Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities
Abstract
:1. Introduction
2. Current Status of Nanocapsules: Materials and Formulation Techniques
2.1. Common Materials for Preparing Polymeric Nanocapsules
2.1.1. Polymeric Shell
2.1.2. Liquid/Solid/Hollow Core
2.2. Polymeric Nanocapsule Formulation
2.2.1. Interfacial Deposition Method
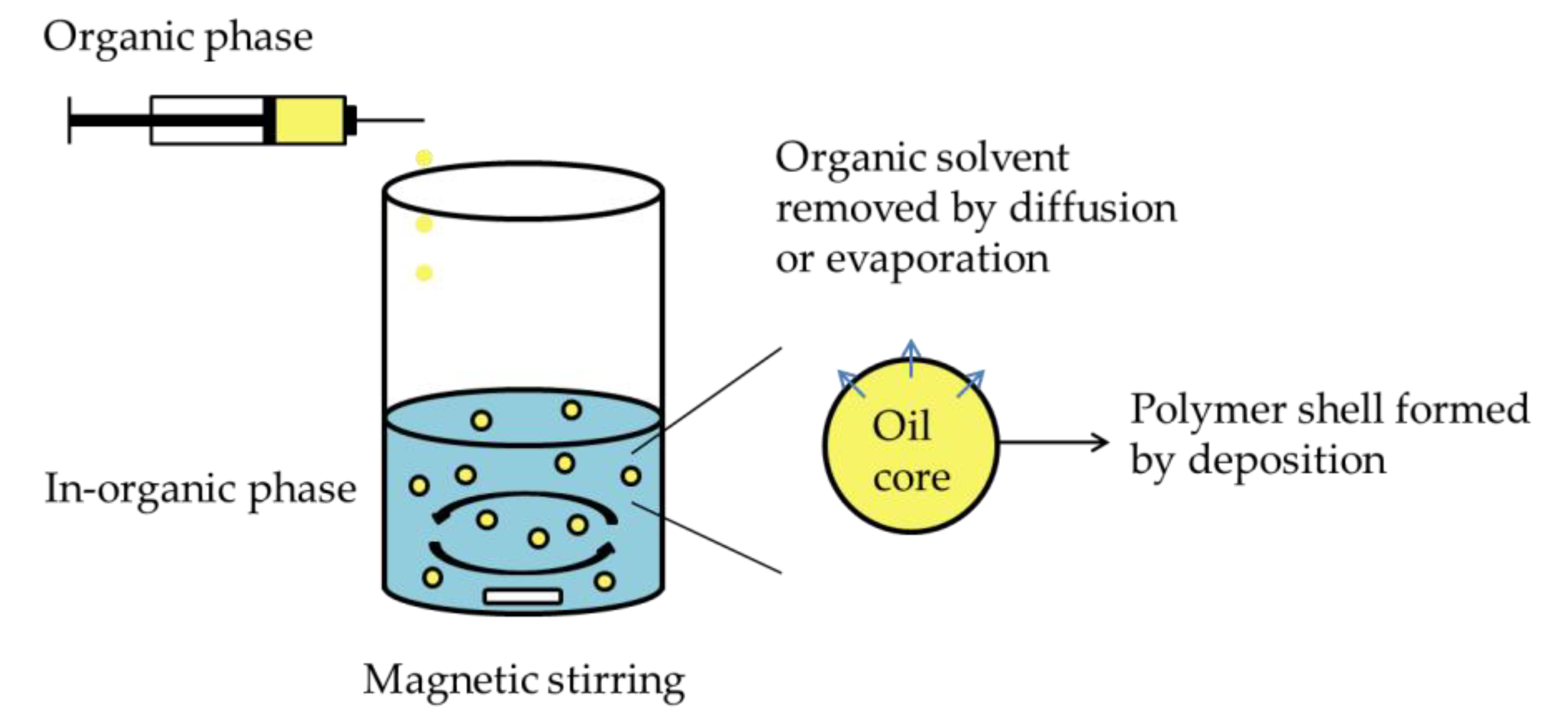
2.2.2. Nano-emulsion Template Method
A. Emulsion–diffusion/Evaporation Method
B. Emulsion–coacervation Method
C. Double Emulsion Method
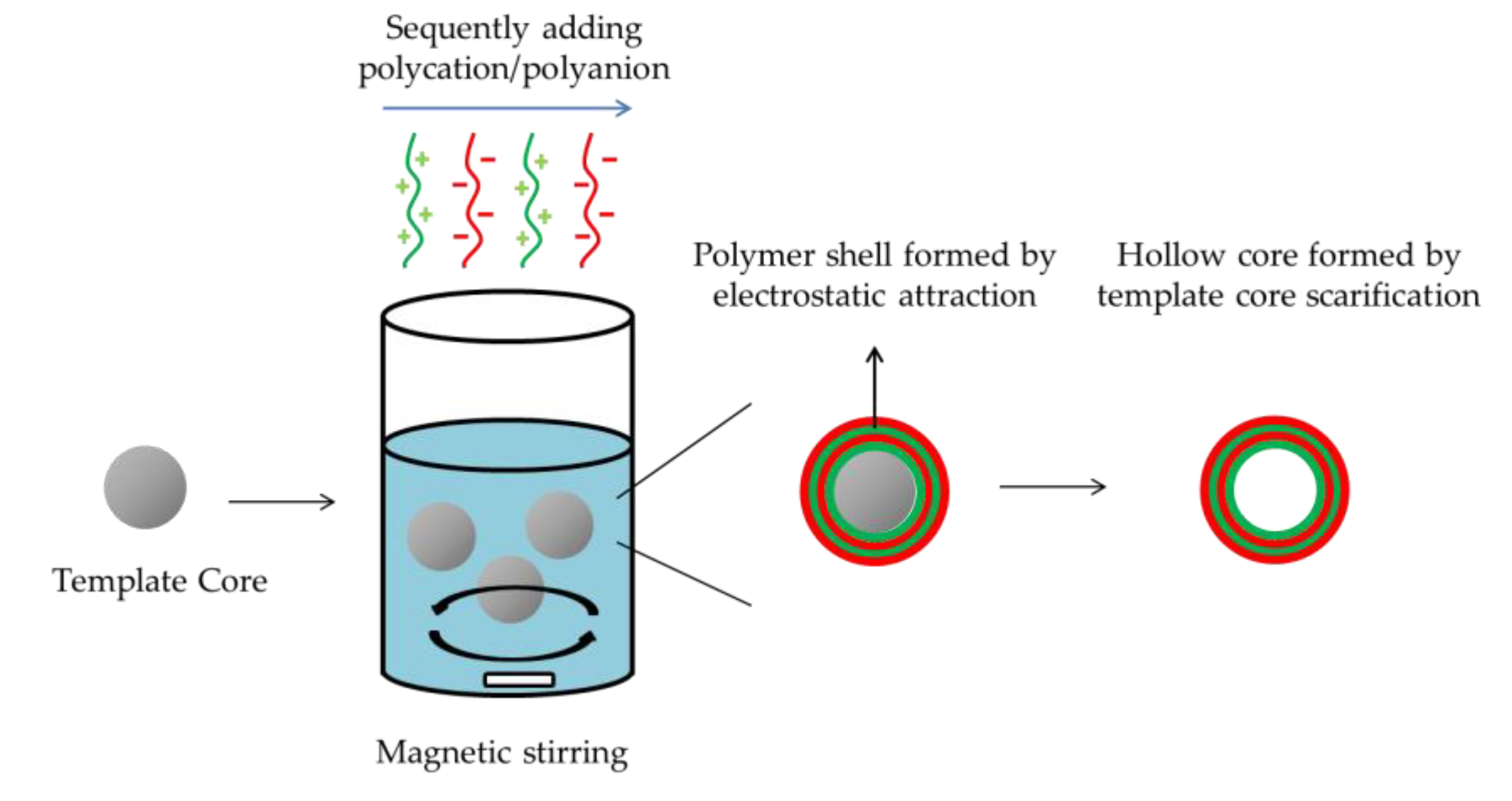
2.2.3. Layer-by-Layer Method
3. Highlighted Applications of Nanocapsules as Drug Delivery System
3.1. Improved Bioavailability of Poorly Soluble Drugs
3.2. Sustained Delivery
3.3. Targeted Delivery
3.4. Stabilization under Harsh Environment
3.5. Toxicity Moderation
4. Challenge of Nanocapsules as Drug Delivery System
4.1. Thickness Characterization of Polymeric Shell
4.2. Organic Solvent Free Formation
4.3. Aggregation and Storage
4.4. Sterilization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.M.-C.; Cuda, G.; Bunimovich, Y.L.; Gaspari, M.; Heath, J.R.; Hill, H.D.; Mirkin, C.A.; Nijdam, A.J.; Terracciano, R.; Thundat, T. Nanotechnologies for biomolecular detection and medical diagnostics. Curr. Opin. Chem. Biol. 2006, 10, 11–19. [Google Scholar] [CrossRef]
- Jain, K.K. Nanodiagnostics: Application of nanotechnology in molecular diagnostics. Expert Rev. Mol. Diagn. 2003, 3, 153–161. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Chen, Z.G.; Shin, D.M. Application of nanotechnology in cancer therapy and imaging. CA A Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine 2005, 1, 193–212. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Jurgons, R.; Seliger, C.; Hilpert, A.; Trahms, L.; Odenbach, S.; Alexiou, C. Drug loaded magnetic nanoparticles for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2893. [Google Scholar] [CrossRef]
- Tocco, I.; Zavan, B.; Bassetto, F.; Vindigni, V. Nanotechnology-based therapies for skin wound regeneration. J. Nanomater. 2012, 2012, 714134. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Moinard-Checot, D.; Chevalier, Y.; Briançon, S.; Fessi, H.; Guinebretière, S. Nanoparticles for drug delivery: Review of the formulation and process difficulties illustrated by the emulsion-diffusion process. J. Nanosci. Nanotechnol. 2006, 6, 2664–2681. [Google Scholar] [CrossRef]
- Fu, C.; Ding, C.; Sun, X.; Fu, A. Curcumin nanocapsules stabilized by bovine serum albumin-capped gold nanoclusters (BSA-AuNCs) for drug delivery and theranosis. Mater. Sci. Eng. C 2018, 87, 149–154. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Lukasiewicz, S.; Szczepanowicz, K.; Podgorna, K.; Blasiak, E.; Majeed, N.; Ogren, S.O.O.; Nowak, W.; Warszynski, P.; Dziedzicka-Wasylewska, M. Encapsulation of clozapine in polymeric nanocapsules and its biological effects. Colloids Surf. B Biointerfaces 2016, 140, 342–352. [Google Scholar] [CrossRef]
- Frank, L.; Gazzi, R.; De Andrade Mello, P.; Buffon, A.; Pohlmann, A.; Guterres, S. Imiquimod-loaded nanocapsules improve cytotoxicity in cervical cancer cell line. Eur. J. Pharm. Biopharm. 2019, 136, 9–17. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Weiss, V.; Mertins, O.; Da Silveira, N.P.; Guterres, S.S. Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres: Development, stability evaluation and nanostructure models. Eur. J. Pharm. Sci. 2002, 16, 305–312. [Google Scholar] [CrossRef]
- Trindade, I.C.; Pound-Lana, G.; Pereira, D.G.S.; De Oliveira, L.A.M.; Andrade, M.S.; Vilela, J.M.C.; Postacchini, B.B.; Mosqueira, V.C.F. Mechanisms of interaction of biodegradable polyester nanocapsules with non-phagocytic cells. Eur. J. Pharm. Sci. 2018, 124, 89–104. [Google Scholar] [CrossRef]
- Teixeira, M.; Alonso, M.J.; Pinto, M.M.M.; Barbosa, C.M. Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur. J. Pharm. Biopharm. 2005, 59, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The weapons for novel drug delivery systems. Bioimpacts: BI 2012, 2, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Shastri, V.P. Non-degradable biocompatible polymers in medicine: Past, present and future. Curr. Pharm. Biotechnol. 2003, 4, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R.J.N. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Yeh, J.; Wu, X.; Cao, Z.; Wang, Y.A.; Zhang, M.; Yang, L.; Mao, H. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PγMPS copolymer coating. Biomaterials 2010, 31, 5397–5407. [Google Scholar] [CrossRef] [Green Version]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. IJBS 2008, 4, 221–228. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, L.; Jary, D.; Lucía, A.; García-Embid, S.; Serrano-Sevilla, I.; Pérez, D.; Ainsa, J.A.; Navarro, F.P.; De la Fuente, J.M. New active formulations against M. tuberculosis: Bedaquiline encapsulation in lipid nanoparticles and chitosan nanocapsules. Chem. Eng. J. 2018, 340, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Anversa Dimer, F.; De Souza Carvalho-Wodarz, C.; Goes, A.; Cirnski, K.; Herrmann, J.; Schmitt, V.; Patzold, L.; Abed, N.; De Rossi, C.; Bischoff, M.; et al. PLGA nanocapsules improve the delivery of clarithromycin to kill intracellular Staphylococcus aureus and Mycobacterium abscessus. Nanomedicine 2019, 24, 102125. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Oh, M.; Yeo, W.-S.; Galvao, K.N.; Jeong, K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Mansour, O.; Carbonnier, B. Promising sub-100 nm tailor made hollow chitosan/poly(acrylic acid) nanocapsules for antibiotic therapy. J. Colloid Interface Sci. 2018, 522, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Cafaggi, S.; Russo, E.; Stefani, R.; Leardi, R.; Caviglioli, G.; Parodi, B.; Bignardi, G.; De Totero, D.; Aiello, C.; Viale, M. Preparation and evaluation of nanoparticles made of chitosan or N-trimethyl chitosan and a cisplatin–alginate complex. J. Control. Release 2007, 121, 110–123. [Google Scholar] [CrossRef]
- Douglas, K.L.; Tabrizian, M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Wang, T.; Feng, Z.; He, N.; Wang, Z.; Li, S.; Guo, Y.; Xu, L. A novel preparation of nanocapsules from alginate-oligochitosan. J. Nanosci. Nanotechnol. 2007, 7, 4571–4574. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan-a review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Pan, D.; Wan, Y.; Wang, Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr. Polym. 2013, 92, 719–725. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Dhaneshwar, S.S.; Mini, K.; Gairola, N.; Kadam, S. Dextran: A promising macromolecular drug carrier. Indian J. Pharm. Sci. 2006, 68, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Tiyaboonchai, W.; Limpeanchob, N. Formulation and characterization of amphotericin B–chitosan–dextran sulfate nanoparticles. Int. J. Pharm. 2007, 329, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Crecente-Campo, J.; Lorenzo-Abalde, S.; Mora, A.; Marzoa, J.; Csaba, N.; Blanco, J.; Gonzalez-Fernandez, A.; Alonso, M.J. Bilayer polymeric nanocapsules: A formulation approach for a thermostable and adjuvanted E. coli antigen vaccine. J. Control. Release 2018, 286, 20–32. [Google Scholar] [CrossRef]
- Anitha, A.; Deepagan, V.; Rani, V.D.; Menon, D.; Nair, S.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Tekie, F.S.M.; Kiani, M.; Zakerian, A.; Pilevarian, F.; Assali, A.; Soleimani, M.; Dinarvand, R.; Arefian, E.; Atashi, A.; Amini, M. Nano polyelectrolyte complexes of carboxymethyl dextran and chitosan to improve chitosan-mediated delivery of miR-145. Carbohydr. Polym. 2017, 159, 66–75. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Oniszczuk, J.; Pawlak, A.; El Joukhar, I.; Goffin, A.; Varrault, G.; Sahali, D.; Carbonnier, B. Cationic poly(cyclodextrin)/alginate nanocapsules: From design to application as efficient delivery vehicle of 4-hydroxy tamoxifen to podocyte in vitro. Colloids Surf. B Biointerfaces 2019, 179, 128–135. [Google Scholar] [CrossRef]
- Ismail, M.; Du, Y.; Ling, L.; Li, X. Artesunate-heparin conjugate based nanocapsules with improved pharmacokinetics to combat malaria. Int. J. Pharm. 2019, 562, 162–171. [Google Scholar] [CrossRef]
- Abellan-Pose, R.; Teijeiro-Valino, C.; Santander-Ortega, M.J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M.; Csaba, N.; Alonso, M.J. Polyaminoacid nanocapsules for drug delivery to the lymphatic system: Effect of the particle size. Int. J. Pharm. 2016, 509, 107–117. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein−nanoparticle interactions: Opportunities and challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein polymer-based nanoparticles: Fabrication and medical applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaber, M.; Hany, M.; Mokhtar, S.; Helmy, M.W.; Elkodairy, K.A.; Elzoghby, A.O. Boronic-targeted albumin-shell oily-core nanocapsules for synergistic aromatase inhibitor/herbal breast cancer therapy. Mater. Sci. Eng. C 2019, 105, 110099. [Google Scholar] [CrossRef] [PubMed]
- Toita, R.; Murata, M.; Abe, K.; Narahara, S.; Piao, J.S.; Kang, J.-H.; Ohuchida, K.; Hashizume, M. Biological evaluation of protein nanocapsules containing doxorubicin. Int. J. Nanomed. 2013, 8, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Kratz, F.; Wang, S.W. Protein nanocapsules containing doxorubicin as a pH-responsive delivery system. Small 2011, 7, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Júnior, W.F.; De Oliveira Pinheiro, J.G.; Moreira, C.D.; De Souza, F.J.; De Lima, Á.A. Alternative technologies to improve solubility and stability of poorly water-soluble drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–305. [Google Scholar] [CrossRef]
- Yao, M.H.; Ma, M.; Chen, Y.; Jia, X.Q.; Xu, G.; Xu, H.X.; Chen, H.R.; Wu, R. Multifunctional Bi2S3/PLGA nanocapsule for combined HIFU/radiation therapy. Biomaterials 2014, 35, 8197–8205. [Google Scholar] [CrossRef]
- Gomes, M.L.S.; Da Silva Nascimento, N.; Borsato, D.M.; Pretes, A.P.; Nadal, J.M.; Novatski, A.; Gomes, R.Z.; Fernandes, D.; Farago, P.V.; Zanin, S.M.W. Long-lasting anti-platelet activity of cilostazol from poly(epsilon-caprolactone)-poly(ethylene glycol) blend nanocapsules. Mater. Sci. Eng. C 2019, 94, 694–702. [Google Scholar] [CrossRef]
- Fustier, C.; Chang, T.M. PEG-PLA nanocapsules containing a nanobiotechnological complex of polyhemoglobin-tyrosinase for the depletion of tyrosine in melanoma: Preparation and in vitro characterisation. J. Nanomed. Biother. Discov. 2012, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Haznedar, S.; Dortunc, B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int. J. Pharm. 2004, 269, 131–140. [Google Scholar] [CrossRef]
- De Gomes, M.G.; Pando Pereira, M.; Guerra Teixeira, F.E.; Carvalho, F.; Pinto Savall, A.S.; Ferreira Bicca, D.; Monteiro Fidelis, E.; Botura, P.E.; Weber Cibin, F.; Pinton, S.; et al. Assessment of unloaded polymeric nanocapsules with different coatings in female rats: Influence on toxicological and behavioral parameters. Biomed. Pharm. 2020, 121, 109575. [Google Scholar] [CrossRef]
- Diou, O.; Fattal, E.; Delplace, V.; Mackiewicz, N.; Nicolas, J.; Mériaux, S.; Valette, J.; Robic, C.; Tsapis, N. RGD decoration of PEGylated polyester nanocapsules of perfluorooctyl bromide for tumor imaging: Influence of pre or post-functionalization on capsule morphology. Eur. J. Pharm. Biopharm. 2014, 87, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, B.; Gupta, S.; Vullev, V.I.; Anvari, B.; Upadhyayula, S. Effect of polyethylene glycol coatings on uptake of indocyanine green loaded nanocapsules by human spleen macrophages in vitro. J. Biomed. Opt. 2011, 16, 051303. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, L.; Alleva, M.; Serrano-Sevilla, I.; Garcia-Embid, S.; Stepien, G.; Moros, M.; De la Fuente, J.M. Controlling properties and cytotoxicity of chitosan nanocapsules by chemical grafting. Mar. Drugs 2016, 14, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bzowska, M.; Karabasz, A.; Szczepanowicz, K. Encapsulation of camptothecin into pegylated polyelectrolyte nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 36–42. [Google Scholar] [CrossRef]
- Shi, C.; Zhong, S.; Sun, Y.; Xu, L.; He, S.; Dou, Y.; Zhao, S.; Gao, Y.; Cui, X. Sonochemical preparation of folic acid-decorated reductive-responsive epsilon-poly-L-lysine-based microcapsules for targeted drug delivery and reductive-triggered release. Mater. Sci. Eng. C 2020, 106, 110251. [Google Scholar] [CrossRef]
- Rosa, P.; Friedrich, M.L.; Dos Santos, J.; Librelotto, D.R.N.; Maurer, L.H.; Emanuelli, T.; Da Silva, C.B.; Adams, A.I.H. Desonide nanoencapsulation with acai oil as oil core: Physicochemical characterization, photostability study and in vitro phototoxicity evaluation. J. Photochem. Photobiol. B Biol. 2019, 199, 111606. [Google Scholar] [CrossRef]
- Venturini, C.G.; Bruinsmann, F.A.; Contri, R.V.; Fonseca, F.N.; Frank, L.A.; D’Amore, C.M.; Raffin, R.P.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Co-encapsulation of imiquimod and copaiba oil in novel nanostructured systems: Promising formulations against skin carcinoma. Eur. J. Pharm. Sci. 2015, 79, 36–43. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Abulateefeh, S.R.; Alkawareek, M.Y.; Alkilany, A.M. Tunable sustained release drug delivery system based on mononuclear aqueous core-polymer shell microcapsules. Int. J. Pharm. 2019, 558, 291–298. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; De Angelis, F.; Cilurzo, F.; Celia, C.; Di Marzio, L.; Russo, D.; Tsapis, N.; Fattal, E.; Fresta, M. Aqueous-core PEG-coated PLA nanocapsules for an efficient entrapment of water soluble anticancer drugs and a smart therapeutic response. Eur. J. Pharm. Biopharm. 2015, 89, 30–39. [Google Scholar] [CrossRef]
- Vrignaud, S.; Anton, N.; Passirani, C.; Benoit, J.P.; Saulnier, P. Aqueous core nanocapsules: A new solution for encapsulating doxorubicin hydrochloride. Drug Dev. Ind. Pharm. 2013, 39, 1706–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, G.; Fattal, E.; Pinto-Alphandary, H.; Gulik, A.; Couvreur, P. Polyisobutylcyanoacrylate nanocapsules containing an aqueous core for the delivery of oligonucleotides. Int. J. Pharm. 2001, 214, 13–16. [Google Scholar] [CrossRef]
- Hillaireau, H.; Le Doan, T.; Chacun, H.; Janin, J.; Couvreur, P. Encapsulation of mono-and oligo-nucleotides into aqueous-core nanocapsules in presence of various water-soluble polymers. Int. J. Pharm. 2007, 331, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Y.; Li, C.; Liu, X. In vitro release of protein from poly(butylcyanoacrylate) nanocapsules with an aqueous core. Colloid Polym. Sci. 2004, 283, 480–485. [Google Scholar] [CrossRef]
- Gil, P.R.; Loretta, L.; Muñoz_Javier, A.; Parak, W.J. Nanoparticle-modified polyelectrolyte capsules. Nano Today 2008, 3, 12–21. [Google Scholar] [CrossRef]
- Johnston, A.P.; Cortez, C.; Angelatos, A.S.; Caruso, F. Layer-by-layer engineered capsules and their applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 203–209. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghorbani, M.; Nikpay, A.; Soltani, M. Chitosan nanocapsule-mounted cellulose nanofibrils as nanoships for smart drug delivery systems and treatment of avian trichomoniasis. J. Taiwan Inst. Chem. Eng. 2019, 95, 290–299. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Owen, A.; Rannard, S.; McDonald, T.O. Controlled synthesis of calcium carbonate nanoparticles and stimuli-responsivemulti-layered nanocapsules for oral drug delivery. Int. J. Pharm. 2019, 574. [Google Scholar] [CrossRef]
- Menard, M.; Meyer, F.; Parkhomenko, K.; Leuvrey, C.; Francius, G.; Begin-Colin, S.; Mertz, D. Mesoporous silica templated-albumin nanoparticles with high doxorubicin payload for drug delivery assessed with a 3-D tumor cell model. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 332–341. [Google Scholar] [CrossRef]
- Shang, B.; Zhang, X.; Ji, R.; Wang, Y.; Hu, H.; Peng, B.; Deng, Z. Preparation of colloidal polydopamine/Au hollow spheres for enhanced ultrasound contrast imaging and photothermal therapy. Mater. Sci. Eng. C 2020, 106, 110174. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Mohan, P.; Dangi, J.S.; Kesavan, K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocapsules for management of glaucoma: Formulation, characterization and pharmacodynamic study. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Couvreur, P.; Barratt, G.; Fattal, E.; Vauthier, C. Nanocapsule technology: A review. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Lu, Z.; Bei, J.; Wang, S. A method for the preparation of polymeric nanocapsules without stabilizer. J. Control. Release 1999, 61, 107–112. [Google Scholar] [CrossRef]
- Ferranti, V.; Marchais, H.; Chabenat, C.; Orecchioni, A.; Lafont, O. Primidone-loaded poly-ε-caprolactone nanocapsules: Incorporation efficiency and in vitro release profiles. Int. J. Pharm. 1999, 193, 107–111. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Noomun, K.; Jongaroonngamsang, N.; Rojsitthisak, P.; Nimmannit, U. Preparation of alginate nanocapsules containing turmeric oil. Carbohydr. Polym. 2008, 74, 209–214. [Google Scholar] [CrossRef]
- Veragten, A.; Contri, R.V.; Betti, A.H.; Herzfeldt, V.; Frank, L.A.; Pohlmann, A.R.; Rates, S.M.K.; Guterres, S.S. Chitosan-coated nanocapsules ameliorates the effect of olanzapine in prepulse inhibition of startle response (PPI) in rats following oral administration. React. Funct. Polym. 2020, 148. [Google Scholar] [CrossRef]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose acetate—Essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Allémann, E.; Doelker, E.; Fessi, H. Preparation and characterization of nanocapsules from preformed polymers by a new process based on emulsification-diffusion technique. Pharm. Res. 1998, 15, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Niknam, S. Preparation of polyamide nanocapsules of Elaeagnus angustifolia L. delivery with in vivo studies. Ind. Crop. Prod. 2014, 55, 49–55. [Google Scholar] [CrossRef]
- Monika, P.; Basavaraj, B.; Murthy, K.; Ahalya, N.; Gurudev, K. Nanocapsules of catechin rich extract for enhanced antioxidant potential and in vitro bioavailability. J. Appl. Pharm. Sci. 2017, 7, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Sombra, F.M.; Rosa Richter, A.; Rodrigues de Araujo, A.; De Oliveira Silva Ribeiro, F.; De Fatima Souza Mendes, J.; Oliveira Dos Santos Fontenelle, R.; Alves da Silva, D.; Paula, H.C.B.; Feitosa, J.P.A.; Goycoolea, F.M.; et al. Development of amphotericin B-loaded propionate sterculia striata polysaccharide nanocarrier. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Boissenot, T.; Bordat, A.; Larrat, B.; Varna, M.; Chacun, H.; Paci, A.; Poinsignon, V.; Fattal, E.; Tsapis, N. Ultrasound-induced mild hyperthermia improves the anticancer efficacy of both Taxol(R) and paclitaxel-loaded nanocapsules. J. Control. Release 2017, 264, 219–227. [Google Scholar] [CrossRef]
- Ma, J.; Feng, P.; Ye, C.; Wang, Y.; Fan, Y. An improved interfacial coacervation technique to fabricate biodegradable nanocapsules of an aqueous peptide solution from polylactide and its block copolymers with poly (ethylene glycol). Colloid Polym. Sci. 2001, 279, 387–392. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, W.; Zhu, G.; Zhou, R.; Niu, Y. Study of production and the stability of styrallyl acetate nanocapsules using complex coacervation. Flavour Fragr. J. 2016, 31, 283–289. [Google Scholar] [CrossRef]
- Bae, K.H.; Lee, Y.; Park, T.G. Oil-encapsulating PEO−PPO−PEO/PEG shell cross-linked nanocapsules for target-specific delivery of paclitaxel. Biomacromolecules 2007, 8, 650–656. [Google Scholar] [CrossRef]
- Lee, K.; Bae, K.H.; Lee, Y.; Lee, S.H.; Ahn, C.H.; Park, T.G. Pluronic/polyethylenimine shell crosslinked nanocapsules with embedded magnetite nanocrystals for magnetically triggered delivery of siRNA. Macromol. Biosci. 2010, 10, 239–245. [Google Scholar] [CrossRef]
- Steinmacher, F.; Baier, G.; Musyanovych, A.; Landfester, K.; Araújo, P.; Sayer, C. Design of cross-linked starch nanocapsules for enzyme-triggered release of hydrophilic compounds. Processes 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Tian, K.; Zeng, J.; Zhao, X.; Liu, L.; Jia, X.; Liu, P. Synthesis of multi-functional nanocapsules via interfacial AGET ATRP in miniemulsion for tumor micro-environment responsive drug delivery. Colloids Surf. B Biointerfaces 2015, 134, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Forero Ramirez, L.M.; Boudier, A.; Gaucher, C.; Babin, J.; Durand, A.; Six, J.L.; Nouvel, C. Dextran-covered pH-sensitive oily core nanocapsules produced by interfacial Reversible Addition-Fragmentation chain transfer miniemulsion polymerization. J. Colloid Interface Sci. 2020, 569, 57–67. [Google Scholar] [CrossRef]
- Ishizuka, F.; Utama, R.H.; Kim, S.; Stenzel, M.H.; Zetterlund, P.B. RAFT inverse miniemulsion periphery polymerization in binary solvent mixtures for synthesis of nanocapsules. Eur. Polym. J. 2015, 73, 324–334. [Google Scholar] [CrossRef]
- Loiko, O.P.; Van Herk, A.M.; Ali, S.I.; Burkeev, M.Z.; Tazhbayev, Y.M.; Zhaparova, L.Z. Controlled release of Capreomycin sulfate from pH-responsive nanocapsules. e-Polymers 2013, 2013, 189–202. [Google Scholar] [CrossRef]
- Ali, S.I.; Heuts, J.P.; Van Herk, A.M. Vesicle-templated pH-responsive polymeric nanocapsules. Soft Matter 2011, 7, 5382–5390. [Google Scholar] [CrossRef]
- Tallian, C.; Herrero-Rollett, A.; Stadler, K.; Vielnascher, R.; Wieland, K.; Weihs, A.M.; Pellis, A.; Teuschl, A.H.; Lendl, B.; Amenitsch, H.; et al. Structural insights into pH-responsive drug release of self-assembling human serum albumin-silk fibroin nanocapsules. Eur. J. Pharm. Biopharm. 2018, 133, 176–187. [Google Scholar] [CrossRef]
- Artusio, F.; Bazzano, M.; Pisano, R.; Coulon, P.-E.; Rizza, G.; Schiller, T.; Sangermano, M. Polymeric nanocapsules via interfacial cationic photopolymerization in miniemulsion. Polymer 2018, 139, 155–162. [Google Scholar] [CrossRef]
- Benedetti, M.; Congdon, T.R.; Bassett, S.P.; Alauhdin, M.; Howdle, S.M.; Haddleton, D.M.; Pisano, R.; Sangermano, M.; Schiller, T.L. Synthesis of polymeric microcapsules by interfacial-suspension cationic photopolymerisation of divinyl ether monomer in aqueous suspension. Polym. Chem. 2017, 8, 972–975. [Google Scholar] [CrossRef] [Green Version]
- Bilati, U.; Allémann, E.; Doelker, E. Sonication parameters for the preparation of biodegradable nanocapsulesof controlled size by the double emulsion method. Pharm. Dev. Technol. 2003, 8, 1–9. [Google Scholar] [CrossRef]
- Ashjari, M.; Khoee, S.; Mahdavian, A.R. Controlling the morphology and surface property of magnetic/cisplatin-loaded nanocapsules via W/O/W double emulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2012, 408, 87–96. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Oliveira, J.L.; Zavala-Betancourt, S.A.; Ledezma, A.S.; Arias, E.; Moggio, I.; Romero, J.; Fraceto, L.F. Development of stained polymeric nanocapsules loaded with model drugs: Use of a fluorescent poly(phenyleneethynylene). Colloids Surf. B Biointerfaces 2016, 147, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdmann, C.; Mayer, C. Permeability profile of poly(alkyl cyanoacrylate) nanocapsules. J. Colloid Interface Sci. 2016, 478, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Chen, S.Y.; Gao, X. Multifunctional nanocapsules for simultaneous encapsulation of hydrophilic and hydrophobic compounds and on-demand release. ACS Nano 2012, 6, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.S.; Hu, S.H.; Liao, B.J.; Chang, Y.C.; Chen, S.Y. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomedicine 2014, 10, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Dodi, G.; Tudorachi, N.; Ponta, O.; Simon, V.; Butnaru, M.; Verestiuc, L. Doxorubicin-loaded magnetic nanocapsules based on N-palmitoyl chitosan and magnetite: Synthesis and characterization. Chem. Eng. J. 2015, 279, 188–197. [Google Scholar] [CrossRef]
- Cuomo, F.; Lopez, F.; Piludu, M.; Miguel, M.G.; Lindman, B.; Ceglie, A. Release of small hydrophilic molecules from polyelectrolyte capsules: Effect of the wall thickness. J. Colloid Interface Sci. 2015, 447, 211–216. [Google Scholar] [CrossRef]
- Ji, F.; Li, J.; Qin, Z.; Yang, B.; Zhang, E.; Dong, D.; Wang, J.; Wen, Y.; Tian, L.; Yao, F. Engineering pectin-based hollow nanocapsules for delivery of anticancer drug. Carbohydr Polym 2017, 177, 86–96. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Nikitina, A.V.; Semina, I.I.; Nizameev, I.R.; Kadirov, M.K.; Khutoryanskiy, V.V.; Zakharova, L.Y.; Sinyashin, O.G. Polyelectrolyte nanocontainers: Controlled binding and release of indomethacin. J. Mol. Liq. 2018, 272, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Ledo, A.M.; Sasso, M.S.; Bronte, V.; Marigo, I.; Boyd, B.J.; Garcia-Fuentes, M.; Alonso, M.J. Co-delivery of RNAi and chemokine by polyarginine nanocapsules enables the modulation of myeloid-derived suppressor cells. J. Control. Release 2019, 295, 60–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Chi, C.; Huang, X.; Zou, Q.; Li, X.; Chen, L. Starch-based nanocapsules fabricated through layer-by-layer assembly for oral delivery of protein to lower gastrointestinal tract. Carbohydr. Polym. 2017, 171, 242–251. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Khutoryanskiy, V.V.; Charalampopoulos, D. Layer-by-layer coating of alginate matrices with chitosan–alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J. Mater. Chem. B 2013, 1, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, M.C.; Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Hollow chitosan/alginate nanocapsules for bioactive compound delivery. Int. J. Biol. Macromol. 2015, 79, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushirobira, C.Y.; Afiune, L.A.F.; Pereira, M.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Dutasteride nanocapsules for hair follicle targeting: Effect of chitosan-coating and physical stimulus. Int. J. Biol. Macromol. 2020, 151, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.T.; Pedra, N.S.; Soares, M.S.P.; Da Silveira, E.F.; Oliveira, P.S.; Grecco, F.B.; Da Silva, L.M.C.; Ferreira, L.M.; Ribas, D.A.; Gehrcke, M.; et al. Ketoprofen-loaded rose hip oil nanocapsules attenuate chronic inflammatory response in a pre-clinical trial in mice. Mater. Sci. Eng. C 2019, 103, 109742. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Chaves, P.; Frank, L.A.; Torge, A.; Schneider, M.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Spray-dried carvedilol-loaded nanocapsules for sublingual administration: Mucoadhesive properties and drug permeability. Powder Technol. 2019, 354, 348–357. [Google Scholar] [CrossRef]
- Gehrcke, M.; Sari, M.H.M.; Ferreira, L.M.; Barbieri, A.V.; Giuliani, L.M.; Prado, V.C.; Nadal, J.M.; Farago, P.V.; Nogueira, C.W.; Cruz, L. Nanocapsules improve indole-3-carbinol photostability and prolong its antinociceptive action in acute pain animal models. Eur. J. Pharm. Sci. 2018, 111, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.; Cervi, V.F.; Sari, M.H.M.; Barbieri, A.V.; Ramos, A.P.; Copetti, P.M.; De Brum, G.F.; Nascimento, K.; Nadal, J.M.; Farago, P.V.; et al. Diphenyl diselenide loaded poly(epsilon-caprolactone) nanocapsules with selective antimelanoma activity: Development and cytotoxic evaluation. Mater. Sci. Eng. C 2018, 91, 1–9. [Google Scholar] [CrossRef]
- Araújo, R.S.; Garcia, G.M.; Vilela, J.M.C.; Andrade, M.S.; Oliveira, L.A.M.; Kano, E.K.; Lange, C.C.; e Brito, M.A.V.P.; De Mello Brandão, H.; Mosqueira, V.C.F. Cloxacillin benzathine-loaded polymeric nanocapsules: Physicochemical characterization, cell uptake, and intramammary antimicrobial effect. Mater. Sci. Eng. C 2019, 104, 110006. [Google Scholar] [CrossRef]
- Marcondes Sari, M.H.; Zborowski, V.A.; Ferreira, L.M.; Jardim, N.D.S.; Araujo, P.C.O.; Bruning, C.A.; Cruz, L.; Nogueira, C.W. Enhanced pharmacological actions of p,p′-methoxyl-diphenyl diselenide-loaded polymeric nanocapsules in a mouse model of neuropathic pain: Behavioral and molecular insights. J. Trace Elem. Med. Biol. 2018, 46, 17–25. [Google Scholar] [CrossRef]
- Sari, M.H.M.; Ferreira, L.M.; Zborowski, V.A.; Araujo, P.C.O.; Cervi, V.F.; Brüning, C.A.; Cruz, L.; Nogueira, C.W. p,p′-Methoxyl-diphenyl diselenide-loaded polymeric nanocapsules are chemically stable and do not induce toxicity in mice. Eur. J. Pharm. Biopharm. 2017, 117, 39–48. [Google Scholar] [CrossRef]
- Nicolas, S.; Bolzinger, M.A.; Jordheim, L.P.; Chevalier, Y.; Fessi, H.; Almouazen, E. Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int. J. Pharm. 2018, 550, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Omwenga, E.O.; Hensel, A.; Shitandi, A.; Goycoolea, F.M. Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E.coli Top 10 biosensor. Colloids Surf. B Biointerfaces 2018, 164, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.M.; Voss, G.T.; De Oliveira, R.L.; Da Fonseca, C.A.R.; Paltian, J.; Rodrigues, K.C.; Ianiski, F.R.; Vaucher, R.A.; Luchese, C.; Wilhelm, E.A. Topic application of meloxicam-loaded polymeric nanocapsules as a technological alternative for treatment of the atopic dermatitis in mice. J. Appl. Biomed. 2018, 16, 337–343. [Google Scholar] [CrossRef]
- Velasques, K.; Maciel, T.R.; De Castro Dal Forno, A.H.; Teixeira, F.E.G.; Da Fonseca, A.L.; Varotti, F.P.; Fajardo, A.R.; Avila, D.S.; Haas, S.E. Co-nanoencapsulation of antimalarial drugs increases their in vitro efficacy against Plasmodium falciparum and decreases their toxicity to Caenorhabditis elegans. Eur. J. Pharm. Sci. 2018, 118, 1–12. [Google Scholar] [CrossRef]
- Chaves, P.D.; Ourique, A.F.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C. Carvedilol-loaded nanocapsules: Mucoadhesive properties and permeability across the sublingual mucosa. Eur. J. Pharm. Biopharm. 2017, 114, 88–95. [Google Scholar] [CrossRef]
- Franco, C.; Antonow, M.B.; Beckenkamp, A.; Buffon, A.; Ceolin, T.; Tebaldi, M.L.; Silveira, G.P.; Guterres, S.S.; Pohlmann, A.R. PCL-b-P(MMA-co-DMAEMA)2 new triblock copolymer for novel pH-sensitive nanocapsules intended for drug delivery to tumors. React. Funct. Polym. 2017, 119, 116–124. [Google Scholar] [CrossRef]
- Lollo Lollo, G.; Gonzalez-Paredes, A.; Garcia-Fuentes, M.; Calvo, P.; Torres, D.; Alonso, M.J. Polyarginine Nanocapsules as a Potential Oral Peptide Delivery Carrier. J. Pharm. Sci. 2017, 106, 611–618. [Google Scholar] [CrossRef]
- Lopes, L.Q.; Santos, C.G.; Vaucher Rde, A.; Raffin, R.P.; Santos, R.C. Nanocapsules with glycerol monolaurate: Effects on Candida albicans biofilms. Microb. Pathog. 2016, 97, 119–124. [Google Scholar] [CrossRef]
- Lollo, G.; Hervella, P.; Calvo, P.; Aviles, P.; Guillen, M.J.; Garcia-Fuentes, M.; Alonso, M.J.; Torres, D. Enhanced in vivo therapeutic efficacy of plitidepsin-loaded nanocapsules decorated with a new poly-aminoacid-PEG derivative. Int. J. Pharm. 2015, 483, 212–219. [Google Scholar] [CrossRef]
- Matoso Sombra, F.; Richter, A.R.; De Araujo, A.R.; De Oliveira Silva Ribeiro, F.; De Fatima Souza Mendes, J.; Dos Santos Fontenelle, R.O.; Da Silva, D.A.; Beserra de Paula, H.C.; Pessoa de Andrade Feitosa, J.; Martin Goycoolea, F.; et al. Nanocapsules of Sterculia striata acetylated polysaccharide as a potential monomeric amphotericin B delivery matrix. Int. J. Biol. Macromol. 2019, 130, 655–663. [Google Scholar] [CrossRef]
- Zafar, S.; Akhter, S.; Ahmad, I.; Hafeez, Z.; Alam Rizvi, M.M.; Jain, G.K.; Ahmad, F.J. Improved chemotherapeutic efficacy against resistant human breast cancer cells with co-delivery of Docetaxel and Thymoquinone by Chitosan Grafted Lipid Nanocapsules: Formulation optimization, in vitro and in vivo studies. Colloids Surf. B Biointerfaces 2019. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.D.O.F.; Rodrigues, T.H.S.; Pereira, R.D.C.A.; Silva, L.M.A.E.; Cáceres, C.A.; Azeredo, H.M.C.D.; Muniz, C.R.; Brito, E.S.D.; Canuto, K.M. Production and physico-chemical characterization of nanocapsules of the essential oil from Lippia sidoides Cham. Ind. Crop. Prod. 2016, 86, 279–288. [Google Scholar] [CrossRef]
- Youm, I.; Yang, X.; Murowchick, J.B.; Youan, B.-B.C. Encapsulation of docetaxel in oily core polyester nanocapsules intended for breast cancer therapy. Nanoscale Res. Lett. 2011, 6, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marto, J.; Pinto, P.; Fitas, M.; Goncalves, L.M.; Almeida, A.J.; Ribeiro, H.M. Safety assessment of starch-based personal care products: Nanocapsules and pickering emulsions. Toxicol. Appl. Pharm. 2018, 342, 14–21. [Google Scholar] [CrossRef]
- Marto, J.; Ruivo, E.; Lucas, S.D.; Goncalves, L.M.; Simoes, S.; Gouveia, L.F.; Felix, R.; Moreira, R.; Ribeiro, H.M.; Almeida, A.J. Starch nanocapsules containing a novel neutrophil elastase inhibitor with improved pharmaceutical performance. Eur. J. Pharm. Biopharm. 2018, 127, 1–11. [Google Scholar] [CrossRef]
- Boissenot, T.; Fattal, E.; Bordat, A.; Houvenagel, S.; Valette, J.; Chacun, H.; Gueutin, C.; Tsapis, N. Paclitaxel-loaded PEGylated nanocapsules of perfluorooctyl bromide as theranostic agents. Eur. J. Pharm. Biopharm. 2016, 108, 136–144. [Google Scholar] [CrossRef]
- Xavier-Jr, F.H.; Gueutin, C.; Chacun, H.; Vauthier, C.; Egito, E.S.T. Mucoadhesive paclitaxel-loaded chitosan-poly (isobutyl cyanoacrylate) core-shell nanocapsules containing copaiba oil designed for oral drug delivery. J. Drug Deliv. Sci. Technol. 2019, 53. [Google Scholar] [CrossRef]
- Behzadi, S.; Stadler, J.; Hosseinpour, S.; Crespy, D.; Landfester, K. Suppressing non-controlled leakage of hydrophilic payloads from redox-responsive nanocapsules. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 2–7. [Google Scholar] [CrossRef]
- Passlick, D.; Piradashvili, K.; Bamberger, D.; Li, M.; Jiang, S.; Strand, D.; Wich, P.R.; Landfester, K.; Bros, M.; Grabbe, S.; et al. Delivering all in one: Antigen-nanocapsule loaded with dual adjuvant yields superadditive effects by DC-directed T cell stimulation. J. Control. Release 2018, 289, 23–34. [Google Scholar] [CrossRef]
- Yilgor, P.; Hasirci, N.; Hasirci, V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. Part A 2010, 93, 528–536. [Google Scholar] [CrossRef]
- Piotrowski, M.; Jantas, D.; Leśkiewicz, M.; Szczepanowicz, K.; Warszyński, P.; Lasoń, W. Polyelectrolyte-coated nanocapsules containing cyclosporine A protect neuronal-like cells against oxidative stress-induced cell damage. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 264–269. [Google Scholar] [CrossRef]
- Baran, E.T.; Özer, N.; Hasirci, V. Poly (hydroxybutyrate-co-hydroxyvalerate) nanocapsules as enzyme carriers for cancer therapy: An in vitro study. J. Microencapsul. 2002, 19, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Mout, R.; Zhao, Y.; Yeh, Y.-C.; Tang, R.; Jeong, Y.; Duncan, B.; Hardy, J.A.; Rotello, V.M. Co-delivery of protein and small molecule therapeutics using nanoparticle-stabilized nanocapsules. Bioconjugate Chem. 2015, 26, 950–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasetyanto, E.A.; Bertucci, A.; Septiadi, D.; Corradini, R.; Castro-Hartmann, P.; De Cola, L. Breakable hybrid organosilica nanocapsules for protein delivery. Angew. Chem. Int. Ed. 2016, 55, 3323–3327. [Google Scholar] [CrossRef] [PubMed]
- Huu, V.A.N.; Luo, J.; Zhu, J.; Zhu, J.; Patel, S.; Boone, A.; Mahmoud, E.; McFearin, C.; Olejniczak, J.; De Gracia Lux, C. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J. Control. Release 2015, 200, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Hu, Z.; Marquez, M. Physically bonded nanoparticle networks: A novel drug delivery system. J. Control. Release 2005, 103, 21–30. [Google Scholar] [CrossRef]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody fragments as nanoparticle targeting ligands: A step in the right direction. Chem. Sci. 2017, 8, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, R.; Kraig, R.P.; Medintz, I.; Delehanty, J.B.; Stewart, M.H.; Susumu, K.; Huston, A.L.; Dawson, P.E.; Dawson, G. Nanoparticle targeting to neurons in a rat hippocampal slice culture model. Asn Neuro 2012, 4, 383–392. [Google Scholar] [CrossRef]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 239–250. [Google Scholar]
- Kansal, S.; Tandon, R.; Dwivedi, P.; Misra, P.; Verma, P.; Dube, A.; Mishra, P.R. Development of nanocapsules bearing doxorubicin for macrophage targeting through the phosphatidylserine ligand: A system for intervention in visceral leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Farokhzad, O.C. Aptamer-functionalized nanoparticles for medical applications: Challenges and opportunities. ACS Nano 2012, 6, 3670–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.-N.T.; LaVan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, N.; Vetrone, F.; Roy, R.; Capobianco, J.A. Carbohydrate-coated lanthanide-doped upconverting nanoparticles for lectin recognition. J. Mater. Chem. 2010, 20, 7543–7550. [Google Scholar] [CrossRef]
- Williams, S.A.; Schreier, A.M. The effect of education in managing side effects in women receiving chemotherapy for treatment of breast cancer. Oncol. Nurs. Forum 2007, 31, E16–E23. [Google Scholar] [CrossRef]
- Sun, C.C.; Bodurka, D.C.; Weaver, C.B.; Rasu, R.; Wolf, J.K.; Bevers, M.W.; Smith, J.A.; Wharton, J.T.; Rubenstein, E.B. Rankings and symptom assessments of side effects from chemotherapy: Insights from experienced patients with ovarian cancer. Supportive Care Cancer 2005, 13, 219–227. [Google Scholar] [CrossRef]
- Sitzia, J.; Huggins, L. Side effects of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy for breast cancer. Cancer Pract. 1998, 6, 13–21. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Mao, W.; Sun, W.; Tang, J.; Sui, M.; Shen, Y.; Gu, Z. Tumor redox heterogeneity-responsive prodrug nanocapsules for cancer chemotherapy. Adv. Mater. 2013, 25, 3670–3676. [Google Scholar] [CrossRef]
- Lu, S.; Xu, L.; Kang, E.T.; Mahendran, R.; Chiong, E.; Neoh, K.G. Co-delivery of peptide-modified cisplatin and doxorubicin via mucoadhesive nanocapsules for potential synergistic intravesical chemotherapy of non-muscle-invasive bladder cancer. Eur. J. Pharm. Sci. 2016, 84, 103–115. [Google Scholar] [CrossRef]
- Galisteo-Gonzalez, F.; Molina-Bolivar, J.A.; Navarro, S.A.; Boulaiz, H.; Aguilera-Garrido, A.; Ramirez, A.; Marchal, J.A. Albumin-covered lipid nanocapsules exhibit enhanced uptake performance by breast-tumor cells. Colloids Surf. B Biointerfaces 2018, 165, 103–110. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Galisteo-González, F. Olive-oil nanocapsules stabilized by HSA: Influence of processing variables on particle properties. J. Nanoparticle Res. 2015, 17. [Google Scholar] [CrossRef]
- Xavier-Junior, F.H.; Egito, E.S.T.D.; Morais, A.R.D.V.; Alencar, E.D.N.; Maciuk, A.; Vauthier, C. Experimental design approach applied to the development of chitosan coated poly(isobutylcyanoacrylate) nanocapsules encapsulating copaiba oil. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 251–258. [Google Scholar] [CrossRef]
- Bouchemal, K.; Couenne, F.; Briançon, S.; Fessi, H.; Tayakout, M. Polyamides nanocapsules: Modeling and wall thickness estimation. AIChE J. 2006, 52, 2161–2170. [Google Scholar] [CrossRef]
- Nassar, T.; Rom, A.; Nyska, A.; Benita, S. Novel double coated nanocapsules for intestinal delivery and enhanced oral bioavailability of tacrolimus, a P-gp substrate drug. J. Control. Release 2009, 133, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Rouzes, C.; Gref, R.; Leonard, M.; De Sousa Delgado, A.; Dellacherie, E. Surface modification of poly (lactic acid) nanospheres using hydrophobically modified dextrans as stabilizers in an o/w emulsion/evaporation technique. J. Biomed. Mater. Res. 2000, 50, 557–565. [Google Scholar] [CrossRef]
- Sahana, D.K.; Mittal, G.; Bhardwaj, V.; Kumar, M.N. PLGA nanoparticles for oral delivery of hydrophobic drugs: Influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J. Pharm. Sci. 2008, 97, 1530–1542. [Google Scholar] [CrossRef]
- Kumar, V.; Prud’homme, R.K. Nanoparticle stability: Processing pathways for solvent removal. Chem. Eng. Sci. 2009, 64, 1358–1361. [Google Scholar] [CrossRef]
- Jakubiak, P.; Thwala, L.N.; Cadete, A.; Préat, V.; Alonso, M.J.; Beloqui, A.; Csaba, N. Solvent-free protamine nanocapsules as carriers for mucosal delivery of therapeutics. Eur. Polym. J. 2017, 93, 695–705. [Google Scholar] [CrossRef]
- Steelandt, J.; Salmon, D.; Gilbert, E.; Almouazen, E.; Renaud, F.N.; Roussel, L.; Haftek, M.; Pirot, F. Antimicrobial nanocapsules: From new solvent-free process to in vitro efficiency. Int. J. Nanomed. 2014, 9, 4467–4474. [Google Scholar] [CrossRef] [Green Version]
- Villegas, M.R.; Baeza, A.; Usategui, A.; Ortiz-Romero, P.L.; Pablos, J.L.; Vallet-Regi, M. Collagenase nanocapsules: An approach to fibrosis treatment. Acta Biomater. 2018, 74, 430–438. [Google Scholar] [CrossRef]
- Ezhilarasi, P.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Wang, Y.; Kho, K.; Cheow, W.S.; Hadinoto, K. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid–polymer hybrid nanoparticles. Int. J. Pharm. 2012, 424, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Z.; Wang, K.; Tang, C.; Liu, Y.; Li, J. Prebiotic carbohydrates: Effect on physicochemical stability and solubility of algal oil nanoparticles. Carbohydr. Polym. 2020, 228, 115372. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Patel, M.; Suthar, A. Spray drying technology: An overview. Indian J. Sci. Technol. 2009, 2, 44–47. [Google Scholar] [CrossRef]
- Moretton, M.A.; Chiappetta, D.A.; Sosnik, A. Cryoprotection–lyophilization and physical stabilization of rifampicin-loaded flower-like polymeric micelles. J. R. Soc. Interface 2011, 9, 487–502. [Google Scholar] [CrossRef]
- Assegehegn, G.; Brito-de la Fuente, E.; Franco, J.M.; Gallegos, C. The importance of understanding the freezing step and its impact on freeze drying process performance. J. Pharm. Sci. 2018. [Google Scholar] [CrossRef]
- Verdun, C.; Couvreur, P.; Vranckx, H.; Lenaerts, V.; Roland, M. Development of a nanoparticle controlled-release formulation for human use. J. Control. Release 1986, 3, 205–210. [Google Scholar] [CrossRef]
- Konan, Y.N.; Cerny, R.; Favet, J.; Berton, M.; Gurny, R.; Allémann, E. Preparation and characterization of sterile sub-200 nm meso-tetra (4-hydroxylphenyl) porphyrin-loaded nanoparticles for photodynamic therapy. Eur. J. Pharm. Biopharm. 2003, 55, 115–124. [Google Scholar] [CrossRef]
- Allemann, E.; Konan, Y.; Gurny, R.; Boch, R.E. Compositions and Methods for Delivery of Photosensitive Drugs. U.S. Patent 7,455,858B2, 25 November 2008. [Google Scholar]
- Heiati, H.; Tawashi, R.; Phillips, N. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilization. J. Microencapsul. 1998, 15, 173–184. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef]
- Masson, V.; Maurin, F.; Fessi, H.; Devissaguet, J. Influence of sterilization processes on poly (ε-caprolactone) nanospheres. Biomaterials 1997, 18, 327–335. [Google Scholar] [CrossRef]
- Özcan, I.; Bouchemal, K.; Segura-Sánchez, F.; Abaci, Ö.; Özer, Ö.; Güneri, T.; Ponchel, G. Effects of sterilization techniques on the PEGylated poly (γ-benzyl-l-glutamate)(PBLG) nanoparticles. Acta Pharm. Sci. 2009, 51. [Google Scholar] [CrossRef] [Green Version]
- Rollot, J.; Couvreur, P.; Roblot-Treupel, L.; Puisieux, F. Physicochemical and morphological characterization of polyisobutyl cyanoacrylate nanocapsules. J. Pharm. Sci. 1986, 75, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Wu, G.; Chen, S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat. Phys. Chem. 2007, 76, 1126–1131. [Google Scholar] [CrossRef]
- Lamanna, M.; Morales, N.J.; García, N.L.; Goyanes, S. Development and characterization of starch nanoparticles by gamma radiation: Potential application as starch matrix filler. Carbohydr. Polym. 2013, 97, 90–97. [Google Scholar] [CrossRef] [PubMed]

| Active Substance | Polymer Film | Core | Surfactant | Organic Phase | In-organic Phase | Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Loading (mg/mL) | Encapsulation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dutasteride | Poly-(ε-caprolactone) | Sorbitan monostearate Caprylic/capric triglyceride | Lecithin Polysorbate 80 | Acetone Ethanol | Water | 199.0 ± 0.5 | 0.12 | −13.6 ± 0.6 | - | ≥ 96.7 ± 1.8 | [124] |
| Poly-(ε-caprolactone) Chitosan | 224.9 ± 3.4 | 0.23 | +40.2 ± 0.8 | ≥94.7 ± 3.0 | |||||||
| Olanzapine | Poly-(ε-caprolactone) Chitosan | Sorbitan monostearate Caprylic/capric triglycerides | Lipoid® S75 Polysorbate 80 | Acetone Ethanol | Water | 162 ± 12 | 0.24 ± 0.01 | +6.9 ± 0.7 | 1.06 | 42.2 | [89] |
| Clarithromycin | Poly (lactic -co-glycolic acid) | Medium-chain triglycerides | Lipoid® S75 Polysorbate 80 | Acetone Ethanol | Water | 94.9 ± 1.3 | 0.21 ± 0.02 | −28.2 ± 0.7 | 0.99 ± 0.02 | 68 ± 1.1 | [31] |
| Poly (lactic -co-glycolic acid) Chitosan | 120.6 ± 2.1 | 0.15 ± 0.01 | +16.5 ± 0.7 | 0.98 ± 0.02 | 67 ± 0.8 | ||||||
| Desonide | Eudragit® RL 100 | Açai oil | Span® 80 Polysorbate 80 | Acetone | Water | 165 ± 2 | 0.12 ± 0.03 | +13.8 ± 0.3 | 0.26 ± 0.004 | 81.8 ± 1.8 | [67] |
| Medium chain triglyceride | 131 ± 2 | 0.15 ± 0.02 | +6.9 ± 0.7 | 0.26 ± 0.009 | 81.6 ± 0.4 | ||||||
| Imiquimod | Poly (ε-caprolactone) | Copaiba oil | Span® 60 Tween® 80 | Acetone | Water | 242.1 ± 17 | 0.17 ± 0.1 | −8.9 ± 0.3 | 0.49 ± 0.05 | 98 ± 0.2 | [17,68] |
| 5-fluorouracil | Chitosan Poly(nvinylpyrrolidone- alt-itaconic anhydride) | Hollow | Span® 80 Tween® 80 | Dimethyl sulfoxide Acetone | Water | 118 ± 3.48 | 0.32 ± 0.015 | −12.6 ± 0.15 | 0.45 ± 0.01 (g/g) | 30.0 ± 0.57 | [82] |
| AS1411 aptamer-chitosan Poly(nvinylpyrrolidone- alt-itaconic anhydride) | 133 ± 3.46 | 0.33 ± 0.020 | −19.2 ± 0.12 | 0.34 ± 0.01 (g/g) | 22.5 ± 0.86 | ||||||
| Ketoprofen | Eudragit® S100 | Rose hip oil | Span® 80- Tween® 80 | Acetone | Water | 186 ± 14 | 0.19 ± 0.03 | −12.9 ± 2.6 | 1.00 ± 0.02 | 99.1 ± 0.5 | [125] |
| Medium chain triglycerides | 193 ± 9 | 0.19 ± 0.01 | −8.7 ± 1.5 | 0.99 ± 0.03 | 99.0 ± 0.2 | ||||||
| Cilostazol | Poly(ε-caprolactone)- Poly(ethylene glycol) | Capric/caprylic acid triglycerides | Span® 80 Tween® 80 | Acetone | Water | 130 ± 5.80 | 0.18 ± 0.03 | −37.6 ± 1.50 | 11,984.64 ± 0.10 (μg/mL) | 99.87 | [58] |
| Carvedilol | Poly(ε-caprolactone) | Grape seed oil | Polysorbate 80 | Acetone | Water | 182 ± 7 | -- | −7.6 ± 1 | 0.47 ± 0.002 | -- | [126] |
| Eudragit® S100 | 139 ± 6 | +7.3 ± 3 | 0.48 ± 0.01 | ||||||||
| Bedaquiline | Chitosan Poly(ethylene glycol) | Oleic acid | Span® 85 Tween® 20 | Ethanol | Water | 455.6 ± 26 | 0.204 ± 0.020 | −9 ± 2 | 25 ± 2 (%) | -- | [30,64] |
| Chitosan | 328 ± 35 | 0.151 ± 0.027 | +26 ± 4 | 28 ± 2 (%) | 70 ± 7 | ||||||
| Indole-3-carbinol | Eudragit® S100 | Rose hip oil | Span® 8 Tween® 80 | Acetone | Water | 334 ± 1 | < 2.0 | +10.47 ± 0.05 | 0.5 | 44 | [127] |
| Diphenyl diselenide | Poly(ε-caprolactone) | Medium chain triglycerides | Span® 80 Tween® 80 | Acetone | Water | 240 ± 52 | 0.151 ± 0.06 | −10.9 ± 2.2 | 5 | 98.0 | [128] |
| Cloxacillin benzathine | Poly(ε-caprolactone) | Labrafac® CC | Span®80 Pluronic® F68 | Methanol Acetone | Water | 322.4 ± 4.0 | 0.088 ± 0.051 | −28.2 ± 0.6 | 0.183 | 46.73 ± 5.92 | [129] |
| Antimicrobial essential oil | Cellulose acetate | Peppermint | -- | Acetone | Water | ~180 | ~0.30 | ~−40 | 82 (%) | -- | [90] |
| Cinnamon | ~150 | ~0.10 | ~−40 | 14.2 (%) | |||||||
| Lemongrass | ~200 | ~0.20 | ~−35 | 155 (%) | |||||||
| p,p’-methoxyl-diphenyl diselenide | Poly(ε-caprolactone) | Medium chain triglycerides | Span® 80 Tween® 80 | Acetone | Water | 236 ± 4 | 0.16 ± 0.019 | −5.4 ± 0.057 | 2.5 | 98.78 ± 1.54 | [130,131] |
| Calcitriol | Poly(D,L-lactic acid) | Miglyol® 829 | Montanox® VG80 | Acetone | Water | 183 ± 8 | 0.083 ± 0.028 | −21.3 ± 2.4 | -- | 86 ± 2 | [132] |
| Quercetin | Chitosan | Miglyol® 812 | Lecithin | Ethanol | Water | 190 ± 4 | 0.12 | + 48.4 ± 3.46 | -- | 99 ± 1.2 | [133] |
| Baicalein | 187 ± 2 | 0.12 | +48.1 ± 2.03 | 87 ± 5.1 | |||||||
| Meloxicam | Poly(ε-caprolactone) | Miglyol® 810 | Polysorbate 80 | Acetone | Water | 247–212 | 0.14 | −36 | 99.9 (%) | -- | [134] |
| Quinine Curcumin | Poly(ε-caprolactone) | Caprylic/capric triglyceride | Lipoid® S45 | Acetone | Water | 194 ± 1 | 0.119 ± 0.00 | −27.2 ± 0.1 | 98 ± 2.4 (%, Quinine) 96 ± 2.2 (%, Curcumin) | 97 ± 2.1 (Quinine) 90 ± 1.3 (Curcumin) | [135] |
| Carvedilol | Poly(ε-caprolactone) | Grape oil Sorbitan monostearate | Polysorbate 80 | Acetone | Water | 180 ± 3 | 0.08 ± 0.01 | −6.6 ± 0.6 | -- | 99.1 ± 0.21 | [136] |
| Eudragit® RS 100 | Grape oil | 139 ± 6 | 0.14 ± 0.01 | +9.2 ± 2.4 | 88 ± 1.10 | ||||||
| Doxorubicin | Synthesized poly(ε-caprolactone)- poly [(methyl methacrylate)-co-(2-dimethylamino)ethyl methacrylate)2] | Caprylic triglyceride | Polysorbate 80 | Acetone Ethanol | Water | 60.6 ± 0.8 | 0.19 ± 0.01 | +13.3 ± 0.9 | 11 μg/mL | 69.7 | [137] |
| Elisidepsin | Polyarginine | Miglyol® 812 Epikuron® 170 | Poloxamer 188 | Ethanol Acetone | Water | 178 ± 15 | 0.1 | +30 ± 11 | -- | 46 ± 7 | [138] |
| Fluorescent probe DiD-labelled polyarginine | 129 ± 2 | 0.1 | +25 ± 1 | 75 ± 5 | |||||||
| Fluorescein-DHPE-labelled polyarginine | 140 ± 1 | 0.1 | +52 ± 1 | 79 ± 1 | |||||||
| Glycerol monolaurate | Polymeric blende of poly (methyl methacrylate) and poly (ethylene glycol) | Capryc/caprylic triglyceride | Sorbitan monooleate | Acetone | Water | 193.2 ± 4 | 0.044 ± 0.028 | −23.3 ± 3 | -- | -- | [139] |
| Plitidepsin | Poly-aminoacid-poly(ethylene glycol) | Epikuron® 170 Miglyol® | Poloxamer® 188 | Acetone Ethanol | Water | 190 ± 15 | 0.1 | −24 ± 5 | -- | 85 ± 4 | [140] |
| Active Substance | Polymer Film | Core | Surfactant | Organic Phase | In-Organic Phase | Diffusion or Evaporation | Particle Size (nm) | PDI | Zeta Potential (mv) | Drug Loading | Encapsulation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B | Propionated Sterculia striata polysaccharide | Miglyol® L812 | -- | Acetone Methanol | Water | Diffusion (Water) | 274.1 ± 8.6 | 0.181 | −39.8 ± 0,2 | -- | 99.2 ± 1.3 | [95,141] |
| Thymoquinone Docetaxel | Chitosan | Oleic acid- phospholipid | Poloxamer | Ethanol | Water | Diffusion (Water) | 141.7 ± 2.8 | 0.17 ± 0.02 | −8.17 ± 0.1 | 7.7 ± 0.4 (%, Thymoquinone) 4.3 ± 0.4 (%, Docetaxel) | 85.3 ± 3.1 (Thymoquinone) 66.1 ± 3.5 (Docetaxel) | [142] |
| Essential oil from the L. sidoides leaves | Poly(ε-caprolactone) | Essential oil Ethyl laurate | Kolliphor® P188 | Ethyl acetate | Water | Diffusion (Water) | 173.6 | 0.2 | −42 | -- | 70.6 | [143] |
| Docetaxel | Poly (D, L-lactide) | Labrafac® CC | Polyvinyl alcohol | Ethyl acetate | Water | Diffusion (Water) | 115–582 | < 0.05 | −36.5 ± 9 | up to 68.3% | 65–93 | [144] |
| Human neutrophil elastase inhibitor | Pregelatinized modified starch | Capric/caprylic triglycerides | Tween®80 Cetrimide | Ethanol | water | Evaporation | 100–900 | -- | > 30 | > 0.5 (%) | > 75 | [145,146] |
| Paclitaxel Perfluorooctyl bromide | Poly(D, L-lactide-co-glycolide)-poly(ethylene glycol) | Perfluorooctyl bromide | Sodium cholate | Dichloromethane | water | Evaporation | 120 | 0.2 | −18 ± 3 | 6 μg/mg | 15 (Paclitaxel) 40 (Perfluorooctyl bromide) | [96,147] |
| Curcumin | Bovine serum albumin-capped gold nanoclusters | Undecylenic acid | Bovine serum albumin-capped gold nanoclusters (Self assemble) | Dichloromethane | water | Evaporation | 177.54 ± 37.11 | -- | −29.8 ± 4.86 | -- | -- | [13] |
| Catechin rich extract | Eudragit® L 100 | -- | Sodium laureth sulfate | Methanol | Lactose aqueous solution | Evaporation | 100–400 | -- | -- | -- | 40–75 | [94] |
| Active Substance | Polymer Film | Core | Surfactant | Organic Phase | In-organic Phase | Coacervation Method | Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Loading (%) | Encapsulation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coumarin 6 | Folic acid decorated-ε-poly-L-lysine-SH | Soybean oil | -- | Soybean oil | Water | Sonochemical method | ~500 | -- | -- | -- | -- | [66] |
| Methotrexate | Human serum albumin Silk fibroin | n-dodecane | -- | n-dodecane | Potassium phosphate buffer | Sonochemical method | 438–888 | -- | −8.81 - −13.43 | 76.80 ± 17.20 | 98.78 ± 0.08 | [107] |
| Brinzolamide | Chitosan Pectin | Aqueous core | Tween® 80 | -- | Water | Electrostatic interaction | 240.05 ± 0.08 | 0.32 ± 0.08 | +27.6 ± 0.03 | 18.92 ± 0.25 | 92.20 ± 0.12 | [83] |
| Paclitaxel | Chitosan Poly(isobutyl cyanoacrylate) (Monomer: isobutyl cyanoacrylate) | Copaiba oil | -- | Ethanol | Water | Interfacial polymerization | 486 ± 3 | 0.17 | +37.1 ± 0.3 | 1.70 ± 0.02 | 74 ± 1 | [148] |
| -- | Monomer: triethylene glycol divinely ether | N-hexadecane | Sodiumdodecyl sulfate | N-hexadecane | Water | Photopolymerization | 218 | 0.18 | −71.5 | -- | -- | [108,109] |
| Polyvinyl pyrrolidone | 293 | 0.27 | −15.1 | |||||||||
| Pluronic® PE 6100 | 308 | 0.12 | −26.8 | |||||||||
| Rhodamine B | Monomer: hydrophilic deprotonated cystamine 2,4-toluene diisocyanate | Aqueous core | Lubrizol U | Cyclohexane | Water | Interfacial Polyaddition reaction | 252–444 | ~0.2 | -- | -- | -- | [149] |
| Sulforhodamine 101 | Hydrophilic potato starch Cross-linker: 2,4-toluene diisocyanate | Aqueous core | Polyglycerol polyricinoleate | Cyclohexane | Sodium chloride aqueous solution | Interfacial polymerization | ~200 | -- | -- | -- | ~70–100 | [101] |
| Coumarin 1 | Monomer: Methyl methacrylate and 2-(diethylamino)ethyl methacrylate Initiator: 2,2-azobis (isobutyronitrile) dextran-based transurf | Miglyol® 810 | Amphiphilic dextran-based transurf | Methyl methacrylate | Water | RAFT polymerization | 198 ± 9 | 0.15 | an almost neutral surface | 1.7 | 99 | [103] |
| Bovine serum albumin | Monomer: styrene MacroRAFT agent: Polystyrene-co-polyN-(2-Hydroxypropyl)methacrylamide Crosslinker: divinylbenzene Initiator: 2,2’-azobis(isobutyronitrile) | Aqueous core | Polystyrene-co-polyN-(2-Hydroxypropyl)methacrylamide | Toluene n-hexane | Sodium chloride aqueous solution | RAFT polymerization | 33–202 | 0.1–0.22 | -- | -- | -- | [104] |
| Capreomycin sulfate | Monomers: N,N-(dimethylamino)ethyl methacrylate or tertiary butyl methacrylate with methyl methacrylate MacroRAFT agent: Poly (butyl acrylate)-co- acrylic acid Crosslinker: Ethylene glycol dimethacrylate Initiator: azo-initiator 4,4‘ - azobis(4-cyanovaleric acid) | Aqueous core | Dimethyldioctadecyl ammonium bromide | -- | Water | RAFT polymerization | ~130 | -- | -- | 70 | -- | [105,106] |
| Doxorubicin | Monomer: Tert-butyl acrylate Macroinitiator: Folate-poly(ethylene glycol)-b-poly(tert-butyl acrylate) Reducing agent: ascorbic acid Crosslinker: N,N’-bis(acryloyl) cystamine Catalyst: CuBr2 | Hollow | Folate-poly(ethylene glycol)-b-poly(tert-butyl acrylate) | Cyclohexanone/hexadecane | Water | AGET ATRP polymerization | ~150 | -- | -- | 39.7 | 99.2 | [102] |
| Active Substance | Polymer Film | Core | In-organic Phase 1/Surfactant | Organic Phase/Surfactant | In-organic Phase 2/Surfactant | Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Loading (%) | Encapsulation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gemcitabine hydrochloride | Poly(ethylene glycol) Poly(L, lactide) | Aqueous core | Water/ Span | Chloroform Dichloromethane | Water/ Sodium deoxycholate hydrate | 210 ± 11 | 0.09 ± 0.01 | ~−30 | ~22 | 88.6 | [71] |
| Resiquimod (R848) Muramyl dipeptide (MDP) | Acetalated dextran | Aqueous core | Phosphate buffered saline | Dichloromethane | Phosphate buffered saline/ Polyvinyl alcohol | ~150 | -- | ~−30 | 0.42 (μg/mg, R848) 1.41 (μg/mg, MDP) | -- | [150] |
| Doxorubicin hydrochloride Magnetic nanoparticles | N-palmitoyl chitosan | Aqueous core | Water/Tween® 80 | Chloroform | Water/Tween® 80 | 215 ± 23.33 | -- | +15.8 ± 0.42 | 1.54 | 73 | [116] |
| Doxorubicin Paclitaxel Magnetic nanoparticles | Poly(methacrylic acid) Polyvinyl alcohol | Aqueous core | Water/ Polyvinyl alcohol | Chloroform | Water/ Polyvinyl alcohol | 184 ± 10 | -- | ~10 (at pH 4–5) −15 & −30 (at pH 7) | 65.21 (μg/mL, Doxorubicin) 21.74 (μg/mL Paclitaxel) | 72 (Doxorubicin) 91 (Paclitaxel) | [115] |
| Plasmid DNA PEGylated quantum dots Pyrene Doxorubicin | Poly(styrene allyl alcohol) | Aqueous core | Water | Chloroform /Oleic acid | Water/ Polyvinyl alcohol | 263 ± 42 | -- | -- | -- | ~30–70 (Plasmid DNA) ~40–70 (PEGylated quantum dots) ~80–90 (Pyrene) ~10–60 (Doxorubicin) | [114] |
| Bovine serum albumin | Poly(lactic acid-co-glycolic acid) | Aqueous core | Water | Dichloromethane | Water/ Polyvinyl alcohol | 327 | -- | -- | -- | 84.75 ± 1.47 | [151] |
| Poly(3-hydroxybutyrateco- 3-hydroxyvalerate) | 438 | 16.72 ± 1.06 | |||||||||
| Fungicides tebuconazole Carbendazim | Synthesized poly(phenyleneethynylene) fluorescent polymer Poly(ε-caprolactone) | Hollow | Acetone (Organic solvent as first phase) | Chloroform | Water/Polyvinyl alcohol | ~430 | <0.2 | ~−13 | -- | -- | [112] |
| Active Substance | Cationic Layer | Anionic Layer | Core | Phase | Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Loading (%) | Encapsulation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Curcumin | Poly-L-arginine (ARG) Chitosan (CS) | Sodium alginate (ALG) Eudragit® L100 (EUD) | CaCO3 (Sacrificial template) | 0.05 M NaCl aqueous solution | 400 ± 50 | ~0.2 | −43 ± 1.75 | 5.1 | 70 | [79] |
| Composition sequence: ARG-ALG-ARG-ALG-CS-EUD | ||||||||||
| Pyrene 4-hydroxy-tamoxifen | Poly(cyclodextrin) (PCD) | Sodium alginate (ALG) | Gold (Sacrificial template) | Water | ~60 | -- | ~+20 | -- | -- | [47] |
| Composition sequence: (PCD/ALG)×4-PCD | ||||||||||
| RNAi Chemokines CCL2 | Polyarginine (ARG) | Hyaluronic acid (HA) | Glyceryl-monolete cubic gel | Water | ~ 150 | ~0.2 | −40 | -- | 46.0 ± 1.0 (CCL2) 68.2 ± 3.2 (RNAi) | [120] |
| Composition sequence: ARG-RNAi-ARG-HA | ||||||||||
| Amoxicillin | Chitosan (CS) | Poly(acrylic acid) (PAA) | Gold (Sacrificial template) | -- | <100 | -- | ~+40 (CS out layer) ~-20 (PAA out layer) | 3.5–5.5 | 62–75 | [33] |
| Composition sequence: (CS/PAA)×4 or 8 | ||||||||||
| Camptothecin | Poly-L-lysine (PLL) | Poly-L-glutamic acid sodium salt (PGA) | Hollow (Aionic surfactant docusate sodium salt) | 0.015 M NaCl aqueous solution | ~120 | <0.2 | ~−5 | 3.2 (μg/ml) | 98 | [65] |
| Composition sequence: (PLL/PGA)×2-PLL-PEG | ||||||||||
| IutA antigen | Chitosan (CS) | Dextran sulfate (DEX) | Vitamin E (Oleic core) | Water | ~200 | ~0.2 | ~−40 | -- | ~70 | [44] |
| CS-DEX | ||||||||||
| Indomethacin | Chitosan orPoly(ethylene imine) | Poly(acrylic acid) | Cetyltrimethylammonium bromide or hexamethylene-1,6-bis(dimethylhexadecylammoniumdibromide (Oleic core) | Water | ~90–200 | -- | −50 – −55 | ~9–18 | ~50–60 | [119] |
| (PAA/PEI)×3 or 5 or 7 | ||||||||||
| Cyclosporine A | Poly-L-lysine (PLL) | Poly(L-glutamic acid) sodium salt (PGA) | Hollow (Aionic surfactant docusate sodium salt) | 0.015 M NaCl aqueous solution | 157.5 (PLL) 160 (PLL/PGA) | 0.224 (PLL) 0.117 (PLL-PGA) | +43 −41 | -- | -- | [152] |
| PLL PLL-PGA | ||||||||||
| Doxorubicin hydrochloride | Chitosan (CS) | Pectin (Pec) | SiO2 (Sacrificial template) | Water | 473.10 ± 3.03 | 0.169 | −28.33 ± 3.01 | 20.32 ± 0.33 | 76.51 ± 1.53 | [118] |
| (Pec/CS)×2-Pec | ||||||||||
| Bovine serum albumin | Cationic quaternary ammonium starch (QAS) | Anionic carboxymethyl starch (CMS) | Bovine serum albumin particles | Water | 20–300 | -- | 0 – −10 | -- | up to 45.52 | [121] |
| CMS/QAS/CMS | ||||||||||
| Clozapine | Poly-L-lysine (PLL) | Poly-L-glutamic acid (PGA) | Hollow (Aionic surfactant docusate sodium salt) | Water | ~100 | <0.2 | −4 ± 6 | -- | -- | [16] |
| (PLL/PGA)×4-PGA-PEG | ||||||||||
| Active Substance | Therapy | Main Achieved Advantage | Polymer Membrane | Core | Formulation Method | Ref. |
|---|---|---|---|---|---|---|
| Olanzapine | Inhibition of startle response (PPI) in rats following oral administration | Chitosan coated increase the mucoadhesion and the brain delivery | Poly (ε-caprolactone) Chitosan | Sorbitan monostearate and caprylic/capric triglycerides | Interfacial deposition | [89] |
| Clarithromycin | Aerosol delivery | Improved bioavailability of poorly soluble drugs | Poly (lactic-co-glycolic acid) | Medium-chain triglycerides | Interfacial deposition | [31] |
| Desonide | Anti-inflammatory | Protecting active substance from hash environment (Photosensitive) | Eudragit® RL 100 | Açai oil or medium chain triglyceride | Interfacial deposition | [67] |
| Imiquimod | Cervical cancer | Improved bioavailability of poorly soluble drugs | Poly (ε-caprolactone) | Copaiba oil | Interfacial deposition | [17,68] |
| 5-fluorouracil | Anti-cancer therapy | Toxicity moderation Targeting delivery (AS1411 aptamer) Improved bioavailability of poorly soluble drugs | Chitosan Poly (Nvinylpyrrolidone- alt-itaconic anhydride) | Hollow | Interfacial deposition | [82] |
| Ketoprofen | Anti-inflammatory | Protecting active substance from hash environment (Photo) Toxicity moderation | Eudragit® S100 | Rose hip oil | Interfacial deposition | [125] |
| Cilostazol | Peripheral arterial disease | Sustained delivery | Poly(ε-caprolactone)- poly (ethylene glycol) | Capric/caprylic acid triglycerides | Interfacial deposition | [58] |
| Carvedilol | Sublingual administration Heart failure, hypertension and coronary artery diseases | Improved bioavailability of poorly soluble drugs | Eudragit® S100 Poly(ε-caprolactone) | Grape seed oil | Interfacial deposition | [126] |
| Bedaquiline | Mycobacterium tuberculosis | Toxicity moderation Improved bioavailability of poorly soluble drugs | Chitosan–poly (ethylene glycol) | Oleic acid | Interfacial deposition | [30,64] |
| Indole-3-carbinol | Antinocieptive | Protecting active substance from hash environment (Photo, temperature and pH) | Eudragit® S100 | Rose hip oil | Interfacial deposition | [127] |
| Diphenyl diselenide | Malignant cutaneous melanoma | Improved bioavailability of poorly soluble drugs Protecting active substance from hash environment (Photosensitive) | Poly(ε-caprolactone) | Medium chain triglycerides | Interfacial deposition | [128] |
| Cloxacillin benzathine | Mastitis | Improved bioavailability of poorly soluble drugs | Poly(ε-caprolactone) | Labrafac® CC | Interfacial deposition | [129] |
| Curcumin | Breast cancer therapy | Improved bioavailability of poorly soluble drugs Sustained delivery | Human serum albumin | Olive oil | Interfacial deposition | [172,173] |
| Amphotericin B | Systemic fungal infection and leishmania | Toxicity moderation Improved bioavailability of poorly soluble drugs | Synthesized propionated Sterculia striata polysaccharide | Miglyol® L812 | Emulsion–diffusion/evaporation | [95,141] |
| Thymoquinone Docetaxel | Anti-cancer therapy | Toxicity moderation Improved bioavailability of poorly soluble drugs | Chitosan | Oleic acid-phospholipid | Emulsion–diffusion/evaporation | [142] |
| Brinzolamide | Glaucoma treatment Ocular delivery | Sustained delivery Improved bioavailability of poorly soluble drugs | Chitosan Pectin | Aqueous core | Emulsion–coacervation | [83] |
| Coumarin 6 | Cancer | Targeting delivery (folic acid as ligand) | Folic acid decorated reductive-responsive ε-poly-L-lysine | Soybean oil | Emulsion–coacervation | [66] |
| Paclitaxel | Anti-cancer drug delivery via oral administration | Chitosan coated nanocapsules for oral delivery | Chitosan Poly(isobutyl cyanoacrylate) | Copaiba oil | Emulsion–coacervation | [148] |
| Copaiba oil | Potential mucoadhesive nanoparticles | Improved bioavailability of poorly soluble drugs | Chitosan–poly(isobutylcyanoacrylate) | Copaiba oil | Emulsion–coacervation | [174] |
| Curcumin | Oral administration Selective drug delivery in the colon | Protecting active substance from hash environment | Poly-L-arginine Sodium alginate Chitosan Eudragit® L100 | Hollow (Template CaCO3) | Layer-by-layer | [79] |
| Pyrene 4-hydroxy-tamoxifen | Anti-estrogenic delivery | Improved bioavailability of poorly soluble drugs | Poly(cyclodextrin) Sodium alginate | Hollow (Template Gold) | Layer-by-layer | [47] |
| RNAi Chemokines CCL2 | Cancer immunotherapy | Improved bioavailability of poorly soluble drugs | Polyarginine and hyaluronic acid | Glyceryl-monooleate cubic gel | Layer-by-layer | [120] |
| Amoxicillin | Antibiotic therapy | Protecting active substance from hash environment (Photosensitive) | Chitosan Poly(acrylic acid) | Hollow (Template Gold) | Layer-by-layer | [33] |
| Camptothecin | Anti-cancer therapy | Improved bioavailability of poorly soluble drugs | Poly-L-lysine Poly-L-glutamic acid sodium salt | Hollow | Layer-by-layer | [65] |
| IutA antigen | — | Protecting active substance from hash environment (biochemical degradation) Improved bioavailability of poorly soluble drugs | Chitosan Dextran sulfate | Vitamin E | Layer-by-layer | [44] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. https://doi.org/10.3390/nano10050847
Deng S, Gigliobianco MR, Censi R, Di Martino P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials. 2020; 10(5):847. https://doi.org/10.3390/nano10050847
Chicago/Turabian StyleDeng, Siyuan, Maria Rosa Gigliobianco, Roberta Censi, and Piera Di Martino. 2020. "Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities" Nanomaterials 10, no. 5: 847. https://doi.org/10.3390/nano10050847







