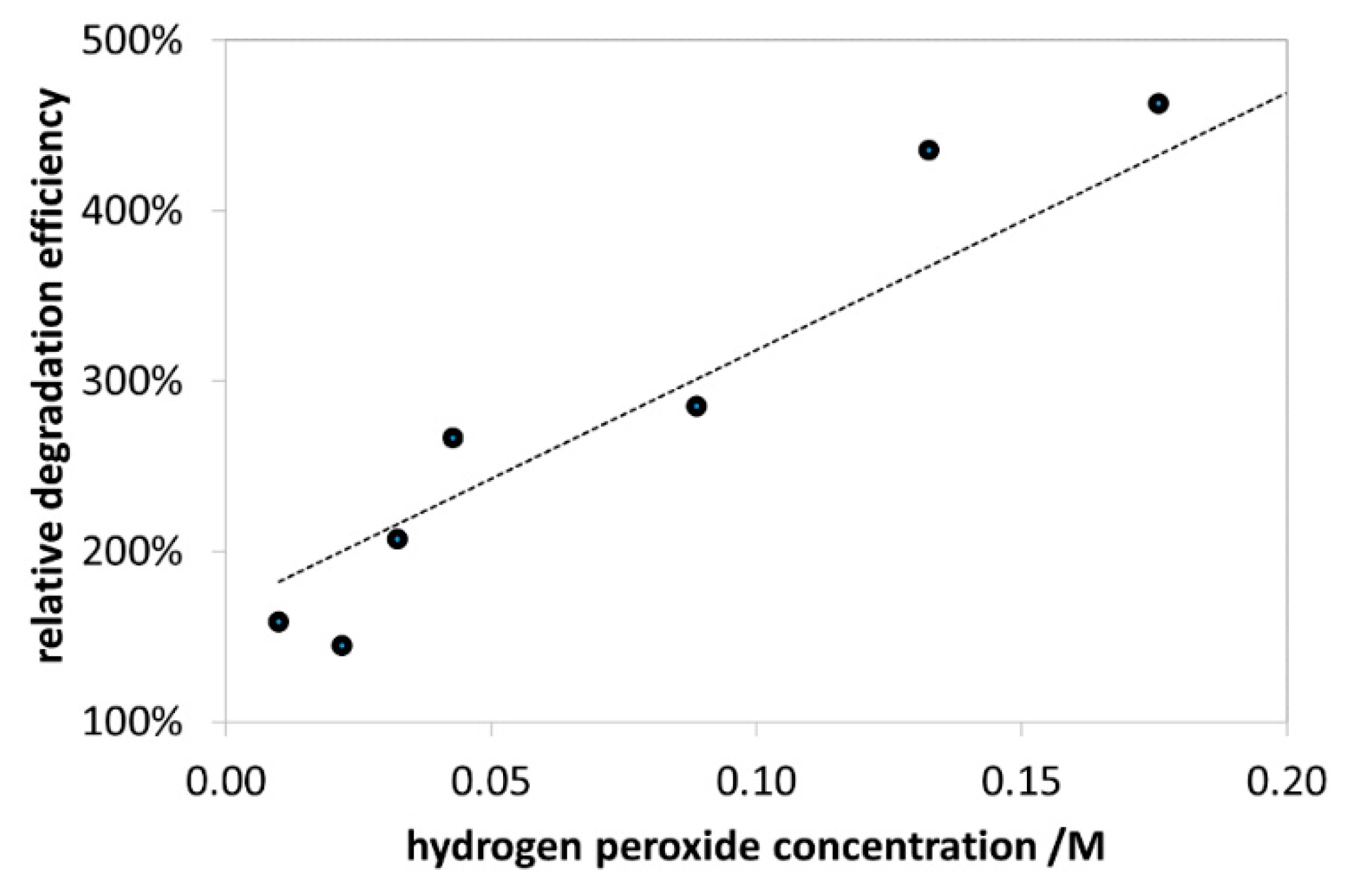
3.2.2. Effect of H2O2 Concentration
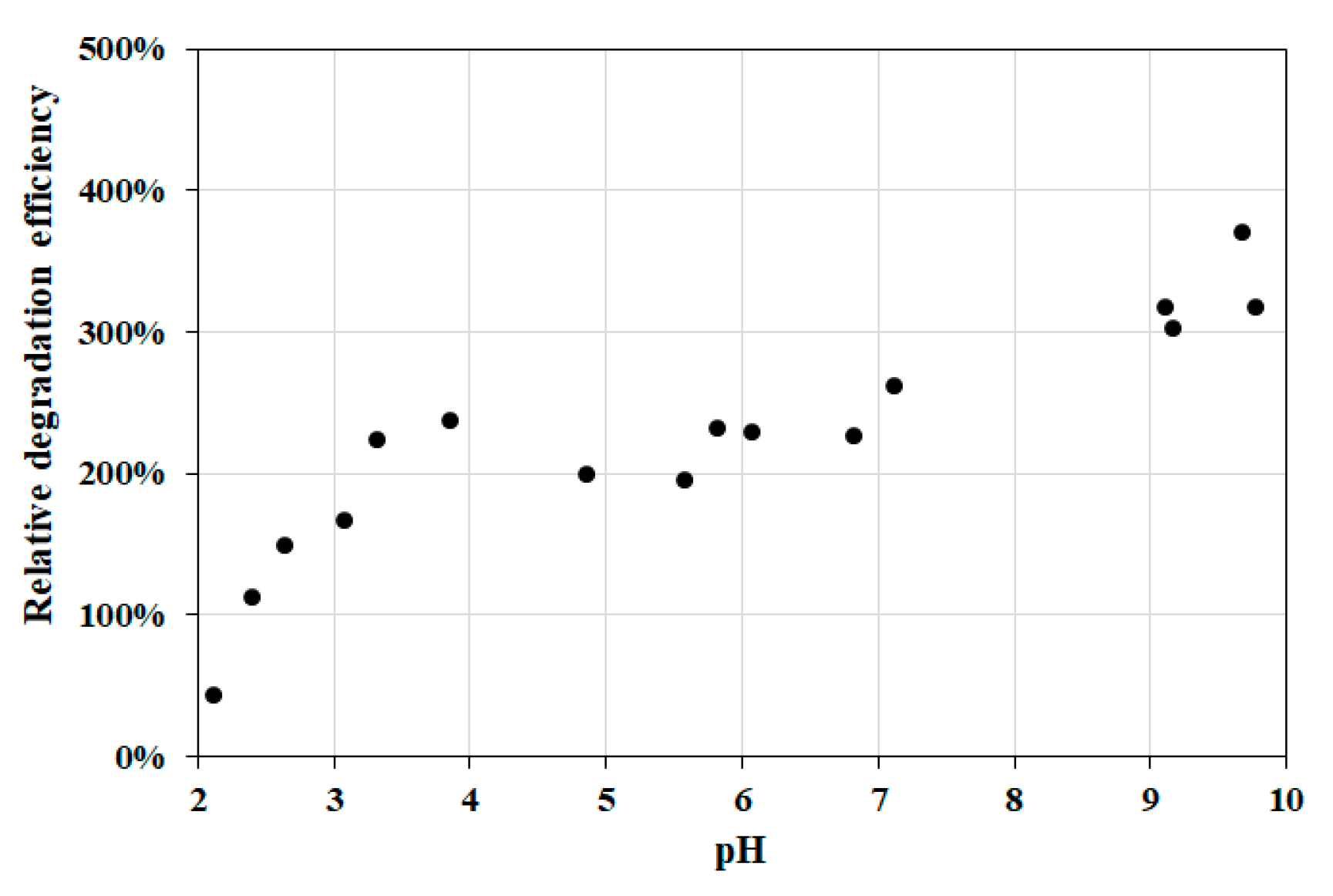
3.2.3. Effect of pH
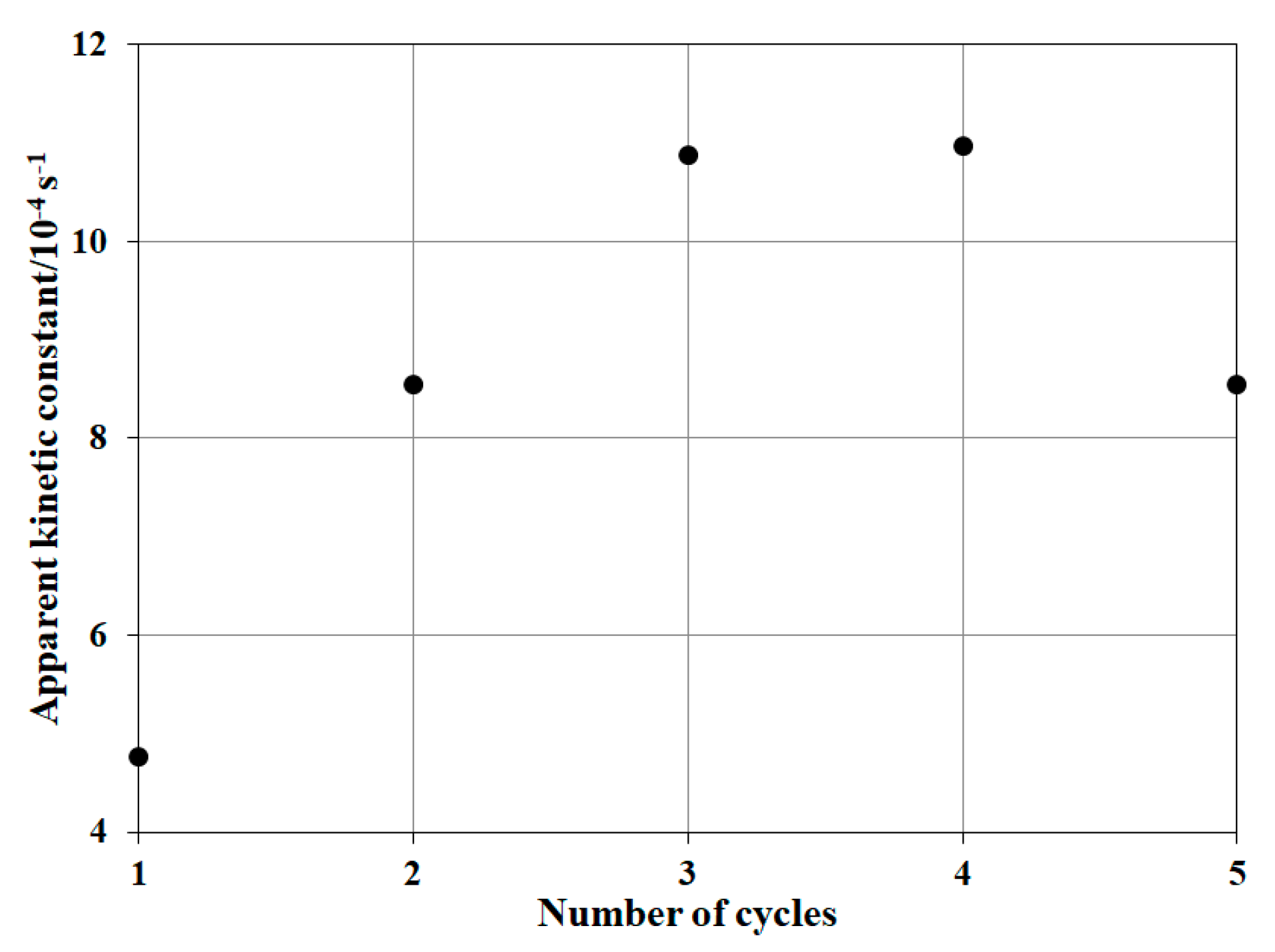
3.2.4. Reusability of NP-3 (CuII0.4FeII0.6FeIII2O4)
3.2.5. Summarizing the Optimized Photocatalytic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, X.T.; Dastan, D.; Wu, F.Y.; Li, J. Facile synthesis of SnO2/LaFeO3− XNX composite: Photocatalytic activity and gas sensing performance. Nanomaterials 2019, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Rupa, E.J.; Kaliraj, L.; Abid, S.; Yang, D.C.; Jung, S.K. Synthesis of a Zinc Oxide Nanoflower Photocatalyst from Sea Buckthorn Fruit for Degradation of Industrial Dyes in Wastewater Treatment. Nanomaterials 2019, 9, 1692. [Google Scholar] [CrossRef] [PubMed]
- Valero Luna, C.; Palomares Sanchéz, S.; Ruíz, F. Catalytic activity of the barium hexaferrite with H2O2/visible light irradiation for degradation of Methylene Blue. Catal. Today 2016, 266, 110–119. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Kallel, M.; Belaid, C.; Mechichi, T.; Ksibi, M.; Elleuch, B. Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem. Eng. J. 2009, 150, 391–395. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Liu, X.; Chen, X. Photocatalytic and photo-Fenton catalytic degradation activities of Z-scheme Ag2S/BiFeO3 heterojunction composites under visible-light irradiation. Nanomaterials 2019, 9, 399. [Google Scholar] [CrossRef]
- Amat, A.M.; Arques, A.; Beneyto, H.; Garcıa, A.; Miranda, M.A.; Seguı́, S. Ozonisation coupled with biological degradation for treatment of phenolic pollutants: A mechanistically based study. Chemosphere 2003, 53, 79–86. [Google Scholar] [CrossRef]
- Lee, E.; Lee, H.; Kim, Y.; Sohn, K.; Lee, K. Hydrogen peroxide interference in chemical oxygen demand during ozone based advanced oxidation of anaerobically digested livestock wastewater. Int. J. Environ. Sci. Technol. 2011, 8, 381–388. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Removal of COD from olive mill wastewater by Fenton‘s reagent: Kinetic study. J. Hazard. Mater. 2009, 168, 1253–1259. [Google Scholar] [CrossRef]
- El Hajjouji, H.; Barje, F.; Pinelli, E.; Bailly, J.R.; Richard, C.; Winterton, P.; Revel, J.C.; Hafidi, M. Photochemical UV/TiO2 treatment of olive mill wastewater (OMW). Bioresour. Technol. 2008, 99, 7264–7269. [Google Scholar] [CrossRef]
- Karunakaran, C.; Anilkumar, P. Semiconductor-catalyzed solar photooxidation of iodide ion. J. Mol. Catal. A-Chem. 2007, 265, 153–158. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Sun, S.P.; Zeng, X.; Li, C.; Lemley, A.T. Enhanced heterogeneous and homogeneous Fenton-like degradation of carbamazepine by nano-Fe3O4/H2O2 with nitrilotriacetic acid. Chem. Eng. J. 2014, 244, 44–49. [Google Scholar] [CrossRef]
- Huang, Y.M.; Li, M.Y.; Yang, L.; Zhai, B.G. Eu2+ and Eu3+ doubly doped ZnWO4 nanoplates with superior photocatalytic performance for dye degradation. Nanomaterials 2018, 8, 765. [Google Scholar] [CrossRef]
- Mesquita, I.; Matos, L.C.; Duarte, F.; Maldonado Hódar, F.; Mendes, A.; Madeira, L.M. Treatment of azo dye-containing wastewater by a Fenton-like process in a continuous packed-bed reactor filled with activated carbon. J. Hazard. Mater. 2012, 237, 30–37. [Google Scholar] [CrossRef]
- Duarte, F.; Maldonado Hódar, F.; Madeira, L.M. Influence of the characteristics of carbon materials on their behaviour as heterogeneous Fenton catalysts for the elimination of the azo dye Orange II from aqueous solutions. Appl. Catal. B Environ. 2011, 103, 109–115. [Google Scholar] [CrossRef]
- Arslan Alaton, I. Degradation of a commercial textile biocide with advanced oxidation processes and ozone. J. Environ. Manage. 2007, 82, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Tekin, H.; Bilkay, O.; Ataberk, S.S.; Balta, T.H.; Ceribasi, I.H.; Sanin, F.D.; Dilek, F.B.; Yetis, U. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 2006, 136, 258–265. [Google Scholar] [CrossRef]
- Bianco, B.; De Michelis, I.; Vegliò, F. Fenton treatment of complex industrial wastewater: Optimization of process conditions by surface response method. J. Hazard. Mater. 2011, 186, 1733–1738. [Google Scholar] [CrossRef]
- Badawy, M.I.; Ghaly, M.Y.; Gad Allah, T.A. Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 2006, 194, 166–175. [Google Scholar] [CrossRef]
- Catalkaya, E.C.; Kargi, F. Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: A comparative study. J. Hazard. Mater. 2007, 139, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Mascolo, G.; Detomaso, A.; Lovecchio, G.; Villani, G. Temperature activated degradation (mineralization) of 4-chloro-3-methyl phenol by Fenton’s reagent. Chemosphere 2005, 59, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Gou, N.; Alshawabkeh, A.N.; Gu, A.Z. Efficient degradation of contaminants of emerging concerns by a new electro-Fenton process with Ti/MMO cathode. Chemosphere 2013, 93, 2796–2804. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S.; Yavuz, H.I.; Erbatur, O. Degradation of 4-chloro-2-methylphenol in aqueous solution by electro-Fenton and photoelectro-Fenton processes. Appl. Catal. B Environ. 2006, 63, 243–248. [Google Scholar] [CrossRef]
- Sirés, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E. Degradation of clofibric acid in acidic aqueous medium by electro-Fenton and photoelectro-Fenton. Chemosphere 2007, 66, 1660–1669. [Google Scholar] [CrossRef]
- Brillas, E.; Banos, M.A.; Skoumal, M.; Cabot, P.L.; Garrido, J.A.; Rodríguez, R.M. Degradation of the herbicide 2, 4-DP by anodic oxidation, electro-Fenton and photoelectro-Fenton using platinum and boron-doped diamond anodes. Chemosphere 2007, 68, 199–209. [Google Scholar] [CrossRef]
- Masomboon, N.; Ratanatamskul, C.; Lu, M.C. Mineralization of 2, 6-dimethylaniline by photoelectro-Fenton process. Appl. Catal. A Gen. 2010, 384, 128–135. [Google Scholar] [CrossRef]
- Garcia Segura, S.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.L.; Centellas, F.; Arias, C.; Brillas, E. Mineralization of flumequine in acidic medium by electro-Fenton and photoelectro-Fenton processes. Water Res. 2012, 46, 2067–2076. [Google Scholar] [CrossRef]
- Brillas, E.; Banos, M.A.; Garrido, J.A. Mineralization of herbicide 3, 6-dichloro-2-methoxybenzoic acid in aqueous medium by anodic oxidation, electro-Fenton and photoelectro-Fenton. Electrochim. Acta. 2003, 48, 1697–1705. [Google Scholar] [CrossRef]
- Fernandez, J.; Bandara, J.; Kiwi, J.; Lopez, A.; Albers, P. Efficient photo-assisted Fenton catalysis mediated by Fe ions on Nafion membranes active in the abatement of non-biodegradable azo-dye. Chem. Commun. 1998, 14, 1493–1494. [Google Scholar] [CrossRef]
- Kasiri, M.B.; Aleboyeh, H.; Aleboyeh, A. Mineralization of CI Acid Red 14 azo dye by UV/Fe-ZSM5/H2O2 process. Environ. Technol. 2010, 31, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Jiang, J.; Zhang, Y.; Lin, X.; Du, C.; Xiong, Y. FeVO4 as a highly active heterogeneous Fenton-like catalyst towards the degradation of Orange II. Appl. Catal. B Environ. 2008, 84, 468–473. [Google Scholar] [CrossRef]
- Pham, A.L.T.; Lee, C.; Doyle, F.M.; Sedlak, D.L. A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environ. Sci. Technol. 2009, 43, 8930–8935. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Gonçalves, M.; Guerreiro, M.; Ramalho, T.; Fabris, J.; Pereira, M.; Sapag, K. A new catalyst material based on niobia/iron oxide composite on the oxidation of organic contaminants in water via heterogeneous Fenton mechanisms. Appl. Catal. A Gen. 2007, 316, 117–124. [Google Scholar] [CrossRef]
- Singh, C.; Goyal, A.; Singhal, S. Nickel-doped cobalt ferrite nanoparticles: Efficient catalysts for the reduction of nitroaromatic compounds and photo-oxidative degradation of toxic dyes. Nanoscale 2014, 6, 7959–7970. [Google Scholar] [CrossRef]
- Han, L.; Zhou, X.; Wan, L.; Deng, Y.; Zhan, S. Synthesis of ZnFe2O4 nanoplates by succinic acid-assisted hydrothermal route and their photocatalytic degradation of rhodamine B under visible light. J. Environ. Chem. Eng. 2014, 2, 123–130. [Google Scholar] [CrossRef]
- Borhan, A.I.; Samoila, P.; Hulea, V.; Iordan, A.R.; Palamaru, M.N. Effect of Al3+ substituted zinc ferrite on photocatalytic degradation of Orange I azo dye. J. Photochem. Photobio.l A Chem. 2014, 279, 17–23. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Zinc ferrite nanoparticle as a magnetic catalyst: Synthesis and dye degradation. Mater. Res. Bull. 2013, 48, 4255–4260. [Google Scholar] [CrossRef]
- Patil, S.; Naik, H.B.; Nagaraju, G.; Viswanath, R.; Rashmi, S. Sugarcane juice mediated eco-friendly synthesis of visible light active zinc ferrite nanoparticles: Application to degradation of mixed dyes and antibacterial activities. Mater. Chem. Phys. 2018, 351–362. [Google Scholar] [CrossRef]
- Abroshan, E.; Farhadi, S.; Zabardasti, A. Novel magnetically separable Ag3PO4/MnFe2O4 nanocomposite and its high photocatalytic degradation performance for organic dyes under solar-light irradiation. Sol. Energy Mater. Sol. Cells. 2018, 178, 154–163. [Google Scholar] [CrossRef]
- Högfeldt, E. Stability Constants of Metal-Ion Complexes: Part A: Inorganic Ligands; Pergamon Pr.: Oxford, UK, 1982; p. 21. [Google Scholar]
- Tatarchuk, T.; Bououdina, M.; Macyk, W.; Shyichuk, O.; Paliychuk, N.; Yaremiy, I.; Al Najar, B.; Pacia, M. Structural, optical, and magnetic properties of Zn-doped CoFe2O4 nanoparticles. Nanoscale Res. Lett. 2017, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tian, A.; You, J.; Yu, Z.; Yang, H.; Xue, X. Fe2SiS4 nanoparticle—A new heterogeneous Fenton reagent. Mater. Lett. 2016, 169, 153–156. [Google Scholar] [CrossRef]
- Shi, X.; Tian, A.; You, J.; Yang, H.; Wang, Y.; Xue, X. Degradation of organic dyes by a new heterogeneous Fenton reagent-Fe2GeS4 nanoparticle. J. Hazard. Mater. 2018, 353, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, Y.; Xu, H.; Fu, J.; Li, X.; Zhao, Q.; Hou, Y. Facile preparation of sphere-like copper ferrite nanostructures and their enhanced visible-light-induced photocatalytic conversion of benzene. Mater. Res. Bull. 2013, 48, 4216–4222. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Gadri, A.; Ammar, S. Structural, optical and morphological characterization of Cu-doped α-Fe2O3 nanoparticles synthesized through co-precipitation technique. J. Mol. Struct. 2017, 1148, 276–281. [Google Scholar] [CrossRef]
- Litter, M.I.; Blesa, M.A. Photodissolution of iron oxides. IV. A comparative study on the photodissolution of hematite, magnetite, and maghemite in EDTA media. Can. J. Chem. 1992, 70, 2502–2510. [Google Scholar] [CrossRef]
- Dhineshbabu, N.; Rajendran, V.; Nithyavathy, N.; Vetumperumal, R. Study of structural and optical properties of cupric oxide nanoparticles. Appl. Nanosci. 2016, 6, 933–939. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P. Degradation and mineralization of methylene blue using a heterogeneous photo-Fenton catalyst under visible and solar light irradiation. Catal. Sci. Technol. 2016, 6, 1222–1232. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Jimmy, C.Y. Enhanced photo-Fenton degradation of rhodamine B using graphene oxide–amorphous FePO4 as effective and stable heterogeneous catalyst. J. Colloid Interf. Sci. 2015, 448, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
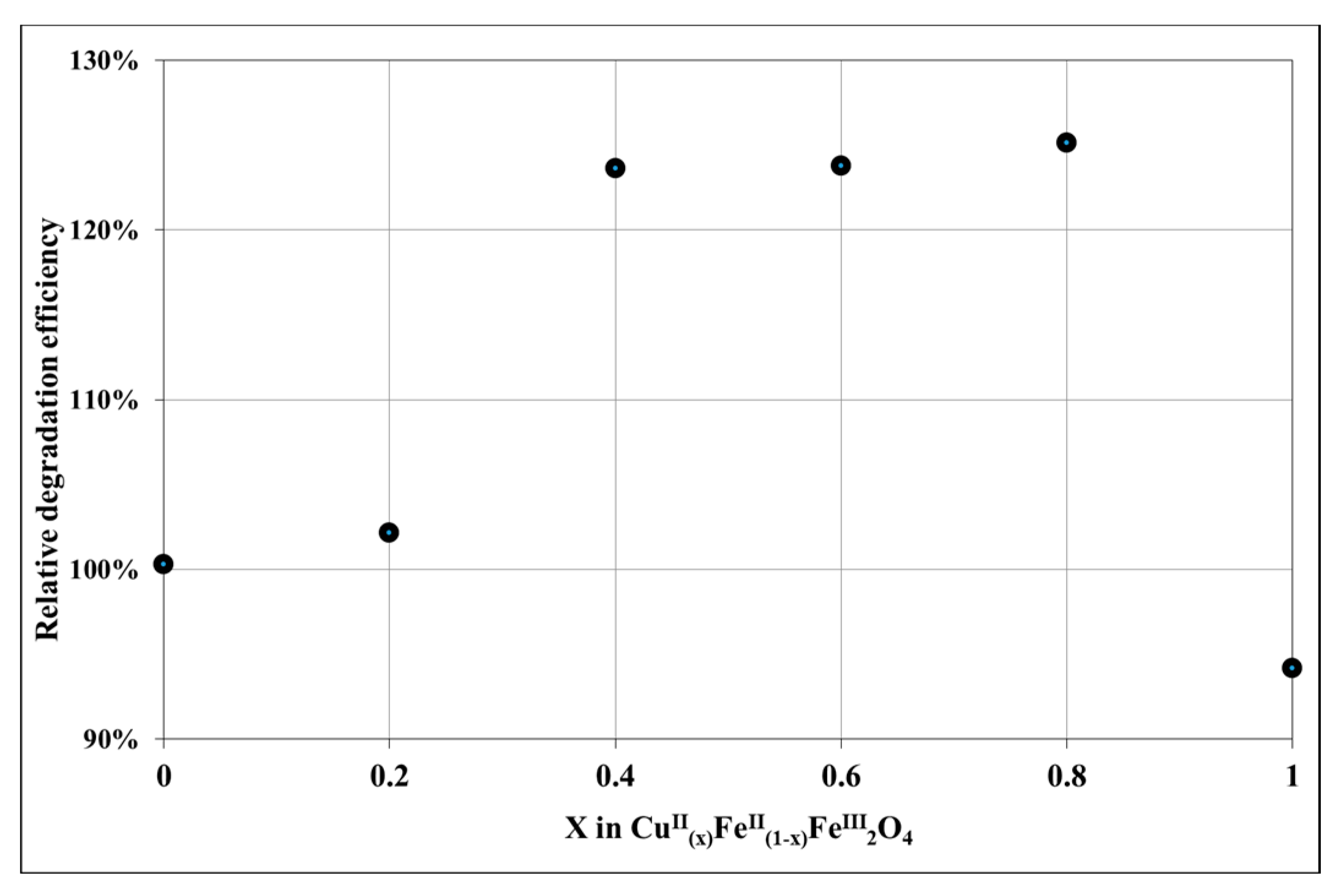
| CuII(x)FeII(1-x)FeIII2O4 | x = 0 | x = 0.2 | x = 0.4 | x = 0.6 | x = 0.8 | x = 1 |
|---|---|---|---|---|---|---|
| Sample name | NP-1 | NP-2 | NP-3 | NP-4 | NP-5 | NP-6 |
| Fe(NH4)2(SO4)2∙6H2O (g) | 1.961 | 1.569 | 1.176 | 0.784 | 0.392 | 0.000 |
| FeCl3∙6H2O (g) | 2.703 | 2.703 | 2.703 | 2.703 | 2.703 | 2.703 |
| CuSO4 (g) | 0.000 | 0.160 | 0.319 | 0.479 | 0.638 | 0.798 |
| Experiment | Reaction Rate (M/s) | Relative Efficiency of Degradation |
|---|---|---|
| MB + NPs + Light | 1.24 × 10−10 | 41.8% |
| MB + Light | 1.13 × 10−10 | 38.3% |
| MB + H2O2 | 2.58 × 10−11 | 8.7% |
| MB + H2O2 + Light | 2.95 × 10−10 | 100.0% (the basis of comparison) |
| MB + NPs + H2O2 + Light | 3.96 × 10−10 | 133.9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Valicsek, Z.; Horváth, O. Synthesis, Characterization and Application of Iron(II) Doped Copper Ferrites (CuII(x)FeII(1-x)FeIII2O4) as Novel Heterogeneous Photo-Fenton Catalysts. Nanomaterials 2020, 10, 921. https://doi.org/10.3390/nano10050921
Khan A, Valicsek Z, Horváth O. Synthesis, Characterization and Application of Iron(II) Doped Copper Ferrites (CuII(x)FeII(1-x)FeIII2O4) as Novel Heterogeneous Photo-Fenton Catalysts. Nanomaterials. 2020; 10(5):921. https://doi.org/10.3390/nano10050921
Chicago/Turabian StyleKhan, Asfandyar, Zsolt Valicsek, and Ottó Horváth. 2020. "Synthesis, Characterization and Application of Iron(II) Doped Copper Ferrites (CuII(x)FeII(1-x)FeIII2O4) as Novel Heterogeneous Photo-Fenton Catalysts" Nanomaterials 10, no. 5: 921. https://doi.org/10.3390/nano10050921
APA StyleKhan, A., Valicsek, Z., & Horváth, O. (2020). Synthesis, Characterization and Application of Iron(II) Doped Copper Ferrites (CuII(x)FeII(1-x)FeIII2O4) as Novel Heterogeneous Photo-Fenton Catalysts. Nanomaterials, 10(5), 921. https://doi.org/10.3390/nano10050921