Nanocarriers as Magic Bullets in the Treatment of Leukemia
Abstract
1. Introduction
2. Nanoparticles as Drug Delivery Systems
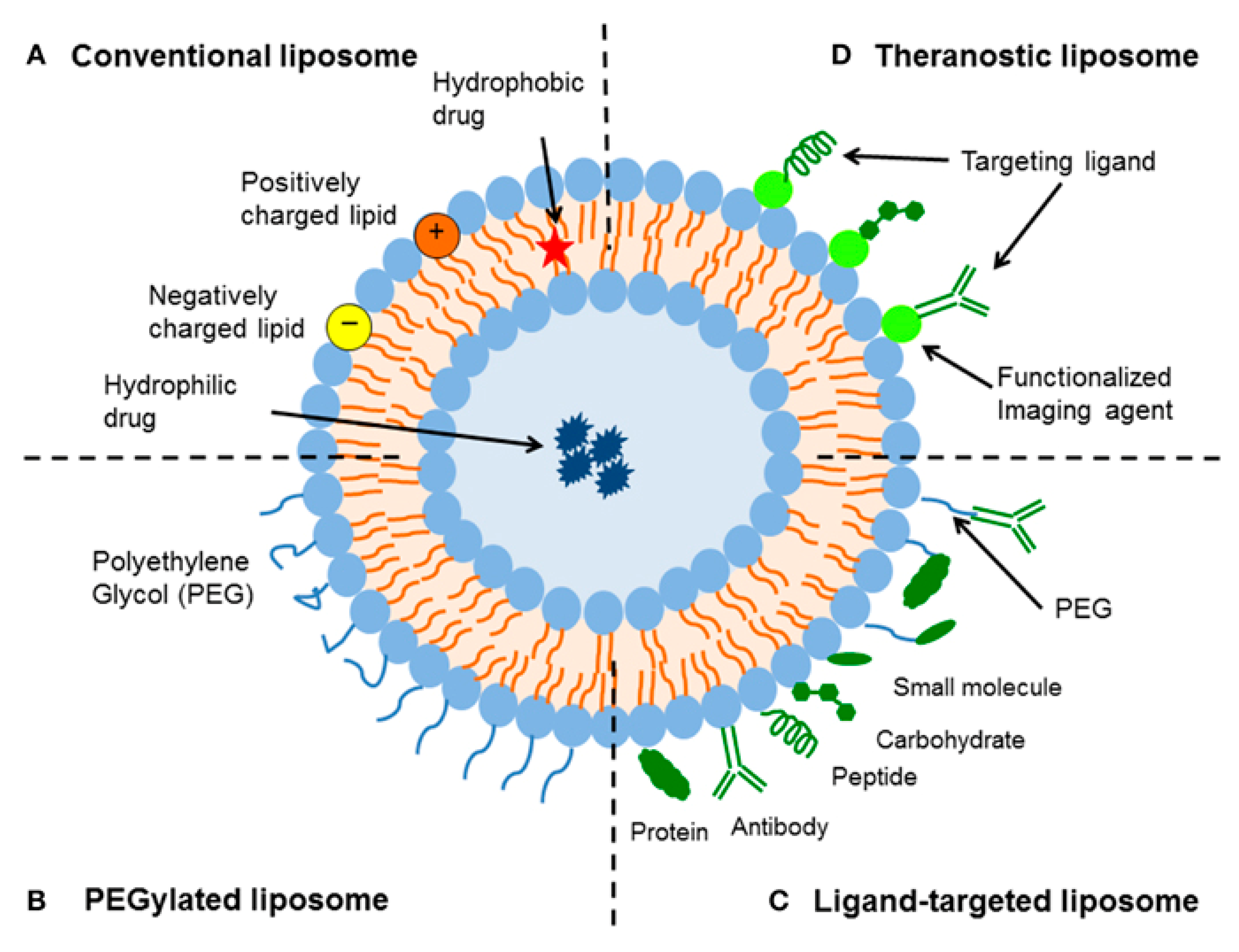
2.1. Liposomes
2.2. Micelles
2.3. Polymeric Nanoparticles
2.4. Solid Lipid Nanoparticles (SLNs)
2.5. Inorganic Nanoparticles
3. Targeted Cancer Therapy: Advantages in Comparison to Conventional Treatments
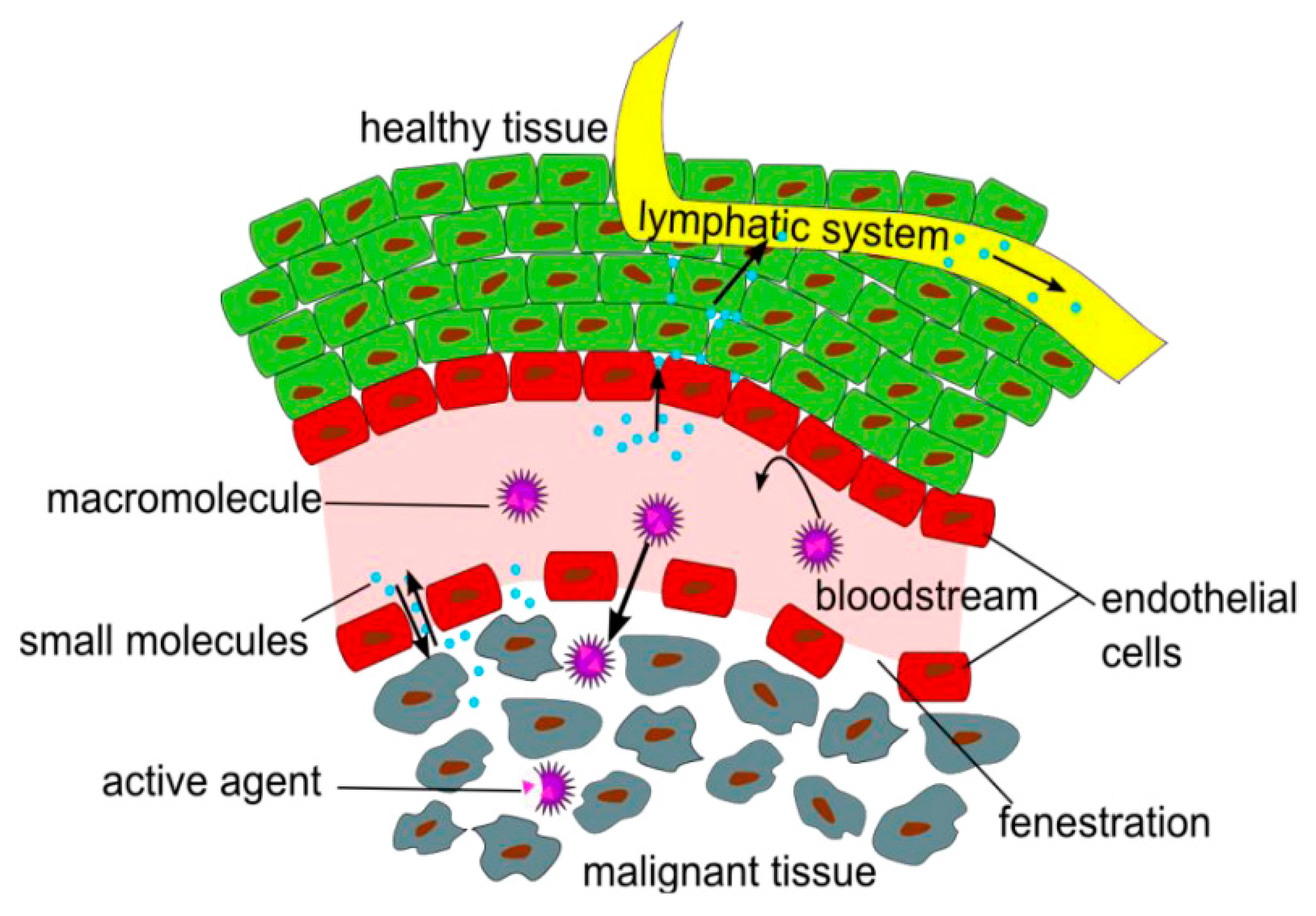
3.1. Passive Targeting
3.2. Active Targeting
4. Nanosystems in the Treatment of Myeloid Malignancies
5. Nanosystems in the Treatment of Lymphoid Malignancies
6. Clinical Trials
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| AML-MRC | AML with myelodysplastic related changes |
| APL | Acute promyelocytic leukemia |
| ATRA | All-trans-retinoic acid |
| BCR | Breakpoint cluster region |
| CLL | Chronic lymphocytic leukemia |
| CMC | Critical micelle concentration |
| CML | Chronic myeloid leukemia |
| DMPG | Dimyristoyl phosphatidylglycerol |
| DOPC | Dioleoyl phosphatidylcholine |
| DOPE | Dioleoyl phosphatidylethanolamine |
| DOPE | Dioleoylphosphatidyl ethanolamine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| DPPC | Dipalmitoyl phosphatidylcholine |
| DPPG | Dipalmitoyl phosphatidylglycerol |
| EMA | European Medicines Agency |
| EPR | Enhanced permeability and retention |
| FDA | Food and Drug Administration |
| FLT3 | Fms-related tyrosine kinase 3 |
| LSC | Leukemic stem cell |
| MRI | Magnetic resonance imaging |
| NLCs | Nanostructured lipid carriers |
| OA | Oleic acid |
| OS | Overall survival |
| PDGF-Rs | Receptors for platelet-derived growth factor |
| PEG | Polyethylene glycol |
| PGA | Poly glycolic acid |
| PLA | Poly lactic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PTL | Parthenolide |
| RES | Reticuloendothelial system |
| RGD | Arginylglycylaspartic acid |
| ROR1 | Tyrosine-protein kinase transmembrane receptor |
| SLNs | Solid lipid nanoparticles |
| SYK | Spleen tyrosine kinase |
| t-AML | Therapy-related AML |
| TKIs | Tyrosine kinase inhibitors |
| Tm | Phase transition temperature |
| VEGFRs | Vascular endothelial growth factor receptors |
| VSLI | Vincristine sulfate liposome injection |
References
- Miranda-Filho, A.; Pineros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 2017, 130, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wu, Y.; Jiang, X.; Zhang, W.; Xu, L. Clinical symptoms and chemotherapy completion in elderly patients with newly diagnosed acute leukemia: A retrospective comparison study with a younger cohort. BMC Cancer 2011, 11, 224. [Google Scholar] [CrossRef]
- Krause, D.S.; Van Etten, R.A. Right on target: Eradicating leukemic stem cells. Trends Mol. Med. 2007, 13, 470–481. [Google Scholar] [CrossRef]
- Rothenberg-Thurley, M.; Amler, S.; Goerlich, D.; Kohnke, T.; Konstandin, N.P.; Schneider, S.; Sauerland, M.C.; Herold, T.; Hubmann, M.; Ksienzyk, B.; et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia 2018, 32, 1598–1608. [Google Scholar] [CrossRef]
- Perl, A.E. The role of targeted therapy in the management of patients with AML. Blood Adv. 2017, 1, 2281–2294. [Google Scholar] [CrossRef]
- Jabbour, E.; Cortes, J.E.; Ghanem, H.; O’Brien, S.; Kantarjian, H.M. Targeted therapy in chronic myeloid leukemia. Expert Rev. Anticancer Ther. 2008, 8, 99–110. [Google Scholar] [CrossRef]
- Bae, K.H.; Chung, H.J.; Park, T.G. Nanomaterials for cancer therapy and imaging. Mol. Cells 2011, 31, 295–302. [Google Scholar] [CrossRef]
- ‘Plenty of room’ revisited. Nat. Nanotechnol. 2009, 4, 781. [CrossRef]
- Fornaguera, C.; Garcia-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal. Transduct Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- Coukell, A.J.; Brogden, R.N. Liposomal amphotericin B. Therapeutic use in the management of fungal infections and visceral leishmaniasis. Drugs 1998, 55, 585–612. [Google Scholar] [CrossRef]
- Adler-Moore, J.; Proffitt, R.T. AmBisome: Liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 2002, 49, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Fluhmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Muhlebach, S. Nanomedicines: The magic bullets reaching their target? Eur. J. Pharm. Sci. 2019, 128, 73–80. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs 1998, 56, 747–756. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Zhao, W.; Zhuang, S.; Qi, X.R. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 2011, 6, 3087–3098. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazu, S.; Kiselev, M.A. Soft Interaction in Liposome Nanocarriers for Therapeutic Drug Delivery. Nanomaterials 2016, 6, 125. [Google Scholar] [CrossRef]
- Felgner, P.L.; Ringold, G.M. Cationic liposome-mediated transfection. Nature 1989, 337, 387–388. [Google Scholar] [CrossRef]
- Pedroso de Lima, M.C.; Neves, S.; Filipe, A.; Duzgunes, N.; Simoes, S. Cationic liposomes for gene delivery: From biophysics to biological applications. Curr. Med. Chem. 2003, 10, 1221–1231. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Chen, E.C.; Fathi, A.T.; Brunner, A.M. Reformulating acute myeloid leukemia: Liposomal cytarabine and daunorubicin (CPX-351) as an emerging therapy for secondary AML. Onco Targets Ther. 2018, 11, 3425–3434. [Google Scholar] [CrossRef]
- Garello, F.; Terreno, E. Sonosensitive MRI Nanosystems as Cancer Theranostics: A Recent Update. Front. Chem. 2018, 6, 157. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: state of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar] [CrossRef]
- Besse, H.C.; Barten-van Rijbroek, A.D.; van der Wurff-Jacobs, K.M.G.; Bos, C.; Moonen, C.T.W.; Deckers, R. Tumor Drug Distribution after Local Drug Delivery by Hyperthermia, In Vivo. Cancers 2019, 11, 512. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef]
- Soleimani Zohr Shiri, M.; Henderson, W.; Mucalo, M.R. A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh. Materials 2019, 12, 1896. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng 2019, 3, 011502. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. A Review on Preparation, Properties and Applications of Polymeric Nanoparticle-Based Materials. Polym.-Plast. Technol. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Sadat Tabatabaei Mirakabad, F.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Dordunoo, S.K.; Jackson, J.K.; Arsenault, L.A.; Oktaba, A.M.; Hunter, W.L.; Burt, H.M. Taxol encapsulation in poly(epsilon-caprolactone) microspheres. Cancer Chemother. Pharmacol. 1995, 36, 279–282. [Google Scholar] [CrossRef]
- Najlaoui, F.; Pigeon, P.; Aroui, S.; Pezet, M.; Sancey, L.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; De Waard, M.; Busser, B.; et al. Anticancer properties of lipid and poly(epsilon-caprolactone) nanocapsules loaded with ferrocenyl-tamoxifen derivatives. J. Pharm. Pharmacol. 2018, 70, 1474–1484. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, F.; Li, Y.; Yang, X.; Huang, J.; Lv, T.; Zhang, Y.; Chen, J.; Chen, H.; Gao, Y.; et al. Chitosan-based nanoparticles for improved anticancer efficacy and bioavailability of mifepristone. Beilstein J. Nanotechnol. 2016, 7, 1861–1870. [Google Scholar] [CrossRef]
- Kamath, P.R.; Sunil, D. Nano-Chitosan Particles in Anticancer Drug Delivery: An Up-to-Date Review. Mini. Rev. Med. Chem. 2017, 17, 1457–1487. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Colazo, J.; Berg, D.; Mugo, S.M.; Serpe, M.J. Multiresponsive Nanogels for Targeted Anticancer Drug Delivery. Mol. Pharm. 2017, 14, 2624–2628. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi. Pharm. J. 2016, 24, 133–139. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Allen, C. Polymer-drug compatibility: A guide to the development of delivery systems for the anticancer agent, ellipticine. J. Pharm. Sci. 2004, 93, 132–143. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef]
- Serpe, L.; Catalano, M.G.; Cavalli, R.; Ugazio, E.; Bosco, O.; Canaparo, R.; Muntoni, E.; Frairia, R.; Gasco, M.R.; Eandi, M.; et al. Cytotoxicity of anticancer drugs incorporated in solid lipid nanoparticles on HT-29 colorectal cancer cell line. Eur. J. Pharm. Biopharm. 2004, 58, 673–680. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody fragments as nanoparticle targeting ligands: a step in the right direction. Chem. Sci. 2017, 8, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Herranz, B.; Santos, H.A. Nanostructured porous Si-based nanoparticles for targeted drug delivery. Biomatter 2012, 2, 296–312. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomedicine 2016, 12, 317–332. [Google Scholar] [CrossRef]
- Fang, M.; Peng, C.W.; Pang, D.W.; Li, Y. Quantum dots for cancer research: Current status, remaining issues, and future perspectives. Cancer Biol. Med. 2012, 9, 151–163. [Google Scholar] [CrossRef]
- Bosch, F.; Rosich, L. The contributions of Paul Ehrlich to pharmacology: A tribute on the occasion of the centenary of his Nobel Prize. Pharmacology 2008, 82, 171–179. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Farber, S.; Diamond, L.K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J. Med. 1948, 238, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Baselga, J. Targeting tyrosine kinases in cancer: The second wave. Science 2006, 312, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Moen, M.D.; McKeage, K.; Plosker, G.L.; Siddiqui, M.A. Imatinib: A review of its use in chronic myeloid leukaemia. Drugs 2007, 67, 299–320. [Google Scholar] [CrossRef]
- Papaetis, G.S.; Syrigos, K.N. Sunitinib: A multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs 2009, 23, 377–389. [Google Scholar] [CrossRef]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Marks, L. The birth pangs of monoclonal antibody therapeutics: The failure and legacy of Centoxin. MAbs 2012, 4, 403–412. [Google Scholar] [CrossRef]
- Liu, J.K. The history of monoclonal antibody development - Progress, remaining challenges and future innovations. Ann. Med. Surg. (Lond.) 2014, 3, 113–116. [Google Scholar] [CrossRef]
- Carter, P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer 2001, 1, 118–129. [Google Scholar] [CrossRef]
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Claret, F.X. Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 163. [Google Scholar] [CrossRef]
- Arachchige, M.C.; Reshetnyak, Y.K.; Andreev, O.A. Corrigendum to “Advanced targeted nanomedicine” [J. Biotechnol. 202 (2015) 88-97]. J. Biotechnol. 2016, 225, 68. [Google Scholar] [CrossRef]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Stockhofe, K.; Postema, J.M.; Schieferstein, H.; Ross, T.L. Radiolabeling of Nanoparticles and Polymers for PET Imaging. Pharmaceuticals 2014, 7, 392–418. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Vinhas, R.; Mendes, R.; Fernandes, A.R.; Baptista, P.V. Nanoparticles-Emerging Potential for Managing Leukemia and Lymphoma. Front. Bioeng. Biotechnol. 2017, 5, 79. [Google Scholar] [CrossRef]
- Barui, A.K.; Oh, J.Y.; Jana, B.; Kim, C.; Ryu, J.-H. Cancer-Targeted Nanomedicine: Overcoming the Barrier of the Protein Corona. Adv. Ther. N/A 2019, 1900124. [Google Scholar] [CrossRef]
- Friedman, A.D.; Claypool, S.E.; Liu, R. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Chen, Z.G.; Shin, D.M. Application of nanotechnology in cancer therapy and imaging. CA Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Attarwala, H. Role of antibodies in cancer targeting. J. Nat. Sci. Biol. Med. 2010, 1, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, Y.; Liu, Y.; Meng, F.; Lee, R.J. Clinical translation of immunoliposomes for cancer therapy: Recent perspectives. Expert Opin. Drug Deliv. 2018, 15, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Immunoliposomes for cancer therapy. Curr. Opin. Mol. Ther. 2006, 8, 39–45. [Google Scholar] [PubMed]
- Frejd, F.Y.; Kim, K.T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef]
- Bi, Y.; Hao, F.; Yan, G.; Teng, L.; Lee, R.J.; Xie, J. Actively Targeted Nanoparticles for Drug Delivery to Tumor. Curr. Drug Metab. 2016, 17, 763–782. [Google Scholar] [CrossRef]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted nanoparticles for cancer therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmuller, M.; Rader, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef]
- Mayer, G. The chemical biology of aptamers. Angew. Chem. Int. Ed. Engl. 2009, 48, 2672–2689. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.E.; Tanaka, T. Aptamer Therapeutics in Cancer: Current and Future. Cancers 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- De Kouchkovsky, I.; Abdul-Hay, M. ‘Acute myeloid leukemia: A comprehensive review and 2016 update’. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Gardin, C. An update of current treatments for adult acute myeloid leukemia. Blood 2016, 127, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Doucette, K.; Norsworthy, K. Recent drug approvals for acute myeloid leukemia. J. Hematol. Oncol. 2019, 12, 100. [Google Scholar] [CrossRef]
- Stein, E.M.; Tallman, M.S. Emerging therapeutic drugs for AML. Blood 2016, 127, 71–78. [Google Scholar] [CrossRef]
- Godley, L.A.; Larson, R.A. Therapy-related myeloid leukemia. Semin. Oncol. 2008, 35, 418–429. [Google Scholar] [CrossRef]
- Vardiman, J.; Reichard, K. Acute Myeloid Leukemia With Myelodysplasia-Related Changes. Am. J. Clin. Pathol. 2015, 144, 29–43. [Google Scholar] [CrossRef]
- Mayer, L.D.; Tardi, P.; Louie, A.C. CPX-351: A nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Small, D. FLT3 mutations: Biology and treatment. Hematol. Am. Soc. Hematol. Educ. Program. 2006, 2006, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bugno, J.; Hu, C.; Yang, Y.; Herold, T.; Qi, J.; Chen, P.; Gurbuxani, S.; Arnovitz, S.; Strong, J.; et al. Eradication of Acute Myeloid Leukemia with FLT3 Ligand-Targeted miR-150 Nanoparticles. Cancer Res. 2016, 76, 4470–4480. [Google Scholar] [CrossRef]
- Garzon, R.; Heaphy, C.E.; Havelange, V.; Fabbri, M.; Volinia, S.; Tsao, T.; Zanesi, N.; Kornblau, S.M.; Marcucci, G.; Calin, G.A.; et al. MicroRNA 29b functions in acute myeloid leukemia. Blood 2009, 114, 5331–5341. [Google Scholar] [CrossRef]
- Huang, X.; Schwind, S.; Yu, B.; Santhanam, R.; Wang, H.; Hoellerbauer, P.; Mims, A.; Klisovic, R.; Walker, A.R.; Chan, K.K.; et al. Targeted delivery of microRNA-29b by transferrin-conjugated anionic lipopolyplex nanoparticles: A novel therapeutic strategy in acute myeloid leukemia. Clin. Cancer Res. 2013, 19, 2355–2367. [Google Scholar] [CrossRef]
- Mahotka, C.; Bhatia, S.; Kollet, J.; Grinstein, E. Nucleolin promotes execution of the hematopoietic stem cell gene expression program. Leukemia 2018, 32, 1865–1868. [Google Scholar] [CrossRef]
- Deng, R.; Ji, B.; Yu, H.; Bao, W.; Yang, Z.; Yu, Y.; Cui, Y.; Du, Y.; Song, M.; Liu, S.; et al. Multifunctional Gold Nanoparticles Overcome MicroRNA Regulatory Network Mediated-Multidrug Resistant Leukemia. Sci. Rep. 2019, 9, 5348. [Google Scholar] [CrossRef]
- Degos, L.; Wang, Z.Y. All trans retinoic acid in acute promyelocytic leukemia. Oncogene 2001, 20, 7140–7145. [Google Scholar] [CrossRef] [PubMed]
- Garattini, E.; Terao, M. Atypical retinoids: An expanding series of anti-leukemia and anti-cancer agents endowed with selective apoptotic activity. J. Chemother. 2004, 16, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Garattini, E.; Parrella, E.; Diomede, L.; Gianni, M.; Kalac, Y.; Merlini, L.; Simoni, D.; Zanier, R.; Ferrara, F.F.; Chiarucci, I.; et al. ST1926, a novel and orally active retinoid-related molecule inducing apoptosis in myeloid leukemia cells: Modulation of intracellular calcium homeostasis. Blood 2004, 103, 194–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Houjeiri, L.; Saad, W.; Hayar, B.; Aouad, P.; Tawil, N.; Abdel-Samad, R.; Hleihel, R.; Hamie, M.; Mancinelli, A.; Pisano, C.; et al. Antitumor Effect of the Atypical Retinoid ST1926 in Acute Myeloid Leukemia and Nanoparticle Formulation Prolongs Lifespan and Reduces Tumor Burden of Xenograft Mice. Mol. Cancer Ther. 2017, 16, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Yan, J.; Ma, B.; Li, S.; Shao, Y.; He, P.; Zhang, W.; He, W.; Ma, P.X.; Lu, W. Lanthanide-doped nanoparticles conjugated with an anti-CD33 antibody and a p53-activating peptide for acute myeloid leukemia therapy. Biomaterials 2018, 167, 132–142. [Google Scholar] [CrossRef]
- Sun, S.; Zou, H.; Li, L.; Liu, Q.; Ding, N.; Zeng, L.; Li, H.; Mao, S. CD123/CD33 dual-antibody modified liposomes effectively target acute myeloid leukemia cells and reduce antigen-negative escape. Int. J. Pharm. 2019, 568, 118518. [Google Scholar] [CrossRef]
- Houshmand, M.; Blanco, T.M.; Circosta, P.; Yazdi, N.; Kazemi, A.; Saglio, G.; Zarif, M.N. Bone marrow microenvironment: The guardian of leukemia stem cells. World J. Stem. Cells 2019, 11, 476–490. [Google Scholar] [CrossRef]
- Laverdiere, I.; Boileau, M.; Neumann, A.L.; Frison, H.; Mitchell, A.; Ng, S.W.K.; Wang, J.C.Y.; Minden, M.D.; Eppert, K. Leukemic stem cell signatures identify novel therapeutics targeting acute myeloid leukemia. Blood Cancer J. 2018, 8, 52. [Google Scholar] [CrossRef]
- Ho, T.C.; LaMere, M.; Stevens, B.M.; Ashton, J.M.; Myers, J.R.; O’Dwyer, K.M.; Liesveld, J.L.; Mendler, J.H.; Guzman, M.; Morrissette, J.D.; et al. Evolution of acute myelogenous leukemia stem cell properties after treatment and progression. Blood 2016, 128, 1671–1678. [Google Scholar] [CrossRef]
- Bausch-Fluck, D.; Hofmann, A.; Bock, T.; Frei, A.P.; Cerciello, F.; Jacobs, A.; Moest, H.; Omasits, U.; Gundry, R.L.; Yoon, C.; et al. A mass spectrometric-derived cell surface protein atlas. PLoS ONE 2015, 10, e0121314. [Google Scholar] [CrossRef]
- Lin, T.Y.; Zhu, Y.; Li, Y.; Zhang, H.; Ma, A.H.; Long, Q.; Keck, J.; Lam, K.S.; Pan, C.X.; Jonas, B.A. Daunorubicin-containing CLL1-targeting nanomicelles have anti-leukemia stem cell activity in acute myeloid leukemia. Nanomedicine 2019, 20, 102004. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Sen, S.; Zhang, G.; Mu, C.; Albayati, Z.F.; Gorenstein, D.G.; Liu, X.; Ferrari, M.; Crooks, P.A.; Roboz, G.J.; et al. In vivo targeting of leukemia stem cells by directing parthenolide-loaded nanoparticles to the bone marrow niche. Leukemia 2016, 30, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Baranello, M.P.; Bauer, L.; Jordan, C.T.; Benoit, D.S.W. Micelle Delivery of Parthenolide to Acute Myeloid Leukemia Cells. Cell Mol. Bioeng. 2015, 8, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Simonetti, G.; Circosta, P.; Gaidano, V.; Cignetti, A.; Martinelli, G.; Saglio, G.; Gale, R.P. Chronic myeloid leukemia stem cells. Leukemia 2019, 33, 1543–1556. [Google Scholar] [CrossRef]
- Houshmand, M.; Circosta, P.; Saglio, G. Immature CML cells implement a BMP autocrine loop to escape TKI treatment. Transl. Cancer Res. 2018, 7, S722–S725. [Google Scholar] [CrossRef]
- Ross, D.M.; Masszi, T.; Gomez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; Garcia-Gutierrez, V.; Gattermann, N.; le Coutre, P.D.; Martino, B.; et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J. Cancer Res. Clin. Oncol. 2018, 144, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xing, J.; Xiong, F.; Yang, F.; Gu, N. Preparation and in vivo safety evaluations of antileukemic homoharringtonine-loaded PEGylated liposomes. Drug Dev. Ind. Pharm. 2017, 43, 652–660. [Google Scholar] [CrossRef]
- Yang, X.; Pang, J.; Shen, N.; Yan, F.; Wu, L.C.; Al-Kali, A.; Litzow, M.R.; Peng, Y.; Lee, R.J.; Liu, S. Liposomal bortezomib is active against chronic myeloid leukemia by disrupting the Sp1-BCR/ABL axis. Oncotarget 2016, 7, 36382–36394. [Google Scholar] [CrossRef]
- Jyotsana, N.; Sharma, A.; Chaturvedi, A.; Budida, R.; Scherr, M.; Kuchenbauer, F.; Lindner, R.; Noyan, F.; Suhs, K.W.; Stangel, M.; et al. Lipid nanoparticle-mediated siRNA delivery for safe targeting of human CML in vivo. Ann. Hematol. 2019, 98, 1905–1918. [Google Scholar] [CrossRef]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef]
- Paul, S.; Kantarjian, H.; Jabbour, E.J. Adult Acute Lymphoblastic Leukemia. Mayo. Clin. Proc. 2016, 91, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Uludag, H.; Brandwein, J.M. Advances in biology of acute lymphoblastic leukemia (ALL) and therapeutic implications. Am. J. Blood Res. 2018, 8, 29–56. [Google Scholar] [PubMed]
- Dudeja, S.; Gupta, S.; Sharma, S.; Jain, A.; Sharma, S.; Jain, P.; Aneja, S.; Chandra, J. Incidence of vincristine induced neurotoxicity in children with acute lymphoblastic leukemia and its correlation with nutritional deficiencies. Pediatr. Hematol. Oncol. 2019, 36, 344–351. [Google Scholar] [CrossRef]
- Mora, E.; Smith, E.M.; Donohoe, C.; Hertz, D.L. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am. J. Cancer Res. 2016, 6, 2416–2430. [Google Scholar]
- Davis, T.; Farag, S.S. Treating relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: Liposome-encapsulated vincristine. Int. J. Nanomed. 2013, 8, 3479–3488. [Google Scholar] [CrossRef][Green Version]
- Schiller, G.J.; Damon, L.E.; Coutre, S.E.; Hsu, P.; Bhat, G.; Douer, D. High-Dose Vincristine Sulfate Liposome Injection, for Advanced, Relapsed, or Refractory Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia in an Adolescent and Young Adult Subgroup of a Phase 2 Clinical Trial. J. Adolesc. Young Adult Oncol. 2018, 7, 546–552. [Google Scholar] [CrossRef]
- Liu, D.; Mamorska-Dyga, A. Syk inhibitors in clinical development for hematological malignancies. J. Hematol. Oncol. 2017, 10, 145. [Google Scholar] [CrossRef]
- Uckun, F.M.; Qazi, S. SYK as a New Therapeutic Target in B-Cell Precursor Acute Lymphoblastic Leukemia. J. Cancer Ther. 2014, 5, 124–131. [Google Scholar] [CrossRef]
- Uckun, F.M.; Myers, D.E.; Cheng, J.; Qazi, S. Liposomal Nanoparticles of a Spleen Tyrosine Kinase P-Site Inhibitor Amplify the Potency of Low Dose Total Body Irradiation Against Aggressive B-Precursor Leukemia and Yield Superior Survival Outcomes in Mice. EBioMedicine 2015, 2, 554–562. [Google Scholar] [CrossRef][Green Version]
- Wang, K.; Wei, G.; Liu, D. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wolff, M.J.; Unternaehrer, J.; Mellman, I.; Mamula, M.J. Targeting antigen to CD19 on B cells efficiently activates T cells. Int. Immunol. 2005, 17, 869–877. [Google Scholar] [CrossRef]
- Myers, D.E.; Yiv, S.; Qazi, S.; Ma, H.; Cely, I.; Shahidzadeh, A.; Arellano, M.; Finestone, E.; Gaynon, P.S.; Termuhlen, A.; et al. CD19-antigen specific nanoscale liposomal formulation of a SYK P-site inhibitor causes apoptotic destruction of human B-precursor leukemia cells. Integr. Biol. 2014, 6, 766–780. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Li, S.; Liao, C.; Guo, X. Targeting of the B-lineage leukemia stem cells and their progeny with norcantharidin encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8 in vitro. J. Drug Target. 2010, 18, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Thompson, P.; Ferrajoli, A.; Nabhan, C.; Mato, A.R.; O’Brien, S. Approaches to Chronic Lymphocytic Leukemia Therapy in the Era of New Agents: The Conundrum of Many Options. Am. Soc. Clin. Oncol. Educ. Book 2018, 10, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Strati, P.; Jain, N.; O’Brien, S. Chronic Lymphocytic Leukemia: Diagnosis and Treatment. Mayo Clin. Proc. 2018, 93, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.F.; Ribeiro, G.N.; Pinto Neto, J.V.; Campos, L.T.; Hamerschlak, N. BCL-2 as therapeutic target for hematological malignancies. J. Hematol. Oncol. 2018, 11, 65. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Koziner, B. Potential therapeutic applications of oblimersen in CLL. Oncology (Williston Park) 2004, 18, 32–38. [Google Scholar]
- Yu, B.; Mao, Y.; Bai, L.Y.; Herman, S.E.; Wang, X.; Ramanunni, A.; Jin, Y.; Mo, X.; Cheney, C.; Chan, K.K.; et al. Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood 2013, 121, 136–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiang, C.L.; Goswami, S.; Frissora, F.W.; Xie, Z.; Yan, P.S.; Bundschuh, R.; Walker, L.A.; Huang, X.; Mani, R.; Mo, X.M.; et al. ROR1-targeted delivery of miR-29b induces cell cycle arrest and therapeutic benefit in vivo in a CLL mouse model. Blood 2019, 134, 432–444. [Google Scholar] [CrossRef]
- Alfayez, M.; Kantarjian, H.; Kadia, T.; Ravandi-Kashani, F.; Daver, N. Emerging drug profile: CPX-351 (vyxeos) in AML. Leuk. Lymphoma 2019, 1–10. [Google Scholar] [CrossRef]
- Baron, J.; Wang, E.S. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559. [Google Scholar] [CrossRef]
- Eghtedar, A.; Verstovsek, S.; Estrov, Z.; Burger, J.; Cortes, J.; Bivins, C.; Faderl, S.; Ferrajoli, A.; Borthakur, G.; George, S.; et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood 2012, 119, 4614–4618. [Google Scholar] [CrossRef]
- Winer, E.S.; Stone, R.M. Novel therapy in Acute myeloid leukemia (AML): Moving toward targeted approaches. Ther. Adv. Hematol. 2019, 10, 2040620719860645. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Stella, S.; Tirro, E.; Romano, C.; Pennisi, M.S.; Puma, A.; Manzella, L.; Zanghi, A.; Stagno, F.; Di Raimondo, F.; et al. Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia. Mol. Cancer 2018, 17, 56. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Tallman, M.S.; de Botton, S.; Kantarjian, H.M.; Collins, R.; Stein, A.S.; Frattini, M.G.; Xu, Q.; Tosolini, A.; See, W.L.; et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia 2019, 33, 2575–2584. [Google Scholar] [CrossRef]
- Phuphanich, S.; Maria, B.; Braeckman, R.; Chamberlain, M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J. Neurooncol. 2007, 81, 201–208. [Google Scholar] [CrossRef]
- Jurczak, W.; Kroll-Balcerzak, R.; Giebel, S.; Machaczka, M.; Giza, A.; Ogorka, T.; Fornagiel, S.; Rybka, J.; Wrobel, T.; Kumiega, B.; et al. Liposomal cytarabine in the prophylaxis and treatment of CNS lymphoma: toxicity analysis in a retrospective case series study conducted at Polish Lymphoma Research Group Centers. Med. Oncol. 2015, 32, 90. [Google Scholar] [CrossRef]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- Levato, L.; Molica, S. Rituximab in the management of acute lymphoblastic leukemia. Expert Opin. Biol. Ther. 2018, 18, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Tobinai, K.; Klein, C.; Oya, N.; Fingerle-Rowson, G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies. Adv. Ther. 2017, 34, 324–356. [Google Scholar] [CrossRef] [PubMed]
- Shepard, R. Liposomal Annamycin—A New Generation Anthracycline That Overcomes MDR and Has No Cardiac Toxicity for the Second Line Treatment of R/R AML. Clin. Lymphoma Myeloma Leuk. 2018, 18, S197–S198. [Google Scholar] [CrossRef]
- Shah, N.N.; Merchant, M.S.; Cole, D.E.; Jayaprakash, N.; Bernstein, D.; Delbrook, C.; Richards, K.; Widemann, B.C.; Wayne, A.S. Vincristine Sulfate Liposomes Injection (VSLI, Marqibo(R)): Results From a Phase I Study in Children, Adolescents, and Young Adults With Refractory Solid Tumors or Leukemias. Pediatr. Blood Cancer 2016, 63, 997–1005. [Google Scholar] [CrossRef]
- Thomas, X.; Paubelle, E. Grb2 inhibition: a new potential targeted therapy for myeloid malignancies? Lancet Haematol. 2018, 5, e128–e129. [Google Scholar] [CrossRef]
- Ohanian, M.; Tari Ashizawa, A.; Garcia-Manero, G.; Pemmaraju, N.; Kadia, T.; Jabbour, E.; Ravandi, F.; Borthakur, G.; Andreeff, M.; Konopleva, M.; et al. Liposomal Grb2 antisense oligodeoxynucleotide (BP1001) in patients with refractory or relapsed haematological malignancies: A single-centre, open-label, dose-escalation, phase 1/1b trial. Lancet Haematol. 2018, 5, e136–e146. [Google Scholar] [CrossRef]
- Huang, H.-Q.; Huang, Y.; Yan, G.; Yang, H.; Zhang, Q.; Yang, R.; Zhou, M.; Li, Y.; Li, Y.; Liu, L.; et al. Safety and Efficacy of Mitoxantrone Hydrochloride Liposome in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma and Extranodal NK/T-Cell Lymphoma: A Multicenter, Single-Arm, Open-Label, Phase 2 Clinical Trial. Blood 2019, 134, 2838. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Y.; Li, C.; Gui, L.; Zhao, X.; Liu, P.; Han, X.; Song, Y.; Li, N.; Du, P.; et al. Phase I clinical trial of pegylated liposomal mitoxantrone plm60-s: Pharmacokinetics, toxicity and preliminary efficacy. Cancer Chemother. Pharmacol. 2014, 74, 637–646. [Google Scholar] [CrossRef]
- Floc’h, N.; Ashton, S.; Taylor, P.; Trueman, D.; Harris, E.; Odedra, R.; Maratea, K.; Derbyshire, N.; Caddy, J.; Jacobs, V.N.; et al. Optimizing Therapeutic Effect of Aurora B Inhibition in Acute Myeloid Leukemia with AZD2811 Nanoparticles. Mol. Cancer Ther. 2017, 16, 1031–1040. [Google Scholar] [CrossRef]
- Ashton, S.; Song, Y.H.; Nolan, J.; Cadogan, E.; Murray, J.; Odedra, R.; Foster, J.; Hall, P.A.; Low, S.; Taylor, P.; et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci. Transl. Med. 2016, 8, 325ra317. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Shin, E.; Wang, H.; Nolan, J.; Low, S.; Parsons, D.; Zale, S.; Ashton, S.; Ashford, M.; Ali, M.; et al. A novel in situ hydrophobic ion paring (HIP) formulation strategy for clinical product selection of a nanoparticle drug delivery system. J. Control. Release 2016, 229, 106–119. [Google Scholar] [CrossRef] [PubMed]



| Brand Name | Nanotechnology | Drug | Indications | Approval |
|---|---|---|---|---|
| Abraxane | Albumin-Nanoparticles | Paclitaxel | Breast cancer | 2005 |
| Non-small-cell lung carcinoma | 2012 | |||
| Pancreatic cancer | 2013 | |||
| DaunoXome® | Liposome | Daunorubicin citrate | Kaposi’s sarcoma | 1996 |
| DepoCyt® | Liposome | Cytarabine | Neoplastic meningitis | 1999 |
| Doxil®/CaelyxTM | PEGylated Liposome | Doxorubicin hydrochloride | Kaposi’s sarcoma | 1995 |
| Multiple myeloma | 2004 | |||
| ovarian cancer | 2005 | |||
| Eligard® | PLGA [poly(lactic-co-glycolic acid)] | Leuprolide acetate | Prostate cancer | 2002 |
| Marqibo® | Liposome | Vincristine sulfate | Acute lymphoblastic leukemia | 2012 |
| Mepact® | Liposomal | Mifamurtide | Osteosarcoma | 2009 |
| Myocet® | Liposome | Doxorubicin | Metastatic breast cancer | 2000 |
| Nanotherm® | Iron oxide NPs | n.a. | Glioblastoma | 2010 |
| Onivyde® | Liposome | Irinotecan | Pancreatic cancer | 2015 |
| NANOSYSTEM | ENCAPSULATED DRUG | SINGLE/ COMBINATION | DISEASE | PHASE | FIRST/ LAST POSTED | NOTES | REF | TRIAL ID1 |
|---|---|---|---|---|---|---|---|---|
| Liposome | Annamycin | Single | AML | Phase I/II | 2017/2019 | [Gil et al., 2019] | NCT03315039 | |
| Liposome | Cytarabine (L-ARA-C, Depocyt®) | Rituximab | Lymphoma | Phase II | 2013–2019 | [Jurczak et al., 2015] | NCT01859819 | |
| Liposome | Cytarabine (L-ARA-C, Depocyt®) | Obinutuzumab or Ifosfamide, Carboplatin, Etoposide (ICE) | Lymphoma | Phase II | 2015–2019 | [Jurczak et al., 2015] | NCT02393157 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Single | Refractory AML | Phase II | 2019 | [Mayer et al., Chen 2018 et al.] | NCT04049539 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Single | ALL, Refractory ALL, Recurrent ALL | Phase II | 2018/2019 | [Mayer et al., Chen 2018 et al.] | NCT03575325 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Enasidenib | Recurrent AML | Phase II | 2019 | [Mayer et al., Chen 2018 et al.] | NCT03825796 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos) | Gemtuzumab Ozogamicin | AML, CML, Recurrent AML, Refractory AML | Phase II | 2018/2019 | [Mayer et al., Chen 2018 et al., Baron et al.] | NCT03672539 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Palbociclib | AML | Phase I/II | 2019 | [Mayer et al., Chen 2018 et al., Winer et al., 2019] | NCT03844997 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Ruxolitinib | AML, ALL | Phase I/II | 2019 | [Mayer et al., Chen 2018 et al., Eghtedar et al., 2012] | NCT03878199 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Venetoclax | AML | Phase Ib | 2019 | CPX-351 Lower Intensity Therapy (LIT) | [Mayer et al., Chen 2018 et al., Massimino et al., 2018] | NCT04038437 |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Venetoclax | AML | Phase II | 2018/2019 | [Mayer et al., Chen 2018 et al., Massimino et al., 2018]] | NCT03629171 | |
| Liposome | Daunorubicin-Cytarabine (CPX-351, Vyxeos®) | Venetoclax or Midostaurin or Enasidenib | AML | Phase I | 2019 | [Mayer et al., Chen 2018 et al., Massimino et al., 2018]] | NCT04075747 | |
| Liposome | Grb2 Antisense Oligonucleotide (BP1001) | Dasatinib | AML, CML | Phase I/II | 2016–2019 | [Thomas et al., 2018; Ohanian et al., 2018] | NCT02923986 | |
| Liposome | Mitoxantrone Hydrochloride | Single | Peripheral T-cell and NK/T-cell Lymphoma | Phase II | 2018 | [Huang et al. 2019] | NCT03776279 | |
| Liposome | Vincristine | Venetoclax | ALL | Phase I/II | 2018–2019 | [Pathak et al., 2014] | NCT03504644 | |
| Liposome | Vincristine sulfate (Marquibo®) | Single | AML | Phase II | 2015/2019 | The study was stopped early due to futility | [Shah et al., 2016] | NCT02337478 |
| Liposome | Vincristine sulfate (Marquibo®) | Bortezomib, Clofarabine, Cyclophosphamide, Dexamethasone, Etoposide, Ofatumumab, Pegfilgrastim, Rituximab | ALL, Burkitt Leukemia, Burkitt Lymphoma | Phase II | 2017–2019 | [Shah et al., 2016] | NCT03136146 | |
| Liposome | Vincristine sulfate (Marquibo®) | Dexamethasone, Mitoxantrone and Asparaginase (UK ALL R3) | ALL | Phase I | 2016–2019 | [Shah et al., 2016] | NCT02879643 | |
| Liposome | Vincristine sulfate (Marquibo®) | Inotuzumab Ozogamicin | ALL | Phase I/II | 2019 | [Shah et al., 2016, Al-Salama ZT 2018] | NCT03851081 | |
| Liposome | Vincristine sulfate (Marquibo®) | Rituximab Bendamustine | Indolent B cell Lymphoma | Phase I | 2014–2019 | [Shah et al., 2016] | NCT02257242 | |
| Nanoparticle | AZD2811 (Barasertib) | Azacitidine | AML | Phase I/II | 2017–2019 | [Floc’h et al., 2017] | NCT03217838 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houshmand, M.; Garello, F.; Circosta, P.; Stefania, R.; Aime, S.; Saglio, G.; Giachino, C. Nanocarriers as Magic Bullets in the Treatment of Leukemia. Nanomaterials 2020, 10, 276. https://doi.org/10.3390/nano10020276
Houshmand M, Garello F, Circosta P, Stefania R, Aime S, Saglio G, Giachino C. Nanocarriers as Magic Bullets in the Treatment of Leukemia. Nanomaterials. 2020; 10(2):276. https://doi.org/10.3390/nano10020276
Chicago/Turabian StyleHoushmand, Mohammad, Francesca Garello, Paola Circosta, Rachele Stefania, Silvio Aime, Giuseppe Saglio, and Claudia Giachino. 2020. "Nanocarriers as Magic Bullets in the Treatment of Leukemia" Nanomaterials 10, no. 2: 276. https://doi.org/10.3390/nano10020276
APA StyleHoushmand, M., Garello, F., Circosta, P., Stefania, R., Aime, S., Saglio, G., & Giachino, C. (2020). Nanocarriers as Magic Bullets in the Treatment of Leukemia. Nanomaterials, 10(2), 276. https://doi.org/10.3390/nano10020276






