Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(lactide-co-glycolide) after Subchronic Oral Exposure of Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
Synthesis and Characterization
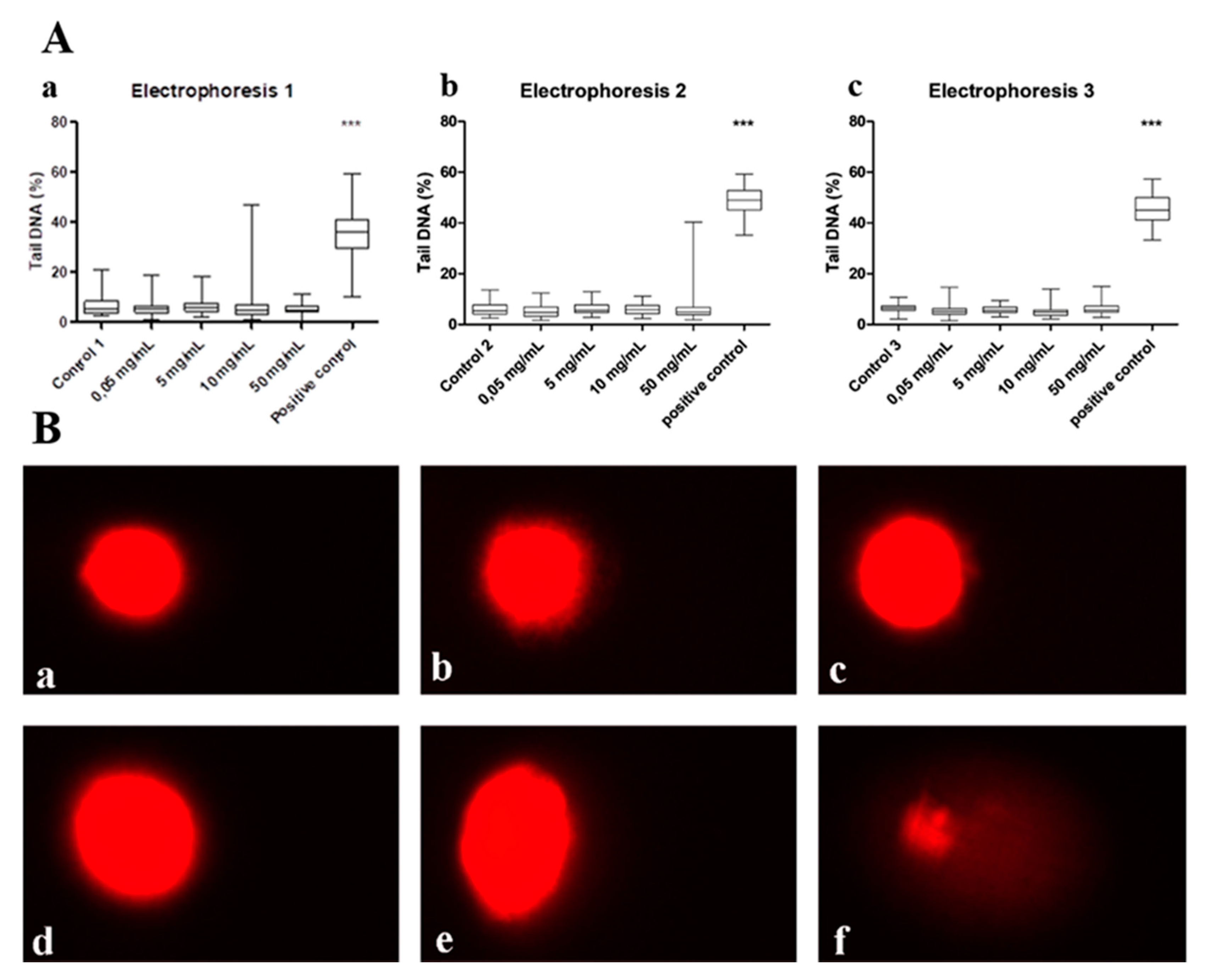
2.2. Genotoxicity Investigations In Vitro
2.2.1. Cell Exposure and Viability Evaluation
2.2.2. Alkaline Comet Assay
2.3. Systemic Subchronic Toxicity Investigation
2.3.1. Experimental Protocol
2.3.2. Blood Analysis
2.4. Histological and Stereological Analysis
2.5. Immunohistochemical Analysis
2.6. Statistical Analyses
3. Results
3.1. Genotoxicity Results
3.2. Systemic Subchronic Toxicity Results
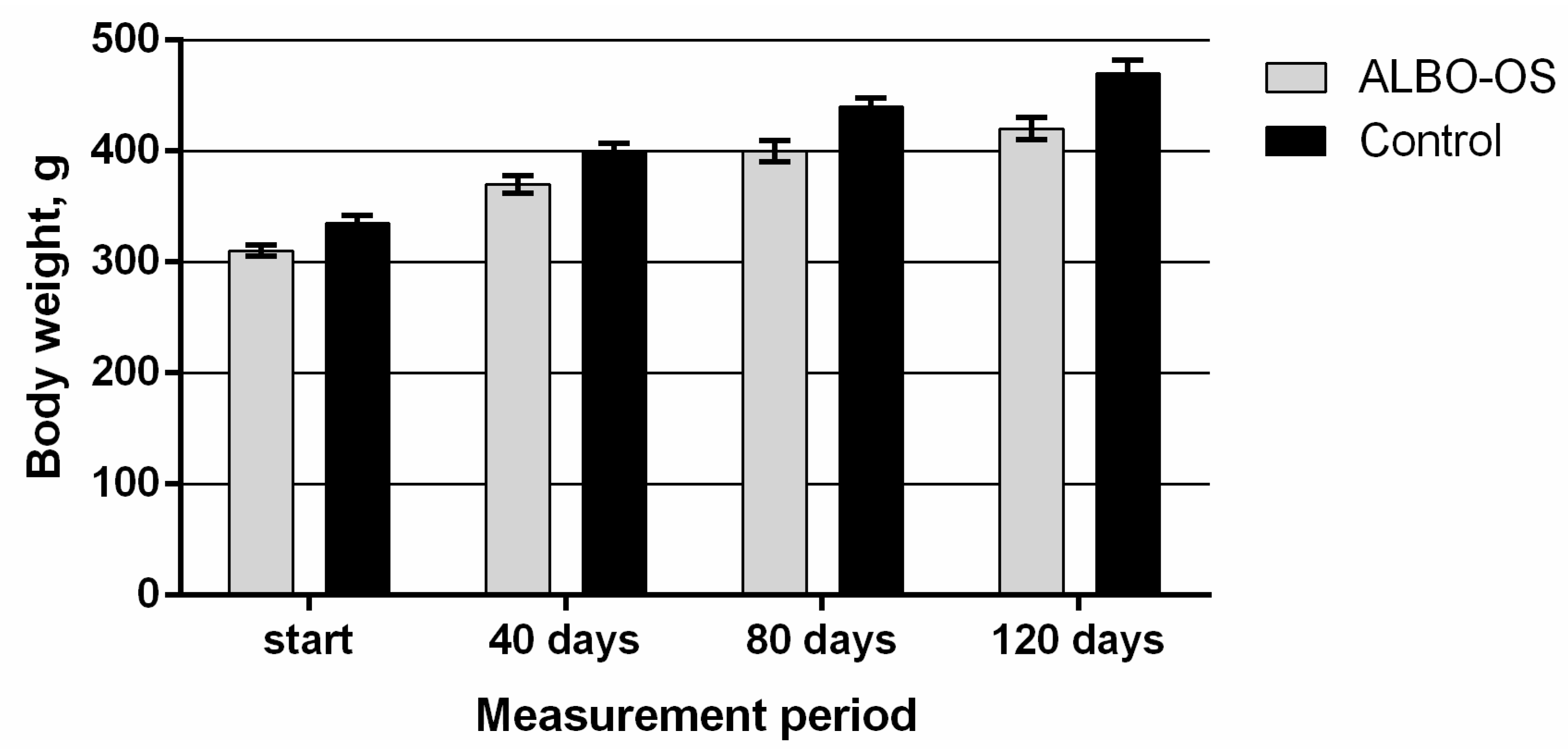
3.2.1. Main Clinical Symptoms and Observations
3.2.2. Results of Blood Analysis
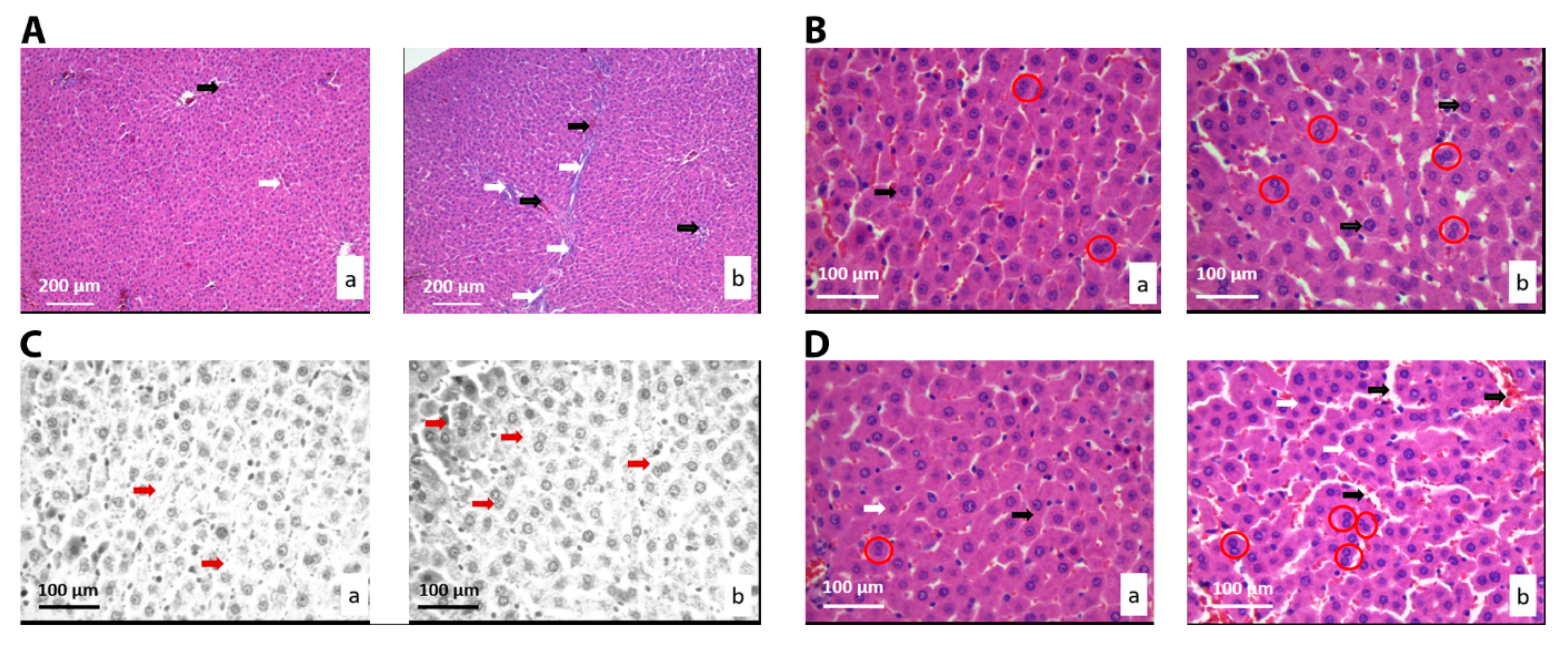
3.2.3. Histological and Stereological Analysis of the Liver Tissue
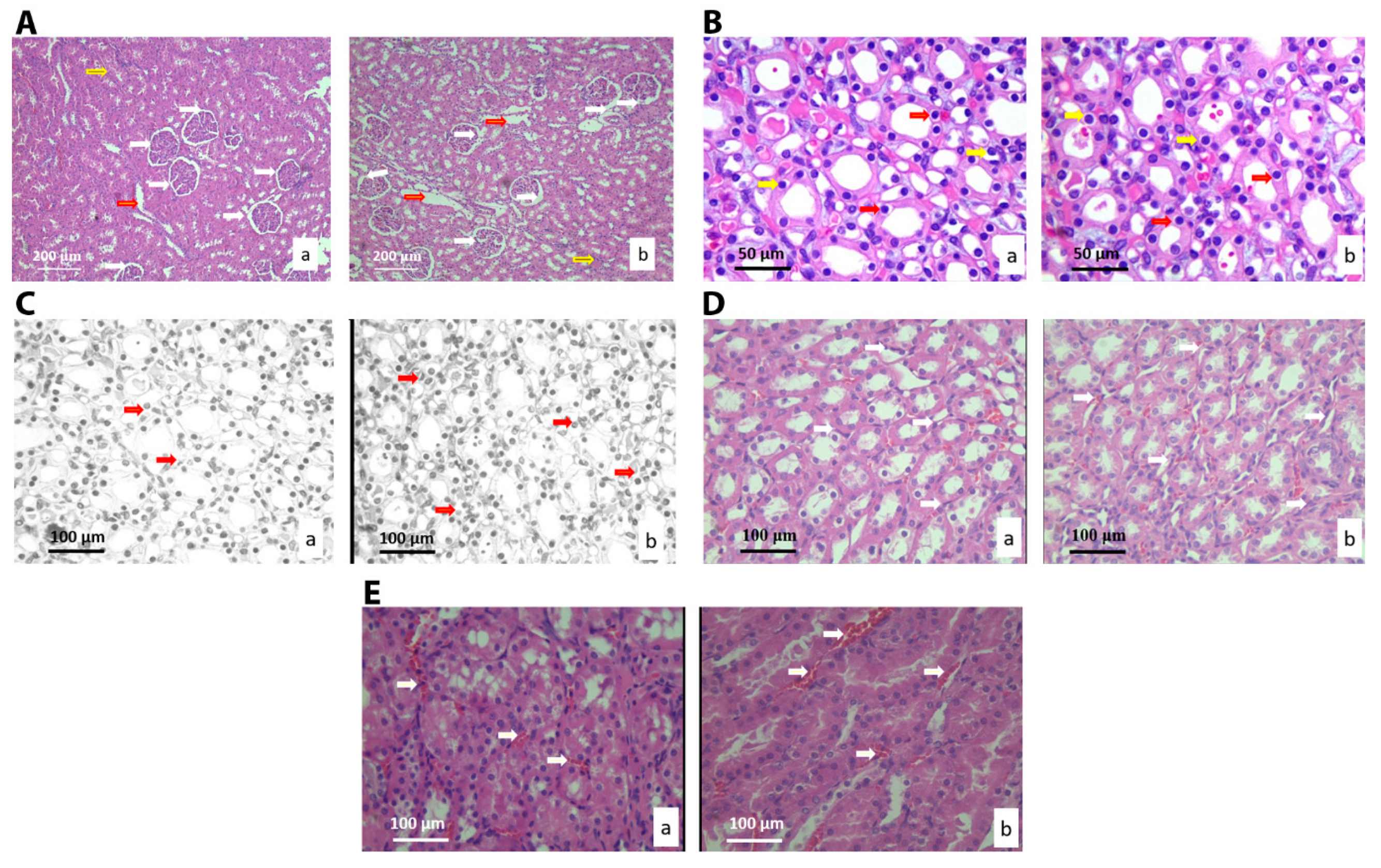
3.2.4. Histological and Stereological Analysis of the Kidney Tissue
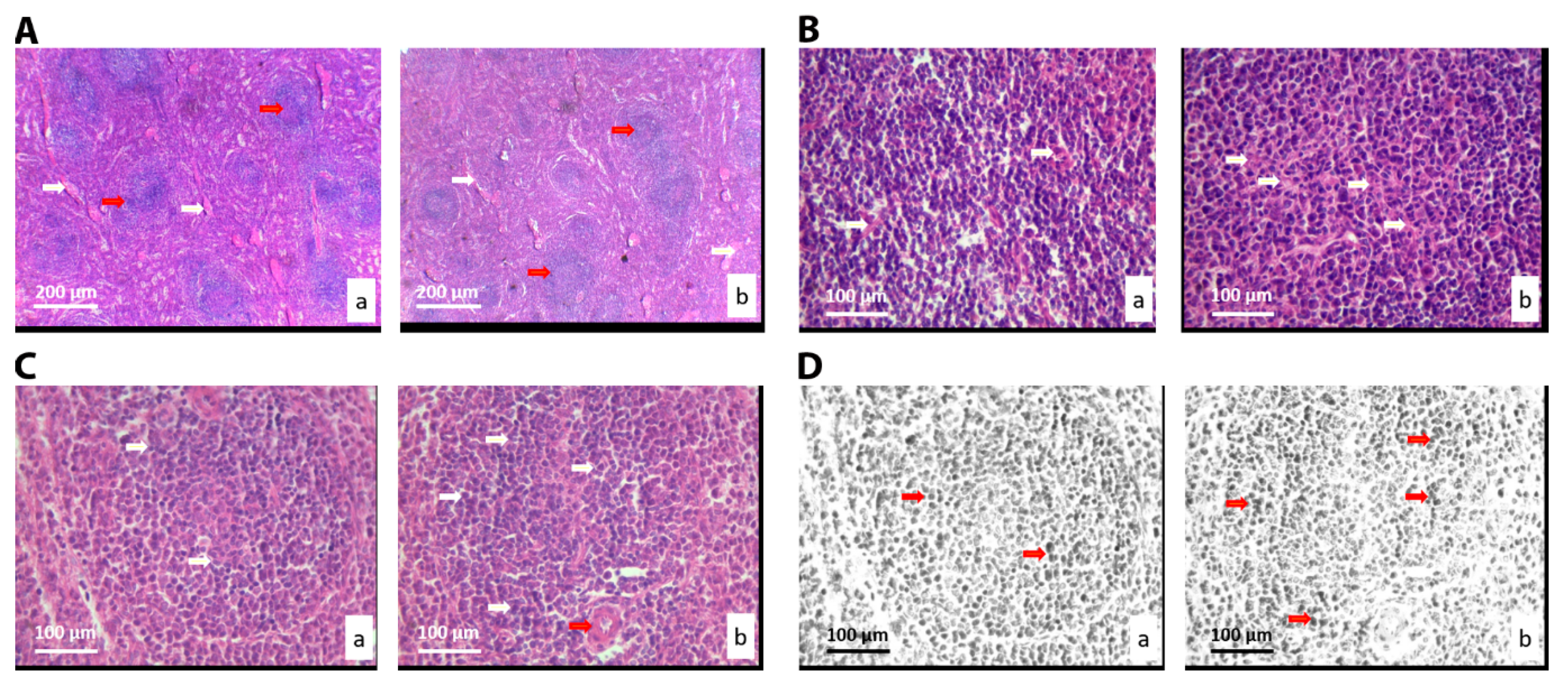
3.2.5. Histological and Stereological Analysis of the Spleen Tissue
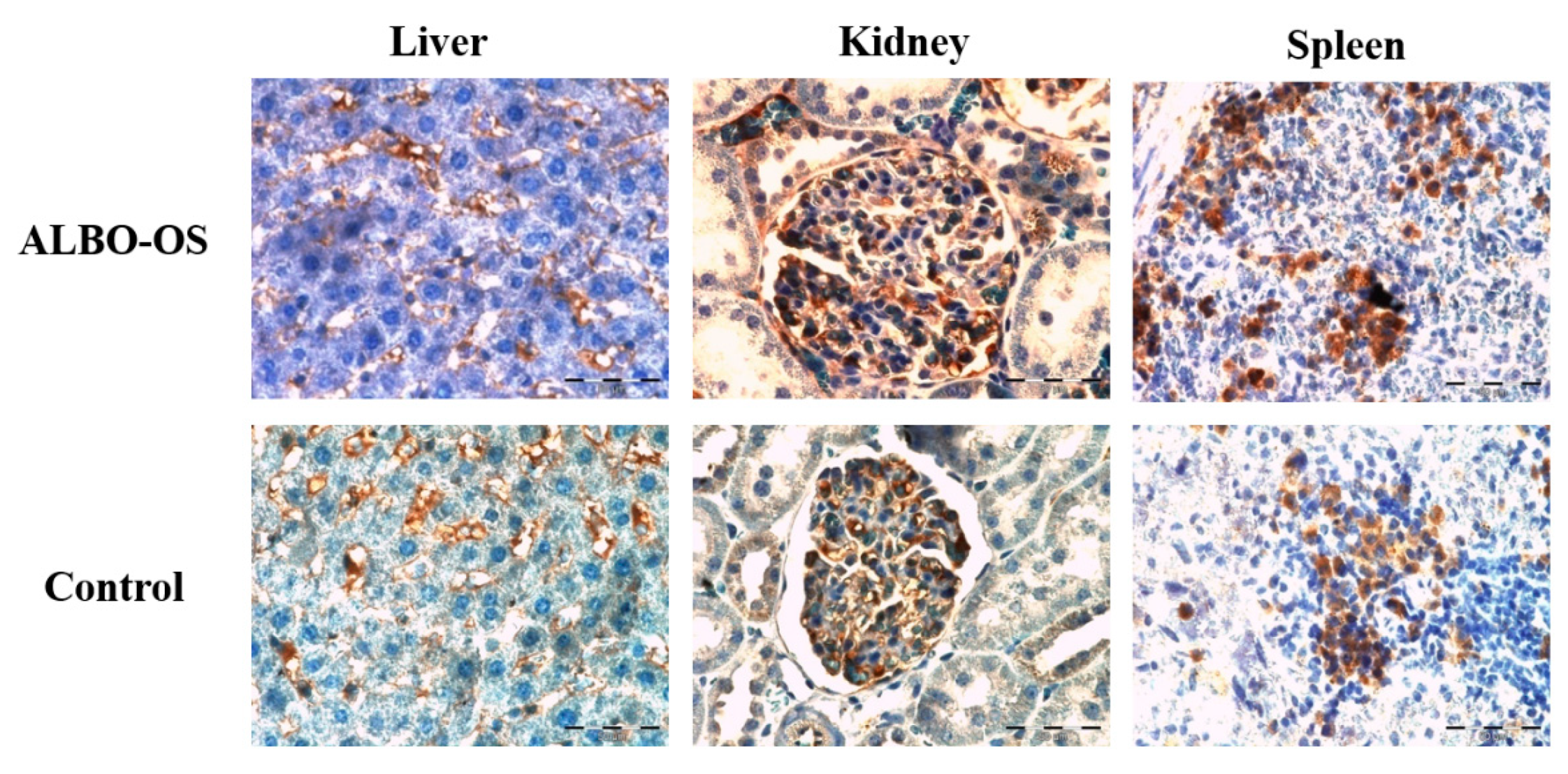
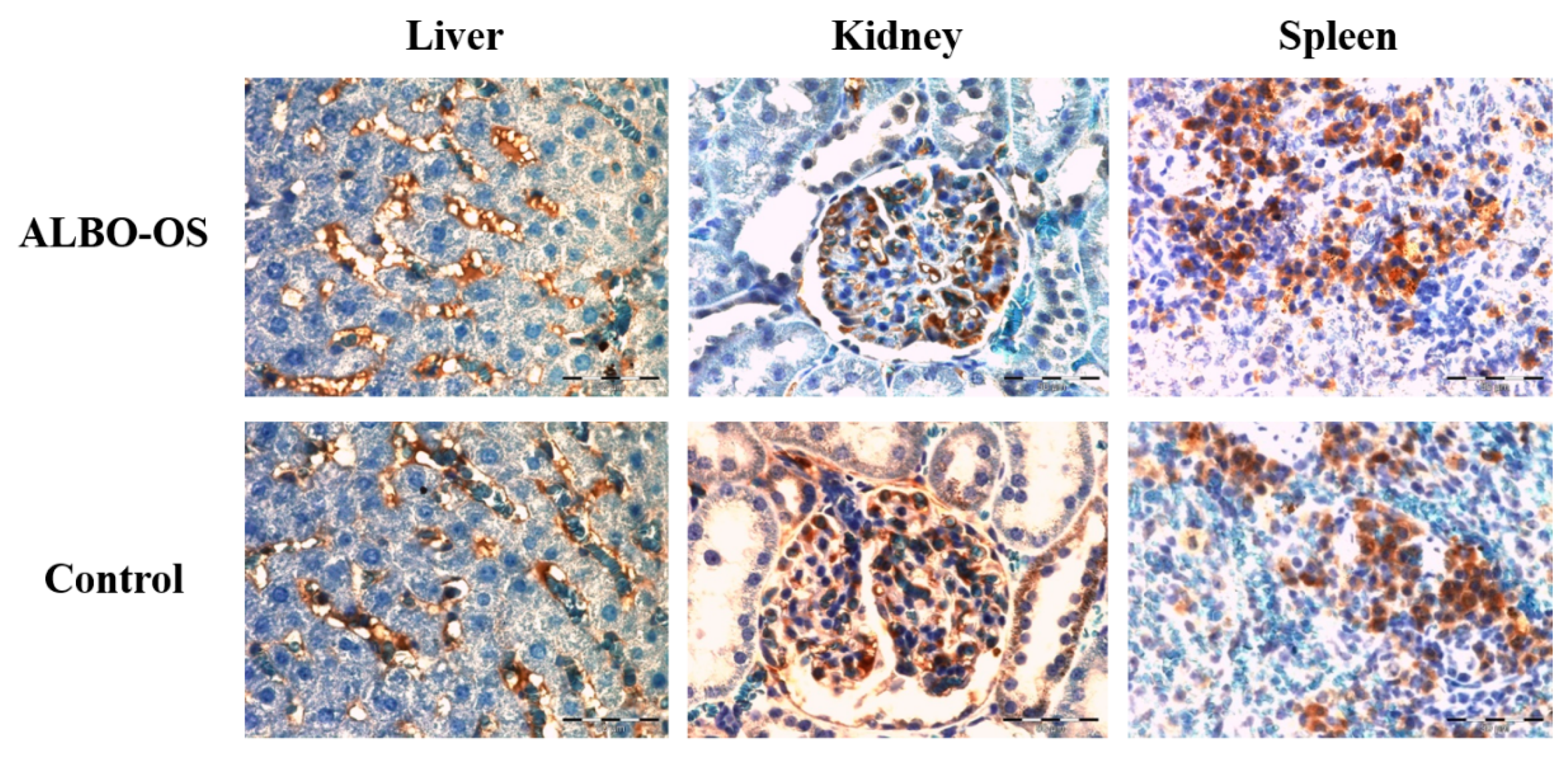
3.2.6. Expression of Proteins Ki67 and CD68 in Liver, Kidney, and Spleen
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Physicochemical Characterization of the Extract of the ALBO-OS Scaffolds
Appendix A.2. A Brief Review of ALBO-OS Structural and Physicochemical Characteristics
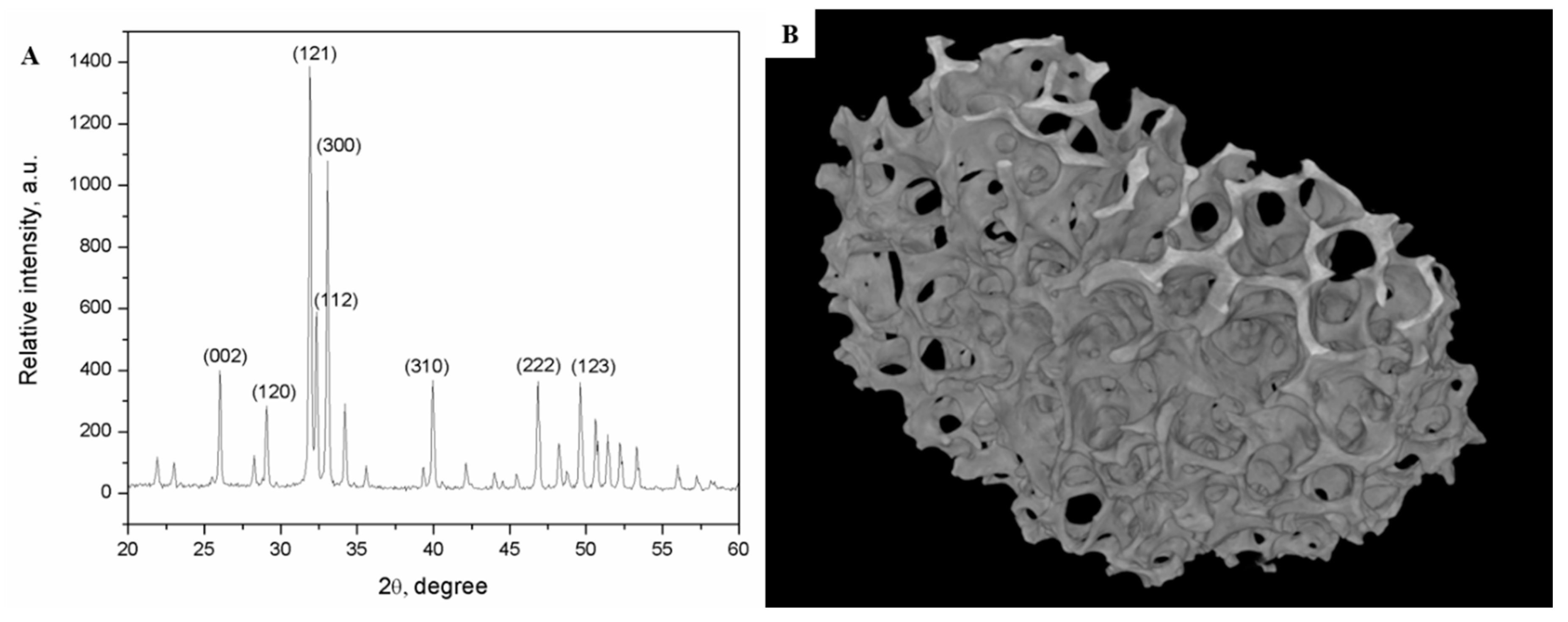
| Pore Sizes | Total Pore Volume (%) | Thickness Distribution of the Scaffold Inner Walls (%) | Number of Pores with Average Radius in the Given Range | Pores of Average Radius in the Given Range in Total Number of Pores (%) |
|---|---|---|---|---|
| 0.06 mm–0.1 mm | 2.52 | 8.90 | 9333 | 61.6 |
| 0.1 mm–0.2 mm | 5.60 | 64.42 | 3294 | 21.7 |
| 0.2 mm–0.3 mm | 5.83 | 26.68 | 737 | 4.8 |
| 0.3 mm–0.4 mm | 11.66 | / | 971 | 6.4 |
| 0.4 mm–0.5 mm | 17.44 | / | 379 | 2.5 |
| 0.5 mm–0.6 mm | 18.18 | / | 216 | 1.4 |
| 0.6 mm–0.7 mm | 16.96 | / | 119 | 0.8 |
| 0.7 mm–0.8 mm | 15.06 | / | 72 | 0.5 |
| More than 0.8 mm | 6.75 | / | 25 | 0.2 |
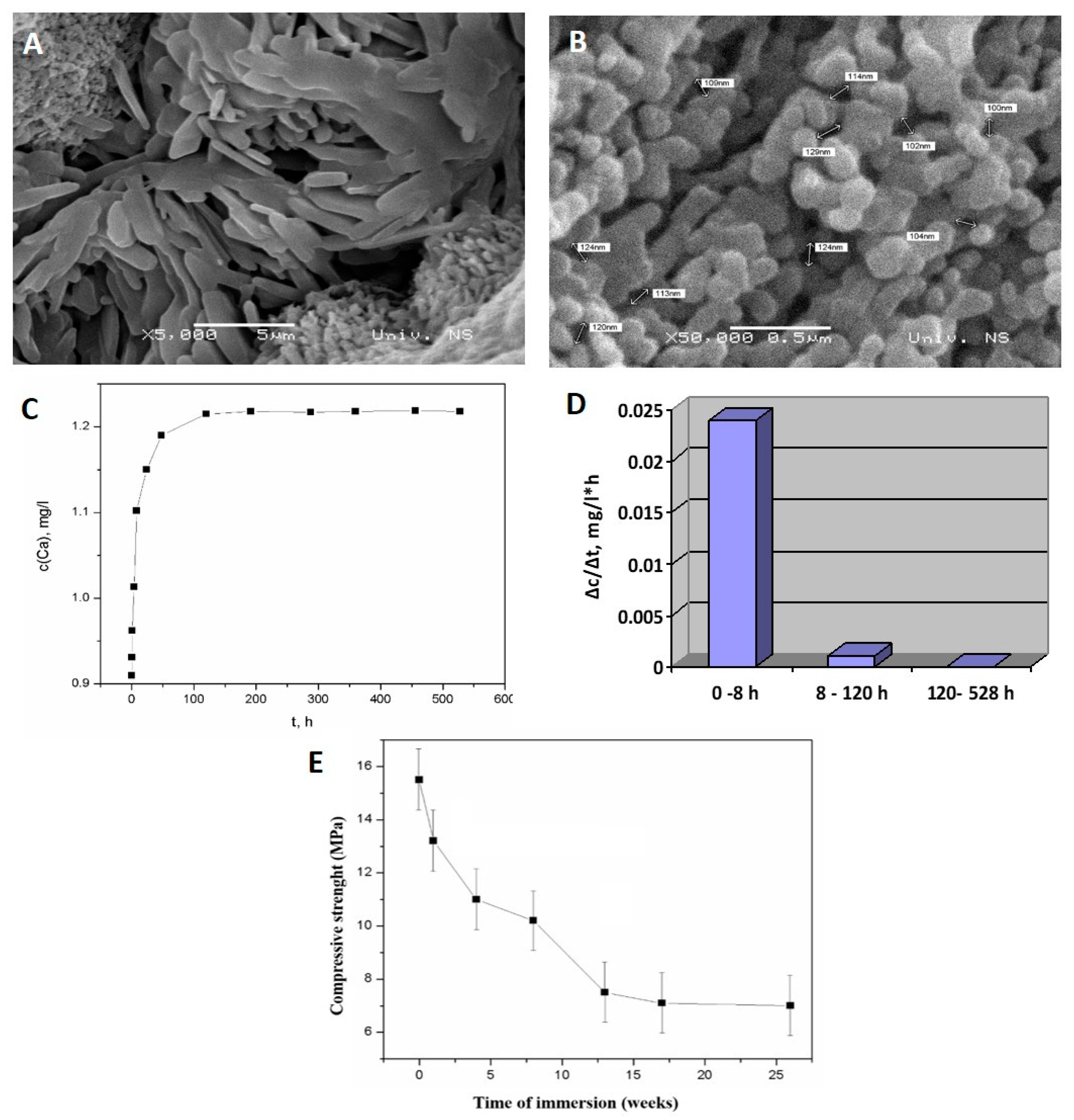
Appendix A.2.1. Rate of ALBO-OS Degradation
Appendix A.2.2. Change of Compressive Strength during ALBO-OS Degradation
References
- Boga, J.C.; Miguel, S.P.; de Melo-Diogo, D.; Mendonça, A.G.; Louro, R.O.; Correia, I.J. In Vitro characterization of 3D printed scaffolds aimed at bone tissue regeneration. Colloids Surf. B Biointerfaces 2018, 165, 207–218. [Google Scholar] [CrossRef]
- Erasmus, E.P.; Sule, R.; Johnson, O.T.; Massera, J.; Sigalas, I. In Vitro evaluation of porous borosilicate, borophosphate and phosphate bioactive glasses scaffolds fabricated using foaming agent for bone regeneration. Sci. Rep. 2018, 8, 3699. [Google Scholar] [CrossRef]
- Jeon, O.H.; Elisseeff, J. Orthopedic tissue regeneration: Cells, scaffolds, and small molecules. Drug Deliv. Transl. Res. 2016, 6, 105–120. [Google Scholar] [CrossRef]
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In Vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of growth factors using a smart porous nanocomposite scaffold to repair a mandibular bone defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef]
- Huang, W.; Shi, X.; Ren, L.; Du, C.; Wang, Y. PHBV microspheres—PLGA matrix composite scaffold for bone tissue engineering. Biomaterials 2010, 31, 4278–4285. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Pandey, A.; Midha, S.; Sharma, R.K.; Maurya, R.; Nigam, V.K.; Ghosh, S.; Balani, K. Antioxidant and antibacterial hydroxyapatite-based biocomposite for orthopedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 13–24. [Google Scholar] [CrossRef]
- Boehler, R.M.; Shin, S.; Fast, A.G.; Gower, R.M.; Shea, L.D. A PLG/HAp composite scaffold for lentivirus delivery. Biomaterials 2013, 34, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Dau, M.; Ganz, C.; Zaage, F.; Staedt, H.; Goetze, E.; Gerber, T.; Kämmerer, P.W. In Vivo comparison of a granular and putty form of a sintered and a non-sintered silica-enhanced hydroxyapatite bone substitute material. J. Biomater. Appl. 2019, 088532821987758. [Google Scholar] [CrossRef]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef]
- Jokanović, V.; Čolović, B.; Marković, D.; Petrović, M.; Jokanović, M.; Milosavljević, P.; Sopta, J. In Vivo investigation of ALBO-OS scaffold based on hydroxyapatite and PLGA. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Jokanović, V.; Čolović, B.; Marković, D.; Petrović, M.; Soldatović, I.; Antonijević, D.; Milosavljević, P.; Sjerobabin, N.; Sopta, J. Extraordinary biological properties of a new calcium hydroxyapatite/poly(lactide-co-glycolide)-based scaffold confirmed by in Vivo investigation. Biomed. Eng. Biomed. Tech. 2017, 62, 295–306. [Google Scholar] [CrossRef]
- Sjerobabin, N.; Čolović, B.; Petrović, M.; Marković, D.; Živković, S.; Jokanović, V. Cytotoxicity investigation of a new hydroxyapatite scaffold with improved structural design. Srp. Arh. Celok. Lek. 2016, 144, 280–287. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. In Current Protocols in Immunology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Volume Appendix 3, p. Appendix 3B. [Google Scholar]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for In Vitro and In Vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Nestorovic, N.; Trifunovic, S.; Jaric, I.; Manojlovic-Stojanoski, M.; Ristic, N.; Filipovic, B.; Sosic-Jurjevic, B.; Milosevic, V. Sex steroid application reverses changes in rat castration cells: Unbiased stereological analysis. Arch. Biol. Sci. 2016, 68, 821–828. [Google Scholar] [CrossRef]
- Santos, M.; Marcos, R.; Santos, N.; Malhão, F.; Monteiro, R.A.F.; Rocha, E. An unbiased stereological study on subpopulations of rat liver macrophages and on their numerical relation with the hepatocytes and stellate cells. J. Anat. 2009, 214, 744–751. [Google Scholar] [CrossRef]
- Pridans, C.; Sauter, K.A.; Irvine, K.M.; Davis, G.M.; Lefevre, L.; Raper, A.; Rojo, R.; Nirmal, A.J.; Beard, P.; Cheeseman, M.; et al. Macrophage colony-stimulating factor increases hepatic macrophage content, liver growth, and lipid accumulation in neonatal rats. Am. J. Physiol. Liver Physiol. 2018, 314, G388–G398. [Google Scholar] [CrossRef]
- Wahab, N.F.A.C.; Kannan, T.P.; Mahmood, Z.; Rahman, I.A.; Ismail, H. Genotoxicity assessment of biphasic calcium phosphate of modified porosity on human dental pulp cells using Ames and Comet assays. Toxicol. Vitro 2018, 47, 207–212. [Google Scholar] [CrossRef]
- Żywicka, B.; Krucińska, I.; Garcarek, J.; Szymonowicz, M.; Komisarczyk, A.; Rybak, Z. Biological properties of low-toxic PLGA and PLGA/PHB fibrous nanocomposite scaffolds for osseous tissue regeneration. Evaluation of potential bioactivity. Molecules 2017, 22, 1852. [Google Scholar] [CrossRef]
- Opačić-Galić, V.; Petrović, V.; Živković, S.; Jokanović, V.; Nikolić, B.; Knežević-Vukčević, J.; Mitić-Ćulafić, D. New nanostructural biomaterials based on active silicate systems and hydroxyapatite: Characterization and genotoxicity in human peripheral blood lymphocytes. Int. Endod. J. 2013, 46, 506–516. [Google Scholar] [CrossRef]
- Petrović, V.; Opačić-Galić, V.; Živković, S.; Nikolić, B.; Danilović, V.; Miletić, V.; Jokanović, V.; Mitić-Ćulafić, D. Biocompatibility of new nanostructural materials based on active silicate systems and hydroxyapatite: In Vitro and in Vivo study. Int. Endod. J. 2015, 48, 966–975. [Google Scholar] [CrossRef]
- De Almeida, A.D.; Leite, F.G.; Chaud, M.V.; de Rebelo, M.A.; de Borges, L.C.F.S.; Viroel, F.J.M.; Hataka, A.; Grotto, D. Safety and efficacy of hydroxyapatite scaffold in the prevention of jaw osteonecrosis in Vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1799–1808. [Google Scholar] [CrossRef]
- Tan, Y.-J.; Ren, Y.-S.; Gao, L.; Li, L.-F.; Cui, L.-J.; Li, B.; Li, X.; Yang, J.; Wang, M.-Z.; Lv, Y.-Y.; et al. 28-day oral chronic toxicity study of arctigenin in rats. Front. Pharmacol. 2018, 9, 1077. [Google Scholar] [CrossRef]
- He, Y.; Tao, H.; Zhang, Y.; Jiang, Y.; Zhang, S.; Zhao, C.; Li, J.; Zhang, B.; Song, Y.; Zhang, X. Biocompatibility of bio-Mg-Zn alloy within bone with heart, liver, kidney and spleen. Sci. Bull. 2009, 54, 484–491. [Google Scholar] [CrossRef]
- Khalil, W.A.; Eid, N.F. Biocompatibility of BioAggregate and mineral trioxide aggregate on the liver and kidney. Int. Endod. J. 2013, 46, 730–737. [Google Scholar] [CrossRef]
- Gerlach, C.; Sakkab, D.Y.; Scholzen, T.; Daßler, R.; Alison, M.R.; Gerdes, J. Ki-67 expression during rat liver regeneration after partial hepatectomy. Hepatology 1997, 26, 573–578. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Camasses, A.; Parisis, N.; Nicolas, E.; Llères, D.; Gerbe, F.; Prieto, S.; Krasinska, L.; David, A.; et al. The cell proliferation antigen Ki-67 organises heterochromatin. eLife 2016, 5, e13722. [Google Scholar] [CrossRef]
- Paraš, S.; Janković, O.; Trišić, D.; Čolović, B.; Mitrović-Ajtić, O.; Dekić, R.; Soldatović, I.; Živković Sandić, M.; Živković, S.; Jokanović, V. Influence of nanostructured calcium aluminate and calcium silicate on the liver: Histological and unbiased stereological analysis. Int. Endod. J. 2019, 52, 1162–1172. [Google Scholar]
- Paranjpe, S.; Bowen, W.C.; Tseng, G.C.; Luo, J.H.; Orr, A.; Michalopoulos, G.K. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am. J. Pathol. 2010, 176, 2669–2681. [Google Scholar] [CrossRef]
- Braet, F.; Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: A review. Comp. Hepatol. 2002, 1, 1. [Google Scholar] [CrossRef]
- Shah, V.; Haddad, F.G.; Garcia-Cardena, G.; Frangos, J.A.; Mennone, A.; Groszmann, R.J.; Sessa, W.C. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J. Clin. Investig. 1997, 100, 2923–2930. [Google Scholar] [CrossRef]
- Jokanović, V.; Čolović, B.; Prokić, B.B.; Tomanović, N.; Popović Bajić, M.; Živković, S. Subchronic Systemic toxicity of new endodontic material based on calcium hydroxyapatite and calcium silicates. Adv. Mater. Sci. Eng. 2018, 2018, 8493439. [Google Scholar] [CrossRef]
- Demirkaya, K.; Can Demirdöğen, B.; Öncel Torun, Z.; Erdem, O.; Çetinkaya, S.; Akay, C. In Vivo evaluation of the effects of hydraulic calcium silicate dental cements on plasma and liver aluminium levels in rats. Eur. J. Oral Sci. 2016, 124, 75–81. [Google Scholar] [CrossRef]
- You, Z.; Xin, Y.; Liu, Y.; Han, B.; Zhang, L.; Chen, Y.; Chen, Y.; Gu, L.; Gao, H.; Xuan, Y. Protective effect of Salvia Miltiorrhizae injection on N(G)-nitro-d-arginine induced nitric oxide deficient and oxidative damage in rat kidney. Exp. Toxicol. Pathol. 2012, 64, 453–458. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; He, Y.; Jiang, Y.; Zhang, J.; Niu, J.; Mao, L.; Yuan, G. In Vivo degradation behavior and biocompatibility of Mg-Nd-Zn-Zr alloy at early stage. Int. J. Mol. Med. 2012, 29, 178–184. [Google Scholar]
- Fray, J.C.S. Stretch receptor model for renin release with evidence from perfused rat kidney. Am. J. Physiol. 1976, 231, 936–944. [Google Scholar] [CrossRef]
- Gui, S.; Zhang, Z.; Zheng, L.; Cui, Y.; Liu, X.; Li, N.; Sang, X.; Sun, Q.; Gao, G.; Cheng, Z.; et al. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2011, 195, 365–370. [Google Scholar] [CrossRef]
- Scholz, H.; Hamann, M.; Götz, K.H.; Kurtz, A. Role of calcium ions in the pressure control of renin secretion from the kidneys. Pflugers Arch. 1994, 428, 173–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lichtman, A.H.; Segel, G.B.; Lichtman, M.A. The role of calcium in lymphocyte proliferation. Blood 1983, 61, 413–422. [Google Scholar] [CrossRef] [PubMed]
- McCrady, C.W.; Ely, C.M.; Westin, E.; Carchman, R.A. Coordination and reversibility of signals for proliferative activation and interleukin-2 mRNA production in resting human T lymphocytes by phorbol ester and calcium ionophore. J. Biol. Chem. 1988, 263, 18537–18544. [Google Scholar]
- Han, A.Y.; Zhang, M.H.; Zuo, X.L.; Zheng, S.S.; Zhao, C.F.; Feng, J.H.; Cheng, C. Effect of acute heat stress on calcium concentration, proliferation, cell cycle, and interleukin-2 production in splenic lymphocytes from broiler chickens. Poult. Sci. 2010, 89, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Lee, H.B.; Blaufox, M.D. Blood volume in the rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar]
- Huang, Y.X.; Ren, J.; Chen, C.; Ren, T.B.; Zhou, X.Y. Preparation and properties of poly(lactide-co-glycolide) (PLGA)/ Nano-Hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J. Biomater. Appl. 2008, 22, 409–432. [Google Scholar] [CrossRef]

| ALBO-OS | Control | |
|---|---|---|
| Leucocytes | 4.12 ± 1.94 | 6.83 ± 0.77 *** |
| Hemoglobin | 144 ± 8.26 | 140.8 ± 6.76 |
| Thrombocytes | 566.56 ± 269.54 | 713.20 ± 181.58 |
| ALBO-OS | Control | |
|---|---|---|
| ALT | 74.50 ± 23.07 | 71.50 ± 31.11 |
| AST | 630.50 ± 637.22 | 844.32 ± 838.57 |
| Urea | 5.68 ± 0.81 | 5.21 ± 0.36 |
| Creatinine | 42.30 ± 5.50 | 40.00 ± 4.84 |
| Bilirubin | 1.12 ± 0.51 | 1.01 ± 0.32 |
| Glucose | 6.23 ± 1.70 | 5.48 ± 0.99 |
| ALP | 81.50 ± 13.91 *** | 63.00 ± 9.80 |
| Parameter | ALBO-OS | Control |
|---|---|---|
| Volume density of hepatocytes (mm0) | 0.681 ± 0.032 | 0.656 ± 0.045 |
| Volume density of capillary sinusoids (mm0) | 0.204 ± 0.008 | 0.163 ± 0.005 |
| Volume density of connective tissue (mm0) | 0.138 ± 0.004 | 0.126 ± 0.003 |
| Number of hepatocytes | 273,818.2 ± 24,146.0 | 255,168.4 ± 21,822.7 |
| Numerical density of hepatocytes (mm−3) | 47,887.4 ± 3119.6 | 44,575.3 ± 2632.9 |
| Surface area of hepatocytes (μm2) | 152.8 ± 4.1 | 148.5 ± 2.9 |
| Surface area of hepatic nucleuses (μm2) | 49.2 ± 1.9 | 46.8 ± 2.1 |
| NCO of hepatocytes | 0.383 ± 0.022 | 0.324 ± 0.023 |
| Mitotic index of hepatocytes | 1.77 ± 0.234 | 1.61 ± 0.24 |
| Number of connective tissue cells | 138,442.5 ± 16,443.4 | 127,486.1 ± 17,518.1 |
| Numerical density of connective tissue cells (mm−3) | 24,888.3 ± 3084.8 | 21,934.4 ± 2366.6 |
| Surface area of connective tissue cells (μm2) | 103.4 ± 2.4 | 99.6 ± 3.3 |
| Number of capillary endothelial cells | 328,132.2 ± 32,336.3 * | 275,915.5 ± 29,079.0 |
| Numerical density of capillary endothelial cells (mm−3) | 53,714.3 ± 953.7 * | 48,068.5 ± 2806.7 |
| Surface area of capillary endothelial cells (μm2) | 84.5 ± 4.6 | 75.1 ± 3.9 |
| Parameter | ALBO-OS | Control |
|---|---|---|
| Volume density of collecting ductus’ epithelial cells (mm0) | 0.34 ± 0.04 | 0.33 ± 0.04 |
| Volume density of blood sinusoids (mm0) | 0.26 ± 0.03 | 0.23 ± 0.03 |
| Volume density of connective tissue (mm0) | 0.11 ± 0.01 | 0.10 ± 0.01 |
| Volume density of glomeruli (mm0) | 0.32 ± 0.04 | 0.30 ± 0.04 |
| Number of collecting ducts’ epithelial cells | 139,562 ± 1523 | 138,147 ± 3332 |
| Numerical density of collecting ducts’ epithelial cells (mm−3) | 20,107 ± 4116 | 19,572 ± 2621 |
| Surface area of collecting ducts’ epithelial cells (μm2) | 209 ± 43 | 204 ± 50 |
| Surface area of collecting ducts’ epithelial cells nucleuses (μm2) | 65 ± 3 | 63 ± 3 |
| NCO of collecting ducts’ epithelial cells | 0.28 ± 0.02 | 0.26 ± 0.03 |
| Number of connective tissue cells | 141,133 ± 18,097 | 134,698 ± 12,922 |
| Numerical density of connective tissue cells (mm−3) | 22,990 ± 2366 | 22,133.4 ± 2982.2 |
| Surface area of connective tissue cells (μm2) | 99 ± 11 | 102 ± 6 |
| Number of capillary endothelial cells | 306,126 ± 24,065 | 296,854 ± 26,658 |
| Numerical density of capillary endothelial cells (mm−3) | 39,141 ± 3342 | 37,645 ± 4459 |
| Surface area of capillary endothelial cells (μm2) | 77 ± 4 | 75 ± 4 |
| Surface area of glomeruli (μm2) | 3086 ± 415 | 4060 ± 535 * |
| Bowman’s space | 49 ± 4 | 44 ± 4 |
| Parameter | ALBO-OS | Control |
|---|---|---|
| Volume density of epithelial cells (mm0) | 0.382 ± 0.036 | 0.359 ± 0.029 |
| Volume density of lymphocytes (mm0) | 0.409 ± 0.036 | 0.304 ± 0.058 |
| Volume density of connective tissue (mm0) | 0.119 ± 0.023 | 0.115 ± 0.025 |
| Volume density of blood capillaries (mm0) | 0.217 ± 0.04 | 0.167 ± 0.05 |
| Number of epithelial cells | 223,847.1 ± 27,129.8 | 204,568.3 ± 33,317.8 |
| Numerical density of epithelial cells (mm−3) | 37,498.5 ± 3318.7 | 35,641.8 ± 2625.9 |
| Surface area of epithelial cells (μm2) | 128.6 ± 2.7 | 131.5 ± 4.2 |
| Surface area of epithelial cells’ nucleuses (μm2) | 48.2 ± 5.3 | 41.2 ± 3.1 |
| NCO of epithelial cells | 0.274 ± 0.045 | 0.285 ± 0.052 |
| Number of connective tissue cells | 149,901.1 ± 6287.0 * | 137,006.4 ± 8694.3 |
| Numerical density of connective tissue cells (mm−3) | 26,104.9 ± 2169.8 | 23,146.3 ± 2871.5 |
| Surface area of connective tissue cells (μm2) | 110.0 ± 4.6 | 105.6 ± 5.7 |
| Number of capillary endothelial cells | 241,697.6 ± 40,694.6 | 223,148.5 ± 20,204.4 |
| Numerical density of capillary endothelial cells (mm−3) | 39,905.4 ± 3588.8 | 34,969.4 ± 2599.3 |
| Surface area of capillary endothelial cells (μm2) | 78.6 ± 2.5 | 74.16 ± 2.3 |
| Number of lymphocytes | 432,527.8 ± 37,841.8 * | 348,336.9 ± 23,324.7 |
| Numerical density of lymphocytes (mm−3) | 57,323.4 ± 2201.4 * | 49,131.5 ± 3740.2 |
| Surface area of lymphocytes (μm2) | 97.2 ± 3.5 | 96.4 ± 4.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraš, S.; Trišić, D.; Mitrović Ajtić, O.; Prokić, B.; Drobne, D.; Živković, S.; Jokanović, V. Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(lactide-co-glycolide) after Subchronic Oral Exposure of Rats. Nanomaterials 2020, 10, 918. https://doi.org/10.3390/nano10050918
Paraš S, Trišić D, Mitrović Ajtić O, Prokić B, Drobne D, Živković S, Jokanović V. Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(lactide-co-glycolide) after Subchronic Oral Exposure of Rats. Nanomaterials. 2020; 10(5):918. https://doi.org/10.3390/nano10050918
Chicago/Turabian StyleParaš, Smiljana, Dijana Trišić, Olivera Mitrović Ajtić, Bogomir Prokić, Damjana Drobne, Slavoljub Živković, and Vukoman Jokanović. 2020. "Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(lactide-co-glycolide) after Subchronic Oral Exposure of Rats" Nanomaterials 10, no. 5: 918. https://doi.org/10.3390/nano10050918
APA StyleParaš, S., Trišić, D., Mitrović Ajtić, O., Prokić, B., Drobne, D., Živković, S., & Jokanović, V. (2020). Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(lactide-co-glycolide) after Subchronic Oral Exposure of Rats. Nanomaterials, 10(5), 918. https://doi.org/10.3390/nano10050918








