The Antibacterial Effect of Silver Nanoparticles on Staphylococcus Epidermidis Strains with Different Biofilm-Forming Ability
Abstract
1. Introduction
2. Results and Discussion
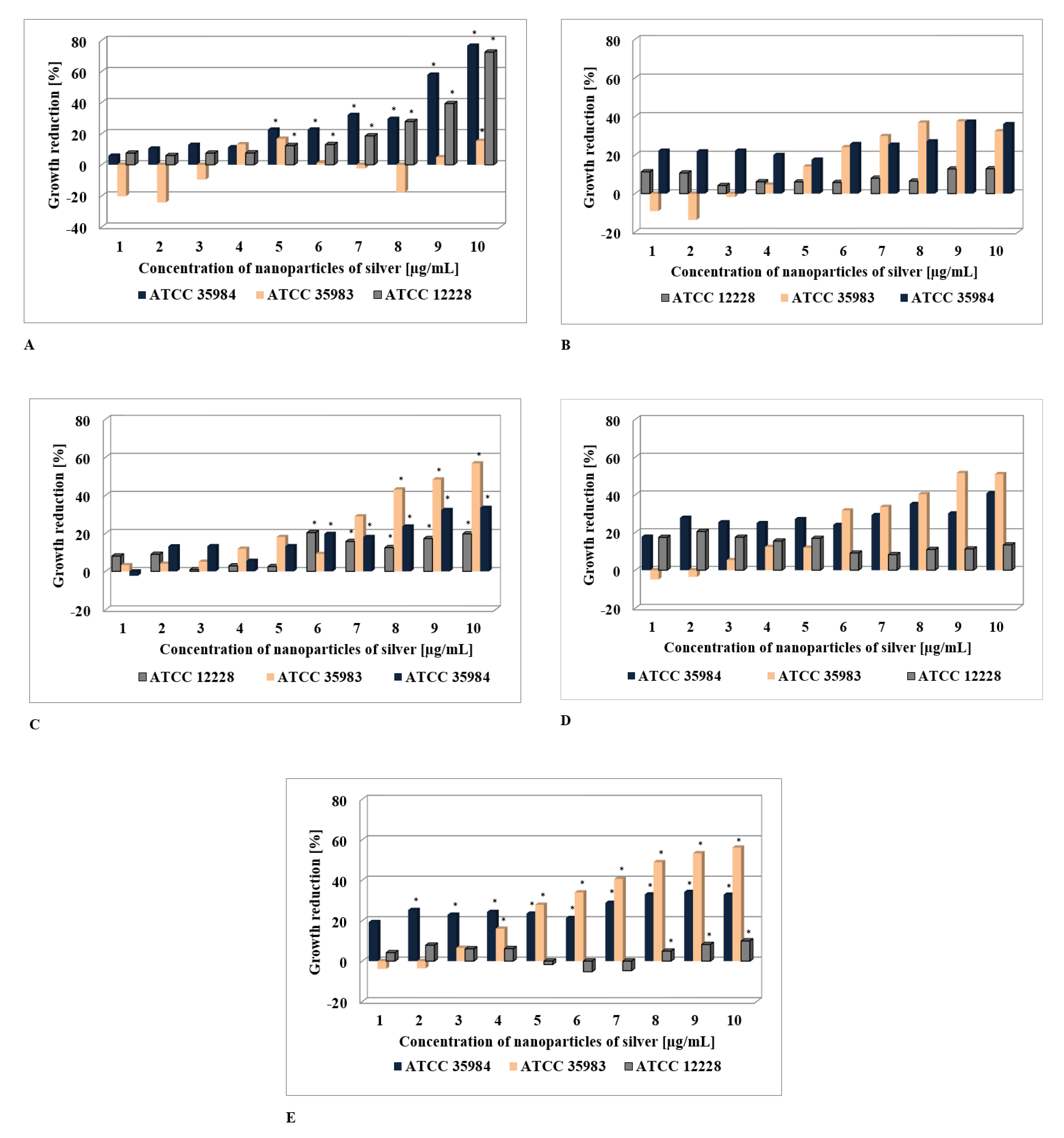
2.1. The Assessment of AgNPs’ Anti-Staphyloccocus Activity
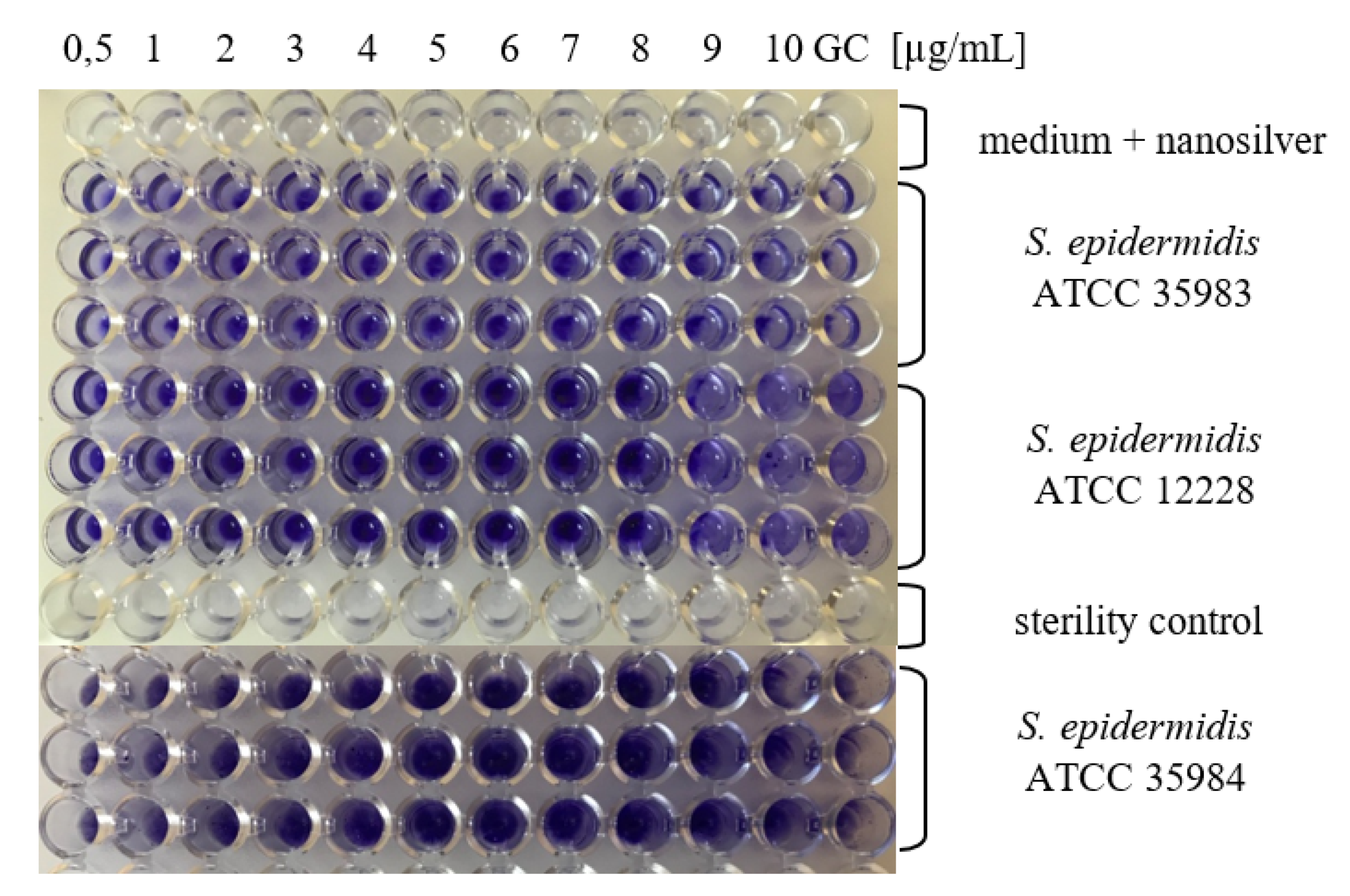
2.2. Determination of MBIC for AgNPs
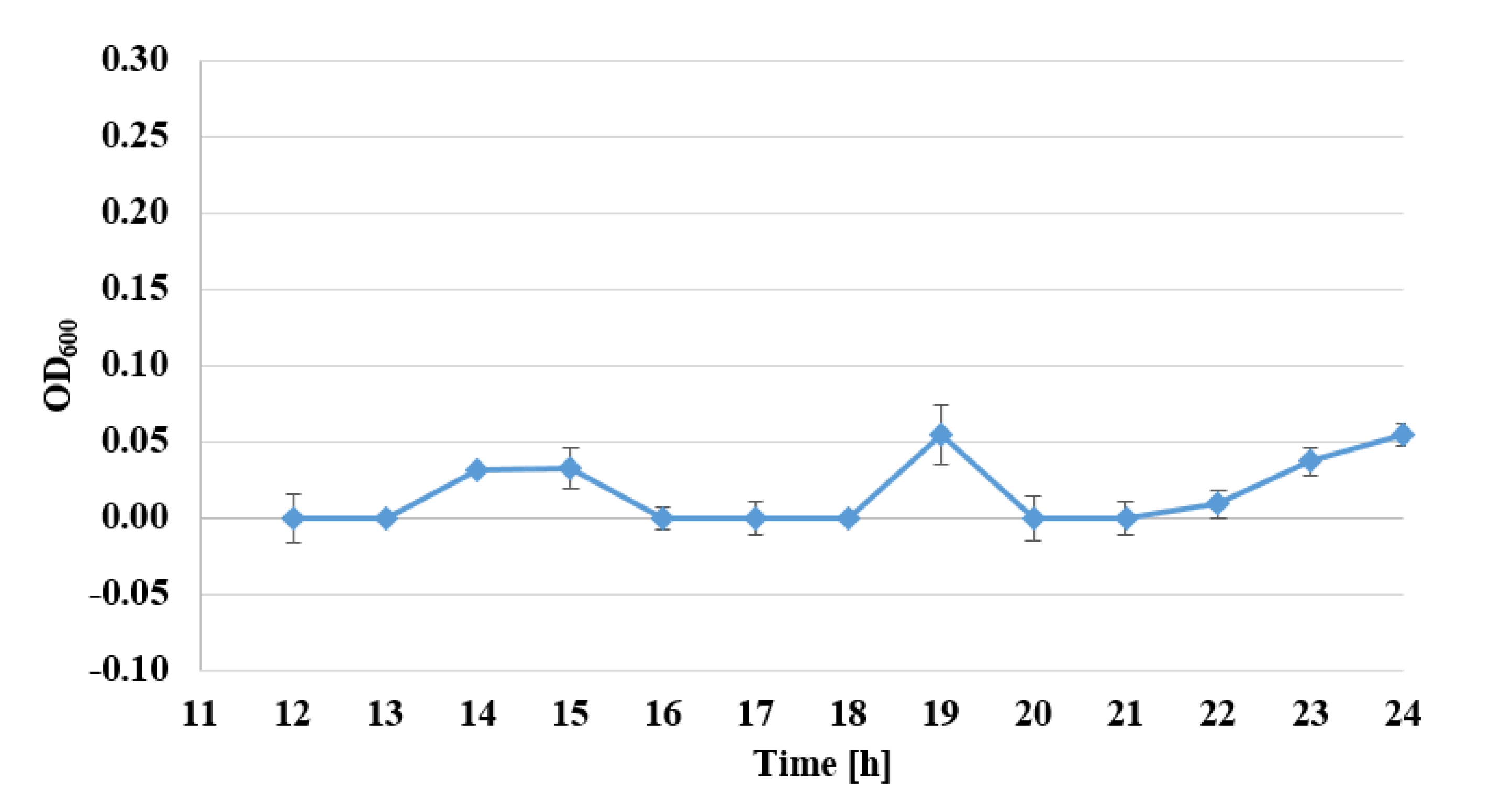
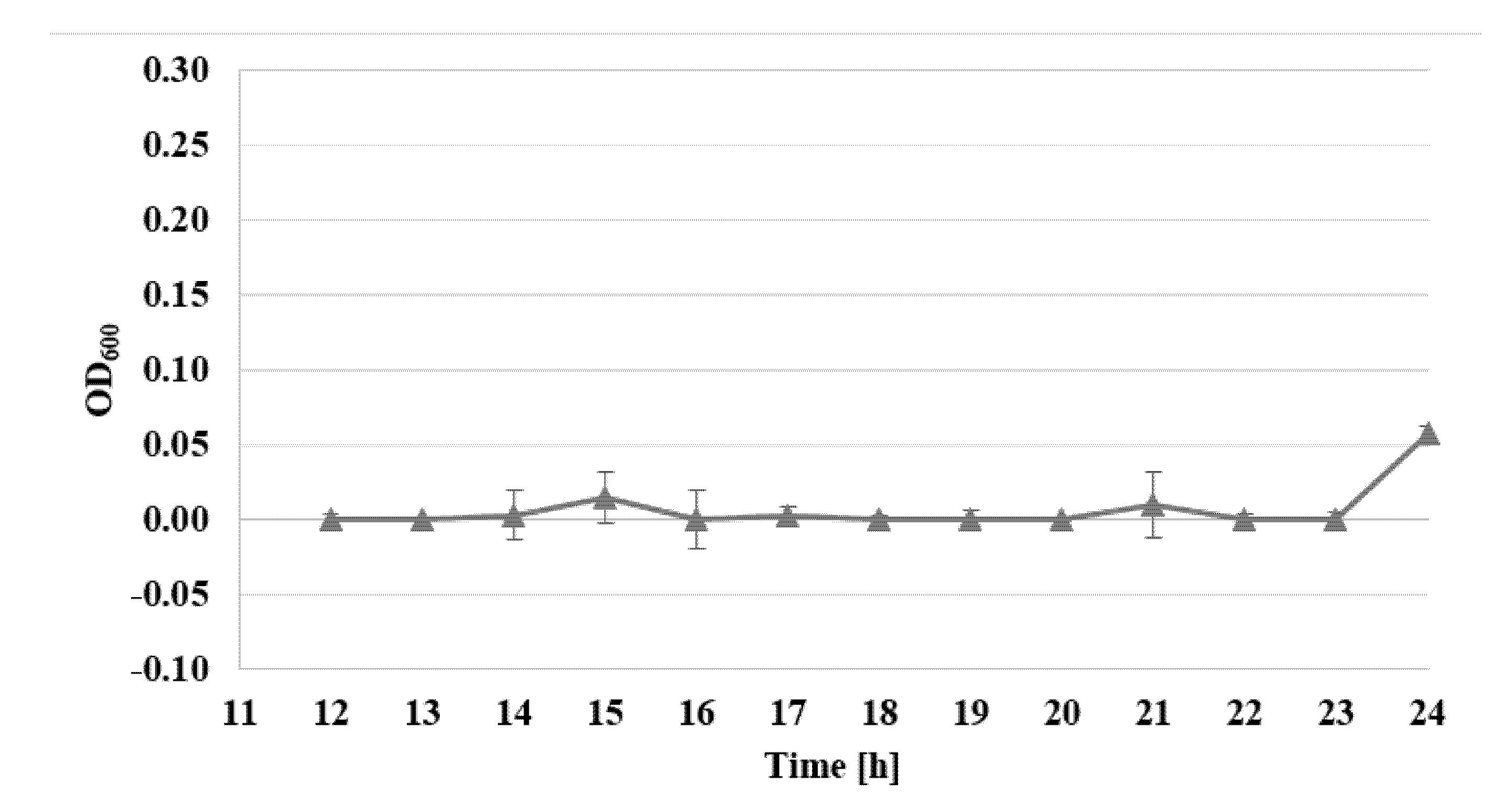
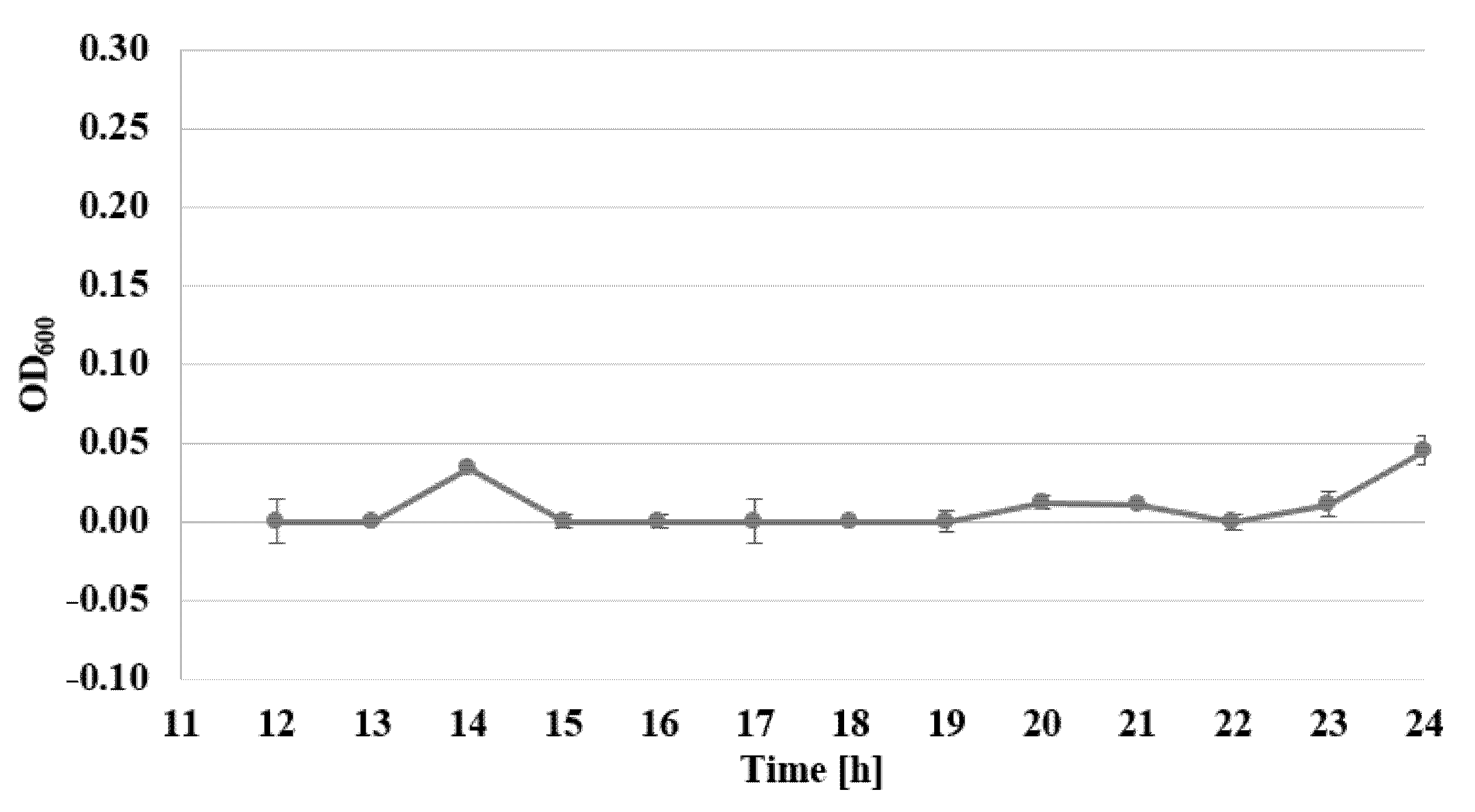
2.3. Determination of Bacterial Cell Viability by Alamar Blue Test
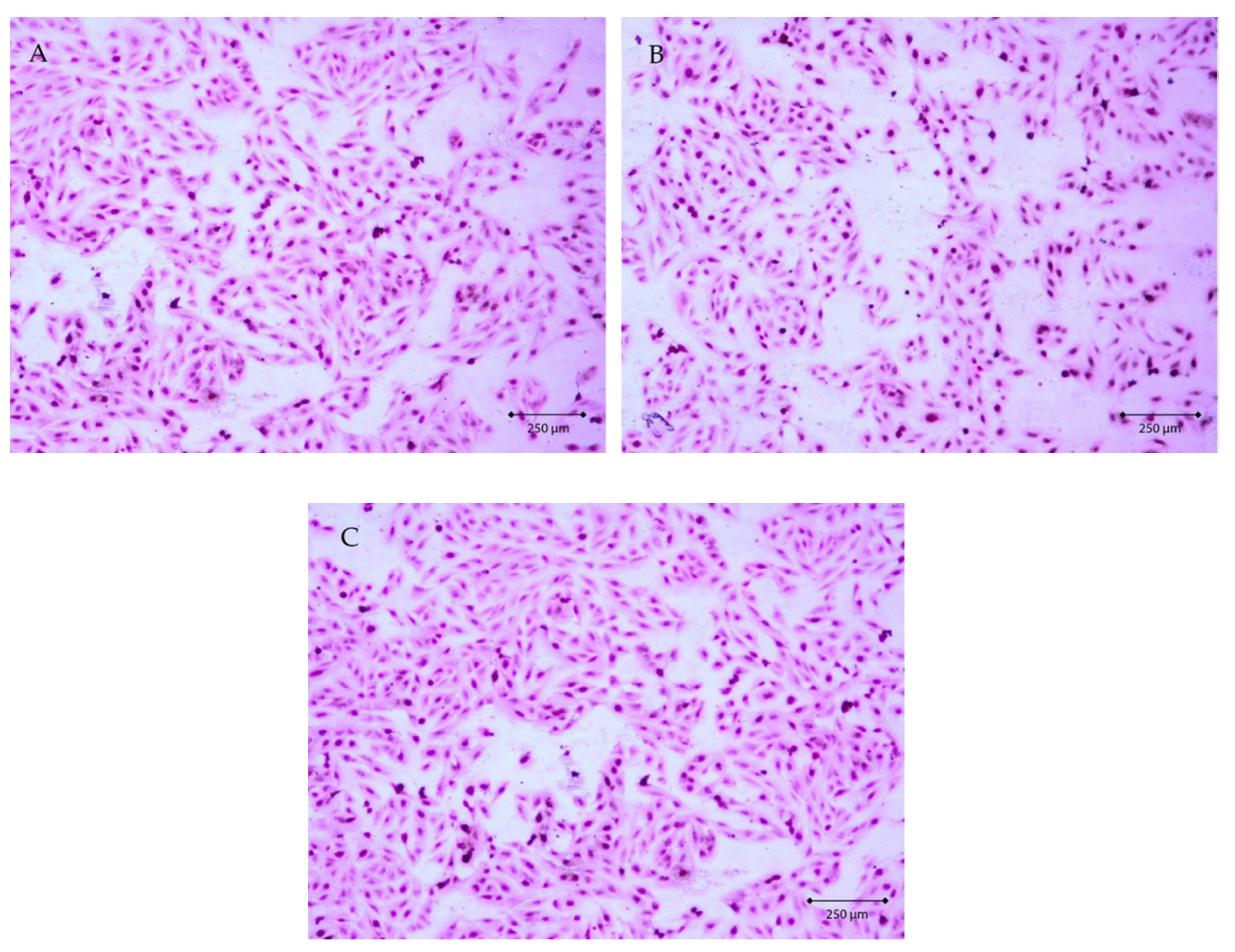
2.4. Evaluation of AgNPs Cytotoxicity Toward Eucariotic Fibroblast Cells of the GM03348 Line
2.5. Statistical Analysis
3. Experimental Section
3.1. Bacterial Strains, Media, and Reagents
3.2. Determination of Antistaphylococcal Activity of AgNPs
- GR—percentage of growth reduction
- ODS—the average value of spectrophotometric measurements at λ = 595 nm for the control sample without the addition of AgNPs
- ODT—the average value of spectrophotometric measurements at λ = 595 nm for the studied sample with the addition of AgNPs at the studied concentration
3.3. Determination of MBIC Values for AgNPs
3.4. Determination of Viability of Bacterial Cells by Alamar Blue Test
3.5. Evaluation of AgNPs’ Cytotoxicity Toward Eucariotic Fibroblast Cells of the GM03348 Line
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, P.; Xie, C.-B.; Sun, F.-H.; Guo, L.-J.; Dai, M.; Cheng, X.; Ma, Y.-X. Molecular characteristics of methicillin-resistant Staphylococcus epidermidis on the abdominal skin of females before laparotomy. Int. J. Mol. Sci. 2016, 17, 992. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Orlewska, K.; Kępa, M.; Idzik, D.; Dziedzic, A.; Mularz, T.; Krawczyk, M.; Miklasińska, M.; Wąsik, T.J. Biofilm Formation and Antimicrobial Susceptibility of Staphylococcus epidermidis strains from a hospital environment. Int. J. Environ. Res. Public Heal. 2014, 11, 4619–4633. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.; Pereira, V.C.; Pinheiro-Hubinger, L.; Riboli, D.; Martins, K.B.; Cunha, M. Antimicrobial Resistance Profile of Planktonic and Biofilm Cells of Staphylococcus aureus and coagulase-negative staphylococci. Int. J. Mol. Sci. 2016, 17, 1423. [Google Scholar] [CrossRef] [PubMed]
- Van Kerckhoven, M.; Hotterbeekx, A.; Lanckacker, E.; Moons, P.; Lammens, C.; Kerstens, M.; Ieven, M.; Delputte, P.; Jorens, P.G.; Malhotra-Kumar, S.; et al. Characterizing the in vitro biofilm phenotype of Staphylococcus epidermidis isolates from central venous catheters. J. Microbiol. Methods 2016, 127, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; França, Â.; Cerca, N. Staphylococcus epidermidis is largely dependent on iron availability to form biofilms. Int. J. Med Microbiol. 2017, 307, 552–563. [Google Scholar] [CrossRef]
- Pulverer, G.; Peters, G.; Schumacher-Perdreau, F. Coagulase-negative staphylococci. Zentralblatt für bakteriologie, mikrobiologie und hygiene. Series A: Medical microbiology, infectious diseases, virology, parasitology 1987, 264, 1–28. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Ceri, H.; Oliveira, R. Evaluation of antimicrobial activity of certain combinations of antibiotics against in vitro Staphylococcus epidermidis biofilms. Indian J. Med Res. 2012, 135, 542–547. [Google Scholar]
- Singh, N.; Rajwade, J.M.; Paknikar, K. Transcriptome analysis of silver nanoparticles treated Staphylococcus aureus reveals potential targets for biofilm inhibition. Colloids Surf. B Biointerfaces 2019, 175, 487–497. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB). Antibiotics 2018, 7, 69. [Google Scholar] [CrossRef]
- Esmaeillou, M.; Zarrini, G.; Rezaee, M.A.; Mojarrad, J.S.; Bahadori, A. Vancomycin capped with silver nanoparticles as an antibacterial agent against multi-drug resistance bacteria. Adv. Pharm. Bull. 2017, 7, 479–483. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.K.; Morelli, G.; Galdiero, S. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, F.; Yaqub, A.; Tanvir, S.; Anderson, W.A. Poly-L-arginine coated silver nanoprisms and their anti-bacterial properties. Nanomaterials 2017, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: New insight into the antimicrobial action of silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram negative bacteria. J. Colloid Interf. Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Gahlawat, G.; Shikha, S.; Chaddha, B.S.; Chaudhuri, S.R.; Mayilraj, S.; Choudhury, A.R. Microbial glycolipoprotein-capped silver nanoparticles as emerging antibacterial agents against cholera. Microb. Cell Factories 2016, 15, 25. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine: Nanotechnology, Boil. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Electron. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Yu-Guo, Y.; Qiu-Ling, P.; Sangiliyandi, G. Effects of silver nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa from mastitis-infected goats: An alternative approach for antimicrobial therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, S.; Mousavi, S.E.; Ashtiani, H.R.A.; Doust, S.R.H.; Rezayat, S.M. Antibacterial activity of silver nanoparticles and their combination with Zataria multiflora essential oil and methanol extract. Jundishapur J. Microbiol. 2016, 9, 10. [Google Scholar] [CrossRef]
- Habash, M.B.; Goodyear, M.C.; Park, A.J.; Surette, M.D.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Potentiation of tobramycin by silver nanoparticles against Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2017, 61, e00415-17. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef]
| Nanosilver Particle Size | S. epidermidis ATCC 12228 | S. epidermidis ATCC 36983 | S. epidermidis ATCC 35984 |
|---|---|---|---|
| 10 nm | 3 µg/mL | 4 µg/mL | 5 µg/mL |
| 20 nm | BA | 4 µg/mL | 1 µg/mL |
| 40 nm | 6 µg/mL | 4 µg/mL | 1 µg/mL |
| 60 nm | BA | 4 µg/mL | 1 µg/mL |
| 100 nm | BA | 3 µg/mL | 1 µg/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swolana, D.; Kępa, M.; Idzik, D.; Dziedzic, A.; Kabała-Dzik, A.; Wąsik, T.J.; Wojtyczka, R.D. The Antibacterial Effect of Silver Nanoparticles on Staphylococcus Epidermidis Strains with Different Biofilm-Forming Ability. Nanomaterials 2020, 10, 1010. https://doi.org/10.3390/nano10051010
Swolana D, Kępa M, Idzik D, Dziedzic A, Kabała-Dzik A, Wąsik TJ, Wojtyczka RD. The Antibacterial Effect of Silver Nanoparticles on Staphylococcus Epidermidis Strains with Different Biofilm-Forming Ability. Nanomaterials. 2020; 10(5):1010. https://doi.org/10.3390/nano10051010
Chicago/Turabian StyleSwolana, Denis, Małgorzata Kępa, Danuta Idzik, Arkadiusz Dziedzic, Agata Kabała-Dzik, Tomasz J. Wąsik, and Robert D. Wojtyczka. 2020. "The Antibacterial Effect of Silver Nanoparticles on Staphylococcus Epidermidis Strains with Different Biofilm-Forming Ability" Nanomaterials 10, no. 5: 1010. https://doi.org/10.3390/nano10051010
APA StyleSwolana, D., Kępa, M., Idzik, D., Dziedzic, A., Kabała-Dzik, A., Wąsik, T. J., & Wojtyczka, R. D. (2020). The Antibacterial Effect of Silver Nanoparticles on Staphylococcus Epidermidis Strains with Different Biofilm-Forming Ability. Nanomaterials, 10(5), 1010. https://doi.org/10.3390/nano10051010









