Peculiarities of the Crystal Structure Evolution of BiFeO3–BaTiO3 Ceramics across Structural Phase Transitions
Abstract
1. Introduction
2. Materials and Methods
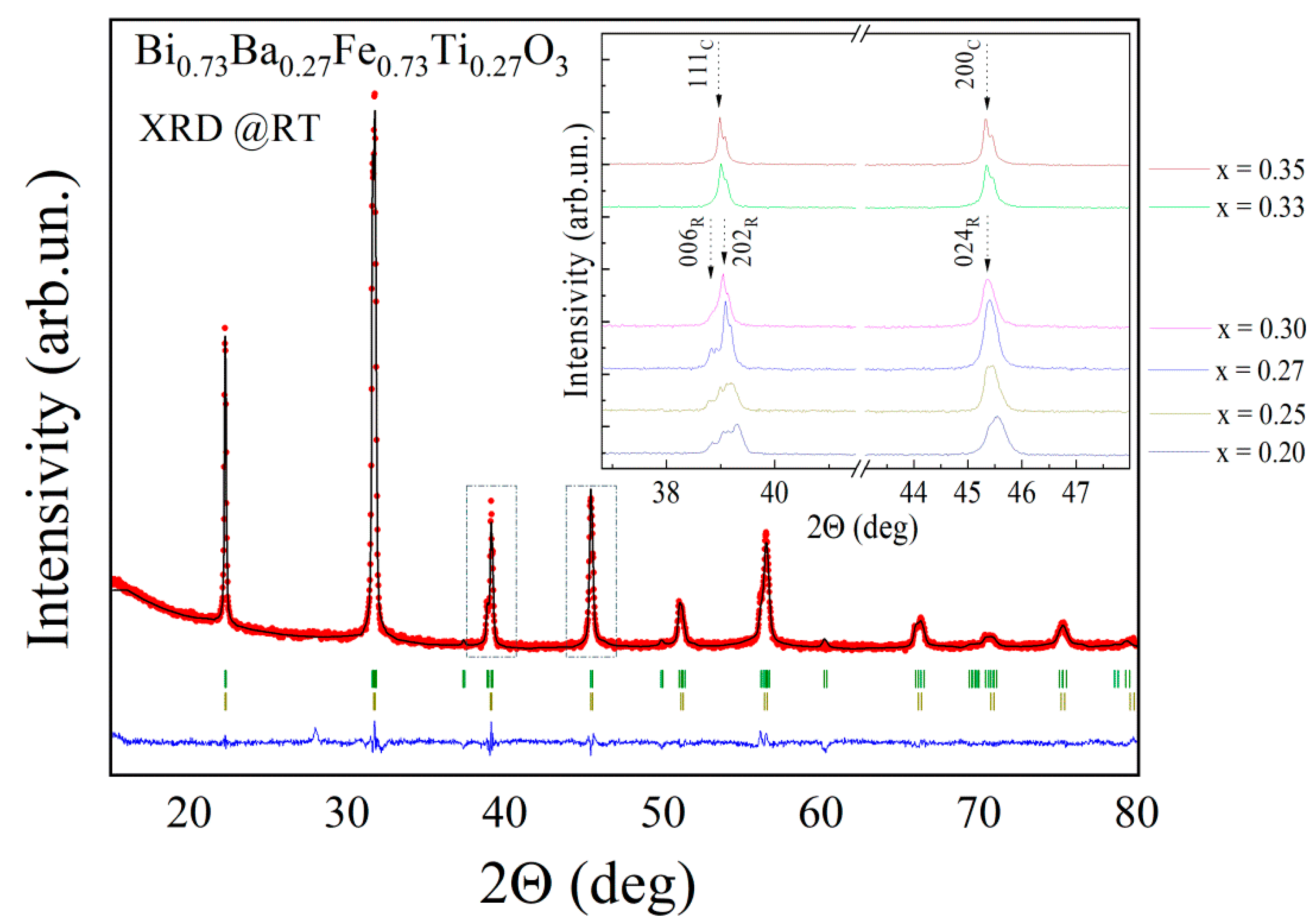
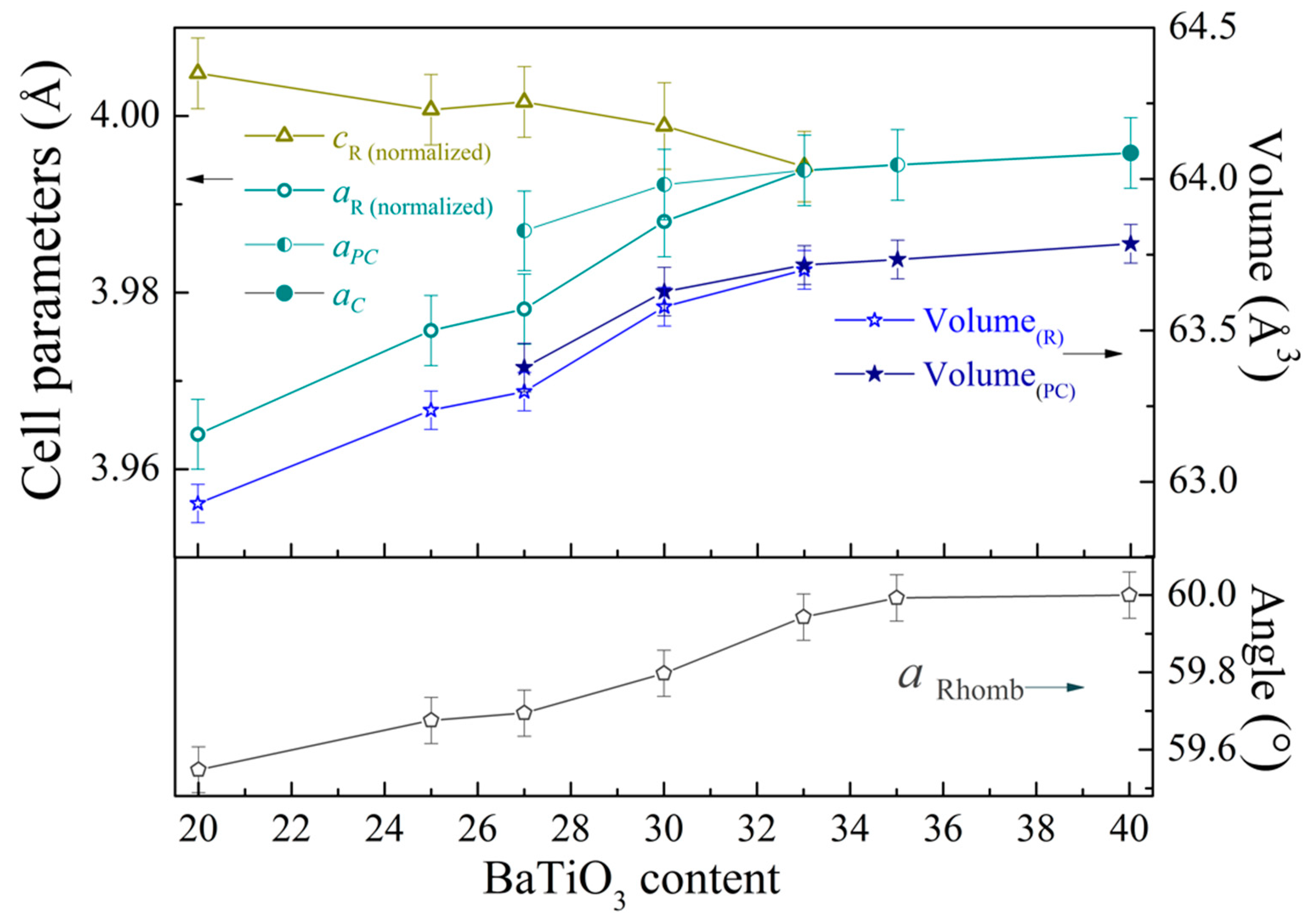
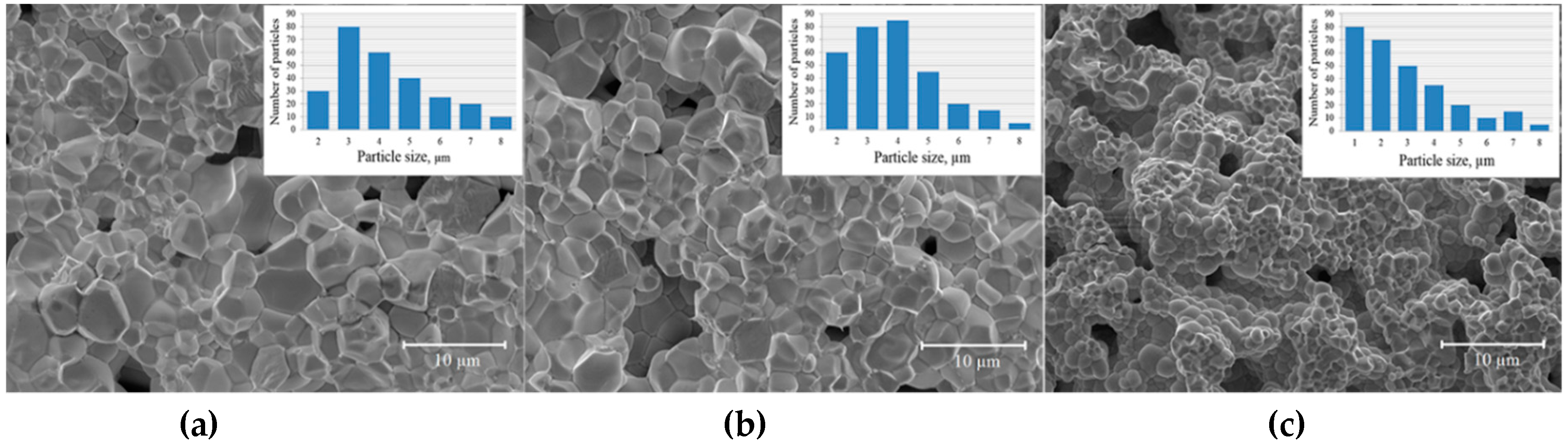
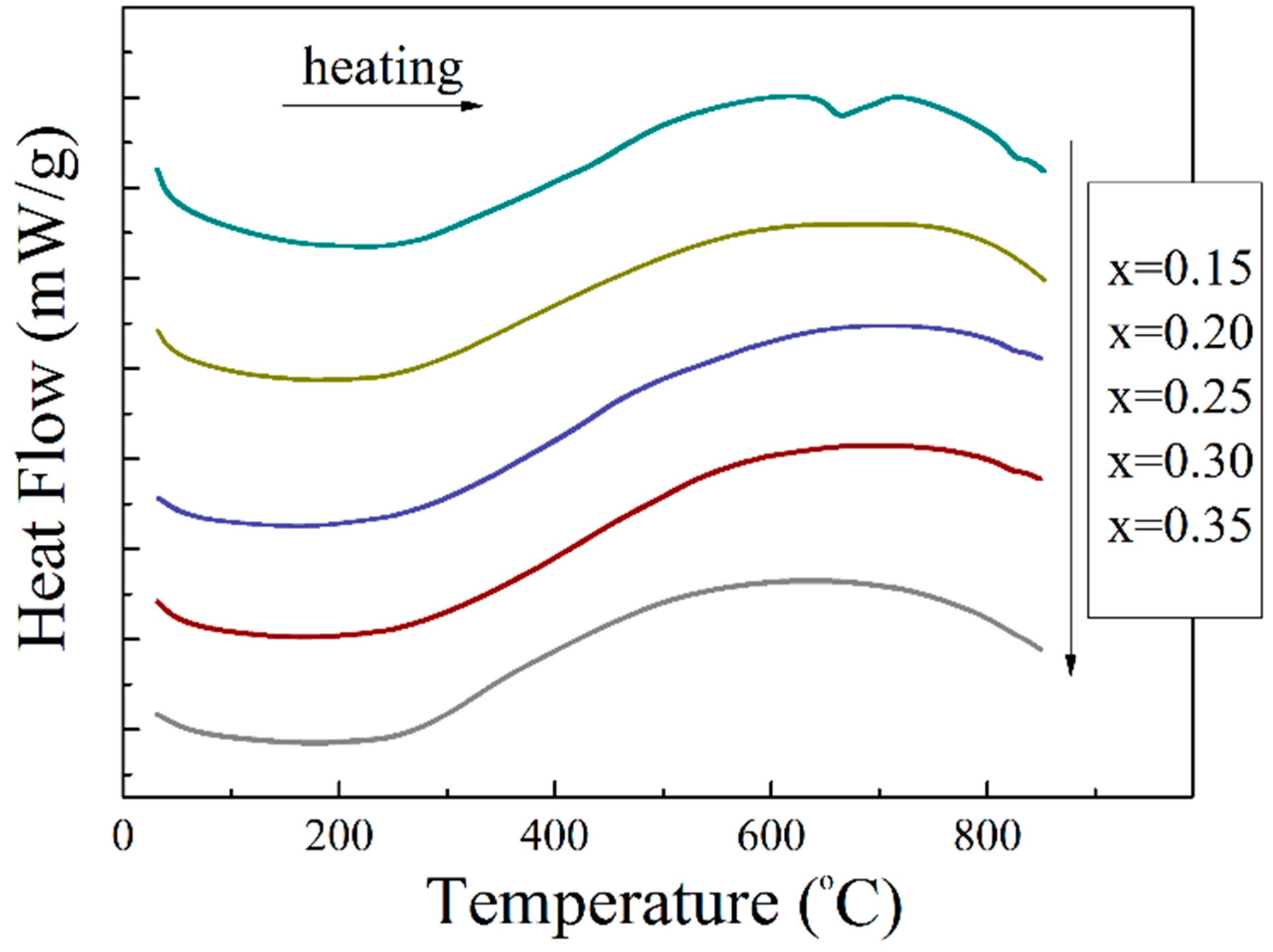
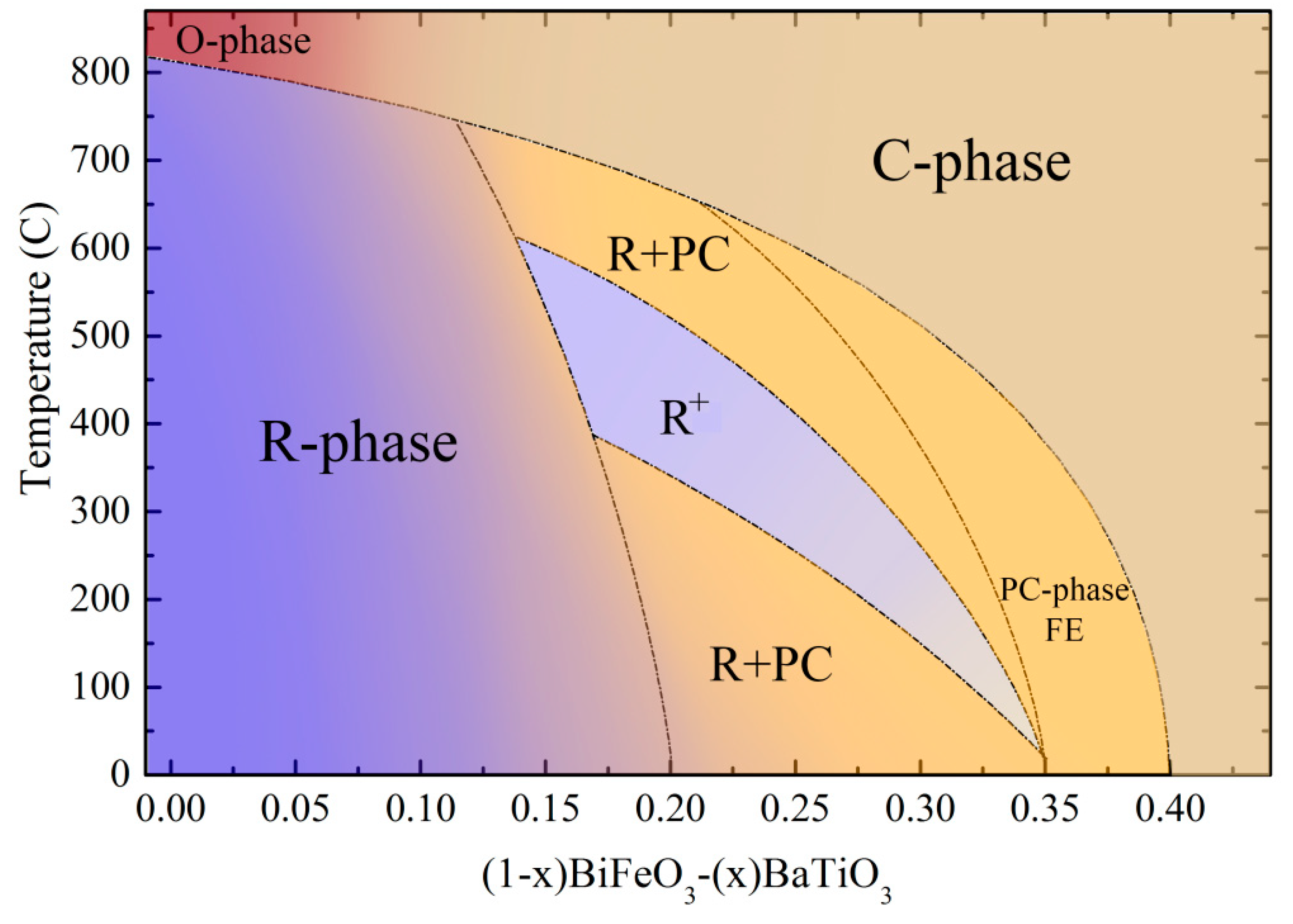
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rao, C.N.R.; Sundaresan, A.; Saha, R. Multiferroic and magnetoelectric oxides: The emerging scenario. J. Phys. Chem. Lett. 2012, 3, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.A. Why are there so few magnetic ferroelectrics? J. Phys. Chem. B 2000, 104, 6694–6709. [Google Scholar] [CrossRef]
- Spaldin, N.A.; Ramesh, R. Advances in magnetoelectric multiferroics. Nat. Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.O.; Bobrikov, I.A.; Trukhanov, S.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Kostishyn, V.G.; Panina, L.V.; Korovushkin, V.V.; Turchenko, V.A.; Thakur, P.; Thakur, A.; Yang, Y.; Vinnik, D.A.; Yakovenko, E.S.; et al. Control of electromagnetic properties in substituted M-type hexagonal ferrites. J. Alloy Compd. 2018, 754, 247–256. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Trukhanov, A.V.; Trukhanova, E.L.; Tishkevich, D.I.; Ivanov, V.M.; Zubar, T.I.; Salem, M.; Kostishyn, V.G.; et al. Polarization origin and iron positions in indium doped barium hexaferrites. Ceram. Int. 2018, 44, 290–300. [Google Scholar] [CrossRef]
- Yakovenko, O.S.; Matzui, L.Y.; Vovchenko, L.L.; Trukhanov, A.V.; Kazakevich, I.S.; Trukhanov, S.V.; Prylutskyy, Y.I.; Ritter, U. Magnetic anisotropy of the graphite nanoplatelet–epoxy and MWCNT–epoxy composites with aligned barium ferrite filler. J. Mater. Sci. 2017, 52, 5345–5358. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Salem, M.M.; Trukhanova, E.L.; Panina, L.V.; Kostishyn, V.G.; Darwish, M.A.; Trukhanov, A.V.; Zubar, T.I.; Tishkevich, D.I.; et al. Preparation and investigation of structure, magnetic and dielectric properties of (BaFe11.9Al0.1O19)1-x—(BaTiO3)x bicomponent ceramics. Ceram. Int. 2018, 44, 21295–21302. [Google Scholar] [CrossRef]
- Salem, M.M.; Panina, L.V.; Trukhanova, E.L.; Darwish, M.A.; Morchenko, A.T.; Zubar, T.I.; Trukhanov, S.V.; Trukhanov, A.V. Structural, electric and magnetic properties of (BaFe11.9Al0.1O19)1-x–(BaTiO3)x composites. Compos. Part B Eng. 2019, 174, 107054. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güngüne, H.; Baykal, A.; Trukhanov, S.V.; Trukhanov, A.V. Manganese/Yttrium codoped strontium nanohexaferrites: Evaluation of magnetic susceptibility and Mössbauer spectra. Nanomaterials 2019, 9, 24. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Trukhanov, A.V.; Slimani, Y.; You, K.Y.; Trukhanov, S.V.; Trukhanova, E.L.; Esa, F.; Sadaqat, A.; Chaudhary, K.; Zdorovets, M.; et al. Correlation between composition and electrodynamics properties in nanocomposites based on hard/soft ferrimagnetics with strong exchange coupling. Nanomaterials 2019, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 nanoparticles for complex targeted delivery and boron neutron capture therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Matzui, L.Y.; Trukhanov, A.V.; Yakovenko, O.S.; Vovchenko, L.L.; Zagorodnii, V.V.; Oliynyk, V.V.; Borovoy, M.O.; Trukhanova, E.L.; Astapovich, K.A.; Karpinsky, D.V.; et al. Functional magnetic composites based on hexaferrites: Correlation of the composition, magnetic and high-frequency properties. Nanomaterials 2019, 9, 1720. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.A.; Trukhanov, A.V.; Senatov, O.S.; Morchenko, A.T.; Saafan, S.A.; Astapovich, K.A.; Trukhanov, S.V.; Trukhanova, E.L.; Pilyushkin, A.A.; Sombra, A.S.B.; et al. Investigation of AC-measurements of epoxy/ferrite composites. Nanomaterials 2020, 10, 492. [Google Scholar] [CrossRef]
- Selbach, S.M.; Tybell, T.; Einarsrud, M.-A.; Grande, T. The ferroic phase transitions of BiFeO3. Adv. Mater. 2008, 20, 3692–3696. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yuan, G.L.; Chen, X.Y.; Liu, J.M.; Liu, Z.G. Electrical and magnetic properties of single-phased and highly resistive ferroelectromagnet BiFeO3 ceramic. J. Phys. D Appl. Phys. 2006, 39, 2019–2023. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Silibin, M.V.; Trukhanov, A.V.; Zhaludkevich, A.L.; Maniecki, T.; Maniukiewicz, W.; Sikolenko, V.; Paixão, J.A.; Khomchenko, V.A. A correlation between crystal structure and magnetic properties in co-doped BiFeO3 ceramics. J. Phys. Chem. Solids 2019, 126, 164–169. [Google Scholar] [CrossRef]
- Wang, D.; Wang, G.; Murakami, S.; Fan, Z.; Feteira, A.; Zhou, D.; Sun, S.; Zhao, Q.; Reaney, I.M. BiFeO3-BaTiO3: A new generation of lead-free electroceramics. J. Adv. Dielect. 2018, 8, 1830004. [Google Scholar] [CrossRef]
- Morozovska, A.N.; Eliseev, E.A.; Glinchuk, M.D.; Fesenko, O.M.; Shvartsman, V.V.; Gopalan, V.; Silibin, M.V.; Karpinsky, D.V. Rotomagnetic coupling in fine-grained multiferroic BiFeO3: Theory and experiment. Phys. Rev. B 2018, 97, 134115. [Google Scholar] [CrossRef]
- Khomchenko, V.A.; Karpinsky, D.V.; Paixão, J.A. Magnetostructural correlations in BiFeO3-based multiferroics. J. Mater. Chem. C 2017, 5, 3623–3629. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Eliseev, E.A.; Xue, F.; Silibin, M.V.; Franz, A.; Glinchuk, M.D.; Troyanchuk, I.O.; Gavrilov, S.A.; Gopalan, V.; Chen, L.-Q.; et al. Thermodynamic potential and phase diagram for multiferroic bismuth ferrite (BiFeO3). NPJ Comput. Mater. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Nomoto, M.; Inoshita, T.; Inoue, Y.; Horibe, Y.; Koyama, Y. Crystallographic features in the vicinity of the morphotropic phase boundary in the multiferroic material Bi1−xSmxFeO3. MRS Adv. 2016, 1, 573–578. [Google Scholar] [CrossRef]
- Shimada, T.; Wang, J.; Ueda, T.; Uratani, Y.; Arisue, K.; Mrovec, M.; Elsässer, C.; Kitamura, T. Multiferroic grain boundaries in oxygen-deficient ferroelectric lead titanate. Nano Lett. 2015, 15, 27–33. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Fesenko, O.M.; Silibin, M.V.; Dubkov, S.V.; Chaika, M.; Yaremkevich, A.; Lukowiak, A.; Gerasymchuk, Y.; Stręk, W.; Pakalniškis, A.; et al. Ferromagnetic-like behavior of Bi0.9La0.1FeO3–KBr nanocomposites. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O.; Deac, I.G.; Diamandescu, L.; Barbu-Tudoran, L. Effect of nickel content on structural, morphological and magnetic properties of NixCo1−xFe2O4/SiO2 nanocomposites. J. Alloys Compd. 2019, 786, 330–340. [Google Scholar] [CrossRef]
- Arnold, D. Composition-driven structural phase transitions in rare-earth-doped BiFeO3 ceramics: A review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2015, 62, 62–82. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Troyanchuk, I.O.; Tovar, M.; Sikolenko, V.; Efimov, V.; Efimova, E.; Shur, V.Y.; Kholkin, A.L. Temperature and composition-induced structural transitions in Bi1−xLa(Pr)xFeO3 ceramics. J. Am. Ceram. Soc. 2014, 97, 2631–2638. [Google Scholar] [CrossRef]
- Mahbub, R.; Fakhrul, T.; Fakhrul Islam, M.; Hasan, M.; Hussain, A.; Matin, M.A.; Hakim, M.A. Structural, dielectric, and magnetic properties of Ba-doped multiferroic bismuth ferrite. Acta Metall. Sin. 2015, 28, 958–964. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Karpinsky, D.V.; Bushinsky, M.V.; Khomchenko, V.A.; Kakazei, G.N.; Araujo, J.P.; Tovar, M.; Sikolenko, V.; Efimov, V.; Kholkin, A.L. Isothermal structural transitions, magnetization and large piezoelectric response in Bi1-xLaxFeO3 perovskites. Phys. Rev. B 2011, 83, 054109. [Google Scholar] [CrossRef]
- Damjanovic, D. A morphotropic phase boundary system based on polarization rotation and polarization extension. Appl. Phys. Lett. 2010, 97, 062906. [Google Scholar] [CrossRef]
- Mostafavi, E.; Ataie, A.; Ahmadzadeh, M.; Palizdar, M.; Comyn, T.P.; Bell, A.J. Synthesis of nano-structured Bi1−xBaxFeO3 ceramics with enhanced magnetic and electrical properties. Mater. Chem. Phys. 2015, 162, 106–112. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Bushinsky, M.V.; Karpinsky, D.V.; Sirenko, V.; Sikolenko, V.; Efimov, V. Structural and magnetic phases of Bi1−xAxFeO3−δ (A = Sr, Pb) perovskites. Eur. Phys. J. B 2010, 73, 375–381. [Google Scholar] [CrossRef]
- Mardani, R. The synthesis of Ba2+-doped multiferroic BiFeO3 nanoparticles using co-precipitation method in the presence of various surfactants and the investigation of structural and magnetic features. Mod. Phys. Lett. B 2017, 31, 1750169. [Google Scholar] [CrossRef]
- Khomchenko, V.A.; Troyanchuk, I.O.; Többens, D.M.; Sikolenko, V.; Paixão, J.A. Composition- and temperature-driven structural transitions in Bi1-xCaxFeO3 multiferroics: A neutron diffraction study. J. Phys. Condens. Matter 2013, 25, 135902. [Google Scholar] [CrossRef]
- Kozakov, A.T.; Kochur, A.G.; Torgashev, V.I.; Googlev, K.A.; Kubrin, S.P.; Trotsenko, V.G.; Bush, A.A.; Nikolskii, A.V. Bi1-xCaxFeO3-δ (0≤x≤1) ceramics: Crystal structure, phase and elemental composition, and chemical bonding from X-ray diffraction, Raman scattering, Mössbauer, and X-ray photoelectron spectra. J. Alloys Compd. 2016, 664, 392–405. [Google Scholar] [CrossRef]
- Kim, S.; Khanal, G.P.; Nam, H.-W.; Fujii, I.; Ueno, S.; Moriyoshi, C.; Kuroiwa, Y.; Wada, S. Structural and electrical characteristics of potential candidate lead-free BiFeO3-BaTiO3 piezoelectric ceramics. J. Appl. Phys. 2017, 122, 164105. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Silibin, M.V.; Trukhanov, A.V.; Zhaludkevich, A.L.; Latushka, S.I.; Zhaludkevich, D.V.; Sikolenko, V.; Khomchenko, V.A. Evolution of crystal structure of Ba and Ti co-doped BiFeO3 ceramics at the morphotropic phase boundary. J. Alloys Compd. 2019, 803, 1136–1140. [Google Scholar] [CrossRef]
- Pakalniškis, A.; Lukowiak, A.; Niaura, G.; Głuchowski, P.; Karpinsky, D.V.; Alikin, D.O.; Abramov, A.S.; Zhaludkevich, A.; Silibin, M.; Kholkin, A.L.; et al. Nanoscale ferroelectricity in pseudo-cubic sol-gel derived barium titanate—Bismuth ferrite (BaTiO3–BiFeO3) solid solutions. J. Alloys Compd. 2020, 830, 154632. [Google Scholar] [CrossRef]
- Singh, A.; Moriyoshi, C.; Kuroiwa, Y.; Pandey, D. Evidence for local monoclinic structure, polarization rotation, and morphotropic phase transitions in (1-x)BiFeO3–(x)BaTiO3 solid solutions: A high-energy synchrotron x-ray powder diffraction study. Phys. Rev. B 2013, 88, 024113. [Google Scholar] [CrossRef]
- Singh, A.; Senyshyn, A.; Fuess, H.; Chatterji, T.; Pandey, D. Neutron powder diffraction study of nuclear and magnetic structures of multiferroic Bi0.8Ba0.2Fe0.8Ti0.2O3: Evidence for isostructural phase transition and magnetoelastic and magnetoelectric couplings. Phys. Rev. B 2011, 83, 054406. [Google Scholar] [CrossRef]
- Leontsev, S.O.; Eitel, R.E. Dielectric and piezoelectric properties in Mn-modified (1−x)BiFeO3–xBaTiO3 ceramics. J. Am. Ceram. Soc. 2009, 92, 2957–2961. [Google Scholar] [CrossRef]
- Tanasescu, S.; Botea, A.; Ianculescu, A. Effects of doping and oxygen nonstoichiometry on the thermodynamic properties of some multiferroic ceramics. In Ferroelectrics–Physical Effects; Lallart, M., Ed.; Intech Open 10: London, UK, 2011. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Balke, N.; Jesse, S.; Yu, P.; Ben, C.; Kalinin, S.V.; Tselev, A. Quantification of surface displacements and electromechanical phenomena via dynamic atomic force microscopy. Nanotechnology 2016, 27, 425707. [Google Scholar] [CrossRef] [PubMed]
- Mote, V.D.; Purushotham, Y.; Dole, B.N. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Khomchenko, V.A.; Ivanov, M.S.; Karpinsky, D.V.; Dubkov, S.V.; Silibin, M.V.; Paixão, J.A. Weak ferromagnetic state in the polar phase of Bi1-xCaxFe1-x/2Nbx/2O3 multiferroics. Mater. Lett. 2019, 235, 46–48. [Google Scholar] [CrossRef]
- Mazilkin, A.A.; Straumal, B.B.; Kilmametov, A.R.; Boll, T.; Baretzky, B.; Kogtenkova, O.A.; Korneva, A.; Zięba, P. Competition for impurity atoms between defects and solid solution during high pressure torsion. Scripta Mater. 2019, 173, 46–50. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpinsky, D.V.; Silibin, M.V.; Trukhanov, S.V.; Trukhanov, A.V.; Zhaludkevich, A.L.; Latushka, S.I.; Zhaludkevich, D.V.; Khomchenko, V.A.; Alikin, D.O.; Abramov, A.S.; et al. Peculiarities of the Crystal Structure Evolution of BiFeO3–BaTiO3 Ceramics across Structural Phase Transitions. Nanomaterials 2020, 10, 801. https://doi.org/10.3390/nano10040801
Karpinsky DV, Silibin MV, Trukhanov SV, Trukhanov AV, Zhaludkevich AL, Latushka SI, Zhaludkevich DV, Khomchenko VA, Alikin DO, Abramov AS, et al. Peculiarities of the Crystal Structure Evolution of BiFeO3–BaTiO3 Ceramics across Structural Phase Transitions. Nanomaterials. 2020; 10(4):801. https://doi.org/10.3390/nano10040801
Chicago/Turabian StyleKarpinsky, Dmitry V., Maxim V. Silibin, Sergei V. Trukhanov, Alex V. Trukhanov, Alexander L. Zhaludkevich, Siarhei I. Latushka, Dmitry V. Zhaludkevich, Vladimir A. Khomchenko, Denis O. Alikin, Alexander S. Abramov, and et al. 2020. "Peculiarities of the Crystal Structure Evolution of BiFeO3–BaTiO3 Ceramics across Structural Phase Transitions" Nanomaterials 10, no. 4: 801. https://doi.org/10.3390/nano10040801
APA StyleKarpinsky, D. V., Silibin, M. V., Trukhanov, S. V., Trukhanov, A. V., Zhaludkevich, A. L., Latushka, S. I., Zhaludkevich, D. V., Khomchenko, V. A., Alikin, D. O., Abramov, A. S., Maniecki, T., Maniukiewicz, W., Wolff, M., Heitmann, V., & Kholkin, A. L. (2020). Peculiarities of the Crystal Structure Evolution of BiFeO3–BaTiO3 Ceramics across Structural Phase Transitions. Nanomaterials, 10(4), 801. https://doi.org/10.3390/nano10040801










