Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Preparation
2.3. Photon Correlation Spectroscopy (PCS)
2.4. Osmolarity and pH
2.5. Stability Studies by Turbiscan® AG Station
2.6. Morphological Analysis by Cryo-TEM
2.7. Raman Spectrometry
2.8. HPLC-UV Analysis
2.9. Encapsulation Efficiency (EE%)
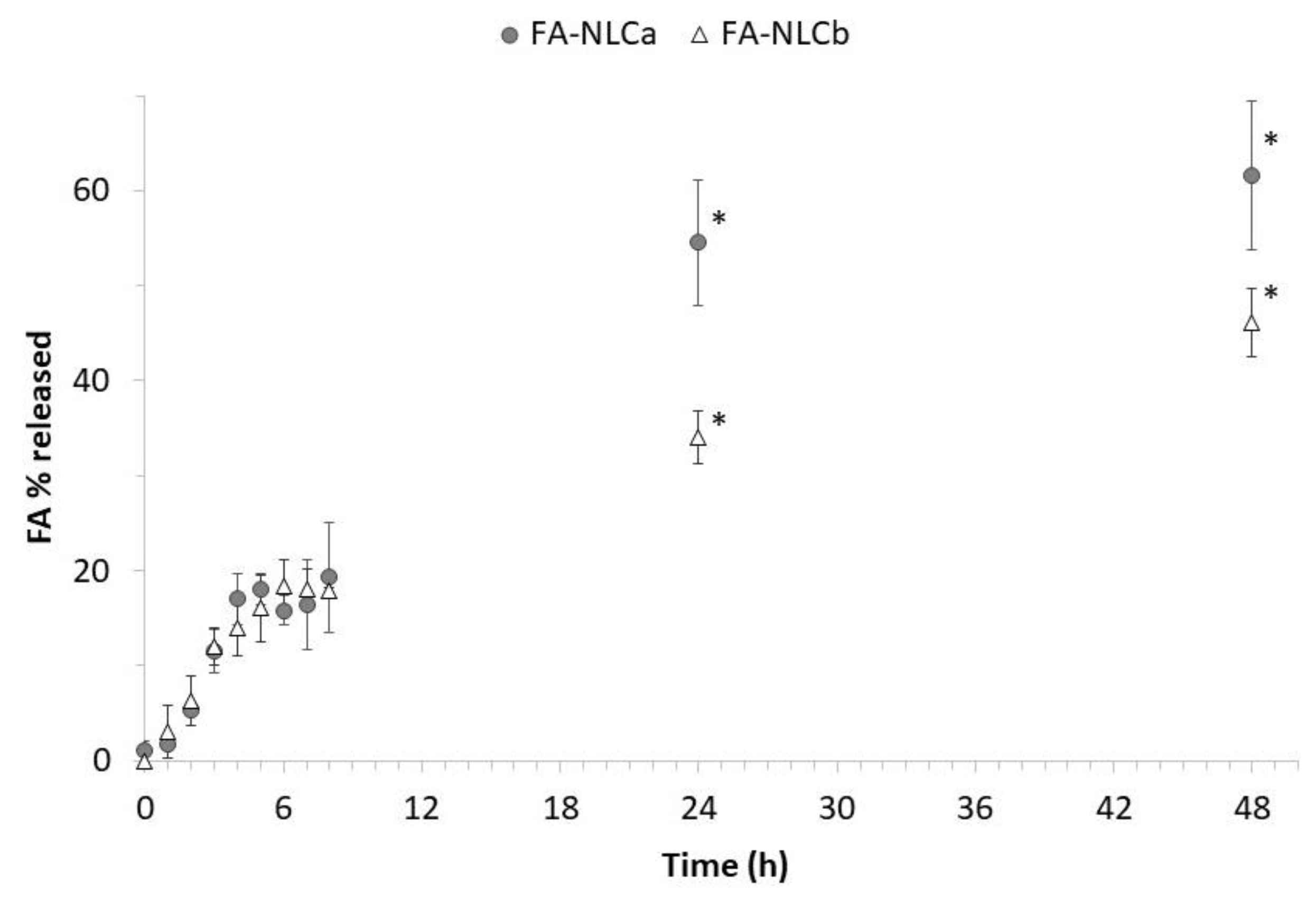
2.10. In Vitro Release Experiments
2.11. Antioxidant Activity: DPPH Assay
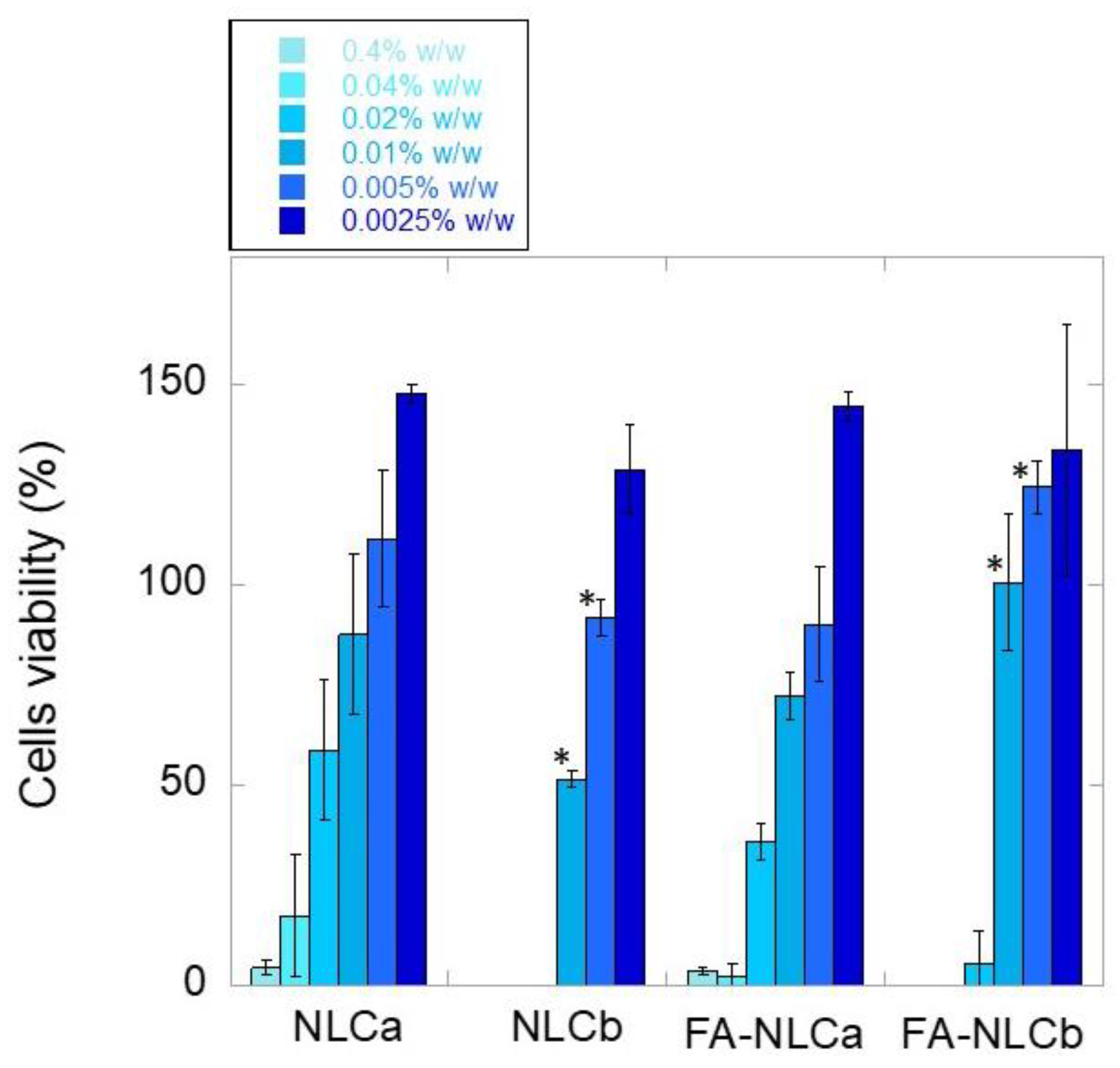
2.12. Cytocompatibility Test
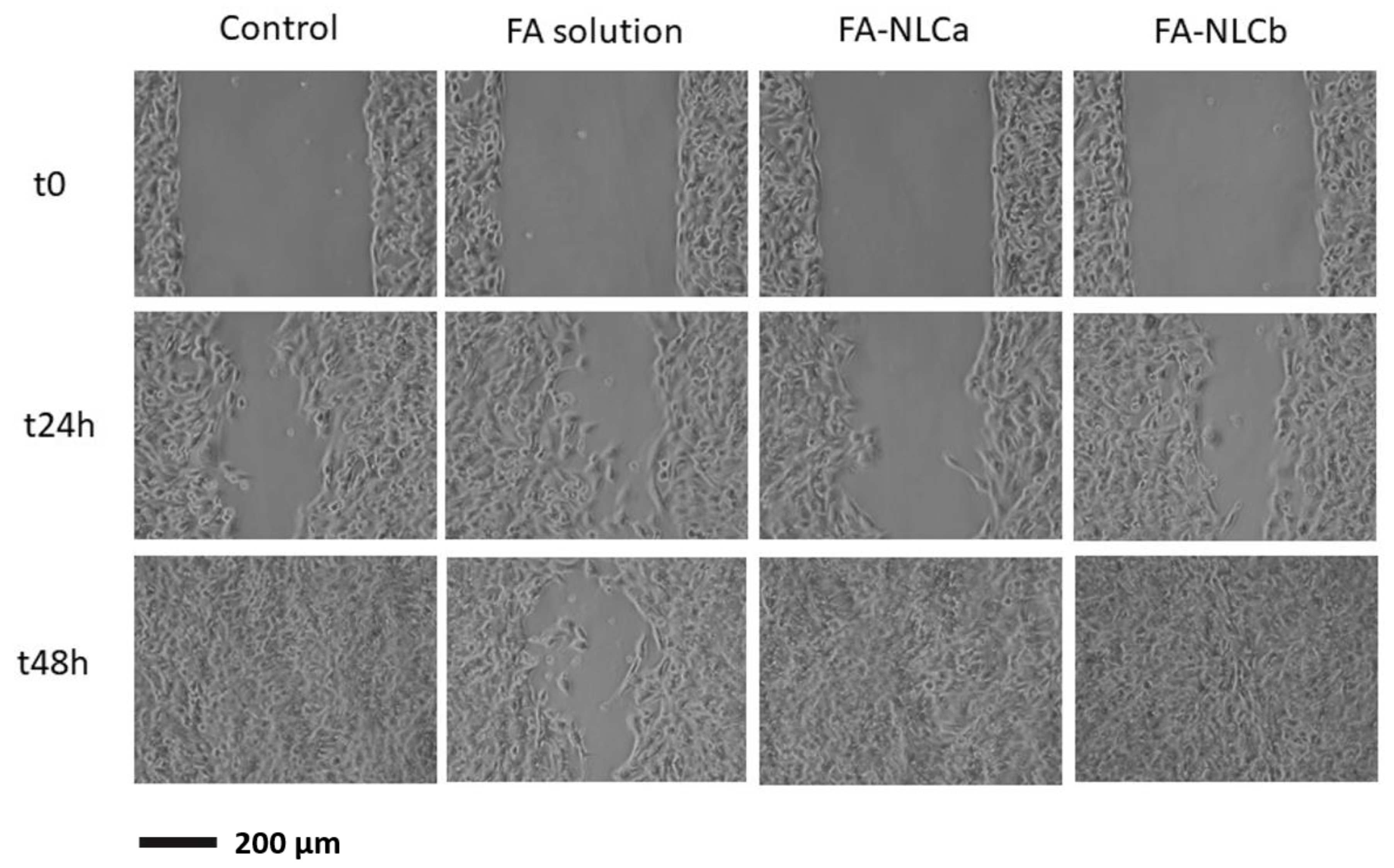
2.13. Migration Test
2.14. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Technological Characterization
3.2. In Vitro Biological Characterization: Cytocompatibility and Wound-Healing Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angelova, A.; Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural Regen. Res. 2017, 12, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kuhne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Liu, R.; Qiao, C.; Lu, Z.; Shi, Y.; Fan, Z.; Zhang, Z.; Zhang, X. Dual-targeting theranostic system with mimicking apoptosis to promote myocardial infarction repair via modulation of macrophages. Theranostics 2017, 7, 4149–4167. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Jain, A.; Hurkat, P.; Jain, S.K. Dual drug delivery using lactic acid conjugated SLN for effective management of neurocysticercosis. Pharm. Res. 2015, 32, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Li, J.; Liu, D.; Wang, C.; Sui, Z. Dual ligands modified double targeted nano-system for liver targeted gene delivery. Pharm. Biol. 2013, 51, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef]
- Zhang, T.; Prasad, P.; Cai, P.; He, C.; Shan, D.; Rauth, A.M.; Wu, X.Y. Dual-targeted hybrid nanoparticles of synergistic drugs for treating lung metastases of triple negative breast cancer in mice. Acta Pharmacol. Sin. 2017, 38, 835–847. [Google Scholar] [CrossRef]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-beta in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Pavan, M.; Novello, G.; Massa, N.; Rocchetti, A.; Berta, G.; Gamalero, E. Sensitivity of Candida albicans to essential oils: Are they an alternative to antifungal agents? J. Appl. Microbiol. 2016, 121, 1530–1545. [Google Scholar] [CrossRef]
- Blass, S.C.; Goost, H.; Tolba, R.H.; Stoffel-Wagner, B.; Kabir, K.; Burger, C.; Stehle, P.; Ellinger, S. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: A PRCT. Clin. Nutr. 2012, 31, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Woung, L.C.; Yen, J.C.; Tseng, P.C.; Chiou, S.H.; Sung, Y.J.; Liu, K.T.; Cheng, Y.H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Amato, G.; Carbone, C.; Diaz-Rodriguez, P.; Musumeci, T.; Concheiro, A.; Alvarez-Lorenzo, C.; Puglisi, G. Micelle-nanogel platform for ferulic acid ocular delivery. Int. J. Pharm. 2020, 576, 118986. [Google Scholar] [CrossRef]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Tee-ngam, P.; Nunant, N.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and rapid determination of ferulic acid levels in food and cosmetic samples using paper-based platforms. Sensors 2013, 13, 13039–13053. [Google Scholar] [CrossRef]
- Dwivedi, S.; Singh, D.; Deshmukh, P.T.; Soni, R.; Trivedi, R. Healing potential of ferulic acid on dermal wound in diabetic animals. Asian J. Mol. Model. 2015, 1, 1–16. [Google Scholar]
- Picone, P.; Bondi, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; Di Carlo, M. Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: Improved delivery by solid lipid nanoparticles. Free Radic. Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mukherjee, P.K.; Bahadur, S.; Biswas, R. Enhanced permeability of ferulic acid loaded nanoemulsion based gel through skin against UVA mediated oxidative stress. Life Sci. 2015, 141, 202–211. [Google Scholar] [CrossRef]
- Carbone, C.; Campisi, A.; Musumeci, T.; Raciti, G.; Bonfanti, R.; Puglisi, G. FA-loaded lipid drug delivery systems: Preparation, characterization and biological studies. Eur. J. Pharm. Sci. 2014, 52, 12–20. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Ferulic acid-loaded nanostructured lipid carriers: A promising nanoformulation against the ischemic neural injuries. Life Sci. 2018, 193, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, C.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Jia, L.; Zhang, Y.; Xu, X. Preparation and in vitro drug release of ferulic acid loaded chitosan microspheres containing liposomes. China J. Chin. Mater. Med. 2010, 35, 2972–2975. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Du, L.; Tnag, Q.; Xu, X. Study on pharmacokinetics of ferulic acid loaded liposome-in-chitosan-microspheres in rats. China J. Chin. Mater. Med. 2011, 36, 1751–1754. [Google Scholar]
- Souto, E.B.; Anselmi, C.; Centini, M.; Muller, R.H. Preparation and characterization of n-dodecyl-ferulate-loaded solid lipid nanoparticles (SLN). Int. J. Pharm. 2005, 295, 261–268. [Google Scholar] [CrossRef]
- Perez-Mosqueda, L.M.; Trujillo-Cayado, L.A.; Carrillo, F.; Ramirez, P.; Munoz, J. Formulation and optimization by experimental design of eco-friendly emulsions based on d-limonene. Colloids Surf. B Biointerfaces 2015, 128, 127–131. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Altaei, D.T. Topical lavender oil for the treatment of recurrent aphthous ulceration. Am. J. Dent. 2012, 25, 39–43. [Google Scholar]
- Koca Kutlu, A.; Cecen, D.; Gurgen, S.G.; Sayin, O.; Cetin, F. A Comparison Study of Growth Factor Expression following Treatment with Transcutaneous Electrical Nerve Stimulation, Saline Solution, Povidone-Iodine, and Lavender Oil in Wounds Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 361832. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; CARBONE, C.C.C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomesagainst inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Caddeo, C.; Pons, R.; Carbone, C.; Fernandez-Busquets, X.; Cardia, M.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr. Polym. 2017, 157, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Campisi, A.; Manno, D.; Serra, A.; Spatuzza, M.; Musumeci, T.; Bonfanti, R.; Puglisi, G. The critical role of didodecyldimethylammonium bromide on physico-chemical, technological and biological properties of NLC. Colloids Surf. B Biointerfaces 2014, 121, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.M.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E.B. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Musumeci, T.; Lauro, M.R.; Puglisi, G. Eco-friendly aqueous core surface-modified nanocapsules. Colloids Surf. B Biointerfaces 2015, 125, 190–196. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Ostacolo, C.; Maria Sommella, E.; Campiglia, P.; Carbone, C.; Drago, F.; Pignatello, R.; Bucolo, C. Ocular formulation based on palmitoylethanolamide-loaded nanostructured lipid carriers: Technological and pharmacological profile. Nanomaterials 2020, 10, 287. [Google Scholar] [CrossRef]
- Carbone, C.; Manno, D.; Serra, A.; Musumeci, T.; Pepe, V.; Tisserand, C.; Puglisi, G. Innovative hybrid vs polymeric nanocapsules: The influence of the cationic lipid coating on the “4S”. Colloids Surf. B Biointerfaces 2016, 141, 450–457. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Egea, M.A.; Souto, E.B.; Calpena, A.C.; Garcia, M.L. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology 2011, 22, 045101. [Google Scholar] [CrossRef]
- Araujo, J.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 167–175. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan coated nanostructured lipid carriers (NLCs) for loading Vitamin D: A physical stability study. Int. J. Boil. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef]
- Iemsam-Arng, J.; Ketchart, O.; Rattana-Amron, T.; Wutikhun, T.; Tapaneeyakorn, S. Modified NLC-loaded coumarin for pharmaceutical applications: The improvement of physical stability and controlled release profile. Pharm. Dev. Technol. 2016, 21, 1015–1022. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Ye, T.; Chen, F.; Yu, S.; Chen, J.; Yang, X.; Yang, N.; Zhang, J.; Liu, J.; et al. Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. Int. J. Nanomed. 2014, 9, 4305–4315. [Google Scholar] [CrossRef]
- Carbone, C.; Tomasello, B.; Ruozi, B.; Renis, M.; Puglisi, G. Preparation and optimization of PIT solid lipid nanoparticles via statistical factorial design. Eur. J. Med. Chem. 2012, 49, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Spiker, R.C.; Levin, I.W. Effect of bilayer curvature on vibrational Raman spectroscopic behavior of phospholipid-water assemblies. Biochim. Biophys. Acta 1976, 455, 560–575. [Google Scholar] [CrossRef]
- Schultz, Z.D.; Levin, I.W. Vibrational spectroscopy of biomembranes. Annu. Rev. Anal. Chem. 2011, 4, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Wurpel, G.W.H.; Schins, J.M.; Muller, M. Direct measurement of chain order in single phospholipid mono- and bilayers with multiplex CARS. J. Phys. Chem. B 2004, 108, 3400–3403. [Google Scholar] [CrossRef]
- Christoph Krafft, T.K.; Richard, H.W. Funk and Reiner Salzer. Identification of organelles and vesicles in single cells by Raman microspectroscopic mapping. Vib. Spectrosc. 2005, 38, 85–93. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Caddeo, C.; Manconi, M.; Fadda, A.M.; Lai, F.; Lampis, S.; Diez-Sales, O.; Sinico, C. Nanocarriers for antioxidant resveratrol: Formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf. B Biointerfaces 2013, 111, 327–332. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.A.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Bastias Venegas, J.; Pauthe, E.; et al. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef]
- Nahr, F.K.; Ghanbarzadeh, B.; Kafil, H.S.; Hamishehkar, H.; Hoseni, M. The colloidal and release properties of cardamom oil encapsulated nanostructured lipid carrier. J. Dispers. Sci. Technol. 2019, 1–9. [Google Scholar] [CrossRef]
- Ben Djemaa, F.G.; Bellassoued, K.; Zouari, S.; El Feki, A.; Ammar, E. Antioxidant and wound healing activity of Lavandula aspic L. ointment. J. Tissue Viability 2016, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; Dos Santos Sampaio, T.I.; Ferreira, I.M.; Lima, E.S.; de Jesus Amazonas da Silva, M.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; da Conceicao, E.C.; et al. Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: In vitro and in zebrafish studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.R.; Syed Alwi, S.S.; Yazan, L.S.; Zakarial Ansar, F.H.; Ong, Y.S. Migration and proliferation effects of thymoquinone-loaded nanostructured lipid carrier (TQ-NLC) and thymoquinone (TQ) on in vitro wound healing models. Evid.-Based Complement. Altern. Med. 2019, 2019, 9725738. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery--liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef]
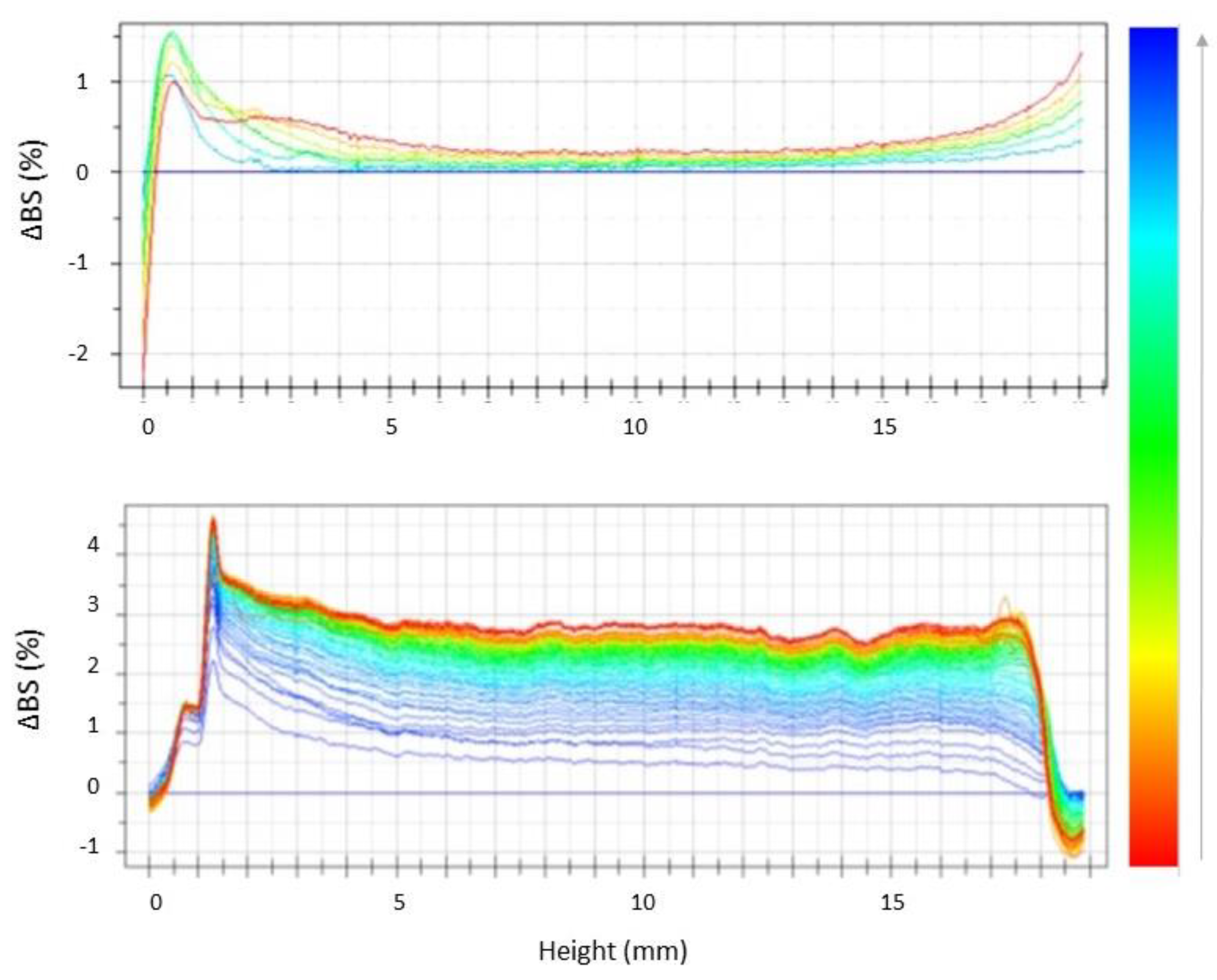
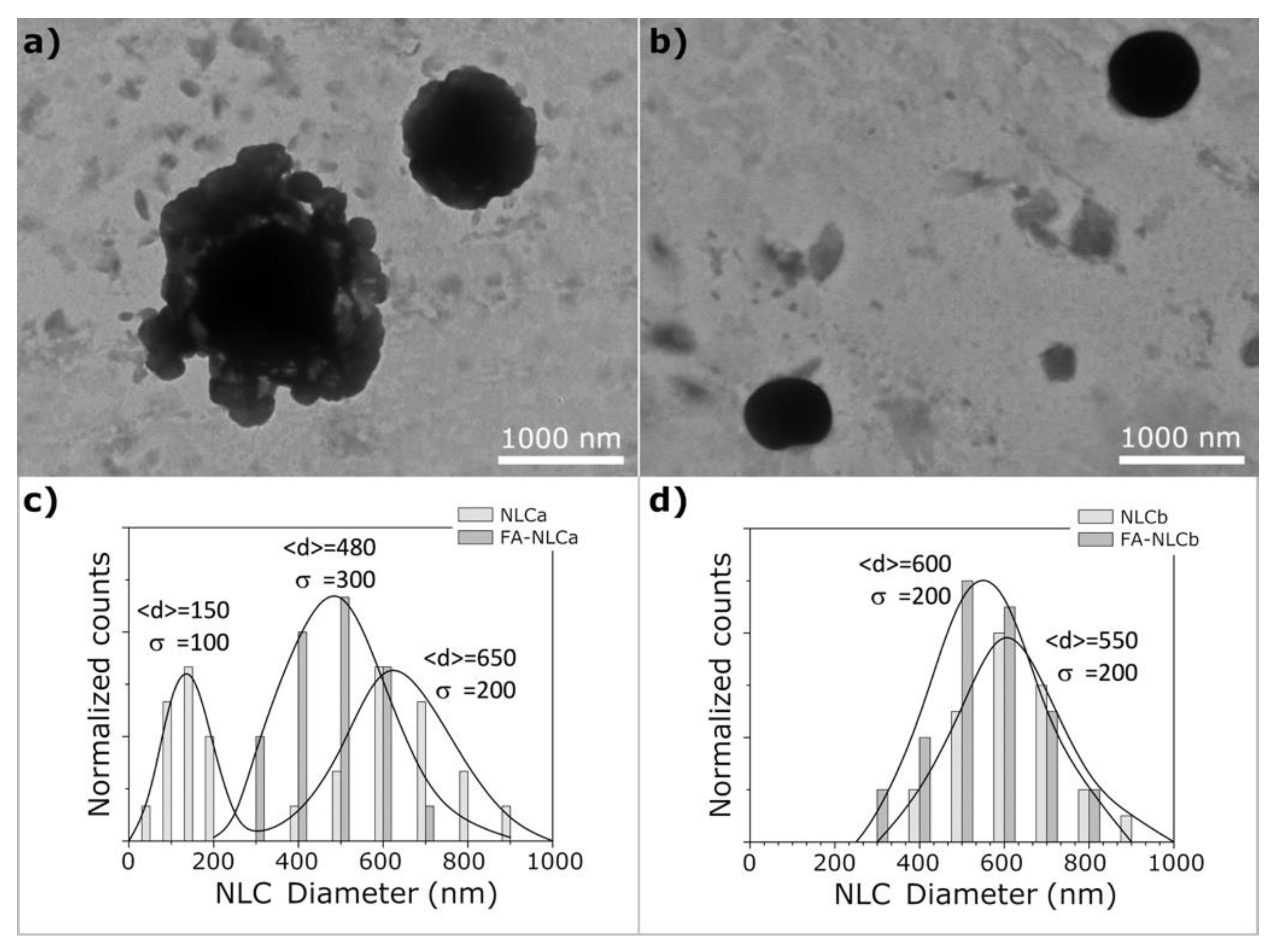
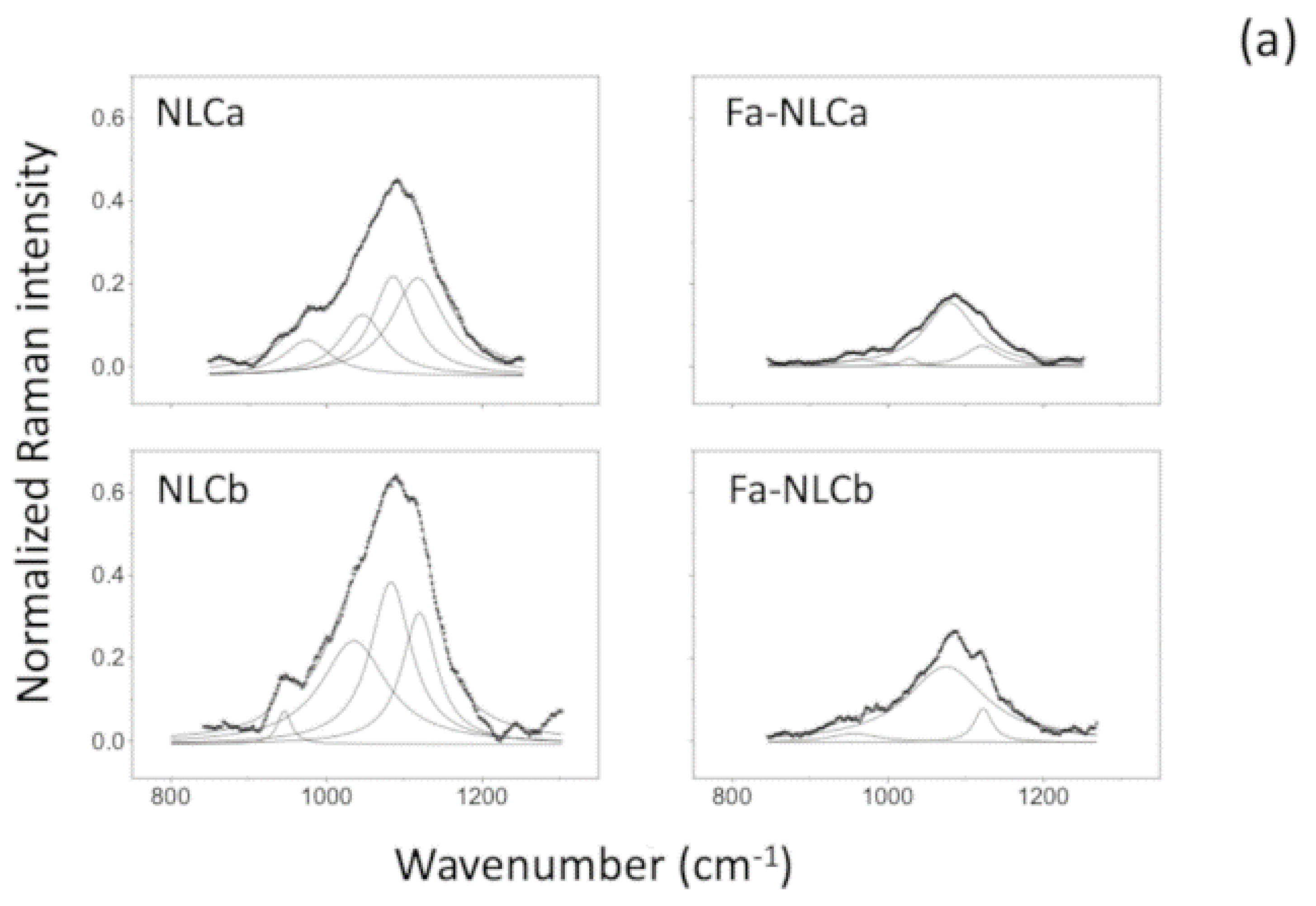
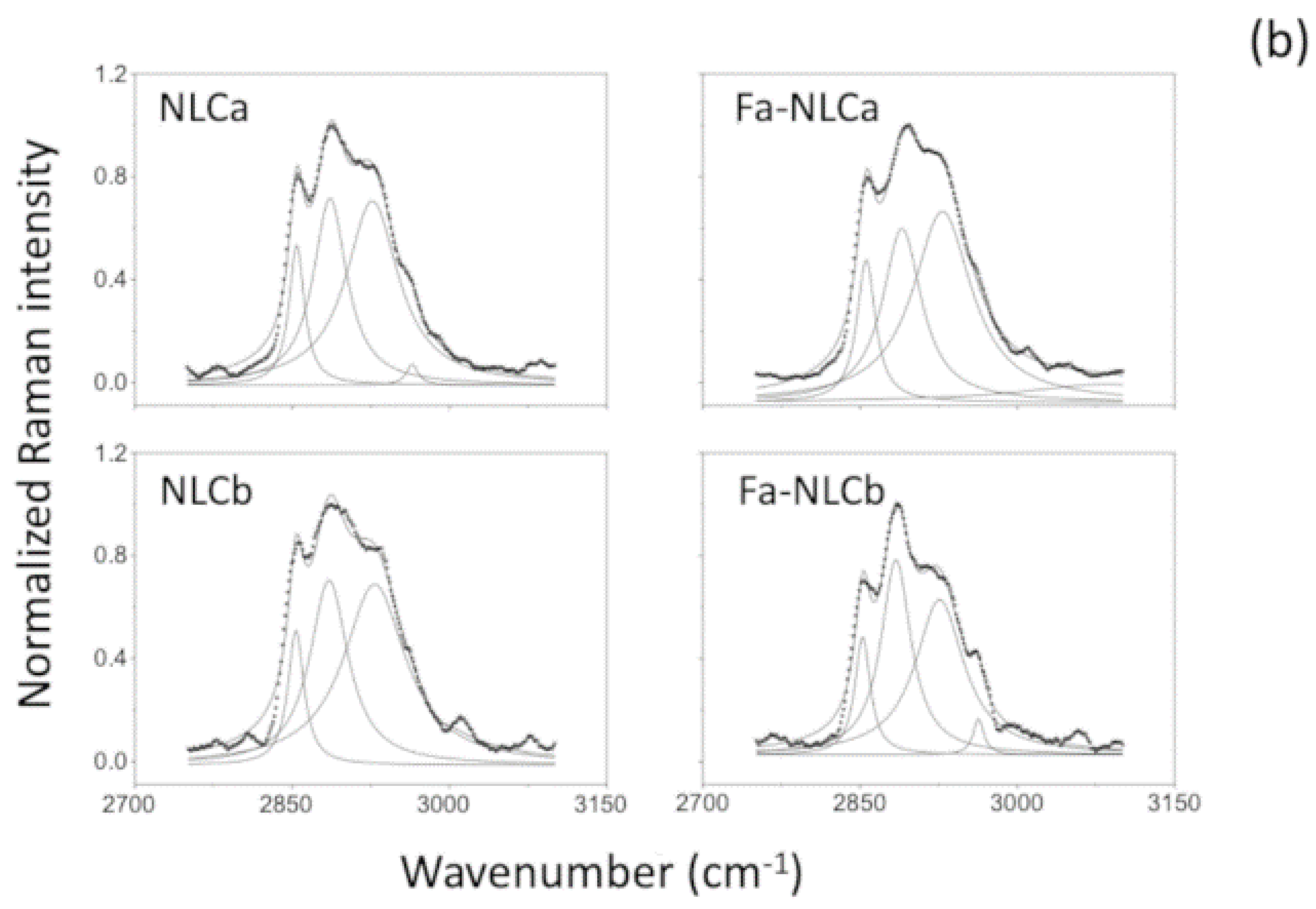
| Batch | Zave (nm) ± SD | PDI ± SD | ZP ± SD | EE% | pH | Osmolarity (mOsm) |
|---|---|---|---|---|---|---|
| NLCa | 122.51 ± 5.98 * | 0.101 ± 0.007 | −4.85 ± 0.15 | - | 7.21 ± 0.02 | 0.282 ± 0.005 |
| NLCb | 99.88 ± 1.33 * | 0.089 ± 0.015 | −5.02 ± 0.02 | - | 7.13 ± 0.05 | 0.289 ± 0.008 |
| FA-NLCa | 87.77 ± 6.30 ** | 0.167 ± 0.061 | −2.53 ± 0.03 * | 86.55 ± 0.95 | 6.02 ± 0.03 | 0.285 ± 0.008 |
| FA-NLCb | 62.86 ± 0.75 ** | 0.056 ± 0.012 | −2.09 ± 0.05 * | 87.02 ± 1.98 | 5.98 ± 0.01 | 0.301 ± 0.007 |
| Sample | Zave ± SD | PDI ± SD |
|---|---|---|
| After Preparation | ||
| NLCa | 122.51 ± 5.98 | 0.101 ± 0.007 |
| NLCb | 99.88 ± 1.33 | 0.089 ± 0.015 |
| After 2 Months of Storage | ||
| NLCa | 163.2 ± 0.8 * | 0.189 ± 0.006 * |
| NLCb | 107.5 ± 0.5 | 0.109 ± 0.005 |
| After 6 Months of Storage | ||
| NLCa | 186.9 ± 0.7 * | 0.218 ± 0.009 * |
| NLCb | 157.2 ± 0.9 * | 0.101 ± 0.007 |
| Sample | I1115/I1050 | I2890/I2850 |
|---|---|---|
| NLCa | 0.92 | 1.25 |
| FA-NLCa | 0.76 | 1.27 |
| NLCb | 0.89 | 1.18 * |
| FA-NLCb | 0.79 | 1.43 * |
| Batch | AA (%) | TEAC (mg of Trolox Equivalents/mL) |
|---|---|---|
| FA-NLCa | 90.8 ± 0.6 | 1.17 ± 0.005 |
| FA-NLCb | 89.3 ± 0.9 | 1.15 ± 0.008 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials 2020, 10, 898. https://doi.org/10.3390/nano10050898
Carbone C, Caddeo C, Grimaudo MA, Manno DE, Serra A, Musumeci T. Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials. 2020; 10(5):898. https://doi.org/10.3390/nano10050898
Chicago/Turabian StyleCarbone, Claudia, Carla Caddeo, Maria Aurora Grimaudo, Daniela Erminia Manno, Antonio Serra, and Teresa Musumeci. 2020. "Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing?" Nanomaterials 10, no. 5: 898. https://doi.org/10.3390/nano10050898
APA StyleCarbone, C., Caddeo, C., Grimaudo, M. A., Manno, D. E., Serra, A., & Musumeci, T. (2020). Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials, 10(5), 898. https://doi.org/10.3390/nano10050898







