Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) Following Long-Term Exposure to Very Small Iron Oxide Particles (VSOPs)
Abstract
1. Introduction
2. Material and Methods
2.1. Characterization of VSOPs
2.2. Isolation and Expansion of hASCs
2.3. Labelling of hASCs with VSOPs
2.4. Detection and Quantification of VSOPs-Labelled hASCs with TEM and Prussian Blue Staining
2.5. Cytotoxicity
2.6. Multidifferentiation Capacity
2.6.1. Histology
2.6.2. Real Time-PCR Analyses
2.7. Expression of Interleukin (IL)-6, IL-8, Vascular Endothelial Growth Factor (VEGF) A and Caspase 3
2.8. Statistical Analyses
3. Results
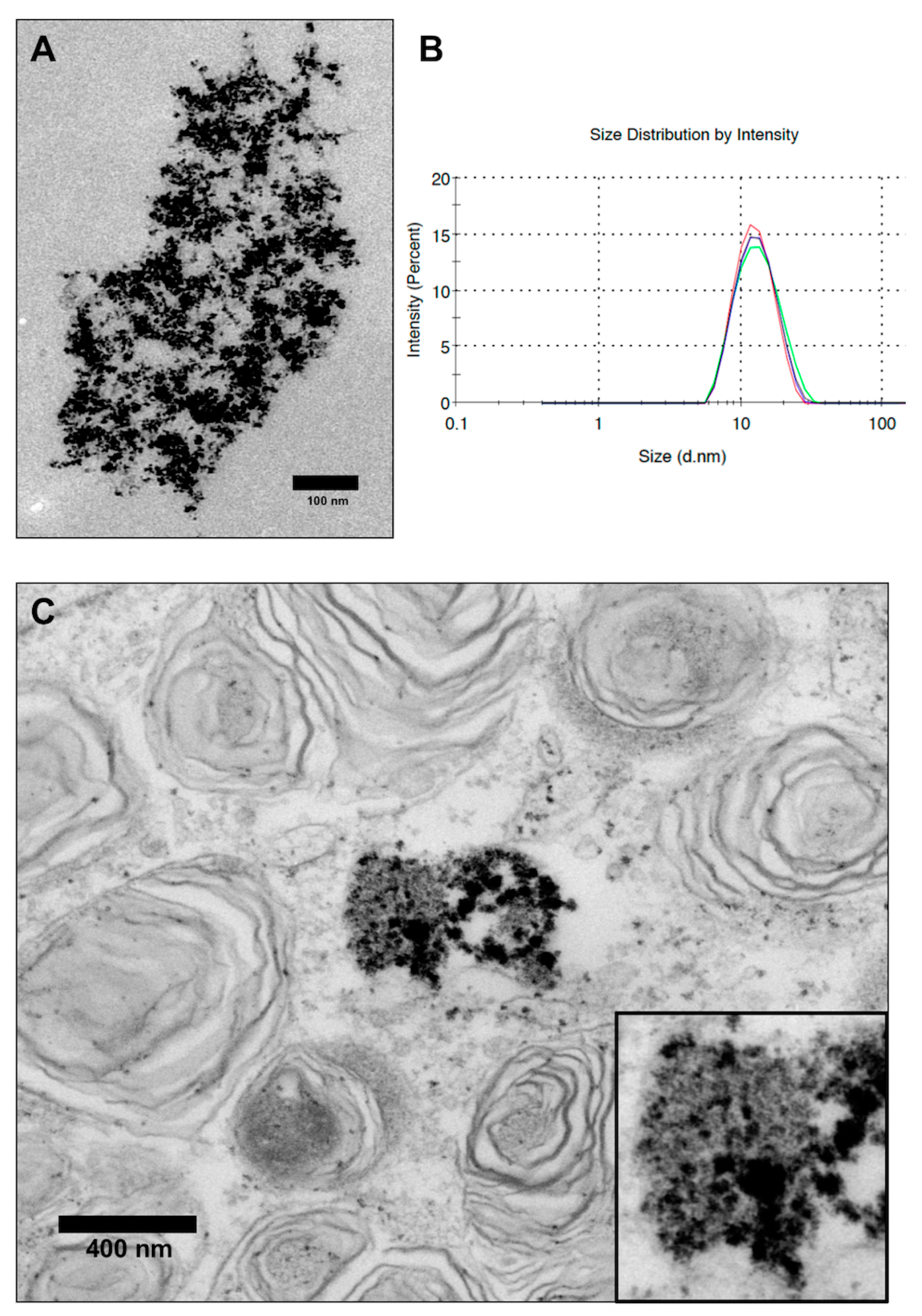
3.1. Characterization of VSOPs
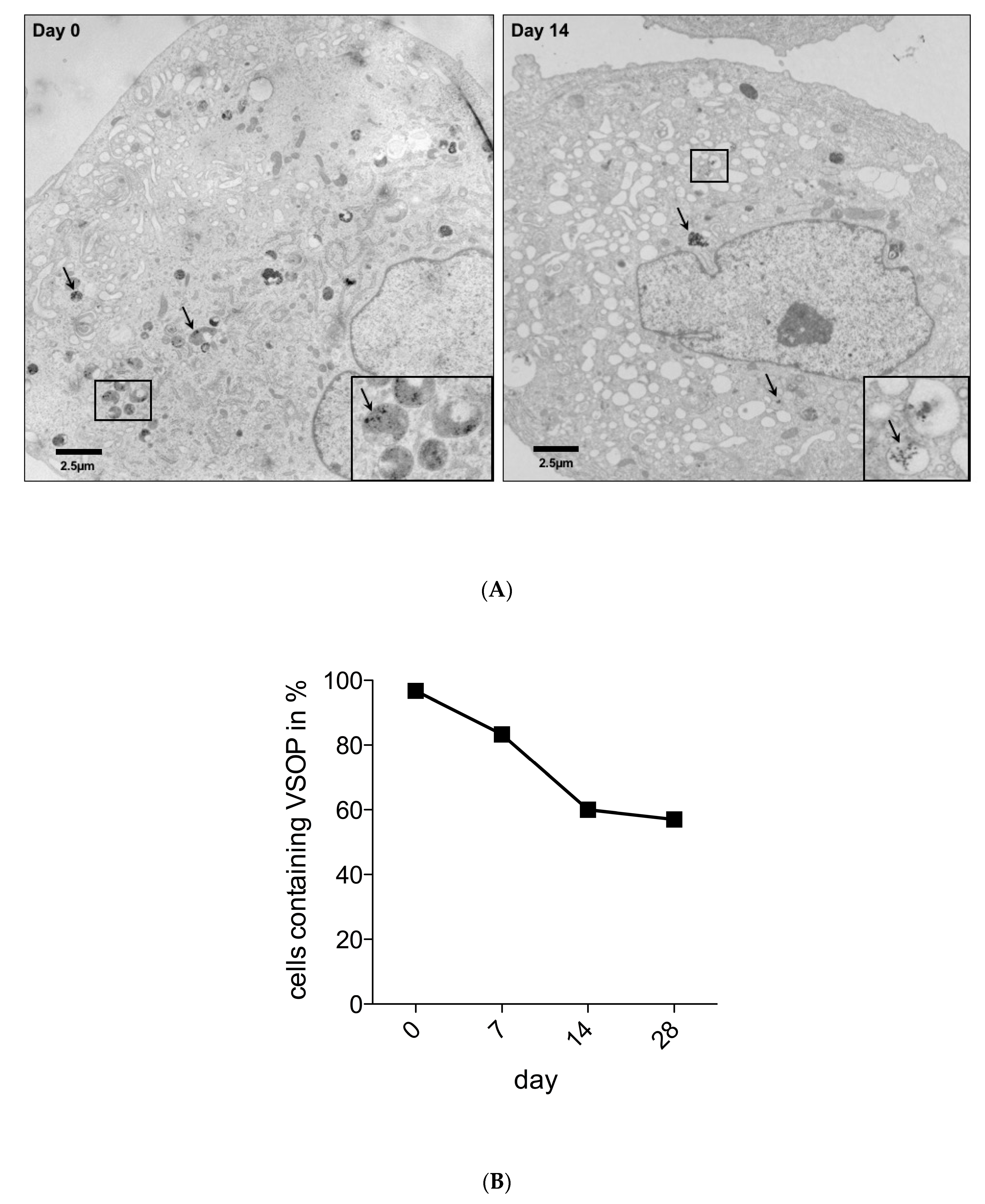
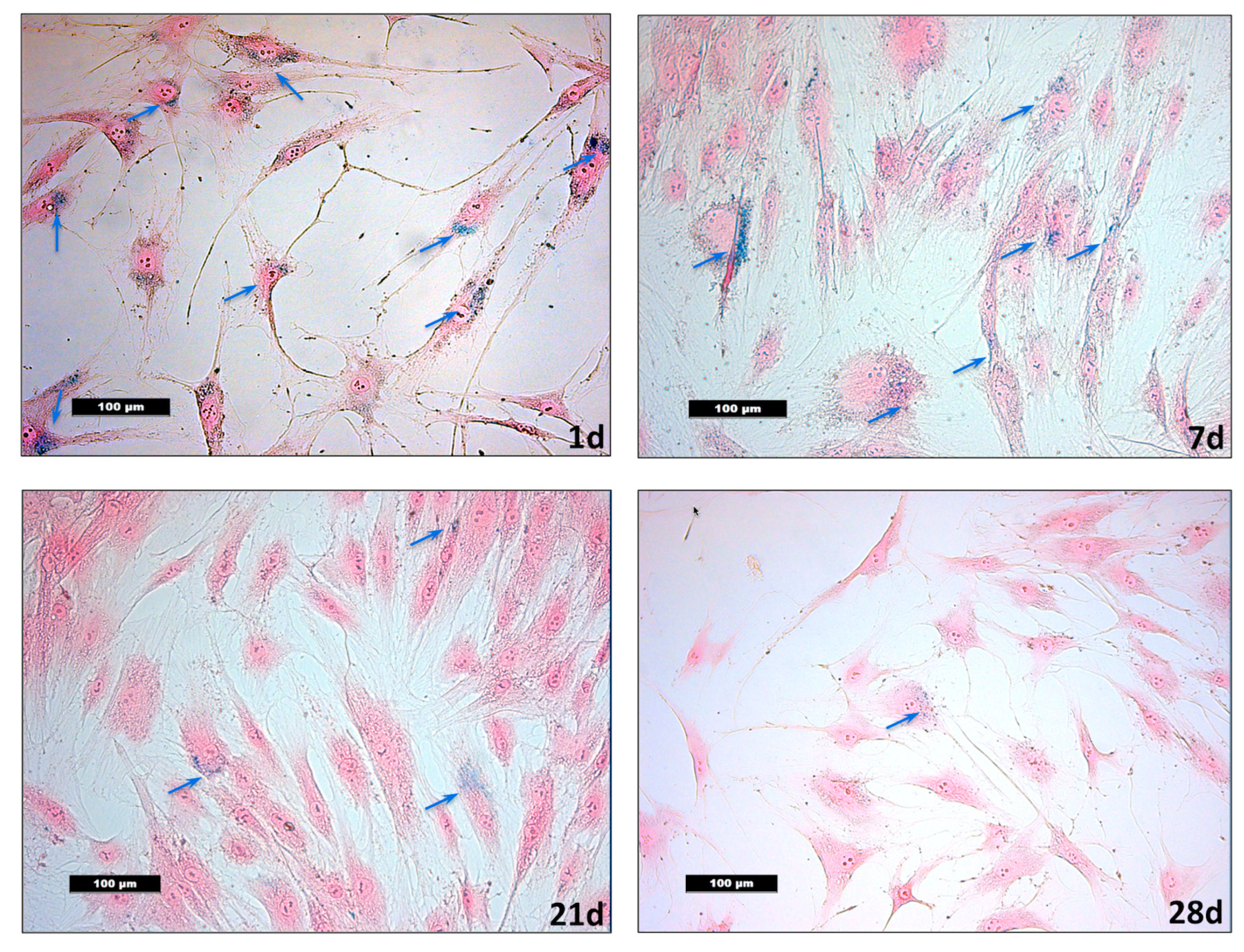
3.2. Detection of VSOPs-Labelled hASCs and Quantification during Expansion
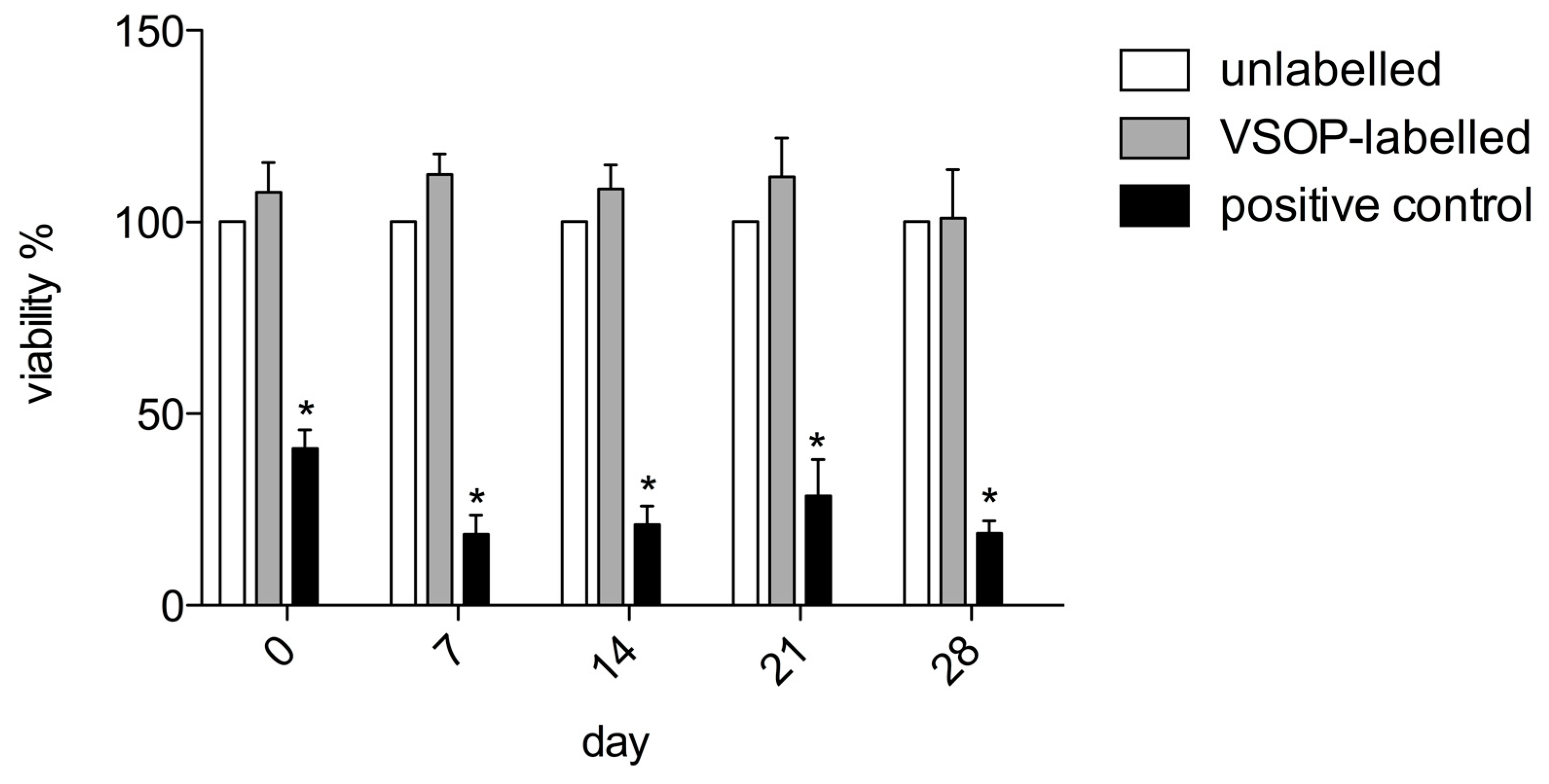
3.3. Cytotoxicity
3.4. Differentiation Capacity
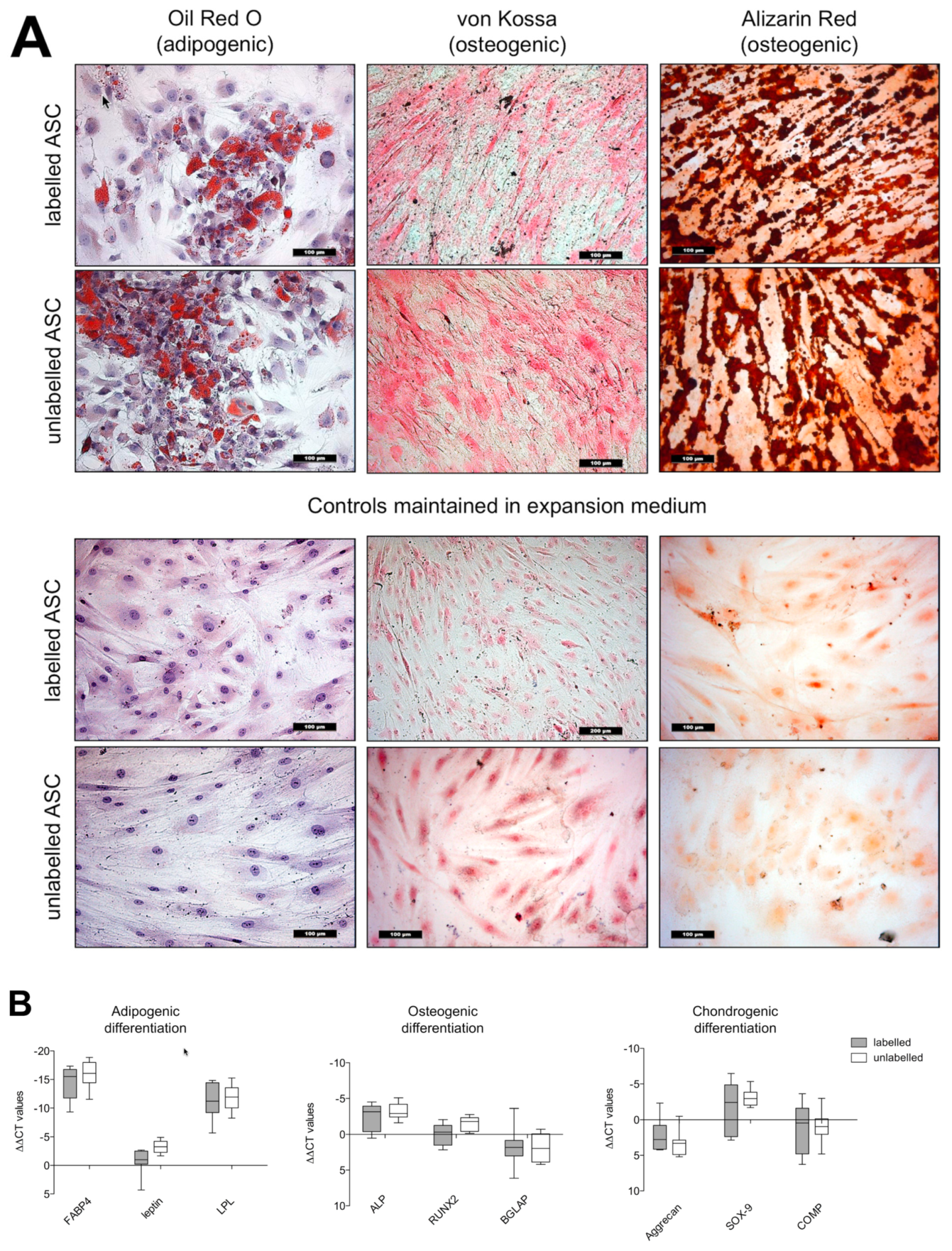
3.4.1. Histology
3.4.2. Real Time-PCR Analyses
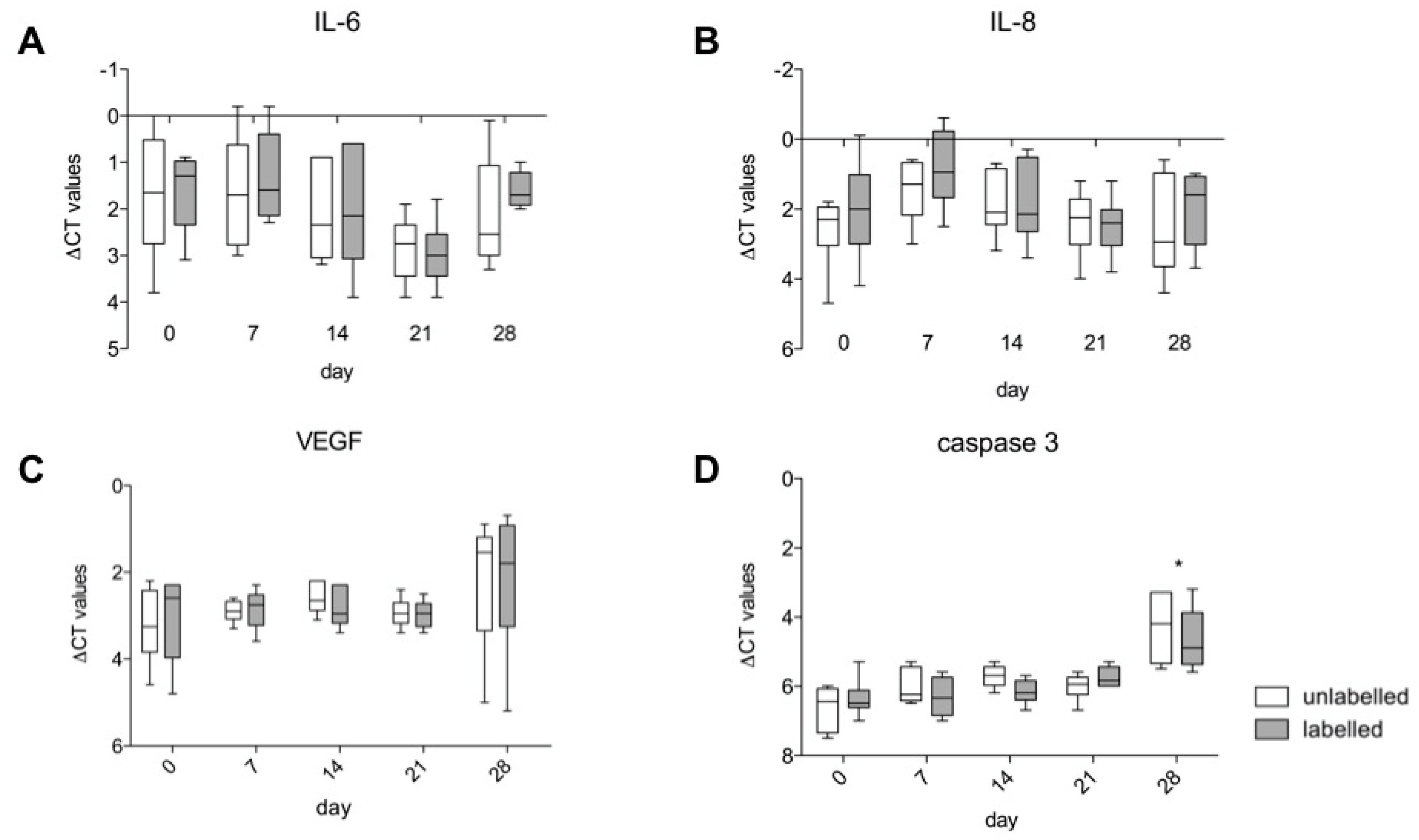
3.5. Gene Expression of IL-6, IL-8, VEGF A and Caspase 3
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soenen, S.J.; Himmelreich, U.; Nuytten, N.; De Cuyper, M. Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 2011, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kilian, T.; Fidler, F.; Kasten, A.; Nietzer, S.; Landgraf, V.; Weiß, K.; Walles, H.; Westphal, F.; Hackenberg, S.; Grüttner, C.; et al. Stem cell labeling with iron oxide nanoparticles: Impact of 3D culture on cell labeling maintenance. Nanomedicine 2016, 11, 1957–1970. [Google Scholar] [CrossRef] [PubMed]
- Stroh, A.; Kressel, J.; Coras, R.; Dreyer, A.Y.; Fröhlich, W.; Förschler, A.; Lobsien, D.; Blümcke, I.; Zoubaa, S.; Schlegel, J.; et al. A Safe and Effective Magnetic Labeling Protocol for MRI-Based Tracking of Human Adult Neural Stem Cells. Front. Neurosci. 2019, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Pöttler, M.; Fliedner, A.; Bergmann, J.; Bui, L.K.; Mühlberger, M.; Braun, C.; Graw, M.; Janko, C.; Friedrich, O.; Alexiou, C.; et al. Magnetic Tissue Engineering of the Vocal Fold Using Superparamagnetic Iron Oxide Nanoparticles. Tissue Eng. Part A. 2019, 25, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Duguet, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.M. Magnetic nanoparticles and their applications in medicine. Nanomedicine 2006, 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Park, I.K.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Meena, R.; Rajamani, P. Biodistribution, Clearance and morphological alterations of intravenously administered iron oxide nanoparticles in male Wistar rats. Int. J. Nanomed. 2019, 14, 9677–9692. [Google Scholar] [CrossRef]
- Mok, H.; Zhang, M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin. Drug Deliv. 2013, 10, 73–87. [Google Scholar] [CrossRef]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Lobsien, D.; Dreyer, A.Y.; Stroh, A.; Boltze, J.; Hoffmann, K.T. Imaging of VSOP Labeled Stem Cells in Agarose Phantoms With Susceptibility Weighted and T2* Weighted MR Imaging at 3T: Determination of the Detection Limit. PLoS ONE 2013, 8, e62644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heymer, A.; Haddad, D.; Weber, M.; Gbureck, U.; Jakob, P.M.; Eulert, J.; Nöth, U. Iron oxide labelling of human mesenchymal stem cells in collagen hydrogels for articular cartilage repair. Biomaterials 2008, 29, 1473–1483. [Google Scholar] [CrossRef]
- Stroh, A.; Zimmer, C.; Gutzeit, C.; Jakstadt, M.; Marschinke, F.; Jung, T.; Pilgrimm, H.; Grune, T. Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic. Biol. Med. 2004, 36, 976–984. [Google Scholar] [CrossRef]
- Stroh, A.; Boltze, J.; Sieland, K.; Hild, K.; Gutzeit, C.; Jung, T.; Kressel, J.; Hau, S.; Reich, D.; Grune, T.; et al. Impact of Magnetic Labeling on Human and Mouse Stem Cells and Their Long-Term Magnetic Resonance Tracking in a Rat Model of Parkinson Disease. Mol. Imaging 2009, 8, 166–178. [Google Scholar] [CrossRef]
- Pilgrimm, H. Superparamagnetic Particles with Increased R1 Relaxivity, Process for Producing Said Particles and Use Thereof. U.S. Patent No. US6638494, 28 October 2003. [Google Scholar]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010. [Google Scholar] [CrossRef]
- Oedayrajsingh-Varma, M.J.; van Ham, S.M.; Knippenberg, M.; Helder, M.N.; Klein-Nulend, J.; Schouten, T.E.; Ritt, M.J.; van Milligen, F.J. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006, 8, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Froelich, K.; Setiawan, L.E.; Technau, A.; Tirado, M.R.; Hackenberg, S.; Hagen, R.; Staudenmaier, R.; Kleinsasser, N.H. Influence of different growth factors on chondrogenic differentiation of adipose-derived stem cells in polyurethane-fibrin composites. Int. J. Artif. Organs 2012, 35, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Hong, K.Y.; Minn, K.W.; Chang, H. Chondrogenesis of adipose-derived stem cells on irradiated cartilage. Plast. Reconstr. Surg. 2020, 145, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Froelich, K.; Hagen, R.; Kleinsasser, N. Adipose-derived stromal cells (ASC)—Basics and therapeutic approaches in otorhinolaryngology. Laryngorhinootologie 2014, 93, 369–380. [Google Scholar]
- Wittmann, K.; Storck, K.; Muhr, C.; Mayer, H.; Regn, S.; Staudenmaier, R.; Wiese, H.; Maier, G.; Bauer-Kreisel, P.; Blunk, T. Development of volume-stable adipose tissue constructs using polycaprolactone-based polyurethane scaffolds and fibrin hydrogels. J. Tissue Eng. Regener. Med. 2016, 10, E409–E418. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef]
- Zielins, E.R.; Luan, A.; Brett, E.A.; Longaker, M.T.; Wan, D.C. Therapeutic applications of human adipose-derived stromal cells for soft tissue reconstruction. Discov. Med. 2015, 19, 245–253. [Google Scholar]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Lizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol. Surg. 2008, 34, 1178–1185. [Google Scholar] [CrossRef]
- Froelich, K.; Mickler, J.; Steusloff, G.; Technau, A.; Ramos Tirado, M.; Scherzed, A.; Hackenberg, S.; Radeloff, A.; Hagen, R.; Kleinsasser, N. Chromosomal aberrations and deoxyribonucleic acid single-strand breaks in adipose-derived stem cells during long-term expansion in vitro. Cytotherapy 2013, 15, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. In Vitro 2011, 25, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Radeloff, K.; Radeloff, A.; Tirado, M.R.; Scherzad, A.; Hagen, R.; Kleinsasser, N.H.; Hackenberg, S. Long-Term Impact of Zinc Oxide Nanoparticles on Differentiation and Cytokine Secretion of Human Adipose-Derived Stromal Cells. Materials 2019, 12, 182. [Google Scholar] [CrossRef]
- Ittrich, H.; Lange, C.; Dahnke, H.; Zander, A.R.; Adam, G.; Nolte-Ernsting, C. Labeling of mesenchymal stem cells with different superparamagnetic particles of iron oxide and detectability with MRI at 3T. Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2005, 177, 1151–1163. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chen, D.; Xia, D.; Pan, Z.; Xu, D.; Zhou, Y.; Wu, Y.; Cai, N.; Tang, Q.; Wang, C.; Yan, M.; et al. Metformin Protects Against Apoptosis and Senescence in Nucleus Pulposus Cells and Ameliorates Disc Degeneration in Vivo. Cell Death Dis. 2016, 7, e2441. [Google Scholar] [CrossRef]
- Froelich, K.; Steussloff, G.; Schmidt, K.; Ramos Tirado, M.; Technau, A.; Scherzed, A.; Hackenberg, S.; Radeloff, A.; Hagen, R.; Kleinsasser, N. DiI labeling of human adipose-derived stem cells: Evaluation of DNA damage, toxicity and functional impairment. Cells Tissues Organs 2013, 197, 384–398. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, D.J.; Moorman, M.A.; Simonetti, D.W.; Craig, S.M.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Nöth, U.; Osyczka, A.M.; Tuli, R.; Hickok, N.J.; Danielson, K.G.; Tuan, R.S. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J. Orthop. Res. 2002, 20, 1060–1069. [Google Scholar] [CrossRef]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell Biochem. 1997, 64, 295–312. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.J.; Maitra, A.; Prashant, C.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Kostura, L.; Kraitchman, D.L.; Mackay, A.M.; Pittenger, M.F.; Bulte, J.W. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004, 17, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Kasten, A.; Grüttner, C.; Kühn, J.P.; Bader, R.; Pasold, J.; Frerich, B. Comparative in vitro study on magnetic iron oxide nanoparticles for MRI tracking of adipose tissue-derived progenitor cells. PLoS ONE 2014, 9, e108055. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Froelich, K.; Hagen, R.; Kleinsasser, N. Functional Responses of Human Adipose Tissue-Derived Mesenchymal Stem Cells to Metal Oxide Nanoparticles In Vitro. J. Biomed. Nanotechnol. 2013, 9, 86–95. [Google Scholar] [CrossRef]
- Ickrath, P.; Wagner, M.; Scherzad, A.; Gehrke, T.; Burghartz, M.; Hagen, R.; Radeloff, K.; Kleinsasser, N.; Hackenberg, S. Time-Dependent Toxic and Genotoxic Effects of Zinc Oxide Nanoparticles after Long-Term and Repetitive Exposure to Human Mesenchymal Stem Cells. Int. J. Environ. Res. Public Health 2017, 14, 1590. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S.; Alhader, M.S. Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol. Trace Elem. Res. 2014, 159, 416–424. [Google Scholar] [CrossRef]
- Novotna, B.; Jendelova, P.; Kapcalova, M.; Rossner, P.; Turnovcova, K.; Bagryantseva, Y.; Babic, M.; Horak, D.; Sykova, E. Oxidative damage to biological macromolecules in human bone marrow mesenchymal stromal cells labeled with various types of iron oxide nanoparticles. Toxicol. Lett. 2012, 210, 53–63. [Google Scholar] [CrossRef]
- Fan, J.; Tan, Y.; Jie, L.; Wu, X.; Yu, R.; Zhang, M. Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Res. Ther. 2013, 4, 44. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsiao, J.K.; Liu, H.M.; Lai, I.Y.; Yao, M.; Hsu, S.C.; Ko, B.S.; Chen, Y.C.; Yang, C.S.; Huang, D.M. The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2010, 245, 272–279. [Google Scholar] [CrossRef]
- Balakumaran, A.; Pawelczyk, E.; Ren, J.; Sworder, B.; Chaudhry, A.; Sabatino, M.; Stroncek, D.; Frank, J.A.; Robey, P.G. Superparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their “stemness”. PLoS ONE 2010, 5, e11462. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Zhang, Y.L.; Qian, S.P.; Yu, X.B.; Xie, H.Y.; Zhou, L.; Zheng, S.S. Assessment of biological characteristics of mesenchymal stem cells labeled with superparamagnetic iron oxide particles in vitro. Mol. Med Rep. 2012, 5, 317–320. [Google Scholar] [PubMed]
- Wang, N.; Zhao, J.Y.; Guan, X.; Dong, Y.; Liu, Y.; Zhou, X.; Wu, R.; Du, Y.; Zhao, L.; Zou, W.; et al. Biological characteristics of adipose tissue-derived stem cells labeled with amine-surface-modified superparamagnetic iron oxide nanoparticles. Cell Biol. Int. 2015, 39, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Ferraraccio, F.; Prati, M.V.; Annunziata, S.; Bianco, A.; Mezzogiorno, A.; Liguori, G.; Angelillo, I.F.; Cazzola, M. Effects of diesel exhaust particles on human lung epithelial cells: An in vitro study. Respir. Med. 2007, 101, 1155–1162. [Google Scholar] [CrossRef]
- Scherzad, A.; Steber, M.; Gehrke, T.; Rak, K.; Froelich, K.; Schendzielorz, P.; Hagen, R.; Kleinsasser, N.; Hackenberg, S. Human Mesenchymal Stem Cells Enhance Cancer Cell Proliferation via IL-6 Secretion and Activation of ERK1/2. Int. J. Oncol. 2015, 47, 391–397. [Google Scholar] [CrossRef]
- Gojova, A.; Guo, B.; Kota, R.S.; Rutledge, J.C.; Kennedy, I.M.; Barakat, A.I. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: Effect of particle composition. Environ. Health Perspect. 2007, 115, 403–409. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeloff, K.; Radeloff, A.; Ramos Tirado, M.; Scherzad, A.; Hagen, R.; Kleinsasser, N.H.; Hackenberg, S. Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) Following Long-Term Exposure to Very Small Iron Oxide Particles (VSOPs). Nanomaterials 2020, 10, 741. https://doi.org/10.3390/nano10040741
Radeloff K, Radeloff A, Ramos Tirado M, Scherzad A, Hagen R, Kleinsasser NH, Hackenberg S. Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) Following Long-Term Exposure to Very Small Iron Oxide Particles (VSOPs). Nanomaterials. 2020; 10(4):741. https://doi.org/10.3390/nano10040741
Chicago/Turabian StyleRadeloff, Katrin, Andreas Radeloff, Mario Ramos Tirado, Agmal Scherzad, Rudolf Hagen, Norbert H. Kleinsasser, and Stephan Hackenberg. 2020. "Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) Following Long-Term Exposure to Very Small Iron Oxide Particles (VSOPs)" Nanomaterials 10, no. 4: 741. https://doi.org/10.3390/nano10040741
APA StyleRadeloff, K., Radeloff, A., Ramos Tirado, M., Scherzad, A., Hagen, R., Kleinsasser, N. H., & Hackenberg, S. (2020). Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) Following Long-Term Exposure to Very Small Iron Oxide Particles (VSOPs). Nanomaterials, 10(4), 741. https://doi.org/10.3390/nano10040741




