Abstract
Since 2004, we have been developing nanomaterials with antimicrobial properties, the so-called nanoantimicrobials. When the coronavirus disease 2019 (COVID-19) emerged, we started investigating new and challenging routes to nanoantivirals. The two fields have some important points of contact. We would like to share with the readership our vision of the role a (nano)materials scientist can play in the fight against the COVID-19 pandemic. As researchers specifically working on surfaces and nanomaterials, in this letter we underline the importance of nanomaterial-based technological solutions in several aspects of the fight against the virus. While great resources are understandably being dedicated to treatment and diagnosis, more efforts could be dedicated to limit the virus spread. Increasing the efficacy of personal protection equipment, developing synergistic antiviral coatings, are only two of the cases discussed. This is not the first nor the last pandemic: our nanomaterials community may offer several technological solutions to challenge the ongoing and future global health emergencies. Readers’ feedback and suggestions are warmly encouraged.
Keywords:
COVID-19; SARS-CoV-2; nanoantiviral; PPE; copper; silver; nanomedicine; contagion; mask; air conditioning Dear Editor,
“Are we ready for pandemic influenza?”
With an as-titled article, published in Science in 2003 and heavily cited later, Webby and Webster proposed some interrogatives about the threat of emerging infectious diseases [1]. After the global spread of severe acute respiratory syndrome caused by a novel coronavirus (SARS-CoV) in 2002 and the H5N1 (avian) influenza virus in the 2003, the scientific community tried to set some medical key points to better respond to potential future pandemic diseases. The demand for a sufficiently large supply of antiviral drugs and vaccines was clear. Other infectious diseases arose over the years, including the pandemic H1N1 influenza (swine flu), the Middle East respiratory syndrome coronavirus (MERS-CoV), and the current pandemic, named coronavirus disease 19 (COVID-19).
In late 2019, a novel pneumonia caused by an unknown pathogen emerged in the city of Wuhan, central China [2]. After few months, and specifically on 19 April 2020, the pandemic counts more than 2,200,000 contagions around the world, according to the World health Organization (WHO) daily reports [3].
Currently, many different antiviral agents, including re-purposed drugs, are under testing in clinical trials to assess their efficacy against the new virus, but the quest for an effective treatment against COVID-19 is still open [4,5,6,7].
Different strategies were pursued, to treat coronavirus related to MERS and SARS infections, including the use of inhibitors of viral and host proteases, interferons (IFNs), and host-directed therapies. One of the first antiviral drugs tested, the ribavirin, a nucleoside analog that acts as RNA polymerase inhibitor [8], was used on patients with SARS and MERS [9]. However, ribavirin monotherapy had limited activity against SARS-CoV and led to significant hemolysis collateral effect [10]. Ribavirin is more commonly used together with lopinavir, ritonavir and HIV protease inhibitors [11]. These combinations were demonstrated to improve the outcome of patients with SARS [9].
Based on antiviral treatment against previous coronavirus SARS and MERS, lopinavir/ritonavir combination was tested [12] too. There are also signs of the benefit of both interferon-α (IFN-α) and interferon-β (IFN-β) treatments [13]. In addition, some studies in vitro and in non-human primates’ trials demonstrated that the combination treatment with ribavirin and INFs improves clinical outcomes in MERS-CoV infection [14]. Translating the findings from these studies into clinical trials remains of particular importance, especially taking into account drugs availability, pharmacokinetic properties, and possible side effects, including long term or permanent ones.
The vital role of the spike protein (S protein) of coronaviruses makes this glycoprotein an important therapeutic target, because it guides coronavirus entry into host cells, by providing the binding and the fusion of virus on the host cell membrane. S protein is composed of two subunits: S1 recognizes and binds to host receptors, and S2 facilitates fusion between the viral envelope and the host cell membrane [15]. Numerous studies have explored how to target this first stage of the virus lifecycle. These methods mainly involve peptidic fusion inhibitors, anti-CoV neutralizing monoclonal antibodies, and entry receptor antagonists. However, none of these curative agents is approved for commercial use in humans [16]. Regarding the SARS-CoV-2 infection, in the absence of a clinically proved effective antiviral therapy against COVID-19, a combination of different drugs has been often supplemented (Table 1).

Table 1.
Non-exhaustive list of the most common antiviral agents used to fight coronavirus disease 19 (COVID-19).
Remdesivir, an adenosine analogue, is a broad-spectrum antiviral with potent in vitro efficacy against multiple genetically unrelated RNA viruses [20]. It is currently under clinical development for the treatment of Ebola virus infection and it also demonstrated a good antiviral result in vitro against SARS and MERS coronaviruses [6]. Remdesivir also revealed as an effective antiviral agent in vitro against COVID-19 infection [21]. Among small-sized molecular agents approved for viral human diseases, an immune modulator, chloroquine, shows inhibitory effects against SARS-CoV-2 [19]. It is known as a potential broad spectrum antiviral drug, widely used as anti-malarial drug [22]. Chloroquine (CQ) blocks virus infection by increasing the endosomal pH required for the fusion between virus and cell. It also interferes with the glycosylation of cellular receptors of SARS-CoV [5,22]. A recent in vitro study demonstrated that CQ functions at both entry- and at post-entry-stages of the SARS-CoV-2 infection [19,23]. Besides its antiviral activity, CQ also presents immune-modulating activity, which can enhance its antiviral effect in vivo. Notably, CQ could be replaced by the less toxic hydroxychloroquine (HCQ) [23].
In this scenario of non-univocal medical treatments and vaccine unavailability, it is evident that, besides the population above 75-year-old and people with immunodeficiency, or chronic and oncological diseases, the category that is most exposed to COVID-19 is that of hospital personnel [24]. It is imperative to ensure the protection of health-care workers. This is not only crucial to guarantee continuous patient care (in all nosocomial environments), but also to prevent virus transmission by unaware SARS-CoV-2 carriers [25]. Besides, contagion protection for the whole population is mandatory in all those cases when social confinement is not possible due to force majeure (work, health problems, caregiving, etc.). Finally, in the first stages of the removal of confinement measurements, it is envisaged that we will all need safer and more effective personal protective equipment (PPE).
Contagion rates of COVID-19 are much higher than those reported for the well-known SARS [26]. This brought to the fast diffusion of the virus in all continents [27,28]. COVID-19 can easily propagate via cough or respiratory droplets, contact with bodily fluids, or from contaminated surfaces (Figure 1). Secondary infection routes involve touching dirty/contaminated surfaces, followed by self-inoculation of mucous membranes. A study carried out on 29 patients [29] estimated that secondary routes are less probable than direct contagion (including eye-, nose-, oral-, etc.). Nevertheless, this risk is not considered negligible by different other sources, including WHO [30]. Many commonly touched surfaces, along with nosocomial environments and PPE have been tested against this risk. Based on a previously published paper [31], which revealed the persistence of human coronavirus 229E on the surface of common materials, G. Kampf recently reviewed the topic for SARS, MERS and SARS-CoV-2 viruses [32,33].
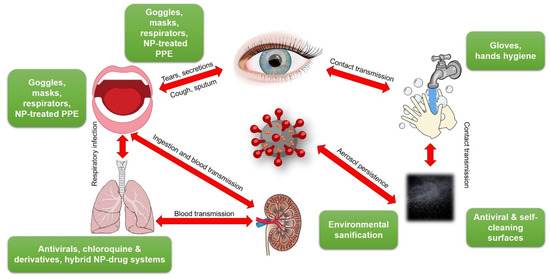
Figure 1.
Transmission pathways of SARS-CoV-2 and possible containment means. NP = nanoparticle; PPE = personal protective equipment. This figure was created using images under the Creative Commons licenses. This figure is a derivative of: “Human anatomy” by Wikimedia Commons, used under CC; “SugarandSkullDesigns” by Pixabay, used under CC; “Blubberfisch“ by Pixabay, used under CC; “Shutterstock” by FreeSVG, used under CC-BY; “OpenClipart” by FreeSVG, used under CC-BY; “James Gathany” CDC Public Health Image library used under CC-BY.
Surface contamination has recently been found to be more significant than originally thought in the spread of this viral disease. In fact, despite the use of proper PPE [34], health-care workers continued to contract SARS-CoV-2, even after barrier precautions were widely implemented. The reason behind this was found in the air, environmental, and PPE contamination in hospitals [35]. Besides common touching surfaces (tables, beds, door handles, light switches, toilet sites, etc.), even anteroom floors and air fans outlets were found to be contaminated [36]. The latter fact is explained by the persistence of SARS-CoV-2 in aerosols, up to several hours [37].
Many resources and biotechnological capabilities are currently directed towards the development of vaccines and treatments against COVID-19. Anyway, given the significance of the surface and air contamination in the spread of the virus, attention should be paid also to the development of antiviral and antibacterial surfaces, along with decontamination equipment and technologies.
We hypothesize that using proper air filtering may reduce the viral load in the environment, sufficiently to decrease the probability of health-care worker infection through flaws in PPE, or in confined public transportation [37]. According to a study of the Purdue University (West Lafayette, IN, USA) [38], air conditioning (AC) systems are not designed to filter out particles as small as the coronavirus. Consequently, the disease could rapidly circulate to other individuals in closed communities as it presumably happened, as an example, in cruise ships [39].
Inverse computational fluid dynamics (CFD) models are available to identify the spread of air particles in passenger vehicles [40]. CFD models, and the airborne nature of SARS-CoV, could be considered to understand the spread of coronavirus in airplane passengers [41].
High-Efficiency Particulate Air (HEPA) filters can limit the spread of airborne fungi, viruses, and bacteria [42,43]. Their use could further benefit from the implementation of a new generation of safe and effective multifunctional antibacterial and antiviral agents.
The contamination of latex/nitrile gloves, N95 respirators, hospital scrubs, overshoes, and floors in a nosocomial environment is considered a serious issue [35,44,45], because it helps the uncontrolled spreading of the disease: health-care workers are anxious about passing the infection to other patients and to their families [46].
Besides nosocomial environments, the contamination of surfaces can be regarded as responsible for many other contagion episodes. As an example, Australian Health Authorities have recently confirmed several cases of COVID-19 among baggage handlers. The circumstances gave rise to concerns about cleaning the luggage and about the virus survival time on hard, smooth materials, such as plastic and metal. The detailed explanation of contamination sources and the current number of contagions among baggage handlers worldwide are still unknown. However, extensive cleaning and disinfecting procedures were implemented in international airports, to reduce the surface contamination of luggage [47].
More generally, many disinfection protocols have been developed so far, which mainly involve the use of sodium hypochlorite [35], 70–85% ethanol [33,45,48], iodine-based and quaternary-ammonium-salt-based disinfectants [32,48,49,50,51]. Van Doremalen et al. [36] tested the viability of SARS-CoV-2 in different environmental conditions (aerosols, plastic, stainless steel, copper, and cardboard). The longest viability was on stainless steel and plastic surfaces; the estimated median virus half-life being approximately 5.6 h on stainless steel and 6.8 h on plastic. Copper was found to be effective in inactivating the virus in a shorter time. These findings are in agreement with what already reported on CoV-229E in 2015 [31]: brasses containing at least 70% copper were very effective at inactivating CoV-229E, and the rate of inactivation was directly proportional to copper percentage. Copper ion release and the generation of reactive oxygen species (ROS) were demonstrated to be responsible for the inactivation of coronaviruses on copper and copper alloy surfaces [36]. Several already-approved biocides based on silver or zinc oxide are just waiting to be tested against SARS-CoV-2 as well.
Based on these findings, we make a heartfelt appeal to the (Nano)Materials Science community: we can exploit the well-known antimicrobial properties of formulations and nanostructures containing copper, silver, and zinc species [52,53] to fight COVID-19 and, more specifically, to prevent and limit both contamination and contagion.
Specifically, the use of copper salt nanoparticles and/or solutions (chloride, iodide, sulfide, etc.), which are known for having an antiviral effect [54,55,56,57,58], could be helpful in the development of PPE with improved shielding properties. As an example, the treatment of non-woven overshoes, surgical gowns, hair cups, respirators, etc. with copper ions could help in preventing the unwanted nosocomial virus spreading by medical personnel. In principle, the presence of metal ions could reduce (or set to zero) the viability of CoV on these substrates, which can be considered as simple carrier interfaces for the infection spread. Analogously, the treatment of common touching surfaces with Cu, or the use of copper brasses for all those surfaces which need to be kept sterile, could be extremely helpful.
Nanotechnology can offer a great support in the design of contamination-safe equipment in this era of pandemic diseases. Metal-loaded nanocomposites are known to be extremely effective in all those cases in which a controlled and long-lasting ionic release is required [59,60]. The controlled release of ionic copper is the key to tune the antimicrobial and antiviral properties of surfaces [61,62].
Metal nanoparticles can act as ion reservoirs for the controlled release of bioactive ions, thus tuning the production of ROS species too. The embedding of metal nanoparticles (copper ones, in principle) in polymer matrices could help in the tuning of metal release properties, and at the same time in minimizing the risk of nanoparticle release into the environment [53]. Moreover, very simple and reliable routes to synergistic nanoparticles combining a copper core and a quaternary ammonium shell (both of which are capable, in principle, of expressing a strong antiviral action) are available in recent literature [63].
To the best of our knowledge, there are few publications about the treatment of nosocomial PPE with copper nanoparticles or copper oxides and salts. It is evident that inadequate PPE and inappropriate PPE guidelines can be responsible for the death of many health-care workers and for viral nosocomial spreading. Bhattacharjee and co-workers reviewed the topic in 2019, before the spreading of SARS-CoV-2, taking into account other viral pandemic diseases like Ebola, SARS, and MERS [64]. They reported that metal-grafted graphene oxide (GO), for the modification of non-woven tissues, showed to have very effective antimicrobial properties. Graphene derivatives have been reported to be used as antimicrobial composites with different metals (Ag, Fe, Cu, Zn, etc.), and photocatalysts (TiO2, CdS, MnS2, etc.) [65]. GO grafted with metal nanoparticles has been investigated as a potential treatment for PPE [66]. Specifically, it is known that silver and copper nano-based systems loaded on GO are very effective against both enveloped and non-enveloped viruses [65,67]. Anti-influenza copper oxide-loaded polypropylene respirators were proposed in 2010; Borkow et al. demonstrated that copper oxide-impregnated masks safely reduced the risk of influenza virus environmental contamination, without altering the filtration capabilities of the masks [68]. However, no reports about the impregnation of masks and other PPE with copper salt solutions were found in literature [68,69]. Their use is actually part of our future research plans, which will involve both nanophases and reference compounds and salts. In order to prevent the potential nano-toxicity of metal nanoparticles on masks and respirators through inhalation, we think that, besides embedding nanoparticles in polymer matrices (vide infra), the best strategy could be the use of copper salts for the impregnation of PPE pieces. In fact, when used at low concentration levels, Cu(II) ions have low cytotoxicity on eukaryotic cells [70].
Recently, polyurethane/CuO nanocomposites were developed, acting as an effective antimicrobial filter for air purification. It is worth noting that microparticles of CuO are a more suitable additive for the modification of polyurethane filters than nanoparticles [71], thus reducing nanotoxicity risks. A recent example of an antiviral air filtering system for transportation was based on the use of SiO2-Ag NPs as active material against MS2 bacteriophage [72].
The importance of Nanotechnology in fighting viruses is not enough explored. One of the possible directions of further investigation is related to the use of nanomaterials to fight virus resistance to conventional therapies; this resistance can be due to the accelerated virus adaptation in peripheral protein sequence, thus resulting in the development of a new viral strain [73].
The antiviral action of metal nanoparticles, notably of silver nanoparticles (AgNPs), is well known. They act as viral reproduction inhibitors, and their viricidal activity depends on the target virus. For example, the AgNP ability to inhibit the viral entry in host cells, in the case of the HIV-1 virus, was reported, demonstrating that AgNPs are able to interact with cell receptors [73]. Regarding double strain RNA (dsRNA) viruses, AgNP interaction with a viral genome was found, inhibiting viral replication [73]. Likewise, gold nanoparticles (AuNPs) stabilized by biocompatible polymers showed antiviral activity against HIV-1 and some subtypes of influenza virus (e.g., H1N1, H3N2, H5N1) [73].
It was also demonstrated that gold nanoparticles coated with sulfated ligands, silver nanoparticles, and hybrid silver-copper nanoparticles are able to bind the HIV envelope glycoprotein gp120 and to inhibit in vitro HIV-1 infection in cellular models [74,75]. In addition, a size-dependent interaction with HIV-1 (size in the range of 1–10 nm) was reported [75]. It was also demonstrated that functionalized AgNPs (e.g., with tannic acid and mercaptoethane-sulfonate) may have the ability to prevent HSV infection by the direct inhibition of virus attachment, penetration and post-infection spread [76,77]. Single-walled carbon nanotubes (SWCNTs) were also proposed as antiviral carriers. Specifically, isoprinosine and ribavirin were chemically linked on the SWCNT surface to carry drugs across biological membranes; then, the increment of the antiviral activity of hybrid materials, compared to that of antiviral drugs, was tested [78,79].
It is known that very small metal NPs (<10 nm) are able to go through the cell membrane and inhibit post-attachment virus replication [80]. As an example, it was recently reported that a nanotechnology-based solution containing titanium dioxide and silver ions was used for street disinfection in Milan, Italy [81].
Given all these premises, the general question is: can inorganic nanoparticles be useful in affecting the early viral lifecycle stages?
The specific question is: is it possible to realize active nanomaterials able to inhibit the binding and fusion of viruses on the host cell?
We believe that it is certainly possible to find a nanotechnological solution for these quests.
Our idea is to realize hybrid antiviral nanomaterials by functionalizing metal nanoparticles with typical antiviral drugs, with enhanced synergistic efficacy. These synergistic nanoantivirals might offer a great help in increasing the efficacy of PPE and improving the safety of common touch surfaces.
They can be used to modify surgical masks and respirators, which are crucial targets in the fight against virus diffusion [82]. Among the WHO-approved antiviral drugs, CQ and HCQ demonstrated their ability to act in the first step of viral lifecycle. CQ and HCQ are aminoquinolines, a class of heterocyclic scaffolds with an amino group. They are able to form metal complexes with Fe(II), Ni(II), Zn(II), and Cu(II) ions [83]. In addition, metal complexes have been investigated as drug delivery agents, leading to reduced side effects and improved pharmacokinetics. It is worth noting that metal complexes of 8-hydroxyquinoline have been already shown to be antiviral agents [84].
Hence, we believe that CQ and HCQ could be proficiently used to functionalize metal nanoparticles. Other antiviral/metal nanostructure combinations could be possible, as well.
This is of course our perspective viewpoint and needs to be tested. We warmly encourage the readers working in this area to investigate the development of hybrid antiviral metal-CQ/HCQ nanomaterials. The resulting synergistic nanoantivirals could be very useful to prevent the diffusion of the virus. They could be implemented in coatings for hard surfaces and used as PPE-modifiers to increase their protective action.
At the present stage, we think that sharing these ideas and triggering our community to develop a deeper level of knowledge about the (unexplored) antiviral properties of nanomaterials could be a good way to fight this pandemic.
It is likely that nanotechnology has enormous potential in the prevention, diagnosis, and treatment of COVID-19. In this communication, we do not cover the diagnostics and treatment outcomes, although we certainly quote the considerable perspectives in (i) designing sensors for developing quick-response COVID-19 tests [85], and (ii) developing novel nanomedicines or theranostic tools [86]. In the future, we could also envisage the development of nanomedicines combining biodegradable nanocarriers [87,88] and one of the aforementioned (nano)antiviral agents, for possible aerosolization in the lung of patients under ventilation. This would allow for a much higher local concentration, i.e., a better efficiency, while limiting the penetration in the bloodstream and thus the side effects.
Reverting to the prevention stage, we strongly believe that contagion-safe PPE (from respirators to surgical gowns, overshoes, hair cups, etc.) and nanotechnology-enabled highly effective antiviral disinfectants, can be considered the most effective way to prevent viruses from spreading.
The readers working in the nanotechnology area are warmly encouraged to contact us with their comments or proposals. Sharing our ideas and setting common actions against this pandemic will be the key to success.
The present pandemic is not the first, nor will it be the last one. As researchers working on nanomaterials for the life sciences, we need to ensure that we have tools in place to deal with the COVID-19, as well as with any future pandemics.
Nanomaterial-based antiviral and antibacterial textiles, non-woven disposable products, packaging solutions, antiviral coatings, synergistic/multifunctional surfaces, air-conditioning filters, PPE, are just few of the possible examples requiring our prompt technological answer. While many good research papers have been published on metal nanoparticles to be used as antibacterial or antiviral agents, the commercialization of novel functional nanomaterials still appears to be limited by nanotoxicology concerns or by several other practical aspects (off-target and/or any other unpredictable effects, costs, production yield, durability, environmental impact, etc.). This is a good time to ask why and how we can facilitate the turning of these research projects into safe and viable products.
In the field of nanoantimicrobials, we have always pursued the use of bioactive nanoparticles as water-insoluble, polymer-confined nano-reservoirs, providing a source of ionic release, without being released as entire nanophases in the contact matrices (e.g., physiological solutions, food, sweat, humid air filtered through air-conditioners, etc.) [52,62].
Blocking the nanoantivirals in an adequately stable embedding matrix could be, again, the right way to ensure safety, preventing a priori nano-toxicological risks. Other technological solutions need to be envisaged in order to promote nanosafety and support technological knowledge transfer and commercialization. This is becoming more and more a part of our academic mission [89,90].
We encourage our nanomaterials community to actively exploit its impressive nanotechnological background to challenge the ongoing global health emergency.
Author Contributions
Conceptualization: M.C.S., R.A.P., N.D. and N.C.; investigation and literature overview: M.C.S., M.I., E.A.K. and S.I.H.; first draft preparation: M.C.S. and M.I.; review and final version: M.C.S., R.A.P., N.D. and N.C.; supervision and funding acquisition: N.C. All authors have read and agreed to the published version of the manuscript.
Funding
The work is part of a project that has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant Agreement No. 813439. Partial financial support is also acknowledged from the Italian MIUR project “E-Design” ARS01_01158.
Acknowledgments
We thank the Academic Editor and the Reviewers for their constructive comments and valuable suggestions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Webby, R.J.; Webster, R.G. Are We Ready for Pandemic Influenza? Science 2003, 302, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Available online: http://www.who.int/en/index.html (accessed on 14 April 2020).
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Rolain, J.-M.; Lagier, J.-C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 105932. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Tung, Y.-A.; Lee, K.-H.; Chen, T.-F.; Hsiao, Y.-C.; Chang, H.-C.; Hsieh, T.-T.; Su, C.-H.; Wang, S.-S.; Yu, J.-Y.; et al. Potential Therapeutic Agents for COVID-19 Based on the Analysis of Protease and RNA Polymerase Docking. Preprints 2020, 2020020242. [Google Scholar] [CrossRef]
- Mercorelli, B.; Palù, G.; Loregian, A. Drug Repurposing for Viral Infectious Diseases: How Far Are We? Trends Microbiol. 2018, 26, 865–876. [Google Scholar] [CrossRef]
- De Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Hui, D.S.C.; Zumla, A. Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect. Dis. Clin. 2019, 33, 869–889. [Google Scholar] [CrossRef]
- Agbowuro, A.A.; Huston, W.M.; Gamble, A.B.; Tyndall, J.D.A. Proteases and protease inhibitors in infectious diseases. Med. Res. Rev. 2018, 38, 1295–1331. [Google Scholar] [CrossRef]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Momattin, H.; Al-Ali, A.Y.; Al-Tawfiq, J.A. A Systematic Review of therapeutic agents for the treatment of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Travel Med. Infect. Dis. 2019, 30, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chafekar, A.; Fielding, B.C. MERS-CoV: Understanding the Latest Human Coronavirus Threat. Viruses 2018, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Lu, Y.; Chen, F.; Zhang, W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends 2020, 14, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Clercq, E.D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, ciaa237. (in press). [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Savarino, A.; Trani, L.D.; Donatelli, I.; Cauda, R.; Cassone, A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006, 6, 67–69. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases. Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Touret, F.; de Lamballerie, X. Of chloroquine and COVID-19. Antivir. Res. 2020, 177, 104762. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Xu, H.; Rebaza, A.; Sharma, L.; Cruz, C.S.D. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir. Med. 2020, 8, e13. [Google Scholar] [CrossRef]
- Cheung, J.C.-H.; Ho, L.T.; Cheng, J.V.; Cham, E.Y.K.; Lam, K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir. Med. 2020, 8, e49. [Google Scholar] [CrossRef]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef]
- Luo, W.; Majumder, M.S.; Liu, D.; Poirier, C.; Mandl, K.D.; Lipsitch, M.; Santillana, M. The role of absolute humidity on transmission rates of the COVID-19 outbreak. medRxiv 2020. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Shao, L.; Ji, M.; Zhao, Q.; Zhou, Y.; Pei, F.; Wang, J.; Wang, M.; Hong, Y.; et al. Transmission Routes of SARS-CoV-2: Based on the Epidemiological and Clinical Characteristics of 29 Cases in Jinan, China. Lancet 2020. [Google Scholar] [CrossRef]
- WHO—World Health Organization Infection Prevention and Control during Health Care When Novel Coronavirus (nCoV) Infection is Suspected—Interim Guidance. Available online: https://extranet.who.int/ (accessed on 24 March 2020).
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef]
- Kampf, G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect. Prev. Pract. 2020, 2, 100044. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Zaloga, D.J.; Friderici, C.S. COVID-19 Personal Protective Equipment (PPE) for the emergency physician. Vis. J. Emerg. Med. 2020, 19, 100740. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, (in press). [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, (in press). [CrossRef]
- Blake, E.; Yaneer, B.-Y. Could Air Filtration Reduce COVID-19 Severity and Spread? Available online: https://necsi.edu/could-air-filtration-reduce-covid19-severity-and-spread (accessed on 14 April 2020).
- Purdue News Service Cruise Ship AC Systems Could Promote Rapid Coronavirus Spread, Prof Says. Available online: https://www.purdue.edu/newsroom/releases/2020/Q1/cruise-ship-ac-systems-could-promote-rapid-coronavirus-spread,-prof-says.html (accessed on 14 April 2020).
- Fang, Z.; Huang, Z.; Li, X.; Zhang, J.; Lv, W.; Zhuang, L.; Xu, X.; Huang, N. How many infections of COVID-19 there will be in the “Diamond Princess”-Predicted by a virus transmission model based on the simulation of crowd flow. arXiv 2020, arXiv:2002.10616. [Google Scholar]
- Zhang, T.F.; Chen, Q. Identification of contaminant sources in enclosed environments by inverse CFD modeling. Indoor Air 2007, 17, 167–177. [Google Scholar] [CrossRef]
- Mazumdar, S.; Poussou, S.B.; Lin, C.-H.; Isukapalli, S.S.; Plesniak, M.W.; Chen, Q. Impact of scaling and body movement on contaminant transport in airliner cabins. Atmos. Environ. 2011, 45, 6019–6028. [Google Scholar] [CrossRef]
- Kte’pi, B. High-Efficiency Particulate Air System|Air Filtration System; Encyclopedia Britannica: Chicago, IL, USA, 2019. [Google Scholar]
- European Standards Agency Standards EN 1822 and EN ISO 29463—EPA, HEPA and ULPA Filters. Available online: https://www.en-standard.eu/set-en-1822-and-en-iso-29463-standards-for-heigh-efficiency-air-filters-epa-hepa-and-ulpa/ (accessed on 14 April 2020).
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef]
- Dowell, S.F.; Simmerman, J.M.; Erdman, D.D.; Wu, J.-S.J.; Chaovavanich, A.; Javadi, M.; Yang, J.-Y.; Anderson, L.J.; Tong, S.; Ho, M.S. Severe Acute Respiratory Syndrome Coronavirus on Hospital Surfaces. Clin. Infect. Dis. 2004, 39, 652–657. [Google Scholar] [CrossRef]
- The Lancet. COVID-19: Protecting health-care workers. Lancet 2020, 395, 922. [Google Scholar] [CrossRef]
- Davies, A. More Qantas Flights Revealed to Have Been Crewed by Staff with Covid-19. Available online: https://www.theguardian.com/business/2020/apr/09/more-qantas-flights-revealed-crewed-staff-covid-19-coronavirus (accessed on 14 April 2020).
- Rabenau, H.F.; Kampf, G.; Cinatl, J.; Doerr, H.W. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005, 61, 107–111. [Google Scholar] [CrossRef]
- Wood, A.; Payne, D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J. Hosp. Infect. 1998, 38, 283–295. [Google Scholar] [CrossRef]
- Kariwa, H.; Fujii, N.; Takashima, I. Inactivation of SARS Coronavirus by Means of Povidone-Iodine, Physical Conditions and Chemical Reagents. Dermatology 2006, 212 (Suppl. 1), 119–123. [Google Scholar] [CrossRef]
- Eggers, M.; Eickmann, M.; Zorn, J. Rapid and Effective Virucidal Activity of Povidone-Iodine Products Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect. Dis. Ther. 2015, 4, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Sportelli, M.C.; Picca, R.A.; Cioffi, N. Recent advances in the synthesis and characterization of nano-antimicrobials. Tractrends Anal. Chem. 2016, 84, 131–138. [Google Scholar] [CrossRef]
- Cioffi, N.; Rai, M. Nano-Antimicrobials: Progress and Prospects, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-24427-8. [Google Scholar]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of copper nanoparticles: An overview of the various methods. Korean J. Chem. Eng. 2014, 31, 1105–1109. [Google Scholar] [CrossRef]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus. Appl. Environ. Microbiol. 2012, 78, 951. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Bien, K.; Orlowski, P.; Celichowski, G.; Grobelny, J. Chapter 12—Tannic acid modification of metal nanoparticles: Possibility for new antiviral applications. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 335–363. ISBN 978-0-323-47720-8. [Google Scholar]
- Broglie, J.J.; Alston, B.; Yang, C.; Ma, L.; Adcock, A.F.; Chen, W.; Yang, L. Antiviral Activity of Gold/Copper Sulfide Core/Shell Nanoparticles against Human Norovirus Virus-Like Particles. PloS ONE 2015, 10, e0141050. [Google Scholar] [CrossRef] [PubMed]
- Sucipto, T.H.; Churrotin, S.; Setyawati, H.; Kotaki, T.; Martak, F.; Soegijanto, S. Antiviral activity of copper(II)chloride dihydrate against dengue virus type-2 in vero cell. Indones. J. Trop. Infect. Dis. 2017, 6, 84–87. [Google Scholar] [CrossRef]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents 2018, 51, 912–917. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Cioffi, N.; Ditaranto, N.; Sabbatini, L.; Tantillo, G.; Torsi, L.; Zambonin, P.G. Bioactive Metal Nanomaterials Stabilized by Bioactive Agents and Preparation Process. European Patent Application EP 2157211 B1, 2 September 2015. [Google Scholar]
- Cioffi, N.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Zambonin, P.G. Nanomaterials for Controlled Metal Release and Process for Their Production. European Patent Application EP 2123797 B1, 25 November 2009. [Google Scholar]
- Sportelli, M.C.; Longano, D.; Bonerba, E.; Tantillo, G.; Torsi, L.; Sabbatini, L.; Cioffi, N.; Ditaranto, N. Electrochemical Preparation of Synergistic Nanoantimicrobials. Molecules 2020, 25, 49. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Joshi, R.; Chughtai, A.A.; Macintyre, C.R. Graphene Modified Multifunctional Personal Protective Clothing. Adv. Mater. Interfaces 2019, 6, 1900622. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral Activity of Graphene–Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef]
- Iyigundogdu, Z.U.; Demir, O.; Asutay, A.B.; Sahin, F. Developing Novel Antimicrobial and Antiviral Textile Products. Appl. Biochem. Biotechnol. 2017, 181, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, S.; Campbell, A. Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicol. Vitr. 2013, 27, 844–851. [Google Scholar] [CrossRef]
- Ungur, G.; Hrůza, J. Modified polyurethane nanofibers as antibacterial filters for air and water purification. RSC Adv. 2017, 7, 49177–49187. [Google Scholar] [CrossRef]
- Krähling, V.; Stein, D.A.; Spiegel, M.; Weber, F.; Mühlberger, E. Severe Acute Respiratory Syndrome Coronavirus Triggers Apoptosis via Protein Kinase R but Is Resistant to Its Antiviral Activity. J. Virol. 2009, 83, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef] [PubMed]
- Di Gianvincenzo, P.; Marradi, M.; Martínez-Ávila, O.M.; Bedoya, L.M.; Alcamí, J.; Penadés, S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorganic Med. Chem. Lett. 2010, 20, 2718–2721. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnology 2005, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic Acid Modified Silver Nanoparticles Show Antiviral Activity in Herpes Simplex Virus Type 2 Infection. PloS ONE 2014, 9, e104113. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of Herpes Simplex Virus Type 1 Infection by Silver Nanoparticles Capped with Mercaptoethane Sulfonate. Bioconjugate Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Huang, A.-G.; Huang, J.-Q.; Huang, Y.-Q.; Wang, G.-X. Anti-betanodavirus activity of isoprinosine and improved efficacy using carbon nanotubes based drug delivery system. Aquaculture 2019, 512, 734377. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.-L.; Ling, F.; Wang, G.-X. Carbon nanotube-based nanocarrier loaded with ribavirin against grass carp reovirus. Antivir. Res. 2015, 118, 29–38. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Nanotech Surface Coronavirus: Nanotech Surface Sanitizes Milan with Nanomaterials Remaining Self-Sterilized for Years|STATNANO. Available online: https://statnano.com//news/67531/Coronavirus-Nanotech-Surface-Sanitizes-Milan-with-Nanomaterials-Remaining-Self-sterilized-for-Years (accessed on 6 April 2020).
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 1–5. [Google Scholar] [CrossRef]
- Fanning, J.C.; Taylor, L.T. Some transition metal complexes of 8-aminoquinoline. J. Inorg. Nucl. Chem. 1965, 27, 2217–2223. [Google Scholar] [CrossRef]
- Phopin, K.; Sinthupoom, N.; Treeratanapiboon, L.; Kunwittaya, S.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Antimalarial and antimicrobial activities of 8-Aminoquinoline-Uracils metal complexes. EXCLI J. 2016, 15, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sona Nanotech. Sona Develops Rapid Screening Test for Coronavirus; Sona Nanotech: Halifax, Canada, 2020. [Google Scholar]
- WHO—World Health Organization WHO|International Clinical Trials Registry Platform (ICTRP). Available online: http://www.who.int/ictrp/en/ (accessed on 27 March 2020).
- Mansour, H.M.; Rhee, Y.S.; Wu, X. Nanomedicine in pulmonary delivery. IJN 2009, 4, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Pontes, J.F.; Grenha, A. Multifunctional Nanocarriers for Lung Drug Delivery. Nanomaterials 2020, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, T.; Ogbogu, U. The commercialization of university-based research: Balancing risks and benefits. BMC Med. Ethics 2015, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, E.; Moen, Ø.; Gulbrandsen, M. Initiatives to promote commercialization of university knowledge. Technovation 2006, 26, 518–533. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).